Abstract
Background/Aim:
The excessive apoptosis of intestinal epithelial cells (IECs) partly accounts for the development of colonic inflammation and eventually results in ulcerative colitis (UC). Humanin, an endogenous anti-apoptotic peptide, has previously been shown to protect against Alzheimer's disease and a variety of cellular insults. The present study aimed to investigate the effects of glysin variant of humanin (HNG) on 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis in rats.
Materials and Methods:
Rats were divided into four groups as follows: Group 1 (n = 8): control; isotonic saline solution 0.1 ml/rat rectally, Group 2 (n = 8): TNBS colitis; 0.1 ml of a 2.5% (w/v) TNBS solution in 50% ethanol rectally, Group 3 (n = 8): 10 μM HNG, and Group 4 (n = 8): 20 μM HNG intraperitoneal (ip) on day 2 and 6 after rectal TNBS administration. Rats were sacrificed 7 days after the induction of colitis. Blood and tissue samples were harvested for biochemical and histopathological analysis.
Results:
HNG treatment significantly ameliorated weight loss and macroscopic and microscopic scores. TNBS-induced colitis significantly increased the colonic mRNA expression of tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), and caspase-3 activities in group II in comparison to the group I. HNG treatment was associated with an inhibition of mRNA expression of TNF-α and IL-1β, and a decrease in caspase-3 activities in colon tissues in group III and IV when compared to group II.
Conclusion:
The results of this study indicate that HNG treatment may exert beneficial effects in UC by decreasing inflammatory reactions and apoptosis.
Key Words: Apoptosis, humanin, ulcerative colitis
Ulcerative colitis (UC) is a chronic, inflammatory disease of the colonic mucosa characterized by a relapsing-remitting course. The pathogenesis of UC is not fully understood at present, however, increasing evidence has shown that the acceleration of epithelial cell apoptosis and inhibition of inflammatory cell apoptosis are associated closely with colonic tissue injury and immunological abnormality in UC.[1,2,3,4] Therefore, apoptosis was reported to play an important role in the loss of epithelial cells in UC.[1,2,3,4]
Humanin (HN) is a polypeptide containing 24 amino acids, originally isolated in the cDNA associated with neuroprotective effects in Alzheimer's disease (AD) patients, and recognized for its anti-apoptotic properties.[5] HN also plays an important role in protecting lymphocytes,[6] pancreatic β-cells,[7] testicular germ cells, and Leydig cells[7,8,9] from apoptosis. Recently, studies used an ox-LDL-induced human aortic endothelial cell apoptosis model and demonstrated that HN expressed in the human vascular endothelial layer[10] reduced intracellular reactive oxgen species and the rate of apoptosis by 50%.[11] Replacement of serine at position 14 of the HN peptide chain (Ser14) with glycine (Gly), leads to the formation of HNG, which has anti-apoptic activity enhanced by 1000-fold when compared with that of HN.[5] The aim of this study was to evaluate the effect of HNG on structural mucosal changes following 2,46-trinitrobenzene sulphonic acid (TNBS)-induced colitis in a rat model and to evaluate the mechanisms by which HNG influences intestinal recovery, including its effect on colonic epithelial cells apoptosis.
MATERIALS AND METHODS
Ethical considerations
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH publication No. 85-23, revised 1996). The protocol was approved by the Committee of Animal Care and Use of School of Medicine, Bulent Ecevit University. All efforts were made to minimize animal suffering.
Animals
Adult male Wistar rats weighing 200 ± 10 g were obtained from Bulent Ecevit University School of Medicine, Experimental Research Laboratory. The rats were maintained in a room at a temperature of 23 ± 2°C under a 12-h light/dark cycle at Bulent Ecevit University School of Medicine, Experimental Animal Laboratory. Before and during the study, they were fed a standard diet, and their weights were monitored daily. The animals were also allowed water ad libitum.
Chemicals
HNG and TNBS were obtained from Sigma Chemical Co. (St. Louis, MD, USA). All Other chemicals were of highest purity and analytical grade. Enzyme-linked immunosorbent assay (ELISA) kits for determination of tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β) were purchased from BioSource International, (Camarillo, CA, USA). Caspase-3 was provided from Lab Vision (Fremont, CA, USA)
Experimental design and treatment protocol
In the current study, 32 rats were randomly divided into four groups (n = 8 per group). Group I (Control group) received physiological saline rectally. Group II (TNBS colitis group) received 30 mg/0.1 ml TNBS and 0.5 ml 50% ethanol rectally. Group III (TNBS + 10 μM HNG) received TNBS rectally + 10 μM HNG intraperitoneally (ip). Group IV (TNBS + 20 μM HNG) received TNBS rectally + 20 μM HNG ip. The selected dose of HNG was based on its previously displayed anti-apoptic actions in an experimental model of lipopolysaccharide induced astrocyte inflammation.[12] The first dose of HNG was given on day 2 and the second dose of HNG was given 6 days after the induction of TNBS colitis. The animals were euthanized using an overdose of anesthesia on the 7th day post TNBS-instillation.
Induction of colitis
TNBS colitis was induced according to the procedures described by Hollenbach et al.[13] Rats were fasted for 24 h and then they were anesthetized through ip injection of sodiumpentobarbital (0.3% solution). A 3.5 F catheter was inserted through the anus into the colon carefully, and the tip was 4 cm proximal to the anal verge. To induce colitis, 0.1 ml of a 2.5% (w/v) TNBS solution in 50% ethanol was injected slowly into the lumen of the colon via a catheter fitted to a 1-ml syringe. In control groups, rats received 0.1 ml of saline only. To ensure the distribution of TNBS throughout the entire colon, including the cecum and appendix, rats were kept vertical for 1 min and then returned to their cages.
Tissue collection and preparation
On the 7th day post TNBS-instillation, rats were anesthetized by intramuscular administration of 80 mg/kg of ketamine hydrochloride (Ketalar, Eczacibasi) and 8 mg/kg of xylazin (Rompun, Bayer). The distal 8 cm portion of the colon was excised, freed of adherent adipose tissue, longitudinally split, and washed with ice-cold saline to remove fecal residues. Then, it was blotted dry, weighed, and macroscopic assessment of colitis was performed. Sections of the distal colon were utilized for histopathological, immunohistochemical, and biochemical investigations.
Assessment of TNBS-induced colitis
Individual body weights of animals were recorded at the beginning of the experiments and on the termination day, and the difference was calculated as the weight loss (%). The severity of colitis was evaluated by an independent observer blind to the identity of treatments according to the criteria of Millar et al.[14] as follows: 0 = No macroscopic changes; 1 = Mucosal erythema only; 2 = Mild mucosal edema, slight bleeding or small erosions; 3 = Moderate edema, bleeding ulcers or erosions; and 4 = Severe ulceration, erosions, edema, and tissue necrosis.
Histopathological examination and microscopic scoring
Regarding the morphologic evaluation of the intestinal specimens, each sample was fixed with 10% formaldehyde immediately after collection freshly. The specimens were washed, dehydrated by alcohol, cleared in xylene, and embedded in paraffin. Sections of 3 mm thickness were cut and stained with hematoxylin and eosin (H and E) and examined under the light microscope (Leica Microsystems, Germany). All histopathologic processing and assessment of specimens were performed by an experienced observer blinded to the identity of the sample being examined to avoid any bias. The colon microscopic damage was scored on a 0–5 scale as described by Galvez et al.[15] as follows: 0 = normal colonic tissue; 1 = inflammation or focal ulceration limited to the mucosa; 2 = focal or extensive ulceration and inflammation limited to the mucosa and the submucosa; 3 = focal or extensive ulceration and inflammation with involvement of muscularis; 4 = focal or extensive ulceration and inflammation with involvement of the serosa; and 5 = extensive ulceration and transmural inflammation with involvement of the serosa.
Determination of cytokine concentrations in the colonic tissue
Total cellular RNA was isolated using the GeneJET RNA Purification Kit (Thermo Scientific) according to manufacturer's instructions. The concentration of the isolated RNA was measured using the Qubit RNA BR assay (Invitrogen) for the Qubit 2.0 fluorometer. First strand cDNA was generated with the RevertAid First Strand cDNA Synthesis Kit (Thermo scientific) with 200 ng of the RNA templates. CDNA was stored at −20°C for later use. Quantitative real time reverse transcriptase-polymerase chain reaction (qRT-PCR) was performed with Applied Biosystems 7500 Real-Time PCR system and TaqMan gene expression products (Applied Biosystems) for IL-1β (assay ID: Rn00580432_m1), and TNF-α (assay ID: Rn01525859_g1) and, as control, ACTB (Rn00667869_m1). All reactions were conducted in triplicate, and each included a nontemplate control. Duplicate CT values were analyzed with the comparative CT (ΔΔCT) method (Applied Biosystems).
Determination of caspase-3 activity
Immunohistochemistry for caspase-3 (rabbit polyclonal antibody, CPP 32, Ab-4, RB-1197-R7, ready to-use for immunohistology, Lab Vision, Fremont, CA, USA) was performed using a combination streptavidin–biotin–peroxidase method and microwave antigen retrieval on formalin-fixed paraffin-embedded tissues. After deparaffinization, sections were treated with 10% hydrogen peroxidase in filtered water to block endogenous peroxidase activity. To retrieve the antigen, slides were boiled with 10 mmol/l citrate buffer (pH 7) for 10 min. After preincubation with Ultra V block (Lab Vision) for 20 min, sections were incubated with the primary antibody for 1 hour at room temperature, followed sequentially by biotinylated goat antipolyvalen (Lab Vision) for 20 min and streptavidin peroxidase complex (Lab Vision) for 20 min. We used 3,3'-diaminobenzidine tetrahydrochloride/DAB (Lab Vision) as the chromagen, and hematoxylin for nuclear counterstain, and then rinsed and mounted. Omission of the primary antibody created the negative control, and tonsil was used as the positive control. The slides were evaluated in a blinded manner by the same investigator. Immunoreactivity was scored using a semiquantitative scale for intensity of staining as follows: 0 (negative, no staining), 1+ (weakly positive), 2+ (moderately positive), and 3+ (strongly positive).
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM) for 8 rats per experimental group. Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS, version 10.0, Chicago, IL, USA) statistical software. One-way analysis of variance (ANOVA) was used to compare the mean values of quantitative variables among the groups. Duncan's multiple range test was used to identify the significance of pairwise comparisons of mean values among the groups.
RESULTS
Effects of HNG on body weight
As shown in Figure 1a, compared to the control group, the body weight of TNBS-treated rats all decreased markedly with loose and bloody stools on 7 day post-TNBS (P < 0.01). The marked weight loss was observed in group II (7.82%). The change of the body weight in groups III and IV were 4.89% and 4.18%, and HNG treatment resulted in less weight loss in groups III and IV compared in group II (P < 0.001). Moreover, there were no significant differences in the percentage of the body weight loss of animals between the groups IV and I.
Figure 1.
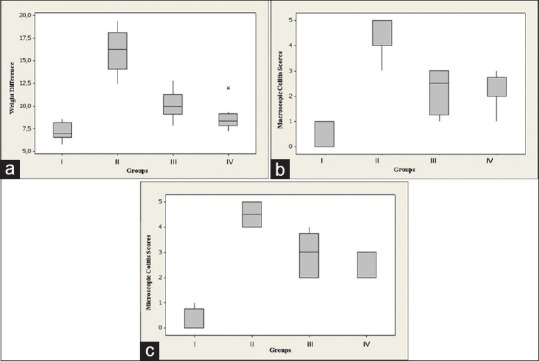
Effects of HNG on (a) body weight, (b) macroscopic, and (c) microscopic colitis scores. (Group I: Control; Group II: TNBS colitis; Group III: TNBS + 10 μM HNG; Group IV: TNBS + 20 μM HNG)
Effects of HNG on macroscopic colitis score
Group I rats showed no macroscopic lesions in the distal colon. The colonic mucosal damage, such as edema, deep ulcerations, and hemorrhage, was easily seen on macroscopic examination in group II rats, which was significantly improved after HNG treatment in groups III and IV as compared with group II. The severity of the lesions in the distal colon was quantified using a macroscopic damage score. In the TNBS-administered rats, macroscopic score was found to be increased significantly compared to group I. The mean macroscopic pathological scores of the Group III and IV were similar; 2.25 (±0.88) and 2.13 (±0.64), whereas the macroscopic pathological score in group II was 4.25 (±0.7) (P = 0.001 and P < 0.001 respectively) [Figure 1b].
Effects of HNG on microscopic colitis score
Histological examination of the colon from group I showed typical features of a normal colon structure [Figure 2a]. TNBS administration in group II caused transmural necrosis, edema, and diffuse infiltration of inflammatory cells (polymorphonuclear leukocytes, lymphocytes, and eosinophils) into the mucosa [Figure 2b]. Although treatment of rats with 10 μM HNG attenuated the extent and severity of the histological signs of the inflammatory response, ulceration of the colonic mucosa and infiltration of inflammatory cells into the muscularis propria was observed in group III [Figure 2c]. The histological evaluation of colons from rats treated with 20 μM HNG revealed a pronounced reduction in the inflammatory response with focal ulceration of the colonic mucosa and inflammation limited to mucosa and submucosa [Figure 2d]. The severity of colonic inflammation in the distal colon was also quantified using a microscopic damage score (range 0–5, as indicated the in Materials and Methods). The mean microscopic scores in the groups I, II, III (10 μM HNG), and IV (20 μM HNG) were 0.25 ± 0.46, 4.5 ± 0.53, 2.88 ± 0.83, and 2.38 ± 0.51, respectively, and in all the TNBS administrated rats microscopic scores were found to be significantly higher when compared to control group [Figure 1c]. It was also observed that treatment with 10 μM (Group I) and 20 μM HNG (Group II) significantly decreased the pathological scores compared to group II (P = 0.002 and P < 0.001 respectively). However, between the groups III and IV microscopic scores were not found statistically different (P = 0.279).
Figure 2.
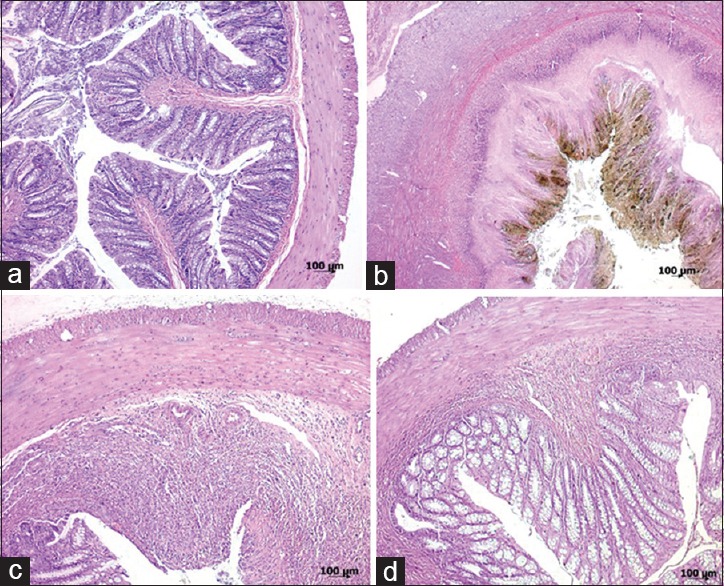
Histopathologic appearances of colon specimens: (a) Group I (control); normal colonic tissue (grade 0); (b) Group II (TNBS colitis); extensive ulceration and transmural inflammation involving the serosa (grade 5); (c) Group III (TNBS + 10 μM HNG); focal ulceration and inflammation involving the muscularis propria (grade 3); (d) Group IV (TNBS + 20 μM HNG); focal ulceration with inflammation limited to the submucosa (grade 2) (H and E; a, ×50; b, ×25; c, d, ×50)
Effects of HNG on colonic TNF-α and IL-1β
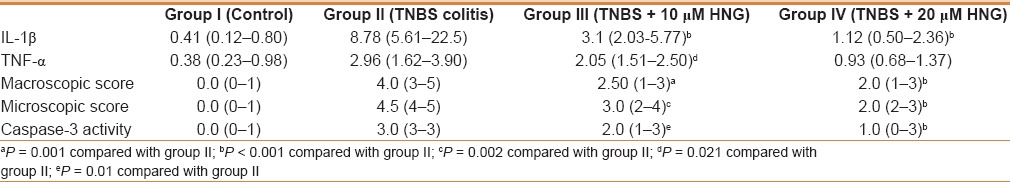
For inflammatory parameters, the mRNA expression levels of TNF-α and IL-1β in colon were measured by qRT-PCR. As shown in Table 1, rats treated with TNBS alone in the group II showed elevated TNF-α and IL-1β mRNA expressions – 2.96 (1.62–3.90) and 8.78 (5.61–22.55). This increase in colonic TNF-α and IL-1β mRNA expression levels was significantly attenuated by treatment with either 10 μM or 20 μM HNG compared to group II, as seen in Table 1. This tendency was most obvious in rats treated with 20 μM HNG in group IV compared to group III. These results proved that the administration of HNG had a direct inhibitory effect on the inflammatory status during the development of colitis in a dose dependent manner.
Table 1.
The median (minimum-maximum) levels of IL-1Beta (IL-1β) and TNF-alpha (TNF-α), microscopic and macroscopic colitis scores, and caspase-3 activities of all groups
Effects of HNG on caspase-3 activity
The activity of caspase-3 was assessed as a marker for colonic apoptosis following the instillation of TNBS. The colon of the control group (Group I) appeared almost normal in microscopic observations except for a slight immunoreaction of the surface columnar epithelium [Figure 3a]. In colon sections from rats receiving TNBS alone (Group II) marked and diffused caspase-3 staining was observed in mucosa [Figure 3b]. Milder immunoreaction and caspase-3 staining were detected in rats treated with 10 μM HNG in the group III [Figure 3c]. The number of positively stained cells markedly decreased in group IV [Figure 3d]. Immunoreactivity of caspase-3 was also scored. The caspase-3 activity in colonic tissue was significantly higher in the groups II, III, and IV than in the group I. Treatment with HNG significantly decreased the caspase-3 activity in colonic tissues from the groups III and IV compared to the group II (P = 0.01 and P < 0.001). However, there was no significant difference observed between the groups III and IV (P = 0.279) [Table 1].
Figure 3.
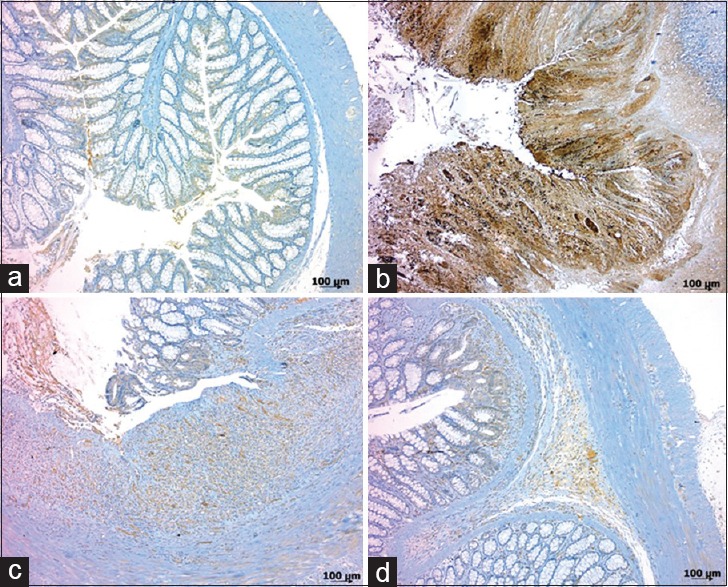
Immunohistochemical staining with caspase-3 of colon tissues: (a) Group I (control); low intensity caspase-3 expression, only on the apical of colonic surface epithelial (grade 1). (b) Group II (TNBS colitis); strong cytoplasmic immunoreactivity (grade 3). (c) Group III (TNBS + 10 μM HNG); moderate cytoplasmic immunoreactivity (grade 2). (d) Group IV (TNBS + 20 μM HNG); weak immunoreactivity (grade 1) (B-SA, 3,3'-diaminobenzidine tetrahydrochloride; a-d, ×50)
DISCUSSION
The causes of UC are still unclear but it is clear that the exaggerated intestinal epithelial apoptosis leads to villus atrophy and epithelial destruction, which plays a central role in the pathogenesis of the disease.[16] Increased rates of intestinal epithelial damage are well known to be commonly associated with increased mucosal cytokine production such as TNF-α and IL-1β. The present study demonstrated that HNG reduced the amount of TNF-α and IL-1β and apoptosis verified by decreased capsase-3 activity in the colonic tissue.
Since it was isolated from a protected lobe of a brain from an Alzheimer's disease patient, HN has been identified in a wide range of tissues including testis, colon, hypothalamus, heart, liver, skeletal muscle, kidney, and vascular wall.[5,17,18,19,20,21] Multiple studies have demonstrated that HN was cytoprotective against injury-induced apoptosis in many tissues including neuronal tissue,[5,22,23,24,25,26,27,28] blood-derived cells,[29] heart and blood vessels,[18,30,31] pancreatic beta cells,[7,32] and testis.[9,19,33] A very recent study provides new evidence for the “anti-inflammatory” effect of HN. A role of HN in the downregulation of inflammatory responses has been demonstrated in vivo and in cell culture systems. Miao et al. first observed that HNG ameliorates Ab25–35- induced neuroinflammatory responses by decreasing the level of IL-6 and TNF-α in mice.[34] Zhang et al. discovered that HN attenuates inflammation by downregulating intrarenal inflammatory markers of monocyte chemoattractant protein-1, TNF-α, and osteopontin and reduces macrophage infiltration in hypercholesterolemic Apo-E deficient mice; thereby decreasing the renal microvascular remodeling, inflammation, and apoptosis in the early stage of kidney disease.[17] Finally, Zhao et al. reported that treatment of HNG partially suppresses the secretion of pro-inflammatory cytokines including IL-6, IL-1β, and TNF-α in a dose-dependent manner in astrocytes induced by lipopolysaccharides (LPS).[12] The pro-inflammatory cytokines IL-1β, IL-6, and TNF-α have been demonstrated to be the regulators of colonic inflammation in UC.[35,36] In this study, we observed that the expression of TNF-α and IL-1β in colonic tissues was significantly higher in TNBS-treated rats compared to the control group, while HNG treatment efficiently reduced the overexpression of these pro-inflammatory cytokines, which was consistent with the alleviation of the inflammatory injuries in the colon tissues of HNG treated rats. In our study, the effects of HNG on mucosal healing and pathological macroscopic and microscopic scores yielded quite remarkable results. The macroscopic and microscopic pathological scores of intestinal tissue from the HNG groups were higher than those of the control group but significantly lower than those of the TNBS group.
During UC, intestinal epithelial cell (IECs) apoptosis is observed in active inflammatory sites.[37,38] The present study supports the significant role of TNF-α in UC by inducing IECs apoptosis. Using a rat model of TNBS-induced colitis that mimics UC, we observed that TNF-α expression in colonic tissues was significantly increased, accompanied by a concomitant increasing of caspase-3 enzyme activity, which are key markers of cell apoptosis. Accumulating evidence has shown that decreased levels of TNF-α in the intestinal tissue and serum reduce IECs apoptosis in UC.[39] In line with this, in this study, we found that treatment with HNG significantly decreased caspase-3 activities compared to that in TNBS-treated rats. Therefore, we speculated that HNG prevented apoptosis caused by TNF-α in intestinal epithelial cells in UC. Our results are consistent with the study of Gottardo et al. showing inhibition of TNF-α induced apoptosis by HN in anterior pituitary cells gonadectomized female and male rats.[40]
CONCLUSION
In conclusion, our results indicate that TNF-α, IL-1β and apoptotic cell death play a very important role in the pathology of colitis. The administration of HNG appears to have beneficial effects on TNBS-induced colitis as indicated by decreased expression of TNF-α and IL-1β in colonic tissues and caspase-3 activity. The cytoprotective effects of HNG seem to be associated with anti-apoptotic and possibly anti-inflammatory effects. However, the precise mechanisms responsible for these anti-apoptotic and anti-inflammatory effects of HNG remain unknown. Therefore, further studies are required to clarify the exact mechanisms mediating the curative effect of HNG in colitis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wu HG, Gong X, Yao LQ, Zhang W, Shi Y, Liu HR, et al. Mechanisms of acapuncture and moxibustion in regulation of epithelial cell apoptosis in rat ulcerative colitis. World J Gastroenterol. 2004;10:682–8. doi: 10.3748/wjg.v10.i5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzon E, Esposito E, Crisafulli C, Riccardi L, Muià C, Di Bella P, et al. Melatonin modulates signal transduction pathways and apoptosis in experimental colitis. J Pineal Res. 2006;41:363–73. doi: 10.1111/j.1600-079X.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 3.Verstege MI, TeVelde AA, Hommes DW. Apoptosis as a therapeutic paradigm in inflammatory bowel diseases. Acta Gastroenterol Belg. 2006;69:406–12. [PubMed] [Google Scholar]
- 4.Karamanolis DG, Kyrlagkitsis I, Konstantinou K, Papatheodoridis GV, Karameris A, Mallas E, et al. The Bcl-2/Bax system and apoptosis in ulcerative colitis. Hepatogastroenterology. 2007;54:1085–8. [PubMed] [Google Scholar]
- 5.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci USA. 2001;98:6336–41. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kariya S, Takahashi N, Hirano M, Ueno S. Humanin improves impaired metabolic activity and prolongs survival of serum-deprived human lymphocytes. Mol Cell Biochem. 2003;254:83–8. doi: 10.1023/a:1027372519726. [DOI] [PubMed] [Google Scholar]
- 7.Hoang PT, Park P, Cobb LJ, Paharkova-Vatchkova V, Hakimi M, Cohen P, et al. The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism. 2010;59:343–9. doi: 10.1016/j.metabol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colon E, Strand ML, Carlsson-Skwirut C, Wahlgren A, Svechnikov KV, Cohen P, et al. Anti-apoptotic factor humanin is expressed in the testis and prevents celldeath in leydig cells during the first wave of spermatogenesis. J Cell Physiol. 2006;208:373–85. doi: 10.1002/jcp.20672. [DOI] [PubMed] [Google Scholar]
- 9.Lue Y, Swerdloff R, Liu Q, Mehta H, Hikim AS, Lee KW, et al. Opposing roles of insulin-like growth factor binding protein 3 and humanin in the regulation of testicular germ cell apoptosis. Endocrinology. 2010;151:350–7. doi: 10.1210/en.2009-0577. [DOI] [PubMed] [Google Scholar]
- 10.Oh YK, Bachar AR, Zacharias DG, Kim SG, Wan J, Cobb LJ, et al. Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice. Atherosclerosis. 2011;219:65–73. doi: 10.1016/j.atherosclerosis.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, et al. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDLinduced oxidative stress. Cardiovasc Res. 2010;88:360–6. doi: 10.1093/cvr/cvq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao ST, Zhao L, Li JH. Neuroprotective Peptide humanin inhibits inflammatory response in astrocytes induced by lipopolysaccharide. Neurochem Res. 2013;38:581–8. doi: 10.1007/s11064-012-0951-6. [DOI] [PubMed] [Google Scholar]
- 13.Hollenbach E, Vieth M, Roessner A, Neumann M, Malfertheiner P, Naumann M. Inhibition of RICK/nuclear factor-kappaB and p38 signaling attenuates the inflammatory response in a murine model of Crohn disease. J Biol Chem. 2005;280:14981–8. doi: 10.1074/jbc.M500966200. [DOI] [PubMed] [Google Scholar]
- 14.Millar AD, Rampton DS, Chander CL, Claxson AW, Blades S, Coumbe A, et al. Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut. 1996;39:407–15. doi: 10.1136/gut.39.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galvez J, Coelho G, Crespo ME, Cruz T, Rodríguez-Cabezas ME, Concha A, et al. Intestinal anti-inflammatory activity of morin on chronic experimental colitis in the rat. Aliment Pharmacol Ther. 2001;15:2027–39. doi: 10.1046/j.1365-2036.2001.01133.x. [DOI] [PubMed] [Google Scholar]
- 16.Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis. 2006;12:413–24. doi: 10.1097/01.MIB.0000217334.30689.3e. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Urbieta-Caceres VH, Eirin A, Bell CC, Crane JA, Tang H, et al. Humanin prevents intra-renal microvascular remodeling and inflammation in hypercholesterolemic ApoE deficient mice. Life Sci. 2012;91:199–206. doi: 10.1016/j.lfs.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muzumdar RH, Huffman DM, Calvert JW, Jha S, Weinberg Y, Cui L, et al. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler Thromb Vasc Biol. 2010;30:1940–8. doi: 10.1161/ATVBAHA.110.205997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moretti E, Giannerini V, Rossini L, Matsuoka M, Trabalzini L, Collodel G. Immunolocalization of humaninin human sperm and testis. Fertil Steril. 2010;94:2888–90. doi: 10.1016/j.fertnstert.2010.04.075. [DOI] [PubMed] [Google Scholar]
- 20.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–42. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colon E, Strand ML, Carlsson-Skwirut C, Wahlgren A, Svechnikov KV, Cohen P, et al. Anti-apoptotic factor humanin is expressed in the testis and prevents cell-death in Leydig cells during the first wave of spermatogenesis. J Cell Physiol. 2006;208:373–85. doi: 10.1002/jcp.20672. [DOI] [PubMed] [Google Scholar]
- 22.Sponne I, Fifre A, Koziel V, Kriem B, Oster T, Pillot T. Humanin rescues cortical neurons from prion-peptide-induced apoptosis. Mol Cell Neurosci. 2004;25:95–102. doi: 10.1016/j.mcn.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Kariya S, Takahashi N, Ooba N, Kawahara M, Nakayama H, Ueno S. Humanin inhibits cell death of serum-deprived PC12h cells. Neuroreport. 2002;13:903–7. doi: 10.1097/00001756-200205070-00034. [DOI] [PubMed] [Google Scholar]
- 24.Kariya S, Hirano M, Nagai Y, Furiya Y, Fujikake N, Toda T, et al. Humanin attenuates apoptosis induced by DRPLA proteins with expanded polyglutamine stretches. J Mol Neurosci. 2005;25:165–9. doi: 10.1385/JMN:25:2:165. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Chua CC, Gao J, Hamdy RC, Chua BH. Humanin is a novel neuroprotective agent against stroke. Stroke. 2006;37:2613–9. doi: 10.1161/01.STR.0000242772.94277.1f. [DOI] [PubMed] [Google Scholar]
- 26.Nishimoto I, Matsuoka M, Niikura T. Unravelling the role of Humanin. Trends Mol Med. 2004;10:102–5. doi: 10.1016/j.molmed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Matsuoka M, Hashimoto Y, Aiso S, Nishimoto I. Humanin and colivelin: Neuronal-death-suppressing peptides for Alzheimer's disease and amyotrophic lateral sclerosis. CNS Drug Rev. 2006;12:113–22. doi: 10.1111/j.1527-3458.2006.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Chua KW, Chua CC, Liu CF, Hamdy RC, Chua BH. Synergistic protective effects of humanin and necrostatin-1 on hypoxia and ischemia/reperfusion injury. Brain Res. 2010;1355:189–94. doi: 10.1016/j.brainres.2010.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D, Li H, Yuan H, Zheng M, Bai C, Chen L, et al. Humanin delays apoptosis in K562 cells by downregulation of P38 MAP kinase. Apoptosis. 2005;10:963–71. doi: 10.1007/s10495-005-1191-x. [DOI] [PubMed] [Google Scholar]
- 30.Jung SS, Van Nostrand WE. Humanin rescues human cerebrovascular smooth muscle cells from Abeta-induced toxicity. J Neurochem. 2003;84:266–72. doi: 10.1046/j.1471-4159.2003.01524.x. [DOI] [PubMed] [Google Scholar]
- 31.Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, et al. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res. 2010;88:360–6. doi: 10.1093/cvr/cvq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, et al. Humanin: A novel central regulator of peripheral insulin action. PLoS One. 2009;4:e6334. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia Y, Lue YH, Swerdloff R, Lee KW, Cobb LJ, Cohen P, et al. The cytoprotective peptide humanin is induced and neutralizes Bax after pro-apoptotic stress in the rat testis. Andrology. 2013;1:651–9. doi: 10.1111/j.2047-2927.2013.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miao J, Zhang W, Yin R, Liu R, Su C, Lei G, et al. S14G-Humanin ameliorates Abeta25-35-induced behavioral deficits by reducing neuroinflammatory responses and apoptosis in mice. Neuropeptides. 2008;42:557–67. doi: 10.1016/j.npep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Neurath MF. IL-23: A master regulator in Crohn disease. Nat Med. 2007;13:26–8. doi: 10.1038/nm0107-26. [DOI] [PubMed] [Google Scholar]
- 36.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–70. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 37.Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis. 2006;12:413–24. doi: 10.1097/01.MIB.0000217334.30689.3e. [DOI] [PubMed] [Google Scholar]
- 38.Hagiwara C, Tanaka M, Kudo H. Increase in colorectal epithelial apoptotic cells in patients with ulcerative colitis ultimately requiring surgery. J Gastroenterol Hepatol. 2002;7:758–64. doi: 10.1046/j.1440-1746.2002.02791.x. [DOI] [PubMed] [Google Scholar]
- 39.Tang R, Yang G, Zhang S, Wu C, Chen M. Opposite effects of interferon regulatory factor 1 and osteopontin on the apoptosis of epithelial cells induced by TNF-α in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1950–6. doi: 10.1097/MIB.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 40.Gottardo MF, Jaita G, Magri ML, Zárate S, Moreno Ayala M, Ferraris J, et al. Antiapoptotic factor humanin is expressed in normal and tumoral pituitary cells and protects them from TNF-α-induced apoptosis. PLoS One. 2014;9:e111548. doi: 10.1371/journal.pone.0111548. [DOI] [PMC free article] [PubMed] [Google Scholar]


