Abstract
Oxidative stress and mitochondrial dysfunction are the main suggested mechanisms for neurodegenerative diseases. In this study, we have evaluated the effects of epicatechin (EC) on mitochondrial damage induced by homocycteine (Hcy) using isolated rat hippocampus mitochondria in vivo. EC (50 mg/kg) was gavaged daily for a period of 10 days, starting 5 days prior to Hcy (0.5 μmol/μL) intra hippocampus injection in rats. Mitochondria were isolated from brain by different centrifuge techniques. Mitochondrial function was assayed by MTT test. Also, mitochondrial swelling and oxidative stress markers, such as reactive oxygen species (ROS), lipid peroxidation and glutathione (GSH), were assayed. Hcy induced mitochondrial dysfunction and swelling. Increase in ROS formation, lipid peroxidation, and decreased GSH were observed after Hcy treatment in isolated brain mitochondria. Furthermore, oral administration of EC significantly decreased the lipid peroxidation and ROS levels and also increased GSH levels. Also, EC treatment significantly improved mitochondrial function. As EC indicated protective effects against oxidative stress and mitochondrial damage induced by Hcy, it is suggested for further trials for prevention or treatments of neurodegenerative disorders such as Alzheimer disease.
Key words: Epicatechin, Homocycteine, Oxidative stress, Mitochondria, Neurodegenerative
INTRODUCTION
Neurodegenerative diseases include a functional loss or sensory dysfunction of nerve cells from brain or spinal cord. The most important pathological causes for aging and neurodegenerative diseases such as Alzheimer’s disease (AD), are mitochondrial dysfunctions, which may show, at first, excitotoxicity changes and, finally, apoptosis(1). Generally, the disturbance of equilibrium between pro-oxidant/antioxidant homeostasis leads to oxidative stress that could further produce reactive oxygen species (ROS) in neuronal cells(2). Moreover, it is known that brain has a high rate of oxidative metabolic activity, high contents of polyunsaturated fatty acid, and low antioxidant capacity, which makes it very susceptible to oxidative damage(3,4). According to many studies, it has been suggested that mitochondrial damage may play an important role in the pathogenesis of AD(5) and several studies have shown that various xenobiotics would increase the risk of AD via mitochondrial dysfunction or oxidative damage(6,8). Therefore, mitochondrial protection, and the subsequent reduction of oxidative damage, can be considered as a therapeutic strategy for the treatment of AD(5,7,8,9). Homocysteine (Hcy) is a sulfur containing amino acid derived from the metabolism of methionine that has a known role in the elevation of risk factor for the generation of oxidative stress and development of various neurodegenerative diseases, such as AD or Parkinson(10).
Tea is a common beverage after water used around the world. This herb contains more than 4000 chemicals which can affect human body in terms of different biological aspects. The components of tea have anti-oxidant, anti-mutagenic and anti-carcinogenic effect, which can protect the human against cancer(11). Furthermore, many healthful effects of tea are due to its source for polyphenols constituents, especially flavonoids, which constitute about 30% of the dry leaves(11). In addition, green tea has protective effects against neurological diseases, such as Parkinson, Alzheimer, and ischemic damages; it has also shown an anti-diabetic effect in insulin resistant animal models. As for other qualities of green tea, antibacterial, anti-HIV, and anti-aging effects, can be noted(12). The anti-oxidant effect of green tea is due to the ability of its polyphenol cathechine components to scavenge ROS, such as hydroxy phenol groups, on the B-ring of non-galolite of epicatechin (EC) and epigallocatechin (EGC) and B-ring and D-ring of galolite of epicatechin 3-gallate (ECG) and epigallocatechin gallate (EGCG). The presence of 3,4,5 tri-hydroxy B-ring is important for antioxidant and radical scavanging properties of cathechines(12). Cathechines in green tea have more antioxidant effect than vitamin C and E(12). In addition to anti-oxidant effects, cathechines in green tea have some effects on molecular and cellular targets in signaling pathways associated with apoptosis or cell viability. These effects have been demonstrated both in epithelial tumors and in neuron cells(12). Also, some studies on mice have shown that EC, through activating nuclear factor erythroid 2 (Nrf2(and hem-oxigenase, can reduce the risks of ischemic heart diseases(12).
Recently, some studies have focused on the potential of neuroprotective effects and antioxidant properties of flavonoids against the neuronal deficits associated with aging or age-related neurodegenerative diseases(13). Flavanols, such as (-)-EC, represent a major class of flavonoids commonly present in some plants, such as Camellia sinensis (green tea)(14). Moreover, studies on rats using EC extracts demonstrated some positive effects against oxidative stress, cognitive function, and memory performance(13). Furthermore, it has been proved that EC and EGCG were more potent than catechin. Due to its simpler structure and more efficient blood–brain barrier penetration properties, EC might be the best therapeutic candidate for neurodegenerative diseases(15). According to these backgrounds and as there have not been precise studies to show protective effect of EC against mitochondrial damages induced by Hcy, this study aimed to better clarify the role of EC against oxidative stress induced by Hcy (as a neurodegenerative model) in mitochondria of hippocampus of rats in vivo.
MATERIALS AND METHODS
Animals
Adult male Wistar rats (n = 24) weighing 180-220 g (at the time of surgery) were obtained from animal house of Mazandaran University of Medical Science (Sari, Iran). The animals were housed at 22 °C in a controlled environment with a 12:12-h light/dark cycle and had free access to standard laboratory food and water. All experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Research and Ethics Committee of Mazandaran University of Medical Science, Iran. (Registration number: 1041).
Materials
(-)-Epicatechin and D-L-homocysteine were purchased from Sigma Chemical Co. (Sigma-Aldrich, Germany). Ketamine and xylazine were obtained from ALFASAN Co, Netherlands.
Animal grouping
Twenty-four rats were randomly divided into four groups of 6 each. 1) Control group: did not receive any injection or gavage; 2) vehicle (sham) group given only the vehicle, phosphate buffered saline (PBS) and normal saline; 3) Hcy alone, and 4) EC with Hcy.
Surgery
Animals were anesthetized with ketamin-xylosin (10 mg/kg i.p.) and placed into a stereotaxic frame (Stoelting Co. Wood Dale, IL). The sterotaxic coordinated to produce a bilateral lesion in the CA1 region of the hippocampus (-4.2 mm posterior to bregma, ± 3.0 mm lateral to bregma, and 2.9 mm ventral to the dura) were standardized based on the stereotaxic atlas of Paxinos and Watson(16). After a sagittal incision, the bregma suture was located and holes were drilled with an electrical drill. Injections of Hcy or vehicle (1 μL) were carried out in the left and right dorsal hippocampus at a rate of 1 μL per 2 min. The cannula was left in situ for a further 5 min following Hcy injection in order to allow for a passive diffusion from the cannula tip and also to minimize the spread into the injection tract. After the surgery, the animals were returned to their cages and monitored constantly during the recovery process.
Drugs preparation and administration
Hcy powder was dissolved in hydrochloric acid (1 M) and diluted with PBS as the vehicle. The PH of the solution was adjusted at 7.4 by the addition of NaOH, 0.1 N. Hcy solution was prepared freshly at a concentration of 0.5 M. 1 μL of Hcy (0.5 μmol/μL) was injected through cannula in the left and right dorsal hippocampus at a rate of 1 μL per 2 min, once a day. A Hamilton syringe with a cannula of 0.3 mm diameter was used to inject Hcy or its vehicle (PBS). Powder of EC was dissolved in normal saline (as vehicle), which was prepared freshly. EC dose (50 mg/kg), was used according to earlier reports about its antioxidant effects(13). EC (50 mg/kg) was gavaged once a day for a period of 10 days, starting 5 days prior to Hcy injection. Also, Hcy was injected one h after the oral gavage of EC. On the other hand, 0.5 mL normal saline was gavaged for a period of 10 days (starting 5 days prior to PBS injection), and PBS (0.5 M) was injected (1 μL) through a cannula in the left and right areas of dorsal hippocampus at a rate of 1 μL per 2 min for one day (one h after normal saline gavage, on the fifth day)(10). To confirm the correct direction of drug injection into hyppocampus (CA1 area), we used metylen blue dye, 05 μL injection into hippocampus area of rat.
Brain sample collection and mitochondrial preparation
Twenty four h after the last treatment, animals were decapitated and the whole brain tissues were rinsed, minced and homogenized in manitol buffer (sucrose 6.4 g, manitol 11.64 g, EDTA 0.009 g) with a glass handheld homogenizer. The nuclei and broken cell debris were precipitated by centrifugation at 1500 × g for 10 min at 4 °C and the pellet was discarded. The supernatant was centrifuged further at 10,000 × g for 10 min and the top layer was carefully discarded. The mitochondrial pellet was washed by gently suspending in the isolation medium and centrifuged again at 10,000 × g for 10 min. Then, samples were suspended in 200 μL Tris buffer (containing 0.05 M Tris–HCl, 0.25 M sucrose, 20 mM KCl, 2.0 mM MgCl2, and 1.0 mM Na2HPO4, pH of 7.4 at 4 °C) except for ROS assay, which were suspended in respiration buffer (0.32 mM sucrose, 10 mMTris, 20 mM Mops, 50 μM EDTA, 0.5 mM MgCl2, 0.1 mM KH2PO4 and 5 mM sodium succinate). Finally, samples were frozen in a freezer (- 20 °C) until use. Also, protein concentrations were determined through the coomassie blue protein-binding method as explained by Bradford(16).
Lipid peroxidation assay
The content of malonyldialdehyde (MDA) was determined based on the method of Shaki, et al.(16) using thiobarbituric acid as the indicator by reading the absorbance at 532 nm with an ELISA reader. Tetramethoxy propane was used as the standard and MDA content was expressed as nmol/mg protein(16).
GSH content assay
GSH content was determined using 5,5’-dithio-bis (2-nitrobenzoic acid) (DTNB) as the indicator by reading the absorbance at 412 nm on a spectrophotometer (UV-1601PC, Shimadzu, Japan). The values were obtained by the interpolation of data with the standard curve of GSH and were expressed as μg/mg protein(17).
ROS assay
ROS were evaluated using dichloro-dihydro-fluorescein diacetate (DCFH-DA) and the results showed fluorescence intensity(18).
Mitochondrial function using MTT assay
The activity of mitochondria was assayed by measuring the reduction of 3-45-dimethylthiazol-2-yl]-2,5- diphenyl tetrazolium bromide(19).
Mitochondrial swelling assay
The analysis of mitochondrial swelling after the isolated mitochondria (0.5 mg protein/mL) was estimated through changes in light scattering as monitored spectrophotometrically at 540 nm at 30 °C. A decrease in absorbance indicates an increase in mitochondrial swelling(19).
Statistical analysis
Results were expressed as mean ± SD. Comparisons between means were analyzed by one-way ANOVA and Tukey test. A value of P < 0.05 was considered statistically significant.
RESULTS
The effect of epicatechin on mitochondrial lipid peroxidation (MDA)
It was found that the injection of Hcy caused a significant increase in the levels of MDA compared with control and vehicle groups (P < 0.001). Moreover, EC treatment significantly decreased Hcy induced lipid peroxidation (P < 0.01) (Fig. 1).
Fig. 1.
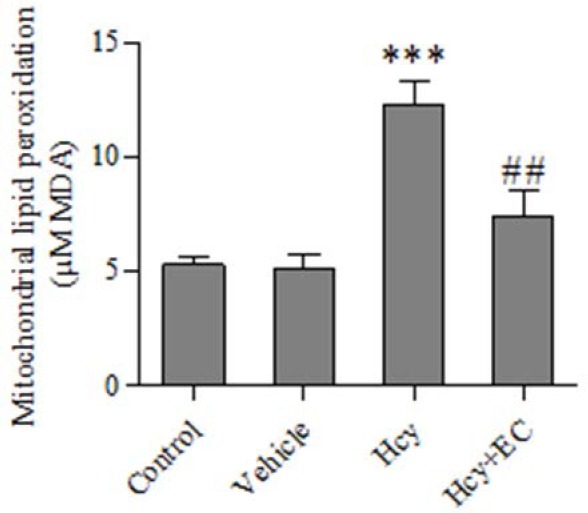
Effect of epicatechin (50 mg/kg) on mitochondrial lipid peroxidation induced by homocycteine. Values are prepresented as mean ± SD (n = 6). ***P < 0.001 compared with control and vehicle groups. ##P < 0.01 compared with homocycteine group. (Hcy) homocysteine, (EC) epicatechin.
The effect of epicatechin on GSH content
Injection of Hcy caused a significant decrease in GSH levels compared with vehicle or control groups (P < 0.01) (Fig. 2). In addition, EC treatment significantly increased the levels of GSH content compared with Hcy group (P < 0.05) (Fig. 2).
Fig. 2.
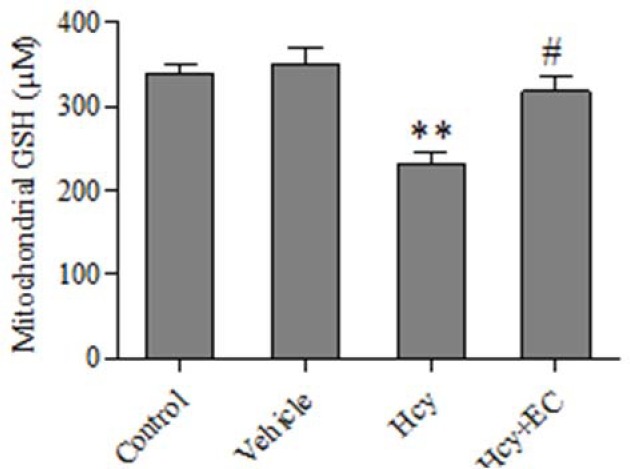
Effect of epicatechin (50 mg/kg) on mitochondrial glutathione oxidation induced by homocycteine. Values are prepresented as mean ± SD (n = 6). **P < 0.01, compared with control and vehicle groups. #P < 0.05 compared with homocycteine group. (Hcy) homocysteine, (EC) epicatechin.
The effect of epicatechin on ROS
Injection of Hcy caused a significant increase in the levels of ROS compared with control and vehicle groups (P < 0.01) (Fig. 3). Moreover, EC treatment significantly decreased the ROS levels induced by Hcy (P < 0.001) (Fig. 3).
Fig. 3.
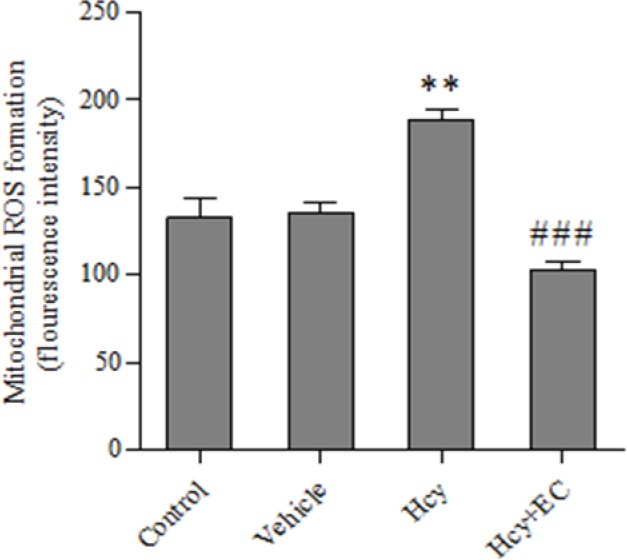
Effect of epicatechin (50 mg/kg) on mitochondrial ROS formation induced by homocycteine. Values are prepresented as mean ± SD (n = 6). **P < 0.01 compared with control and vehicle groups. ###P < 0.001 compared with homocysteine group. (Hcy) homocysteine, (EC) epicatechin.
The effect of epicatechin on mitochondrial function
It was found that the injection of Hcy caused a significant decrease in the MTT absorption compared with control and vehicle groups (P < 0.01). Moreover, EC treatment significantly inhibited Hcy-induced mitochondrial dysfunction (P < 0.05) (Fig. 4).
Fig. 4.
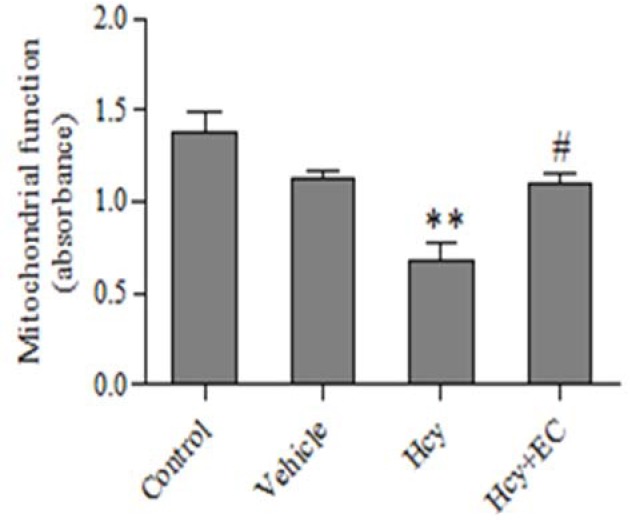
Effect of epicatechin (50 mg/kg) on mitochondrial dysfunction induced by homocycteine. Values are prepresented as mean ± SD (n = 6). **P < 0.01 compared with control and vehicle groups. #P < 0.05 compared with homocysteine group. (Hcy) homocysteine, (EC) epicatechin.
The effect of epicatechin on mitochondrial swelling
It was found that the injection of Hcy caused a significant increase in the mitochondrial swelling compared with control and vehicle groups (P < 0.01). Moreover, EC treatment significantly inhibited Hcy-induced mitochondrial swelling (P < 0.05) (Fig. 5).
Fig. 5.
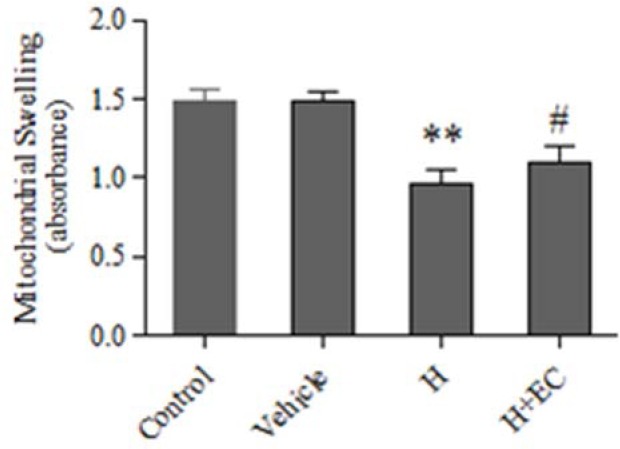
Effect of epicatechin (50 mg/kg) on mitochondrial swelling induced by homocycteine. Values are prepresented as mean ± SD (n = 6). **P < 0.01 compared with control and vehicle groups. #P < 0.05 compared with homocysteine group. (Hcy) homocysteine, (EC) epicatechin.
Confirming the correct direction of drug injection into hyppocampus
After cross sectioning of rat brain, injection (metylen blue dye) into hippocampus area of rat (CA1 area), was confirmed (Fig. 6).
Fig. 6.
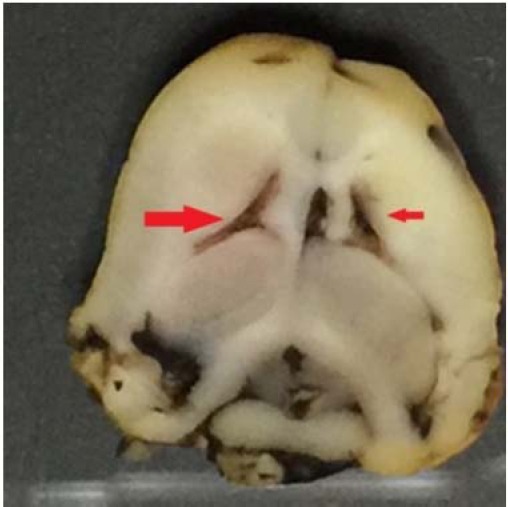
Rat brain after metylene blue (0.5 μL) injection into hippocampus CA1 area.
Histological findings
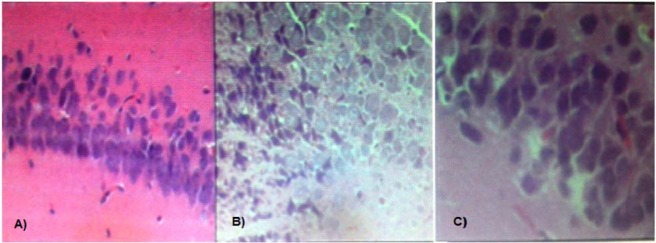
For histopathological studies of drug effects, hippocampal areas of animals were removed and after section preparation and staining with Hematoxyline and Eosine, the slides have been observed with a light microscope with a magnificence of 10× and 40×, and the histological differences between control and Hcy and Ec + Hcy have been assayed. In Control group, normal stratified layers of cells showing smooth and normal nuclear borders (Fig. 7A).
Fig. 7.
Rat hippocampal sections stained with Hematoxylin and Eosin observed optically (× 10 & × 40). (A) Control group, normal stratified layers of cells showing smooth and nuclear borders; (B) homocysteine group, necrotic pyramidal cells characterized by piknotic hyperchromatic nuclei and irregular nuclear borders; (C) epicatechin + homocysteine group, signs of repair characterized by layers of granular cells with distinct cell borders.
In Hcy group, necrotic pyramidal cells were characterized by piknotic hyperchromatic nuclei and irregular nuclear borders, which would explain histological disturbances (Fig. 7B), but after treatment with EC, granular cells with distinct increased cell borders were observed showing tissue improvement process (Fig. 7C).
DISCUSSION
Mitochondria are considered as the major source of ATP via oxidative phosphorylation (OXPHOS) in the most mammalian cell types, and also main organelles involved in the development of oxidative damage as well as apoptosis in various cell types(20). There are strong evidences that mitochondrial abnormality occurs in an early stage of oxidative damage and has a main role in the pathogenesis of many neurodegenerative diseases including AD, Parkinson’s disease, amyotrophic lateral sclerosis, and Huntington’s disease(21,22).
In fact, CNS functions critically depend on normal mitochondrial function, since brain is a high-energy demand tissue with high mitochondria content. On the other hand, the low level of antioxidant system and high content of poly-unsaturated fatty acid (PUFA) in brain tissue make it more susceptible to oxidative damage. It has been shown that both mutations in mitochondrial DNA (mtDNA), and important environmental factors such as free radicals may contribute to the energy failure in mitochondria and promote the genesis and amplification of ROS, which could lead to neurodegenerative diseases(22,23).
Hcy could modulate death via apoptosis or necrosis and might be used as a model for neurodegenerative animal studies(24). Previous studies have shown that Hcy promoted mitochondrial damage, induced oxidative stress and subsequent toxic effects, such as neurotoxicity and atherosclerosis(7,8,9,10). According to previous studies(7,8,9,10), the injection of 1 μL of Hcy (0.5 μmol/μL) in the left and right dorsal hippocampus made neurotoxic effect in rats and the present study has aimed to assay the effects of EC against Hcy-induced mitochondrial dysfunction using isolated rat hippocampus mitochondria in vivo.
The induction of mitochondrial dysfunction by Hcy was determined by MTT assay, which is an indicator of the activity of succinate dehydrogenase (a mitochondrial enzyme). As shown in the results section, Hcy increased mitochondrial dysfunction in isolated rat hippocampus mitochondria. On the other hand, any disturbance in mitochondrial electron transfer chain would lead to OXPHOS failure and subsequently increase ROS production and oxidative stress.
We found that Hcy increased ROS formation and induced oxidative stress, which is shown by an increased lipid peroxidation and decreased GSH content (as the main antioxidant in mitochondria). Oxidative damage to mitochondria could lead to the peroxidation of membrane lipid and mitochondrial membrane injury. Mitochondrial membrane damage could result in a lack of selective permeability of membrane, which was shown by mitochondrial swelling. As demonstrated in the results section, Hcy increased mitochondrial swelling(25).
The use of molecules with antioxidant ability in experimental models has largely contributed to the understanding of the mechanisms involved in the selective damage induced by different neurotoxins. Various studies have used flavonoids as the protective agent in several models of neurotoxicity, such as those produced by 6-hydroxydopamine, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, amyloid-beta peptides and Hcy. It has been proposed that these molecules are able to exert neuroprotective actions through their antioxidant activity(8).
EC is a flavonoid mostly found in green and black tea extract(26,27). It has been demonstrated that the bioavailability of EC is greater than that of the catechin in rat(28). Also, flavonoids are able to transverse the blood-brain-barrier after oral ingestion(29).
In this study, we have shown that the oral administration of EC at 50 mg/kg for a period of 10 days significantly decreased the level of ROS in isolated rat hippocampus mitochondria. According to previous studies, EC has radical scavenging activity(30) and would inhibit cell death caused by hydrogen peroxide(27). Generally, ROS are highly reactive with all macromolecules (e.g. proteins, DNA and lipid)(31). The oxidation of polyunsaturated fatty acids results in the production of multiple aldehydes with different carbon chain lengths, such as MDA(9). On the other hand, peroxides and MDA are the key factors for the initiation and the propagation of neurodegenerative diseases(32). Along with previous studies, EC significantly inhibited lipid peroxidation induced by Hcy in hippocampus isolated mitochondria(11,12,33).
GSH is one of the primary non-enzymatic antioxidant systems against hydrogen peroxide and other ROS, which constitutes nearly 10–15% of total cellular GSH in the mitochondria(34). Therefore, the decline of reduced GSH content in mitochondria could cause severe deficiency in their defense system against oxidative damage, leading to a further rise in lipid peroxidation(19). On the other hand, mitochondrial GSH is necessary for the maintenance of thiol group in inner mitochondrial membrane. In fact, increased ROS level by Hcy would lead to cross linking and oxidation of thiol groups in the mitochondrial membrane protein and the induction of the conformational changes in the pore complex, which would finally result in the mitochondrial permeability transition (MPT)(35). Our findings demonstrated that EC inhibited the mitochondrial swelling as an indicator of MPT, due to Hcy administration. Also, MPT is considered as an early step in apoptosis signaling. In the present study, it was observed that EC treatment, not only inhibited Hcy-induced mitochondrial swelling, but also inhibited mitochondrial dysfunction. Furthermore, it has been proven that EC and EGCG were more potent than catechin as neuroprotectants. Due to its simpler structure and more efficient blood–brain barrier penetration properties, EC might be an effective therapeutic candidate for neurodegenerative diseases(36).
CONCLUSION
In summary, we have demonstrated that EC has protective effects against oxidative stress and mitochondrial damage induced by Hcy using isolated rat hippocampus mitochondria in vivo as a model of neurodegenerative diseases.
ACKNOWLEDGMENTS
This study was extracted from MSc thesis of Yaghoub Shayeste and supported by a grant from the Research Council of Mazandaran University of Medical Sciences, Sari, Iran. We also appreciate Miss Fatemeh Tavassoli for her great editing of the manuscript.
REFERENCES
- 1.Mattson MP. Metal‐catalyzed disruption of membrane protein and lipid signaling in the pathogenesis of neurodegenerative disorders. Ann of the New York Acad of Sci. 2004;1012(1):37–50. doi: 10.1196/annals.1306.004. [DOI] [PubMed] [Google Scholar]
- 2.Lepoivre M, Flaman J-M, Bobé P, Lemaire G, Henry Y. Quenching of the tyrosyl free radical of ribonucleotide reductase by nitric oxide. Relationship to cytostasis induced in tumor cells by cytotoxic macrophages. J Biol Chem. 1994;269(34):21891–21897. [PubMed] [Google Scholar]
- 3.Massimo CMA, Mastrocola R, Gallicchio M, Carolina Rosa A, Dianzani C, Danni O, et al. Modulation of the oxidative stress and inflammatory response by PPAR-γ agonists in the hippocampus of rats exposed to cerebral ischemia/reperfusion. Eur J Pharmacol. 2006;530:70–80. doi: 10.1016/j.ejphar.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 4.Collino M, Aragno M, Mastrocola R, Gallicchio M, Rosa AC, Dianzani C, et al. Modulation of the oxidative stress and inflammatory response by PPAR-γ agonists in the hippocampus of rats exposed to cerebral ischemia/reperfusion. Eur J Pharmacol. 2006;530(1-2):70–80. doi: 10.1016/j.ejphar.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 5.Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc Natl Acad Sci USA. 2010;107(43):18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agnati L, Genedani S, Rasio G, Galantucci M, Saltini S, Filaferro M, et al. Studies on homocysteine plasma levels in Alzheimer’s patients. Relevance for neurodegeneration. J Neural Transm. 2005;112(1):163–169. doi: 10.1007/s00702-004-0154-7. [DOI] [PubMed] [Google Scholar]
- 7.Ataie A, Sabetkasaei M, Haghparast A, Moghaddam AH, Kazeminejad B. Neuroprotective effects of the polyphenolic antioxidant agent, curcumin, against homocysteine-induced cognitive impairment and oxidative stress in the rat. Pharm Biochem Behav. 2010;96(4):378–385. doi: 10.1016/j.pbb.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Cuevas E, Limón D, Pérez-Severiano F, Díaz A, Ortega L, Zenteno E, et al. Antioxidant effects of epicatechin on the hippocampal toxicity caused by amyloid-beta 25-35 in rats. Eur J Pharmacol. 2009;616(1):122–127. doi: 10.1016/j.ejphar.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Soung HS, Wang MH, Tseng HC, Fang HW, Chang KC. (-) Epigallocatechin-3-gallate decreases the stress-induced impairment of learning and memory in rats. Neurosci Lett. 2015;602:27–32. doi: 10.1016/j.neulet.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Streck EL, Vieira PS, Wannmacher CM, Dutra-Filho CS, Wajner M, Wyse AT. In vitro effect of homocysteine on some parameters of oxidative stress in rat hippocampus. Metab Brain Dis. 2003;18(2):147–154. doi: 10.1023/a:1023815119931. [DOI] [PubMed] [Google Scholar]
- 11.Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland BA, Rahman RM, Appleton I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J Nut Biochem. 2006;17(5):291–306. doi: 10.1016/j.jnutbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Shah Zale R, Ahmad AS, Kensler A, Yamamoto M, Biswal S, et al. The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J Cereb Blood Flow Metab. 2010;30(12):1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuevas E, Limón D, Pérez-Severiano F, Díaz A, Ortega L, Zenteno E, et al. Antioxidant effects of epicatechin on the hippocampal toxicity caused by amyloid – beta 25 – 23 in rats. Eur J Pharmacol. 2009;616(1-3):122–127. doi: 10.1016/j.ejphar.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Guzmán M, Jiménez R, Sánchez M, Zarzuelo MJ, Galindo P, Quintela AM, et al. Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension. Free Radic Biol Med. 2012;52:70–79. doi: 10.1016/j.freeradbiomed.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Nath S, Bachani M, Harshavardhana D, Steiner JP. Catechins protect neurons against mitochondrial toxins and HIV proteins via activation of the BDNF pathway. J Neurovirol. 2012;18(6):445–455. doi: 10.1007/s13365-012-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaki F, Hossein MJ, Ghazi-Khansari M, Pourahmad J. Toxicity of depleted uranium on isolated rat kidney mitochondria. Biochim Biophys Acta. 2012;1820(12):1940–1950. doi: 10.1016/j.bbagen.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Sadegh C, Ronald PS. The spectroscopic determination of aqueous sulfite using Ellman’s reagent. MURJ. 2003;8:39–43. [Google Scholar]
- 19.Pérez-Severiano F, Salvatierra-Sánchez R, Rodríguez-Pérez M, Cuevas-Martínez EY, Guevara J, Limón D. S-allylcysteine prevents amyloid-ß peptide-induced oxidative stress in rat hippocampus and ameliorates learning deficits. Eur J Pharmacol. 2004;489(3):197–202. doi: 10.1016/j.ejphar.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Shaki F, Pourahmad J. Mitochondrial toxicity of depleted uranium: protection by beta-glucan. Iran J Pharm Res. 2012;12:131–140. [PMC free article] [PubMed] [Google Scholar]
- 21.Petrozzi L, Ricci G, Giglioli NJ, Siciliano G, Mancuso M. Mitochondria and neurodegeneration. Biosci Rep. 2007;27:87–104. doi: 10.1007/s10540-007-9038-z. [DOI] [PubMed] [Google Scholar]
- 22.Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E. Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci. 2012;322(1-2):254–262. doi: 10.1016/j.jns.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 23.Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agnati LF GS, Rasio G. Studies on homocysteine plasma levels in Alzheimer’s patients for neurodegeneration. J Neural Transm. 2005;112:163–169. doi: 10.1007/s00702-004-0154-7. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Cho SY, Lee JH, Jeong SM, Yoon IS, Lee BH, et al. Neuroprotective effects of ginsenoside Rg3 against homocysteine-induced excitotoxicity in rat hippocampus. Brain Res. 2007;1136:190–199. doi: 10.1016/j.brainres.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 26.Shaki JP, Hosseini MM, Ghazi-Khansari F. Depleted uranium disrupted the bioenergetics of liver mitochondria: a potential mechanism of fatigue syndrome. Res Pharm Sci. 2012;7(5):S131. [Google Scholar]
- 27.Spencer JP, Schroeter H, Crossthwaithe AJ, Kuhnle G, Williams RJ, Rice-Evans C. Contrasting influences of glucuronidation and O-methylation of epicatechin on hydrogen peroxide-induced cell death in neurons and fibroblasts. Free Radic Biol Med. 2001;31(9):1139–1146. doi: 10.1016/s0891-5849(01)00704-3. [DOI] [PubMed] [Google Scholar]
- 28.Baba S, Osakabe N, Natsume M, Muto Y, Takizawa T, Terao J. In vivo comparison of the bioavailability of (+)-catechin, (−)-epicatechin and their mixture in orally administered rats. J Nutr. 2001;131(11):2885–2891. doi: 10.1093/jn/131.11.2885. [DOI] [PubMed] [Google Scholar]
- 29.Mohsen MA, Marks J, Kuhnle G, Rice-Evans C, Moore K, Gibson G, et al. The differential tissue distribution of the citrus flavanone naringenin following gastric instillation. Free Radic Res. 2004;38(12):1329–1340. doi: 10.1080/10715760400017293. [DOI] [PubMed] [Google Scholar]
- 30.Longpré F, Garneau P, Christen Y, Ramassamy C. Protection by EGb 761 against beta-amyloid-induced neurotoxicity: involvement of NF-kappaB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free Radic Biol Med. 2006;41(12):1781–1794. doi: 10.1016/j.freeradbiomed.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Man WW. Thesis of Master in Pharmacy. South Africa: Rhodes University E-Publishing; 2005. An investigation into the neuroprotective effects of estrogen and progesterone in a model of homocysteine – induced neurodegeneration; pp. 65–180. [Google Scholar]
- 32.Praticò D, Delanty N. Oxidative injury in diseases of the central nervous system: focus on Alzheimer’s disease. Am J Med. 2000;109(7):577–585. doi: 10.1016/s0002-9343(00)00547-7. [DOI] [PubMed] [Google Scholar]
- 33.Ban JY, Jeon SY, Bae K, Song KS, Seong YH. Catechin and epicatechin from Smilacis chinae rhizome protect cultured rat cortical neurons against amyloid β protein (25–35)-induced neurotoxicity through inhibition of cytosolic calcium elevation. Life Sci. 2006;79(24):2251–2259. doi: 10.1016/j.lfs.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Zhong Q, Putt DA, Xu F, Lash LH. Hepatic mitochondrial transport of glutathione: studies in isolated rat liver mitochondria and H4IIE rat hepatoma cells. Arch Biochem Biophys. 2008;474(1):119–127. doi: 10.1016/j.abb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosseini MJ, Shaki FS, Ghazi-Khansari M, Pourahmad J. Toxicity of arsenic (III) on isolated liver mitochondria: a new mechanistic approach. Iran J Pharm Res. 2013;12(Suppl):121–138. [PMC free article] [PubMed] [Google Scholar]
- 36.Nath S, Bachani M, Harshavardhana D, Steiner JP. Catechins protect neurons against mitochondrial toxins and HIV proteins via activation of the BDNF pathway. J Neurovirol. 2012;18(6):445–455. doi: 10.1007/s13365-012-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]