Abstract
Transfusion in the treatment of thalassemia gives rise to iron deposits in many organs. Since there are many obstacles in the use of deferoxamin (DFO) as an iron chelating agent, it is important to find another alternative therapy that can act as iron chelation. The study aims to compare the histopathological pictures of the heart and spleen in iron-induced rats after administration of DFO and nanoparticles of green tea extract. The research used experimental research design with a post-test only control group. Experimental nano green teas were divided into four treatment groups; no diet, DFO supplementation, nano green tea supplementation, and a combination of both DFO and green tea. Ferritin and glutathione peroxides were used as biochemical parameters, and histopathological pictures of the heart and spleen were recorded. The study showed that there was significant improvement in the rats receiving DFO and nanoparticles of green tea compared with the rats in the no diet group. The study also reported that nano green tea has an effect comparable to DFO.
Key words: Green tea, Nanoparticle, Thalassemia, Iron chelator, Transfusion
INTRODUCTION
Thalassemia is a monogenic hematological disorder that is found abundantly worldwide, especially in thalassemic belt countries. Until now, there has been no effective treatment for dealing with thalassemia in addition to blood transfusions. Continuous transfusions, however, can cause many complications as a result of iron deposit in many organs leading to cell damage.
Blood transfusion dependence in patients with thalassemia leads to a buildup of iron (Fe), which may cause reactive oxygen species (ROS) via the Haber-Weiss and Fenton, which can damage important organs such as the liver, spleen, and heart(1). Thalassemia patients are also aggravated with the perturbation of ROS activity that can lead to an oxidant-antioxidant enzyme imbalance, including superoxide dismutase (SOD), and glutathione peroxides (GPx)(2).
To date, standard treatment for reducing iron deposition is to use some kind of chelating agent like deferoxamin (DFO). However, these chemical preparations have side effects that are still quite dangerous. In addition, the price is relatively expensive and isn’t affordable for most poor patients(3). Thus, it is important to find an alternative iron chelation therapy which is safe, affordable, and utilizes natural ingredients that are easily available in the patient’s environment(4). Green tea (Camellia sinensis), which is widely consumed in many countries, contains polyphenol compounds that have been proven to reduce the risk of various diseases(5,6).
Green tea is a good source of antioxidant polyphenols, particularly catechins, which make up 30% of the dry weight of tea leaves. The compound can prevent the absorption of Fe, dispose of ROS, bind Fe, and prevent lipid peroxidation, thus preventing cell damage(1). Along with the development of nanotechnology, it is possible to produce medicine in a variety of sizes. Nanotechnology is the application of technology in the field of science and technology to create new materials, including nanoparticles (10-9 m)(7).
The development of the nanotechnology industry into nanoparticles can hypothetically pass active substances of the drug more efficiently into cells(8). In vitro studies revealed that the iron binding capacity of the chitosan-coated magnetite (CCM) nanoparticles in the blood serum was 2.3 mM/g of nanoparticle, more effective than the optimum binding of iron by conventional medicine DFO, which is 1.5 mM/g of the drug(9). This study aims to look at the differences between the biochemical and histopathological effects that green tea extract has in the heart and spleen of induced ferrous sulfate Rattus norvegicus rats. Moreover, this study was conducted to see the effectiveness of green tea nanoparticles as an adjuvant or monotherapy iron chelation in rat models of thalassemia.
MATERIALS AND METHODS
Design
This research was true experimental post-test only, with the control group designed using a completely randomized design. Rattus norvegicus females, Sprague Dawley strain from Gadjah Mada Animal Laboratory were used as experimental animals. Twenty rats aged 2-3 months and with a body weight of 150-250 g were prepared for the experiment. Rats were placed in cages that were identical in shape, material, and size. Ethical clearance regarding the use of animal model was obtained from Ethical Committee, Faculty of Medicine, Jenderal Soedirman University. Animal handling of the study complied with principles of laboratory animal care. Rats were given food and drink ad libitum. Ferrous sulfate (Sigma Aldrich, St Louis, USA) administrated at a dose of 0.5 mg/kg/day for 35 days, as documented in the previous study. Animals were then divided into 4 groups for 30 days treatment. Rats were divided in four experimental groups including group I (normal diet), group II (DFO 5 mg/kg/day), group III (green tea nanoparticles 3 mg/kg/day), and group IV (DFO 5 mg/kg/day + green tea nanoparticles 3 mg/kg/day), as described in the previous study(10). DFO was supplied by Sigma Aldrich, St Louis, USA. All treatments were carried out orally using a stomach probe. Rats were terminated on day 66, then the heart and spleen were retrieved. Organs were then fixed in a 10% buffered formalin solution for histopathological preparations made using Hematoxylin Eosin staining (Sigma Aldrich, St Louis, USA) and Pearls’ Prussian blue (Leicabiosystems, Singapore). Histopathology was read using a light microscope, with a 400 × magnification. Biochemical parameters were taken shortly after the administration of ferrous sulfate and on the last day of diet DFO and green tea preparations.
Nanoparticle preparation
Nanoparticle formulation of the green tea was achieved using the CCM method explained elsewhere(9). Briefly, nanoparticles were prepared by ionic gelation method. Half of mL green tea extract was added to 0.5 mL chitosan solution at pH 5.0. The mixing was homogenized using vortex for 20 s, then added with triphenyl phosphate (TPP) solution 0.03% and homogenized again for 20 s. The nanoparticles were then evaluated for volume of sedimentation to describe the physical stability. The size of nanoparticles was pictured using particle size analyzer (PSA) and transmission electron microscope (TEM).
Oxidative stress measurement
Examination of biochemical parameters of enzyme GPx was done by spectrophotometric method as per manufacturer direction (Abcam, Cambridge, USA). All standard reagents and buffers were prepared at room temperature. Immediately prior to use, reaction mix for each reaction was prepared. Sample, positive controls and reagent control wells were then added 40 μL of reaction mix, and left for 15 min. Glutathione peroxidase (GPx) reaction was achieved after adding 10 μL cumene hydro peroxide solution. The mix then read at 340 nm.
Ferritin measurement
Ferritin levels were measured using Immunochemiluminescent technique (Abcam, Cambridge, USA) as described. Briefly, plasma sample were prepared and performed directly from fresh blood. All reagents, working standards and samples were performed at room temperature. Ninety six wells in microplate that has been precoated ferritin antibody were filled with 50 μL standard ferritin reagent and samples. Liquid were washed five times with 200 μL of 1× wash buffer manually. Fifty μL of 1× biotinylated ferritin antibody was added to each well, incubated for 1 h at room temperature, and followed by washing procedure. 1× SP conjugate was then filled into each well and incubated for 30 min, and followed by washing step. Fifty μL of chromogen substrate was added per well and let the optimal blue color density appeared. The final step was adding stop solution.
After the color was changed from blue to yellow, reading the absorbance was then performed on a microplate reader at a wavelength of 450 nm.
RESULTS
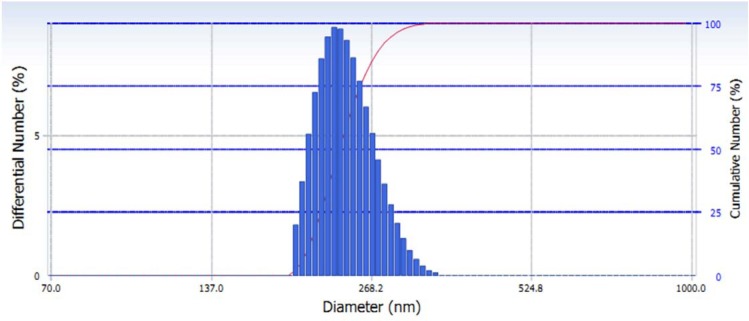
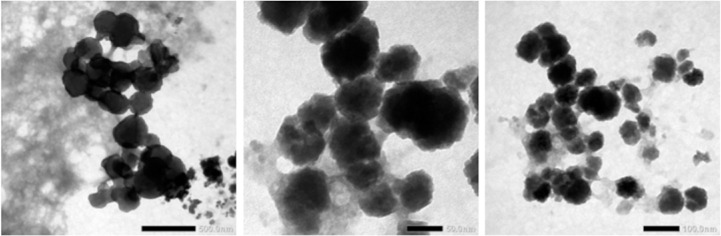
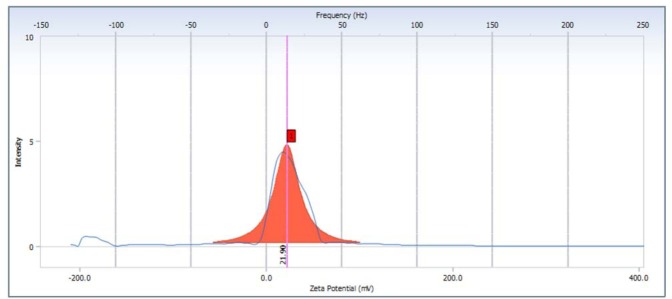
In the physical stability test, nano suspension showed high stability. Study also reported that volume sedimentation test showed there was no precipitation. The size distribution of green tea extract nanoparticles were 194.3–350.8 nm, the average is 241.4 nm (Fig. 1). The morphology of nanosuspension was observed by TEM, the picture seen that the nanoparticles has less uniform spheris (Fig. 2). The nanoparticles of green tea extract has zeta potential value 21.90 mV (Fig. 3). However, the study has limitation since it did not measure the content of the nanoparticles.
Fig. 1.
Size distribution of nanoparticles green tea extract using particle size analyzer (PSA).
Fig. 2.
Nanoparticles of green tea extract images using transmission electron microscopy (TEM).
Figure 3.
The value of zeta potential of green tea extract nanoparticles.
Ferritin level showed an increase from the normal value (1.32 to 2.3 ng/mL) up to 7.04 ng/mL after supplementation of ferrous sulfate, and then they dropped back to normal values after administration of iron chelation, as shown in Fig. 4.
Fig. 4.
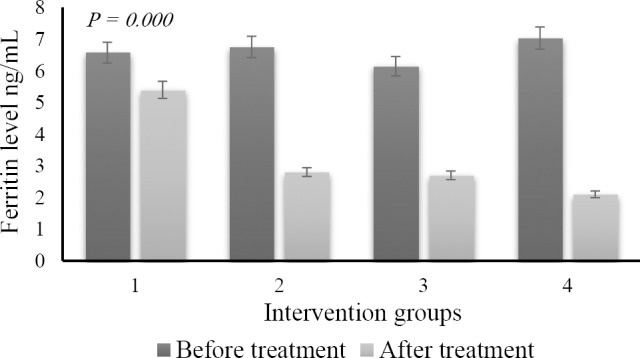
The difference of ferritin level shortly after the induction period of ferrous sulfate (dark color) and after administration of DFO and green tea nanoparticles (light color). (1) control no diet; (2) DFO diet; (3) nano green tea diet; and (4) DFO and nano green tea diet.
Subsequent measurements of the levels of GPx activity were obtained as in Fig. 3. We demonstrated that enzyme activity dramatically dropped after iron supplementation and went back to the normal value after administration of DFO and nano green tea (Fig. 5).
Fig. 5.
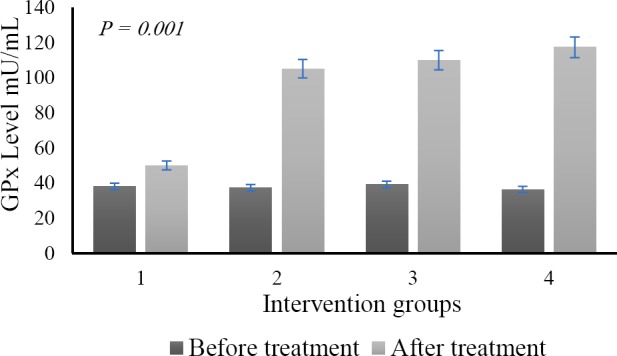
The different level of the glutathione peroxidase (GPx) enzyme shortly after the induction period of ferrous sulfate (dark color) and after administration of DFO and green tea nanoparticles (light color). (1) group with no diet; (2) DFO diet; (3) nano green tea diet; and (4) DFO and nano green tea diet.
Anova test after nano green tea supplementation revealed that the difference of ferritin levels between groups showed a high level of significance (P = 0.000). Tukey post hoc test showed that there was a significant value of inter-group differentiation. The study also confirmed that the GPx enzyme activity expressed a significant improvement compared to the situation prior to the nanoparticle green tea supplementation (P = 0.01). Histopathological examination presented that there were no heart muscle cells containing hemosiderin pigment. The study found other things on the histopathological picture, such as the cell damage caused by the necrosis process (Fig. 6). However, a different effect was found in the spleen. We found hemosiderin in almost the entire field of view of the organ (Fig. 7). The full level of hemosiderin in spleen were presented as follow; group I (66.80 ± 4.09), group II (42.25 ± 2.52), group III (25.50 ± 5.0), and group IV (13.0 ± 5.83).
Fig. 6.
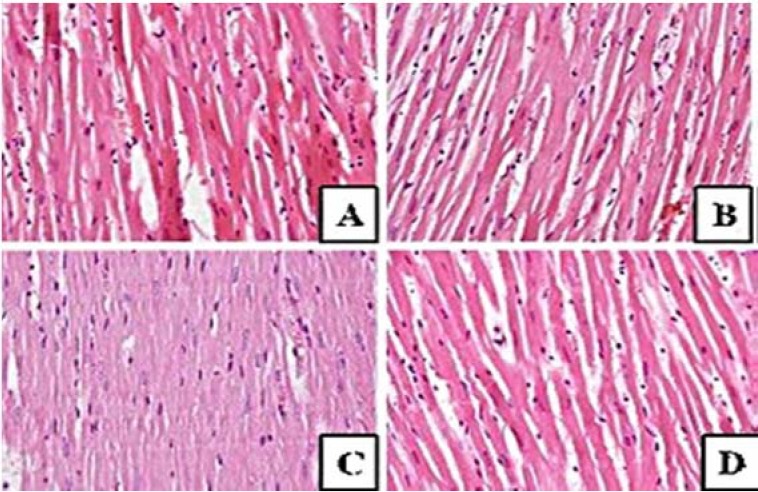
Histopathological features in myocardium hematoxylin and eosin staining. There is no hemosiderosis, only some cells undergo necrosis. (A) Control no diet; (B) DFO diet; (C) nano green tea diet, and (D) DFO and nano green tea diet.
Fig. 7.
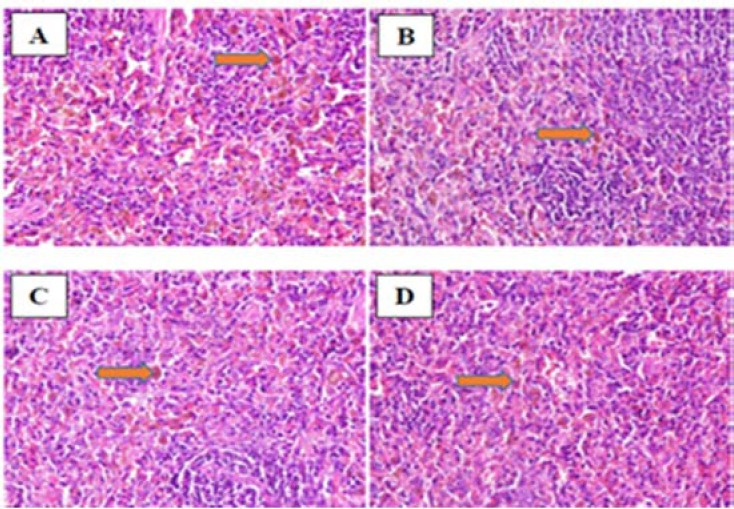
Hemosiderin laden of macrophages in the spleen of mice induced ferrous sulfate. (A) control no diet; (B) DFO diet; (C) nano green tea diet; and (D) DFO and nano green tea diet.
There is a significant improvement in images C and D compared to images A and B in Fig. 7. Image A, belonging to no diet group, showed abundant hemosiderin laden through the parenchymal cells (> 85%). Image B, which treated with DFO diet, showed low density level of the pigments (65%). Image C and D, which fed with nano green tea in combination with DFO showed minimal hemosiderin in the parenchymal cells (30-40%).
DISCUSSION
It has been shown in previous studies using traditional extract, the green tea leaf extract significantly lowers the blood ferritin levels in rat animal models and that it was comparable with DFO(11). In this study, green tea extract was threshed into nanoparticles. The nanoparticles of green tea extract that was prepared in this research has good physical stability and good size distribution. Data showed that administration of green tea in the nanometer size replicated the previous studies. Nanoparticulate green tea was able to work as an iron chelator and had a significant effect, similar to the classic chelating agent DFO (2.6 ng/mL vs. 2.7 ng/mL). Compared to previous studies that only used pure extraction of green tea leaf(11), this new size has a better effect as an adjuvant property. This can be seen from group IV (image D), in which ferritin levels went down to 2.1 ng/mL (Fig. 2). The previous study was only able to lower the threshold to 2.5 ng/mL. The data was also in line with Srichairatanakool, et al. who found that green tea extract and epigallocatechin 3-gallate reduced the labile iron pool and protected oxidative stress in iron-loaded cultured hepatocytes(1). In the other study, the green tea supplementation also had a significant effect on reducing ferittin and increasing plasma oxidant activities on the metabolic syndrome(12).
The effects of nanoparticles proved to have a good ability in terms of depleting the state of hemosiderosis. The ability of nanoparticles, which have more volume ratio areas, could lead an active compound to be associated with epithelium surface, accelerating pinocytosis, extravasation through the endothelium, and also microcapillar penetration. The study showed that by the time iron capacity has accumulated in the body, ferritin levels are at similar levels among the groups; from 6.15 ng/mL to 7.04 ng/mL. Declining level of ferritin was achieved after supplementation, the most of which came from the group that was given nanoparticles of green tea and DFO (2.1 ng/mL), whereas the smaller one was in no diet group (5.4 ng/mL) (Fig. 4). At the peak levels of iron, GPx decreased most significantly from normal values to the level of 36 mU/mL. After supplementation, GPx was augmented, especially in the green tea and DFO group (105.1 mU/mL). The study also indicates that the monotherapy of green tea nanoparticles was favorable, as well as the combination with DFO (Fig. 5). Previous study reported that using green tea extract as chelator, malondialdehyde (MDA) level as ROS marker was significantly reduced after couple days treatment in metabolic syndrome(12). This kind of finding was in line with review data provided by Hatcher, et al.(13). They stated that iron-chelation therapy, whether using synthetic or natural iron chelators as monotherapy or a combination, effectively modulates iron biochemistry and improves the status of antioxidant levels. Such treatments have been reported as a good therapy for many oxidant-antioxidant disturbances such as thalassemia(14), cancer(15), metabolic syndrome(12) and Parkinson’s disease(16). The study showed that staining in the cardiac organ has not shown hemosiderin pigment accumulation in the tissue. This means that the increasing level of blood ferritin has not yet raised hemosiderin buildup in the heart; this finding was not in accordance with the expectations. However, the spleen showed the most hemosiderin heap. This finding was in agreement with the previous data, in which hemosiderin was initially built up in the reticuloendothelial organs(17). Giving a different route of Fe would affect the iron accumulation; oral administration of Fe is more likely to accumulate first in the lien, whereas parenteral administration will accumulate in the liver(18).
DFO as a major iron chelating drug is still used in patients with thalassemia(19). DFO given orally is not as effective when administered parenteral. The short half-time of DFO reduces the effectiveness of the chemical agent(20). This medication binds iron with a ratio of 1:1 and is excreted through urine and feces(21). DFO will bind non transferrin binding iron (NTBI), which in turn would reduce ROS production due to reduced NTBI circulating in the blood(22).
DFO administration of 5 mg/kg/day for 30 days was proven to reduce blood ferritin levels in mice induced with chronic iron ferrous sulfate. Decrease in blood ferritin levels did not differ significantly in the group given green tea extract 3 mg/kg/day(11). Green tea contains compounds of catechins (epigallocatechin gallate (EGCG)), an antioxidant compound that is proven to reduce the absorption of Fe(23), which increases the total plasma antioxidant(24) and acts as an iron chelator(22). This action reduces ROS production and protects cells from damage and cell death.
The study found that control group had the highest hemosiderin cells, while the group given green tea extract and DFO had the lowest hemosiderin cells. Mechanisms that may underlie the presence of hemosiderin laden macrophages in the spleen is caused by excessive iron that is not used in the erythropoiesis process. When hemosiderin forms in the spleen it would be considered a foreign body, leading to macrophages phagocytosis in the splenic cords of red pulp and forming hemosiderin laden macrophages(17).
The group with the administration of green tea extract (group IV) had the lowest average of all the treatment groups. This finding was consistent with the previous study, in that blood ferritin levels will be decreased in a group given both DFO and green tea extract. DFO administration as iron chelation will bind iron (Fe2+) from hemosiderin, ferritin, and transferrin in a 1:1 ratio(3). Catechin molecules in green tea extract is known to form a complex bond catechins-Fe2+, OH--catechins and catalyzing Fe3+ to Fe2(25). The combination of this action will reduce the levels of iron formation of hemosiderin in the spleen. It has been known that green tea is a source of polyphenol antioxidants which are good and effective as an iron chelator(26). Catechins, as the highest component in the dry weight of tea leaves, contain EGCG, the most catechins form of green tea(27). EGCG inhibit the binding activity of the iron by reducing the absorption of non-heme iron because of the Gallate/Galoil (3 hydroxyl groups) component in EGCG(23). Metal ions such as Fe2+ and Fe3+ can join up to three gallate groups; polyphenols made from the gallate groups were more effective in binding iron with a 3:1 ratio(26). Nanoparticles of green tea can be conjugated with an iron chelator without changing the iron chelation ability. This complex can improve the efficacy and reduce the toxicity of iron chelation(28). Green tea also has synergitic effect on antioxidant activity when combine with other nature substrate(29).
CONCLUSION
In conclusion, we reported that green tea nanoparticles are capable of acting as an iron chelator and that these compounds are able to interact with standard iron chelators to reduce the toxicity of the chemical agent.
ACKNOWLEDGEMENTS
Authors acknowledge the contribution of persons who help this study, including Wahyu Mulyono for helping in histology laboratory practices, and Nirwan for animal handling.
REFERENCES
- 1.Srichairatanakool S, Kulprachakarn K, Pangjit K, Pattanapanyasat K, Fuchaeron S. Green tea extract and epigallocatechin 3-gallate reduced labile iron pool and protected oxidative stress in iron-loaded cultured hepatocytes. Adv Biosci Biotechnol. 2012;3(8):1140–1150. [Google Scholar]
- 2.Rujito L, Mulatsih S, M. Sofro AM. Status of superoxide dismutase in transfusion dependent thalassaemia. N Am J Med Sci. 2015;7(5):194–198. doi: 10.4103/1947-2714.157480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhu R, Prabhu V, Prabhu RS. Iron overload in beta thalassemia – a review. J Biosci Tech. 2009;1(1):20–31. [Google Scholar]
- 4.Badria FA, Ibrahim AS, Badria AF, Elmarakby AA. Curcumin attenuates iron accumulation and oxidative stress in the liver and spleen of chronic iron-overloaded rats. PLoS One. 2015;10(7):e0134156. doi: 10.1371/journal.pone.0134156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawada T, Konomi A, Yokoi K. Iron deficiency without anemia is associated with anger and fatigue in young Japanese women. Biol Trace Elem Res. 2014;159(1-3):22–31. doi: 10.1007/s12011-014-9963-1. [DOI] [PubMed] [Google Scholar]
- 6.Crespy V, Williamson G. A review of the health effect of green tea catechins in in vivo animal models. J Nutr. 2004;134(12 suppl):3431S–3440S. doi: 10.1093/jn/134.12.3431S. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht MA, Evans W, Raston CL. Green chemistry and the health implications of nanoparticles. Green Chem. 2006;8(5):417–432. [Google Scholar]
- 8.Suri SS, Fenniri H, Singh B. Nanotechnology-based drug delivery systems. J Occup Med Toxicol. 2007;2:16. doi: 10.1186/1745-6673-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namdeo M, Bajpai SK. Chitosan-coated magnetite (CCM) nanoparticles as novel iron-chelator for treatment of β-Thalassemia by tag and drag (TAD) approach. J Bionanosci. 2007;1(2):131–133. [Google Scholar]
- 10.Khan N, Bharali DJ, Adhami VM, Siddiqui IA, Cui H, Shabana SM, et al. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis. 2014;35(2):415–423. doi: 10.1093/carcin/bgt321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ounjaijean S, Thephinlap C, Khansuwan U, Phisalapong C, Fucharoen S, Porter JB, et al. Effect of green tea on iron status and oxidative stress in iron-loaded rats. Med Chem. 2008;4(4):365–370. doi: 10.2174/157340608784872316. [DOI] [PubMed] [Google Scholar]
- 12.Basu A, Betts NM, Mulugeta A, Tong C, Newman E, Lyons TJ. Green tea supplementation increases glutathione and plasma antioxidant capacity in adults with the metabolic syndrome. Nutr Res. 2013;33(3):180–187. doi: 10.1016/j.nutres.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatcher HC, Singh RN, Torti FM, Torti SV. Synthetic and natural iron chelators: therapeutic potential and clinical use. Future Med Chem. 2009;1(9):1643–1670. doi: 10.4155/fmc.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saewong T, Ounjaijean S, Mundee Y, Pattanapanyasat K, Fucharoen S, Porter JB, et al. Effects of green tea on iron accumulation and oxidative stress in livers of iron-challenged thalassemic mice. Med Chem. 2010;6(2):57–64. doi: 10.2174/157340610791321479. [DOI] [PubMed] [Google Scholar]
- 15.Yi S, Wang Y, Huang Y, Xia L, Sun L, Lenaghan SC, et al. Tea nanoparticles for immunostimulation and chemo-drug delivery in cancer treatment. J Biomed Nanotechnol. 2014;10(6):1016–1029. doi: 10.1166/jbn.2014.1782. [DOI] [PubMed] [Google Scholar]
- 16.Mounsey RB, Teismann P. Chelators in the treatment of iron accumulation in parkinson’s disease. Int J Cell Biol. 2012;1 doi: 10.1155/2012/983245. ID 983245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Tubaikh JA. Internal Medicine: An Illustrated radiological Guide. 1st ed. Berlin: Springer-Verlag New York, LLC; 2010. [Google Scholar]
- 18.Aigner E, Theurl I, Theurl M, Lederer D, Haufe H, Dietze O, et al. Pathways underlying iron accumulation in human nonalcoholic fatty liver disease. Am J Clin Nutr. 2008;87(5):1374–1383. doi: 10.1093/ajcn/87.5.1374. [DOI] [PubMed] [Google Scholar]
- 19.Walker JM. Thalassaemia major and the heart: a toxic cardiomyopathy tamed. Heart? 2013;99:827–834. doi: 10.1136/heartjnl-2012-302857. [DOI] [PubMed] [Google Scholar]
- 20.Aaseth J, Skaug MA, Cao Y, Andersen O. Chelation in metal intoxication--Principles and paradigms. J Trace Elem Med Biol. 2015;31:260–266. doi: 10.1016/j.jtemb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Berdoukas V, Farmaki K, Carson S, Wood J, Coates T. Treating thalassemia major-related iron overload: the role of deferiprone. J Blood Med. 2012;3:119–129. doi: 10.2147/JBM.S27400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srichairatanakool S, Ounjaijean S, Thephinlap C, Khansuwan U, Phisalpong C, Fucharoen S. Iron-chelating and free-radical scavenging activities of microwave-processed green tea in iron overload. Hemoglobin. 2006;30(2):311–327. doi: 10.1080/03630260600642666. [DOI] [PubMed] [Google Scholar]
- 23.Samman S, Sandström B, Toft MB, Bukhave K, Jensen M, Sørensen SS, et al. Green tea or rosemary extract added to foods reduces nonheme-iron absorption. Am J Clin Nutr. 2001;73(3):607–612. doi: 10.1093/ajcn/73.3.607. [DOI] [PubMed] [Google Scholar]
- 24.Mandel S, Weinreb O, Amit T, Youdim MB. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J Neurochem. 2004;88(6):1555–1569. doi: 10.1046/j.1471-4159.2003.02291.x. [DOI] [PubMed] [Google Scholar]
- 25.Pinyou P, Kradtap Hartwell S, Jakmunee J, Lapanantnoppakhun S, Grudpan K. Flow injection determination of iron ions with green tea extracts as a natural chromogenic reagent. Anal Sci. 2010;26(5):619–623. doi: 10.2116/analsci.26.619. [DOI] [PubMed] [Google Scholar]
- 26.Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53(2):75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 27.Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78(18):2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Men P, Harris PL, Rolston RK, Perry G, Smith MA. Nanoparticle iron chelators: a new therapeutic approach in alzheimer disease and other neurologic disorders associated with trace metal imbalance. Neurosci Lett. 2006;406(3):189–193. doi: 10.1016/j.neulet.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Ahosseini M, Abbasian S, Moloudian H, Karimi F, Khoshayand M. Evaluation of antioxidant properties of Iran’s native plants and investigation their synergistic effects with tea. Res Pharm Sci. 2012;7(5):S10. [Google Scholar]