Abstract
Undoped CeO2 and 0.50–5.00 mol% Fe-doped CeO2 nanoparticles were prepared by a homogeneous precipitation combined with homogeneous/impreganation method, and applied as photocatalyst films prepared by a doctor blade technique. The superior photocatalytic performances of the Fe-doped CeO2 films, compared with undoped CeO2 films, was ascribed mainly to a decrease in band gap energy and an increase in specific surface area of the material. The presence of Fe3+ as found from XPS analysis, may act as electron acceptor and/or hole donor, facilitating longer lived charge carrier separation in Fe-doped CeO2 films as confirmed by photoluminescence spectroscopy. The 1.50 mol% Fe-doped CeO2 film was found to be the optimal iron doping concentration for MO degradation in this study.
Organic pollutants from the textile industry represent a major source of water contamination. About 15% of commercially used dyes are released without treatment1, while those that are required either a chemical or energy intensive processing which leads to further strain on the environment. An inherent requirement of most of these dyes is that they are stable for long peroids of time in their application. Unfortunately, this also makes degradation (particularly biodegradation) of their excess difficult. Methyl orange (MO) is a simpleazo dye. Azo dyes are widely used in textile, printing, pharmaceutical, and research laboratories. It can enter the human body through skin, and can cause the death of lung tissues, fast heart rate, and vomiting2. For these reasons, MO was chosen as a model dye for photocatalytic degradation in this research.
Advanced oxidation processes based on semiconductor oxide photocatalysts are an effective method for dye degradation3, with various oxide semiconductors such as TiO2, ZnO, and WO3 being used in heterogeneous photocatalysis4,5,6. CeO2 has been shown to be a particularly effective catalyst, in part due to the redox potential of the Ce4+/Ce3+ couple, as well as its resistance to chemical and photocorrosion, and strong light absorptionin the UV region (absorption edge ~385–400 nm). Unfortunately the large band gap (~3.2 eV) limits further application of CeO27,8. From the AM1.5 spectrum, it can be seen that UV light only composes ~3–5% of the photon flux reaching the earth's surface, while around 45% is in the visible light range. Thus it would be hugely beneficial to extend the light absorption of CeO2 to the visible light region9, and as such, many methods have been investigated to shift the absorption onset by doping or coupling with other materials10,11.
Doping with metal ions, specially Fe3+ was chosen in this research as it is believed to facilitate the Fenton reaction, which help improve the photocatalytic activity by producing the very powerful hydroxyl radicals (OH·) as oxidizers12,13.
The separation of catalyst from the waste water suspension is another major problem which limits practical application of nanomaterials14,15. Dense films lack the high surface area required for effective catalysis. Therefore, the CeO2 nanoparticles are fabricated to form a mesoporous film. The doctor blade deposition technique was employed for film preparation to well adhered, high surface, porous films with a good photocatalytic activity16,17.
In this study, kinetics studies of the photocatalytic degradation of MO over Fe–doped CeO2 films were investigated. Improved electron–hole separation and charge transfer process mechanism was also proposed based on the obtained XPS and UV–vis DRS results. Also the formation of OH· in the photocatalytic process has been confirmed via photoluminescence spectroscopy.
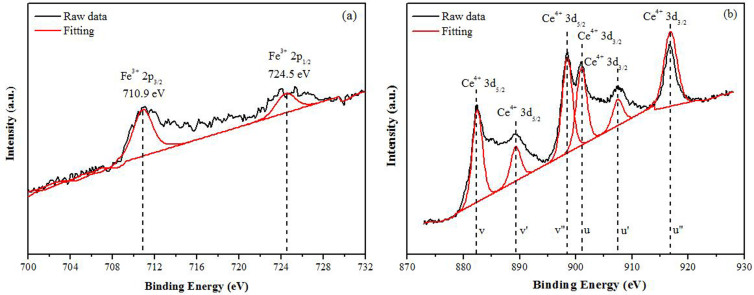
XPS analysis was carried out in order to characterise the valence states of both cerium and iron (5.00 mol% Fe-doped CeO2). The two main peaks of Fe 2p in Figure 1a were observed at 710.9 and 724.5 eV of 2p3/2 and 2p1/2 respectively, which corresponds with theFe3+ species in Fe2O318,19. For the Ce 3d XPS spectrum, the typical six peaks of Ce 3d are presented in Figure 1b. The peaks denoted as V (882.5 eV), V’ (889.3 eV), V”(898.5 eV), U (901.0 eV), U’ (907.5 eV), and U”(916.8 eV) were attributed to Ce4+ state20,21.
Figure 1. The XPS spectra of 5.00 mol% Fe-doped CeO2 (a) Fe 2p and (b) Ce 3d.
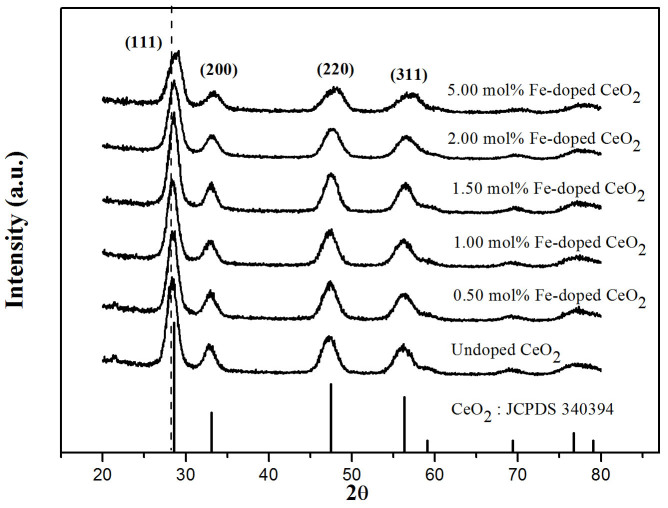
Each of the XRD patterns in Figure 2 indexed as CeO2, with a cubic fluorite structure (JCPDS 3403940)22. No other peaks fromeither metallic iron or FexOy were found in the XRD analysis. Samples with higher doping revealed a shift in the peak associated with the 111 direction (located at 2θ = 28.3899 for the undoped sample), which shifted towards higher values. The calculated crystallite size and lattice parameter decreased slightly with increasing iron content. This may be explained by larger Ce4+ ions (0.101 nm) being replaced by smaller Fe3+ ions (0.064 nm), thus decreasing the lattice parameters, unit cell volume and crystallite size23 (Supplementary Information 1).
Figure 2. XRD patterns of the undoped and Fe-doped CeO2 films fabricated by the doctor blade technique.
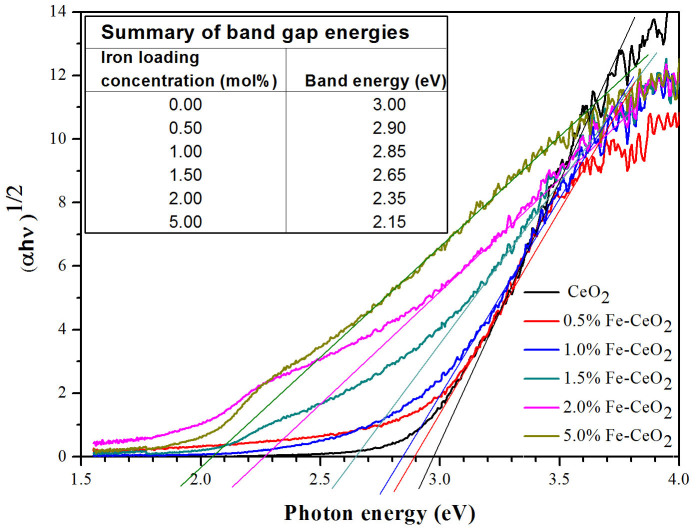
To obtain the band gap energies of the Fe–doped CeO2 films, Schuster-Kubelka-Munk absorption function 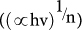 was plotted against the photon energy (hν) according to equation (1)24.
was plotted against the photon energy (hν) according to equation (1)24.
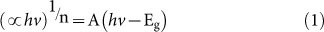 |
where A is a proportionality constant, h is Planck's constant, ν is the frequency of vibration (hence hν is photon energy), and ∝ is an absorption coefficient. The value of n depends on the type of optical transition of the semiconductor (n = 2 for indirect transition)25. The approximated band gap can then be determined from the straight line x–intercept as shown in Figure 3.
Figure 3. Schuster-Kubelka-Munk absorption function of Fe–doped CeO2 films with an inset showing the band gap energies of catalyst films.
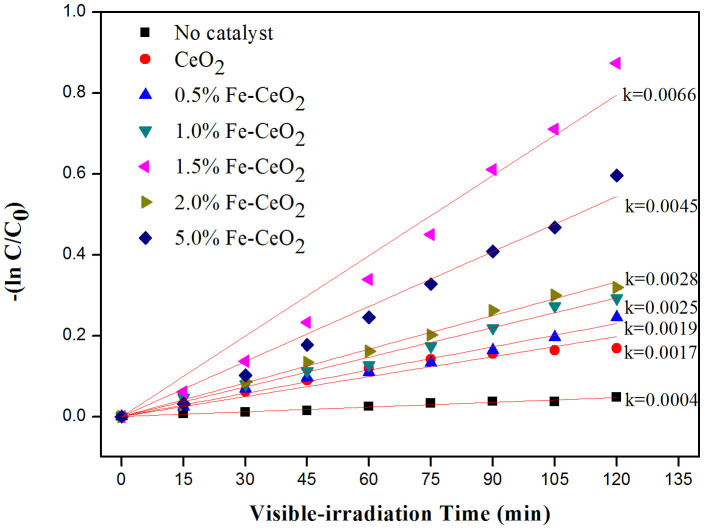
The kinetics of MO decolourisation are presented in Figure 4, and are found to follow pseudo-first order reaction as shown in equation (2)26:
 |
where k is the apparent rate constant (min−1), C0 is the initial concentration of dye and C is the concentration of dye at time (t). As the dye concentration in these experiments remains in the regime where the Beer-Lambert law holds, the concentration can be substituted by the dye absorbance at a given wavelength (typically the peak absorption).
Figure 4. The kinetics plots for pseudo first order reaction of MO decolourisation at pH 5.
Iron modified CeO2 films clearly presented superior photocatalytic activity towards the degradation of MO in pH 5 conditions compared to undoped CeO2, with 1.50 mol% Fe doping providing the most rapid dye degradation. BET analysis (Supplementary Information 2) showed that the 1.50 mol% Fe–doped CeO2 film possessed the highest specific surface area, and hence more active sites are available for the MO degradation reaction27.
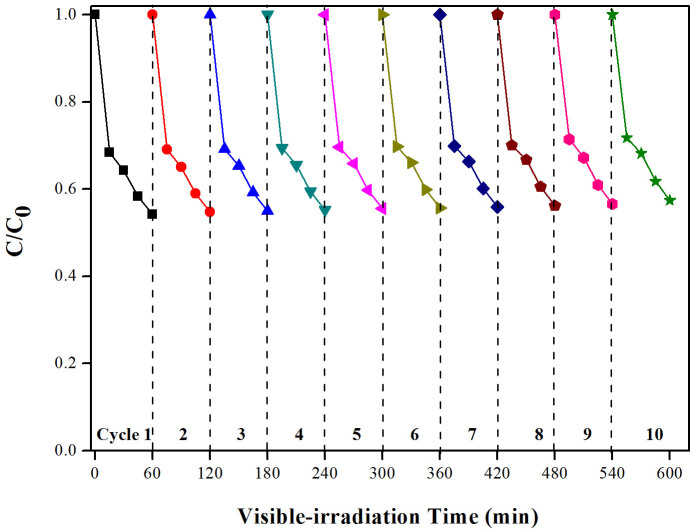
Another important real world consideration for photocatalytic systems is long-term stability. The 1.50 mol% Fe-doped CeO2 was employed to degrade MO solution (pH5) for 2 h before the films was removed and regenerated (heated to 250°C for 120 min) and process repeated (10 cycles in total). The material displayed good stability with regards to photocatalytic performance, with less than 10% decreased from its initial activity during photodegradation process (Figure 5).
Figure 5. Cycling runs of MO decolourisation using 1.50 mol% Fe-doped CeO2 film at pH 5 of MO solution.
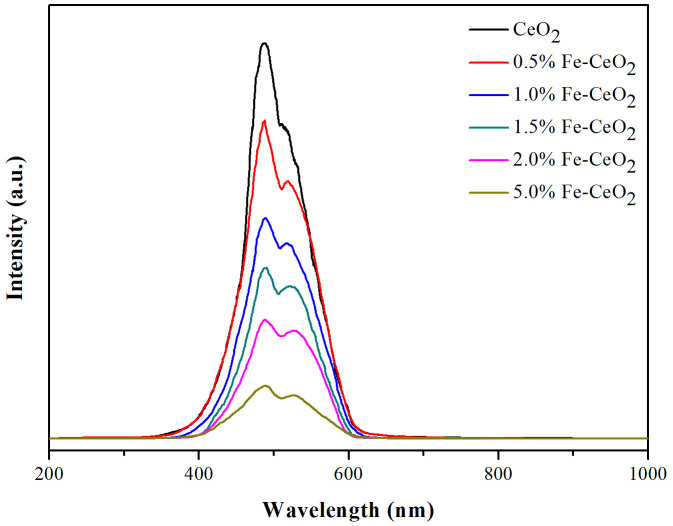
The observation of photoluminescence (PL) was employed to explain the chance of electron–hole pair recombination in catalyst. Since the PL emission spectra was reported from the recombination of excited electrons and holes28,29, thus a higher PL intensity indicated a higher recombination rate of electron–hole pairs under light irradiation. In Figure 6, undoped CeO2 presented the highest intensity, which indicated that undoped CeO2 provided the fastest recombination rate of electrons and holes. After the iron ions were introduced to CeO2, the intensity of the PL emission gradually decreased, which confirmed that the doping with iron ions could delay the recombination rate of photogenerated charge carriers.
Figure 6. PL spectra of CeO2 at differrent amounts of iron doping level.
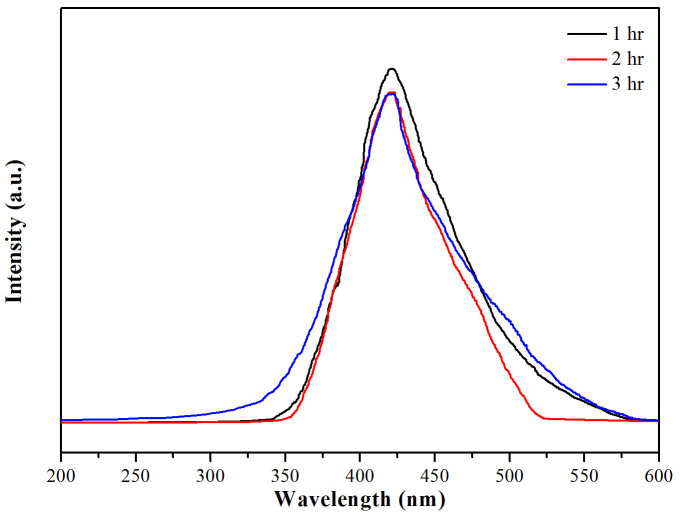
Moreover, the detection of hydroxyl radical (OH·) in photocatalytic reactions have been reported via the reaction of terephthalic acid (TA) with OH·, and formed the fluorescent 2-hydroxy terephthalate (TA–OH·) as a reaction product, which can be detected by fluorescence spectroscopy30. In this study, the 1.50% Fe-doped CeO2 catalyst was employed to detect the generated OH· on the surface. As shown in Figure 7, the PL intensity at approximately 425 nm of TA–OH· increased in the first hour of photocatalytic degradation process and kept constant with increasing time after this one hour, which means that the catalyst presented the highest performance in the first hour of light irradiation.
Figure 7. Fluorescence spectra of a TA–OH· solution generated by 1.50 mol% Fe-doped CeO2 under visible light irradiation.
Discussion
Based on the optical band gap energy values determined above (Figure 3) and equation (3) and (4), below, the conduction band (CB) and valence band (VB) potentials of CeO2 can be calculated31:
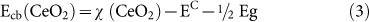 |
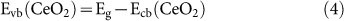 |
Where χ is the absolute electronegativity of the semiconductor (χ is 5.56 eV for CeO2)32,
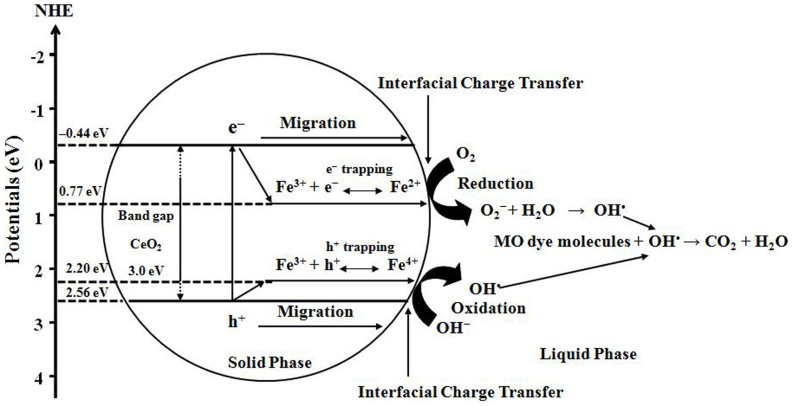
EC is the scaling factor relating the hydrogen electrode scale (NHE) to absolute vacuum scale (AVS) (~4.5 eV vs. AVS for 0 V vs. NHE)32 and Eg is the band gap energy of CeO2 (3.00 eV). The calculated CB and VB potentials of CeO2 are −0.44 and 2.56 eV, respectively.
It is proposed the schematically in Figure 8 that the photogenerated electron-hole pairs are able to be separated for into trap states in the doped material. The presence of Fe3+, as shown in XPS analysis (Figure 1a) may act as electron acceptor (from Fe3+ to Fe2+) and/or hole donor (from Fe3+ to Fe4+) to facilitate charge carrier localisation and hence prolonged separation by trapping at energy levels close to the conduction or valence bands, respectively33. Therefore, the Fe3+ doping could be effective in producing materials that (1) delay electron–hole recombination, thereby increasing the lifetime of the electron–hole separation34 as confirmed in PL results and (2) support the charge carrier transfer to the catalyst surface.
Figure 8. Proposed mechanism for the photoexcited electron–hole separation and transport processes at the Fe-doped CeO2 interface under visible light irradiation.
In summary, Fe-doped CeO2 catalysts with different iron doping concentrations have successfully been fabricated to produce photocatalytic films by a doctor blade technique. Under visible light irradiation, Fe3+ doping can significantly increase the photocatalytic activity of CeO2 towards the degradation of MO, with the best efficiency obtained from a 1.50 mol% iron doping. The enhanced photocatalytic performance upon doping with iron could be ascribed to both the red shift in the light adsorption range and an increased specific surface area, shown in the BET analysis. The presence of Fe3+ observed from XPS analysis may contributed to the Fe3+/Fe2+ and Fe4+/Fe3+ additional levels in the CeO2, and a decrease in band gap energy as a consequence. The PL results confirmed that the iron doping could be effective in delay electron–hole recombination, thereby increasing the lifetime of the electron–hole separation. Since the hydroxyl radical is known to be the key species in photocatalytic reactions, thus the PL results proved that the OH· adduct TA–OH· was formed during the photocatalytic reaction with the presence of catalyst.
Methods
Catalysts powder preparation
Undoped CeO2 and Fe-doped CeO2 powders were prepared by the combination of homogeneous precipitation and impregnation methods. Cerium (III) nitrate hexahydrate (Ce(NO3)3.6H2O) and ferric (III) nitrate nonahydrate (Fe(NO3).9H2O) were used as precursors. Firstly, a 20 mM of Ce(NO3)3.6H2O in 100 mL of 80% ethylene glycol was prepared and stirred at 50°C until fully dissolved. During this time the colour of solution changed from purple to yellow. Addition of ammonia causes the solution to go purple once again, however after the 12 h aging a turbid yellow suspension was produced, which was then washed with deionised water, then ethanol and then dried in a vacuum oven at 70°C for 24 h. Finally, the prepared CeO2 samples were calcined at 500°C for 1 h. Fe-doped CeO2 with different amounts of iron concentration (0.50–5.00 mol%) were prepared by impregnation of undoped CeO2 powders suspensionin water, with the addition of appropriate amount of Fe(NO3)3.9H2O while continuous stirring for 2 h. The sample was then filtered, washed and dried at 70°C overnight and calcined at 500°C for 1 h.
Catalysts film preparation
Films of both doped and undoped CeO2 were prepared by doctor blade technique as described elsewhere35. The obtained film was dried in air at 50°C for about 30 min, and further annealed at 500°C for 2 h in order to remove Triton X-100 binder. The films thickness was ~5.0 ± 0.2 μm as measured by profilometer (Supplementary Information 3).
Film Characterisation
The films thickness was found to be around 5.0 ± 0.2 μm from Profilometer measurement (Veeco Dektak150). The optical properties of the prepared samples were obtained from a photospectrometer (Shimadzu UV-3600) with integrating sphere attachment. The point of zero charge of catalyst powders was measured with the Zeta potential analysis (Malvern, Zetasizer-NanoS90). The crystalline phase of catalyst film was determined by glancing angle X-ray diffraction (GAXRD, Philips X'pert Materials). The porosity and specific surface area (SSA) were determined using Brunauer, Emmett, Teller (BET) analysis of nitrogen adsorption-desorption data (Quantachrome Autosorp 1 MP). The chemical composition and oxidation state of all materials were studied by X-ray photoelectron spectroscopy (XPS) using Mg X-ray source (MgKα, Kratos Axis Ultra DLD). The binding energy of the adventitious carbon (C 1s) line at 285 eV was used for calibration and the position of other peaks was corrected according to the position of the C 1s signal. The thickness cross-section of the films was measured by scanning electron microscopy (FESEM, JSM-6335F, JEOL). The fluorescence spectra of the generated TA–OH· were measured on the fluorescence spectrophotometer at 425 nm (Avantes, AvaSpec-2048 TEC-USB2-2); fluorescence was induced by excitation at 345 nm.
Photocatalytic testing for the degradation of dyes
Photocatalytic degradation processes under visible irradiation were investigated using undopedCeO2 and Fe-dopedCeO2 films through the decolourisation of MO solution with an initial dye concentration of 0.025 mM at pH 5. The adsorption efficiency of MO in dark at different pH value are explained in Supplementary Information 4 and 5. The photoreactor system employed with a halogen lamp (50 watt power) providing a light intensity of ~185 mW cm−2. Decolourisation of dye solutions at different time intervals was determined by measuring the change in absorbance maxima of MO at 464 nm via UV-vis spectrophotometer (Shimadzu UV-1800).
Determination of reactive species (hydroxyl radical, OH·)
In order to measure the relative concentration of hydroxyl radicals, the terephthalic acid (TA) fluorescence method was employed in this study owing to the TA can react with OH· forming highly fluorescent 2-hydroxyterephthalic acid (TA–OH·). The mixture of TA solution was prepared from 5 × 10−4 molar of TA and 6 × 10−3 molar of NaOH in DI water. Then, 0.25 g of the 1.50% Fe-doped CeO2 catalyst was dispersed in 50 mL of the TA aqueous solution. The solution was collected at every one hour during the irradiation procedure in order to estimate the generated TA–OH·, which can be detected by fluorescence spectroscopy at 425 nm36,37.
Supplementary Material
Acknowledgments
This work has been supported by Thailand Research Fund (TRF) through the Royal Golden Jubilee (RGJ)-Ph.D Program. National Research University Project under Thailand's Office of Higher Education Commission; Materials Science Research Center, Department of Chemistry, Faculty of Science and the Graduate School, Chiang Mai University are greatly acknowledged. AN would like to thank the Australian Renewable Energy Agency (ARENA) for financial support. The authors would also like to thank the Australian National Fabrication Facility (ANFF) for access to equipment.
Footnotes
The authors declare no competing financial interests.
Author Contributions D.C. analysed the data and wrote the manuscript. S.P. and B.I. supervised the project, had given valuable advices on the proceeding of this work, and revised the manuscript. A.N. and J.C. designed the concept and the experiment method of the research. S.U. supported the characterisation of the samples. N.W. had provided valuable suggestions on the selection of supplementary information. All authors discussed the results and commented on the manuscript at all stages.
References
- Houas A. et al. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B: Environ. 31, 145–157 (2001). [Google Scholar]
- Ai L., Zhang C. & Meng L. Adsorption of methyl orange from aqueous solution on hydrothermal synthesized Mg-Al layered double hydroxide. J. Chem. Eng. Data. 56, 4217–4225 (2011). [Google Scholar]
- Lathasree S., Nageswara R., Sivasankar B., Sadasivam V. & Rengaraj K. Heterogeneous photocatalytic mineralisation of phenols in aqueous solutions. J. Mol. Catal. A: Chem. 223, 101–105 (2004). [Google Scholar]
- Chen L., Tian J. & Qiu H. Preparation of TiO2 nanofilm via sol-gel process and its photocatalytic activity for degradation of methyl orange. Ceram. Int. 13, 3422–3435 (2009). [Google Scholar]
- Shen W. et al. Photocatalytic degradation for methylene blue using zinc oxide prepared by co-deposition and sol-gel methods. J. Hazard. Mater. 152, 172–175 (2007). [DOI] [PubMed] [Google Scholar]
- Kim S. et al. Photocatalytic activity of metal-inserted WO3 thin films prepared by RF magnetron sputtering. J. Nanosci. Nanotechnol. 10, 7053–7055 (2013). [DOI] [PubMed] [Google Scholar]
- Magesh G., Viswanathan B., Viswanath R. P. & Varadarajan T. K. Photocatalytic behavior of CeO2-TiO2 system for the degradation of methylene blue. Indian J. Chem. B Org. 3, 480–488 (2009). [Google Scholar]
- Truffault L. et al. Application of nanostructured Ca doped CeO2 for ultraviolet filtration. Mater. Res. Bull. 45, 527–535 (2010). [Google Scholar]
- Ho C., Yu J. C., Kwong Y., Mak A. C. & Lai S. Morphology-controllable synthesis of mesoporous CeO2 nano and microstructures. Chem. Mater. 17, 4514–4522 (2005). [Google Scholar]
- Tuprakay S. & Liengcharernsit W. Lifetime and regeneration of immobilized titania for photocatalytic removal of aqueous hexavalent chromium. J. Hazard. Mater. 124, 53–58 (2005). [DOI] [PubMed] [Google Scholar]
- Grätzel M. P. Photoelectrochemical cells. Nature 414, 338–344 (2001). [DOI] [PubMed] [Google Scholar]
- Channei D. et al. Photocatalytic activity under visible light of Fe-doped CeO2 nanoparticles synthesized by flame spray pyrolysis. Ceram. Int. 39, 3129–3134 (2013). [Google Scholar]
- An H. et al. Iron-coated TiO2 nanotubes and their photocatalytic performance. J. Mater. Chem. 20, 603–610 (2010). [Google Scholar]
- Watts R., Kong S. & Lee W. Sedimentation and reuse of titanium dioxide: application to suspended-photocatalyst reactors. J. Environ. Eng. 121, 730–735 (1995). [Google Scholar]
- Wang Z. et al. Synthesis and characterization of SiO2/(PMMA/Fe3O4) magnetic nanocomposites. J.Nanosci. Nanotechnol. 8, 1797–1802 (2008). [PubMed] [Google Scholar]
- Andronic L., Manolache S. & Duta A. Photocatalytic degradation of methyl orange: influence of H2O2 in the TiO2-based system. J. Nanosci. Nanotechnol. 8, 728–732 (2008). [DOI] [PubMed] [Google Scholar]
- Andronic L., Enesca A., Vladuta C. & Duta A. Photocatalytic activity of cadmium doped TiO2 films for photocatalytic degradation of dyes. Chem. Eng. J. 152, 64–71 (2009). [Google Scholar]
- Joshi K. M. & Shrivastava V. S. Removal of methylene blue dye aqueous solution using photocatalysis. Int. J. Nano. Dim. 2, 241–252 (2012). [Google Scholar]
- Yamashita T. & Hayes P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 254, 2441–2449 (2008). [Google Scholar]
- Grosvenor A. P., Kobe B. A., Biesinger M. C. & Mcintyre N. H. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interf. Anal. 36, 1564–1574 (2004). [Google Scholar]
- Pradhan G. K. & Parida K. M. Fabrication of iron-cerium mixed oxide: An efficient photocatalyst for dye degradation. Int. J. Eng. Sci. Tech. 2, 53–65 (2010). [Google Scholar]
- Khare A., Choudhary R. J., Bapna K., Phase D. M. & Sanyal S. P. Resonance photoemission studies of (111) oriented CeO2 thin film grown on Si (100) substrate by pulsed laser deposition. J. Appl. Phys. 108, 1–5 (2010). [Google Scholar]
- Shannon R. D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta. Cryst. Sect. A. 32, 751−767 (1976). [Google Scholar]
- Boonprakob N. et al. Enhanced visible-light photocatalytic activity of g-C3N4/TiO2 films. J. Colloid. Interf. Sci. 417, 402–409 (2014). [DOI] [PubMed] [Google Scholar]
- LÓpez R. & Gómez R. Band-gap energy estimation from diffuse reflectance measurements on sol-gel and commercial TiO2: A comparative study. J. Sol–Gel Sci. Technol. 61, 1–7 (2012). [Google Scholar]
- Olad A., Behboudi S. & Entezami A. A. Preparation, characterization and photocatalytic activity of TiO2/polyaniline core-shell nanocomposite. Bull. Mater. Sci. 35, 801–809 (2012). [Google Scholar]
- Bangashi S. K. & Alam S. Adsorption of acid blue 1 on activated carbon produced from the wood of Ailanthus altissima. Braz. J. Chem. Eng. 26, 275–285 (2009). [Google Scholar]
- Wang X. et al. Novel BiOCl–C3N4 heterojunction photocatalysts: In situ preparation via an ionic-liquid-assisted solvent-thermal route and their visible-light photocatalytic activities. Chem. Eng. J. 234, 361–371 (2013). [Google Scholar]
- Nagaveni K., Hegde M. S. & Madras G. Structure and photocatalytic activity of Ti1-xMxO2-δ (M = W, V, Ce, Zr, Fe, and Cu) synthesized by solution combustion Method. J. Phys. Chem. B. 108, 20204–20212 (2004). [Google Scholar]
- Rawal S. B., Sung S. D. & Lee W. I. Novel Ag3PO4/TiO2 composites for efficient decomposition of gaseous 2-propanol under visible-light irradiation. Catal. Commun. 17, 131–135 (2012). [Google Scholar]
- Xu Y. & Schoonen M. A. A. The absolute energy position of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 85, 543–556 (2000). [Google Scholar]
- Magesh G., Viswanathan B., Viswanath R. P. & Varadarajan T. K. Photocatalytic behavior of CeO2-TiO2 system for the degradation of methylene blue. Indian J. Chem. 48A, 480−488 (2009). [Google Scholar]
- Masui T., Hirai H. & Imanaka N. Synthesis of cerium oxide nanoparticles by hydrothermal crystallization with citric acid. J. Mater. Sci. Lett. 21, 489-491 (2002). [Google Scholar]
- Zhang Y. & Weber W. J. Grain growth and phase stability of nanocrystalline cubic zirconia under ion irradiation. Phys.Rev.B. 82, 184105–184112 (2010). [Google Scholar]
- Belhekar A. A., Awate S. V. & Anand R. Photocatalytic activity of titania modified mesoporous silica for pollution control. Catal. Commun. 3, 453–458 (2002). [Google Scholar]
- Ishibashi K., Fujishima A., Watanabe T. & Hashimoto K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem. Commun. 2, 207–210 (2000). [Google Scholar]
- Li F. T. et al. Photocatalytic oxidative desulfurization of dibenzothiophene under simulated sunlight irradiation with mixed-phase Fe2O3 prepared by solution combustion. Catal. Sci. Technol. 2, 1455–1462 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.