Abstract
Sabin strains used in the manufacture of oral polio vaccine (OPV) replicate in the human organism and can give rise to vaccine-derived polioviruses. The increased neurovirulence of vaccine derivatives has been known since the beginning of OPV use, but their ability to establish circulation in communities has been recognized only recently during the latest stages of the polio eradication campaign. This important observation called for studies of their emergence and evolution as well as extensive surveillance to determine the scope of this phenomenon. Here, we present the results of a study of vaccine-derived isolates from an immunocompromised poliomyelitis patient, the contacts, and the local sewage. All isolates were identified as closely related and slightly evolved vaccine derivatives with a recombinant type 2/type 1 genome. The strains also shared several amino acid substitutions including a mutation in the VP1 protein that was previously shown to be associated with the loss of attenuation. Another mutation in the VP3 protein resulted in altered immunological properties of the isolates, possibly facilitating virus spread in immunized populations. The patterns and rates of the accumulation of synonymous mutations in isolates collected from the patient over the extended period of excretion suggest either a substantially nonuniform rate of mutagenesis throughout the genome, or, more likely, the strains may have been intratypic recombinants between coevolving derivatives with different degrees of divergence from the vaccine parent. This study provides insight into the early stages of the establishment of circulation by runaway vaccine strains.
The use of two highly efficient vaccines, the Sabin live oral polio vaccine (OPV) and the Salk inactivated polio vaccine (IPV), resulted in a dramatic decrease in poliovirus morbidity and led to the virtual disappearance of wild polioviruses from most of the world (38). OPV consists of live attenuated poliovirus strains of three serotypes, while IPV contains formalin-inactivated wild-type polioviruses. In contrast to the live vaccine, IPV does not induce adequate immunity in the gastrointestinal tract and does not prevent cryptic virus circulation in communities immunized with this vaccine (11). The major shortcoming of OPV is its ability to cause rare cases of vaccine-associated paralytic poliomyelitis (VAPP) in vaccine recipients and unimmunized or nonadequately immunized contact persons (36). In recent years most developed, polio-free countries have switched to the exclusive use of IPV, but the great majority of children throughout the rest of the world are still being vaccinated by OPV. In the countries that have already achieved eradication of poliomyelitis but are still using OPV, the only source of rare cases of paralytic poliomyelitis is the vaccine itself. Therefore, according to one of the proposed scenarios for the posteradication period, the use of OPV should completely stop at some point after the world is certified to be free of circulating wild-type polioviruses (11, 40). The basic assumptions underlying this strategy are the absence of natural reservoirs of poliovirus other than in human communities and strictly time-limited circulation of vaccine-derived poliovirus (VDPV) strains (39). The latter assumption is indeed supported by some evidence (13, 40), but other observations also point to some significant risks associated with stopping all polio vaccinations. For example, retrospective analysis of the local cessation of OPV vaccination in 1963 to 1966 after virtual eradication of poliomyelitis by extensive use of Sabin vaccine in the Mogilev region of Byelorussia in the former Soviet Union showed a widespread circulation and evolution of independent lineages of vaccine derivatives (25). Some of these lineages originated from OPV given to 40 children in the community during this intentional gap in immunization. In addition to demonstrating high risks associated with the cessation of OPV vaccination without well-developed global strategy, these results also point to the potential danger of the proposed use of Sabin vaccine to control possible reemergence of poliovirus in the postvaccination period.
The long-term persistence of live vaccine strains in immunocompromised persons and the ability of these strains to evolve into highly diverged immunodeficient VDPVs (iVDPVs) have been well documented (2, 22, 28). Another significant risk of the prolonged persistence and circulation of VDPVs was demonstrated by recent outbreaks of poliomyelitis in Egypt (43), Dominican Republic and Haiti (21), Philippines (7), and Madagascar (37) caused by circulating VDPV (cVDPV) strains that fully regained their virulence and the ability to spread in human populations with poor immunity, apparently resulting from gaps in OPV coverage.
The formulation of rational immunization policies for the final phases of the polio eradication campaign as well as for the posteradication period urgently requires extensive studies of the processes leading to the emergence and evolution of VDPVs (39). Therefore one of the most important tasks of the World Health Organization Global Polio Laboratory Network is the rapid detection and characterization of VDPVs from both clinical specimens and environmental samples (38). Recently we proposed new methods based on the hybridization of viral probes with oligonucleotide microarrays for rapid analysis of genetic variations during the evolution of OPV strains (8). The microarray analysis of viral recombination (MAVR) and the microarray analysis for resequencing and heterogeneity (MARSH) enabled us to generate instant genetic maps of OPV derivatives and to reveal the degree of their evolutionary divergence. Here, we describe the application of these techniques for the analysis of the spread of pathogenic type 2 OPV derivatives that were recently identified in a children's hospital in Russia, the country that was certified polio free in 2002. The results reported here provide insight into the origin and early stages of VDPV evolution.
MATERIALS AND METHODS
Lymphocyte population analysis.
Peripheral blood lymphocytes were isolated in a Ficoll-Verografin density gradient and were labeled with monoclonal antibodies, conjugated with fluorescein isothiocyanate (14). Combinations of monoclonal antibodies were used to detect the following cell markers of differentiation and activation: CD3, CD4, CD8, CD72, CD16, CD20, CD25, and HLA-DR. The lymphocyte subpopulations were analyzed with double staining in a FACscan flow cytometer (Becton Dickinson).
Virus isolation and characterization.
Virus isolation from stool samples and sewage sample was done in RD cells (human rhabdomyosarcoma cell line) by the standard methods (35). The viruses were typed in microneutralization tests with type-specific sera (35). Sampling of sewage was performed locally using the gauze-pad method (31) from the sewage collector of the children's hospital. The gauze-pad exposure period was from 13 to 17 June 2002.
Characterization of poliovirus RNA.
RNA was extracted from cell lysates with Trizol reagent (Life Technologies) and reverse transcribed using random hexanucleotide primers (Boehringer Ingelheim) with avian myeloblastosis virus reverse transcriptase (Promega) at 42°C for 1 h. PCR using Sabin-specific primers was performed as described previously (42). The cDNA copies of the genomic region covering nucleotides 955 to 3384 were sequenced manually or by an ABI Prism 310 genetic analyzer (Applied Biosystems) analyzing both complementary strands.
Oligonucleotide microarray analysis.
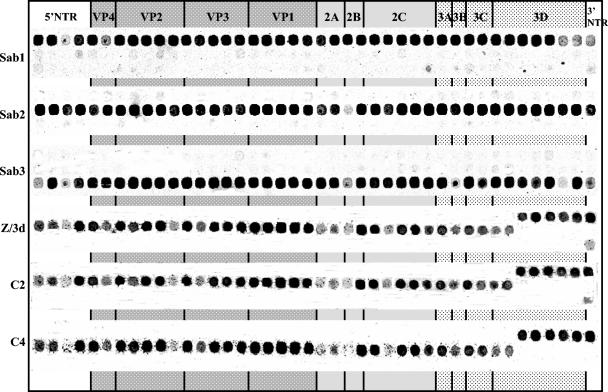
The MAVR and MARSH methods were described in detail previously (8). Briefly, a MAVR microchip contained 42 oligoprobes specific for each of the three Sabin strains spaced ∼150 nucleotides apart along the entire genome and spotted on the microchip according to their location in the genome in three rows corresponding to each serotype (Fig. 1). Hybridization of fluorescently labeled PCR-amplified full-length cDNA was performed in the incubation chamber (ArrayIt) for 1 h at 45°C. Microchip images taken by subsequent confocal laser scanning provided genomic maps revealing the recombination patterns of viruses. The MARSH microarray was created for the identification of emerging point mutations in OPV derivatives. Each microarray contained 102 to 103 (depending on the serotype of poliovirus) oligonucleotides overlapping at half-length and covering the region coding for the VP1 protein. The small size of the oligonucleotides (14 to 24 nucleotides) ensured that their binding would be critically affected by a single nucleotide mismatch. MARSH chips contained four replicates of the microarray to assess reproducibility of results. Hybridization probes were prepared as follows: the VP1-coding region was PCR amplified using the reverse primer that contained a T7 RNA polymerase promoter, PCR products were transcribed in vitro, and RNA was labeled by using a Cy3 Micromax ASAP RNA labeling kit (Perkin Elmer). Two chips were simultaneously hybridized during at least 1 h at 45°C with fluorescently labeled RNA samples prepared from reference Sabin strain and from a test strain. The magnitude of the fluorescent signal from each spot in the reference Sabin strain MARSH image was divided by the magnitude of the respective signal obtained from the hybridization of test sample, and the ratios were used to represent the relative intensity of the hybridization of the microarray with the test sample. Mutations in the sample resulted in decreased binding to the corresponding oligoprobes and thus higher ratio values.
FIG. 1.
The MAVR images of genomes of the PV2/3d, C2, and C4 isolates (the other samples had the identical genomic scheme [data not shown]). The hybridization profiles of three OPV strains are shown for comparison.
ELISA tests.
Intratypic differentiation was performed with cross-absorbed polyclonal antisera (CAP-enzyme-linked immunosorbent assay [ELISA]) following the published procedure (34). Block-ELISA assay of antigenic profiles of polioviruses was described previously (30a) and was based on the use of biotin-labeled polyclonal immunoglobulin G (IgG) and a panel of monoclonal antibodies (MAbs). MAbs specific for site 1 (269, 435, 969, 2b, IIa, and IIo), for site 2 (1251, 1268, IIc, and 1037), and for site 3 (1059, 1103, and 1050) were obtained from the National Institute for Biological Standardization and Control, London, United Kingdom, and from the Institut Pasteur, Paris, France. Briefly, purified poliovirus was captured on ELISA plates coated with polyclonal antiserum and partially blocked by its reaction with a panel of MAbs to different antigenic determinants. The remaining antigen reactivity was revealed by treatment with polyclonal biotin-IgG conjugate and avidin-peroxidase. The decrease in reactivity caused by treatment with a MAb reflects its relative contribution to the overall reactivity of poliovirus. All available MAbs were tested in the block-ELISA assay with the wild-type polioviruses and attenuated Sabin strains. The results were normalized by dividing by the values obtained with specific references, either wild-type or attenuated poliovirus, depending on the specificity of each MAb.
Analysis of sequence relatedness.
Two sequences were considered related, e.g., recently originated from a common predecessor, if the number of common silent mutations was significantly higher than the number expected by chance. If two strains contain k and m mutations out of a total number of n possible silent mutations, the probability of i mutations being present in both strains is as follows: P(i, n, k, m) = kCi × n − kCm − i/nCk, where nCk is the binomial coefficient equal to n!/[k!(n − k)].
The probability that two strains share i or less mutations is equal to the following equation:
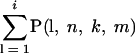 |
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper are available in the GenBank nucleotide sequence database under accession numbers AY649327 to AY649330.
RESULTS
The VAPP case and its epidemiological investigation.
Type 2 poliovirus strain #RUS/2002-040-037-009 (hereinafter referred to as isolate PV2/3d, for brevity) was isolated in June 2002 in a children's hospital in the city of Saratov, Russia, from stool samples of a 20-month-old nonvaccinated child on day 3 after the onset of acute flaccid paralysis (AFP). Prior to the onset of paralytic illness, the child had been placed in the orphanage directly from a maternity clinic. Then, in March 2002, the child had been hospitalized with respiratory viral infection and 3 months later had developed polyradiculoneuropathy. In June 2002, the patient was transferred to another hospital and diagnosed with AFP.
The causative link between the isolate and the paralytic disease was supported by an increase in the level of serum antibodies against type 2 poliovirus (1:16 and 1:64 on days 3 and 21, respectively) and the absence of antibodies against polioviruses of types 1 and 3. Analysis of immunoglobulins performed in September 2002 revealed the level of IgG to be at 340 mg/dl versus the normal range of 490 to 1,250 mg/dl, 82 mg/dl of IgM versus 47 to 178 mg/dl, and 47 mg/dl of IgA versus 35 to 205 mg/dl. The patient also had a decreased count of CD8-positive peripheral blood lymphocytes (T suppressors) and increased count of CD4-positive cells (T helpers). The results of the blood test were consistent with the hypogammaglobulinemia, typical for children with similar health condition and medical history. The immunodeficiency of this patient manifested itself in various unrelated conditions caused by infections, respiratory syndrome, fever, etc., that are not uncommon to those of other children in the orphanage population. The frequency of other immunodeficiency cases in the population is unknown.
Collection of stool samples from the patient was continued for 128 days after the onset of paralysis. The last successful isolation of poliovirus was on day 78. After a period of negative samples, two specimens collected on days 114 and 115 contained a nonpolio enterovirus. Based on the result of this investigation, in August 2002 the case was classified as a contact VAPP case.
An epidemiological study of the case was initiated in the hospital on the third day (17 June 2002) after the admission of the child with signs of paralysis. During these 3 days, the patient shared the ward with five other children (contacts) aged 5 months to 2.5 years. Stools of these contact children as well as a sample from the local sewage were tested. Type 2 polioviruses, named C1, C2, C3, C4, and sewage, were isolated from four contacts and the local sewage, respectively. Only one of the contact children who excreted poliovirus (isolate C2) had been vaccinated with OPV 6 months prior to the date of specimen collection, while the other children had been deferred from polio vaccination for various health reasons.
Overall genome features of the type 2 polio isolates.
Type 2 polioviruses isolated from the VAPP patient, the contacts, and the local sewage were positive in PCR specific for Sabin strains (42), which allowed us to perform rapid analysis by MAVR and MARSH methods (8). MAVR analysis of all these strains showed a type 2/type 1 recombinant scheme of their genomes with the same location of crossover points in the polymerase-coding region (Fig. 1). The exact location of the crossover region was confirmed by sequencing and was found to be between nucleotides 6271 and 6305 (nucleotides numbered according to reference 33). Identical recombination patterns of all these isolates strongly suggested their relatedness. MARSH analysis of the strains initially isolated from the patient, from the contacts, and from the sewage revealed a strong similarity in the mutation patterns in their VP1-coding sequences and only a minor divergence of these strains from their Sabin 2 progenitor (Fig. 2).
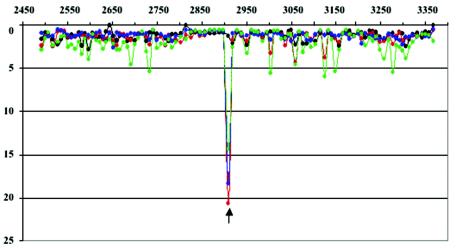
FIG. 2.
Mutational profiles of the VP1-coding region of the isolates from the patient and the contacts. The ratio of signals obtained from hybridization of MARSH with reference sample (Sabin 2) and analyzed strains: Sabin 2, black; isolate PV2/3d, red; contact isolate C1, blue (the other samples C2, C3, and C4 had similar profiles [data not shown]); sewage sample, green. x axis, the nucleotide coordinates on poliovirus genome; y axis, the ratio of fluorescent signals between reference and test samples. The arrow shows the location of mutation U2909→C.
Next, the nucleotide sequences of genomic regions coding for major capsid proteins were determined. All strains collected from the contacts were found to have sequences identical to each other and to the strain isolated from the patient. They had common mutations relative to their Sabin 2 ancestor: two silent mutations in the VP2-coding region, four silent mutations as well as one missense mutation in VP3, and one missense mutation in VP1 (Table 1). The strain obtained from the local sewage had two additional silent mutations and was also clearly related to the strain isolated from the patient on day 3 after the onset of paralysis (Table 1).
TABLE 1.
Antigenic properties and genetic changes observed in the studied isolatesa
| Isolate(s) | CAP-ELISA results | VP2 | VP3 | VP1 |
|---|---|---|---|---|
| PV2/3d, PV2/4d, C1, C2, C3, C4 | NSL | A1104→G, G1704→A | A1990→G (Thr75→Ala), U2077→C, A2205→G, C2398→U, U2430→C | U2909→C (Ile143→Thr) |
| PV2/60d, PV2/61d | NR | A1104→G, G1462→A (Ala170→Thr), C1671→U, G1704→A | A1990→G(Thr75→Ala), (His77→Asn), C1996→A, U2077→C, C2193→A, A2205→G, C2398→U, U2430→C | U2909→C (Ile143→Thr), U3381→C |
| PV2/77d, PV2/78d | NR | A1104→G, C1269→U, A1338→U, C1451→U, U1461→C (Ala166→Val), A1479→G, U1698→C, G1704→A, C1725→U | A1990→G(Thr75→Ala), (His77→Arg), A1997→G, U2077→C, A2205→G, A2325→G, C2398→U, C2418→U, U2430→C | C2543→U (Pro21→Leu), U2909→C (Ile143→Thr), U3090→C, U3163→C, C3243→U |
| Sewage | NSL | A1104→G, C1331→U, G1704→A | C1806→U, A1990→G (Thr75→Ala), U2077→C, A2205→G, C2398→U, U2430→C | U2909→C (Ile143→Thr) |
Common mutations are underlined.
Microarray and sequence analysis of all poliovirus strains collected from the VAPP patient during the entire period of virus excretion was also performed. MAVR analysis of all these strains showed the same type 2/type 1 recombinant scheme of their genomes. The sequence analysis clearly demonstrated the evolution occurring during the period of virus replication leading to accumulation of additional changes (Table 1). The strains isolated on 2 consecutive days had identical genomic sequences, e.g., similar sequences were observed for isolates collected on days 3 and 4, on days 60 and 61, and on days 77 and 78, respectively.
Remarkably, while day 60 and day 78 strains contained all mutations observed on day 3, the additional mutations accumulated over the period of excretion were unique to each strain, raising a question of their relatedness (Table 1 and Fig. 3). Relatedness of coding parts of virus genomes can be analyzed by comparing synonymous (silent) mutations that are less likely (compared to coding mutations) to be fixed in response to a selective pressure, and thus can be considered random markers of evolution. If the number of observed shared synonymous mutations is significantly higher than the number expected by chance, the sequences are likely to be related. The VP2- and VP3-coding regions of day 60 and day 78 isolates were clearly related to each other and to the day 3 isolate since they all contained six common silent mutations, while the probability of even one common mutation (see Materials and Methods) was very low. The VP1-coding region of the day 3 isolate contained no synonymous mutations, making it difficult to trace its history. The absence of common mutations in day 60 and day 78 strains other than those that were already present in the day 3 isolate suggested that the day 78 strain is not a derivative of the day 60 strain, but rather that both of them independently evolved from the day 3 strain.
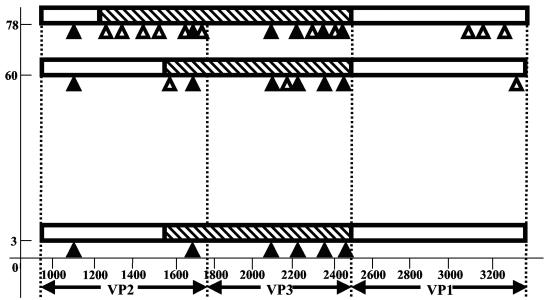
FIG. 3.
Schematic representation of proposed evolution of the patient isolates. Genomic regions coding for the major capsid proteins are presented schematically. Genomic parts originating from proposed older type 2 vaccine derivative are marked by hatched bars; younger Sabin 2 derivative sequences are presented by empty bars. Only approximate positions of the borders between the older and younger segments can be given. The synonymous mutations accumulated are shown by triangles placed under bars; closed and open symbols correspond to common mutations and mutations characteristic of a single isolate, respectively. x axis, nucleotide position numbering; y axis, days after paralysis onset.
The number of silent mutations determined in the genomic regions of day 60 and day 78 strains was not always consistent with the time of virus isolation. We made calculations of the probable “ages” of each of the three analyzed capsid-coding regions of all isolates collected from the VAPP patient assuming a linear rate of fixation of synonymous changes in evolving polioviruses (Table 2). In some cases, the calculated ages significantly exceeded the real time after the illness onset (Table 2), suggesting the coexistence of more than one vaccine-derived lineage with different ages. For instance, the VP3-coding region of the first isolate, PV2/3d, is conspicuously “older” than other slightly evolved genomic regions and supposed to originate from at least 6-month-old vaccine derivative (Table 2 and Fig. 3). The VP3-coding region of the PV2/60d strain isolated 2 months later also demonstrated obvious discrepancy between the predicted time of evolution and time after illness onset (Table 2), despite that it was apparently a descendant of the day 3 isolate (Fig. 3). However, the next strain PV2/78d appeared to also have an older VP2-coding region (Table 2). Taking into account the distribution of common and unique silent mutations, we suggest that the two lineages may have different genomic parts that originated from different vaccine predecessors. Isolate PV2/78d had a significant portion of the VP2-coding region along with the VP3-coding region taken from the older predecessor, and the older insertion in genomes of day 3 and 60 isolates is smaller (Fig. 3). The approximate left border of the older insertions can likely be placed in the VP2-coding region of isolates PV2/60d and PV2/78d somewhere upstream of their uncommon silent mutations. As for the right border of the older insertions in the genomes of the patient isolates, we can suggest its location somewhere downstream of the shared silent mutation U2430→C. Thus, the observed discrepancy in the rates and patterns of the evolution of different genomic parts of these viruses can be best explained by the recombination of coreplicating lineages with various degrees of divergence (Fig. 3), even though dramatic changes in the rate of poliovirus evolution within the patient caused by yet unknown reasons also cannot be excluded.
TABLE 2.
redicted ages of the genomic regions of the patient isolatesa
| Isolate | Time after paralysis onset (mo) | VP2
|
VP3
|
VP1
|
|||
|---|---|---|---|---|---|---|---|
| Ksb | Agec | Ks | Age | Ks | Age | ||
| PV2/3d | 0.1 | 0.7 | 2.8 | 1.7 | 6.8 | 0.0 | |
| PV2/60d | 2 | 1.1 | 4.4 | 2.5 | 10 | 0.3 | 1.2 |
| PV2/78d | 2.6 | 3.4 | 13.6 | 2.6 | 10.4 | 1.2 | 4.8 |
The most conspicuous discrepancies between the calculated ages and the time after paralysis onset are underlined.
Ks, the percentage of mutated synonymous sites among all synonymous sites.
Antigenic properties of the isolates.
The strains isolated from the VAPP patient, the contacts, and from the sewage appear to be antigenically different from the parental Sabin 2 strain as revealed by the CAP-ELISA test. Both strains obtained from the patient on days 3 and 4 and all strains isolated from the contacts and the sewage were classified as non-Sabin-like (NSL), but other isolates collected from the patient at later times had nonreactive (NR) properties (Table 1). We performed analysis of antigenic profiles by a block-ELISA test of the strains isolated from the patient on days 3, 60, and 78 and the strain obtained from the hospital sewage. It demonstrated a clear difference between antigenic profiles of the Sabin 2 strain and these isolates, as well as between consecutive isolates from the patient obtained at different times of excretion (Fig. 4). Therefore, it was interesting to determine the molecular basis of this antigenic drift.
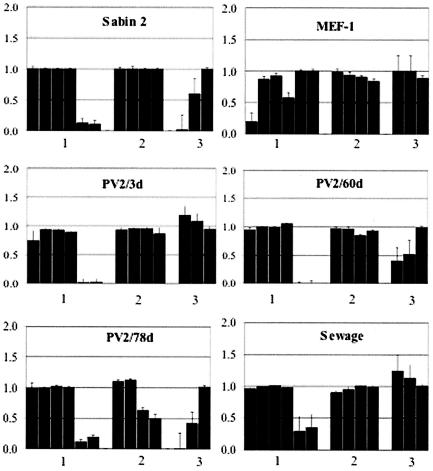
FIG. 4.
Epitope profiles of the PV2/3d, PV2/60d, PV2/78d, and sewage isolates determined by block-ELISA procedure. Profiles of reference strains Sabin 2 and MEF-1 are placed for comparison. x axis, the antigenic sites tested; y axis, the values represent the ratio of block percent in a sample to the respective block percent in a homotypic reference, i.e., MEF-1 for wild-type specific MAbs and Sabin 2 for MAbs specific to attenuated virus, respectively. The order of the monoclonal antibodies used is the same as indicated in Materials and Methods.
The first strains isolated from the VAPP patient and all the strains from the contacts and sewage that demonstrated NSL properties in CAP-ELISA had only one amino acid substitution, Thr75→Ala, located in the VP3 protein next to the AgS3B. No other changes of known antigenic sites were observed (Table 1). The sequencing of the strain isolated on day 60, which had NR properties in CAP-ELISA, revealed additional amino acid substitutions: His77→Asn in AgS3B of VP3 and Ala170→Thr in site 2B of VP2. Surprisingly, the last isolate, PV2/78d, had another substitution, His77→Arg in AgS3B of VP3, as well as mutation Ala166→Val in AgS2B of VP2 (Table 1), further confirming that it evolved independently from the day 60 isolate (see above). In addition to the mutations in and near the antigenic sites, all strains isolated from the patient, contacts, and sewage contained the C→T substitution at nucleotide 2909 resulting in the Ile143→Thr mutation in VP1, which was previously shown to result in the loss of attenuation of Sabin 2 poliovirus (27).
DISCUSSION
Adaptive mutations in poliovirus evolution.
Quantitative and qualitative contributions of individual mutations to poliovirus virulence are poorly understood. Certain changes observed in strains causing VAPP tend to be reversions of known attenuating mutations. For the Sabin 2 strain, it was shown that reversions at just two positions, at nucleotide 481 in the 5′ nontranslated region and at amino acid 143 in the capsid protein VP1, resulted in highly neurovirulent virus (27). Indeed, the VP1 sequence of the type 2 vaccine derivatives isolated from the VAPP patient on day 3 after the onset of paralysis differed from its progenitor only in this neurovirulent mutation. Frequent occurrence of this mutation suggests that it provides the virus with a strong replicative advantage in the organisms of vaccine recipients. It is also reasonable to assume that this is not the only mutation in vaccine derivatives that occurs under strong selective pressure. One obvious mechanism that can explain such selective pressure is the escape from neutralization by antibodies. Amino acid substitution at position 75 of VP3 is located in the immediate vicinity of antigenic site 3B and may lead to the observed switch of antigenic properties from Sabin-like to NSL in both polyclonal and monoclonal ELISA tests. The effect of this mutation on antigenic properties suggests that amino acid residue 75 of VP3 is a functional part of the antigenic site and that the epitope may be larger than previously recognized. This mutation was also frequently observed in other type 2 vaccine-derived isolates including some cVDPV strains (25, 43; our unpublished data), suggesting that it confers adaptive advantage. Rapid antigenic evolution of the isolates leading to conversion from NSL to NR phenotype revealed in CAP-ELISA tests was accompanied by additional amino acid changes that occurred at positions 166 or 170 in VP2 and at position 77 in VP3 in the strains isolated on days 60 and 78 from the onset of VAPP (Table 1). Either or both of these changes may be responsible for the switch in reactivity with polyclonal antiserum. It should be noted that the change His77→Arg in AgS3B was also observed in all studied OPV-related strains with NR properties in CAP-ELISA (our unpublished data), pointing to positive selection of this mutation.
Block-ELISA analysis was performed only with MAbs having neutralizing activity, and therefore the alteration of the antigenic profile revealed by this test suggests that the strain may also have altered neutralization properties. The mutations located in or near the antigenic regions and associated with altered antigenic features may have been fixed as a result of immune evasion. We suggest that emergence of runaway vaccine strains with increased transmissibility and virulence may begin with antigenic drift occurring under immunological pressure from the immune system of a host. This mechanism does not necessarily require poor overall immune status of a given community. Inadequate population immunity simply provides the spreading virus a better chance of establishing circulation and producing outbreaks of paralytic disease, while in adequately vaccinated communities the VDPV strains do not cause disease other than in rare unvaccinated individuals.
Since most of the amino acid substitutions are fixed under selective pressure, they do not represent a good marker of relatedness of VDPV isolates. Inference about phylogenetic relationship can only be made based on analysis of synonymous mutations that emerge under no or much smaller selective pressure. The patient isolates described in this study demonstrate different apparent rates and patterns of mutation accumulation in different genomic regions. This can be due either to spontaneous local burst of mutagenesis (which has never been reliably documented for poliovirus) or by recombination between viruses from two lineages having different degrees of divergence from their vaccine progenitor (Fig. 3). In either case, the divergence observed in the VP2- and VP3-coding regions being higher than that in the VP1 region suggests that the commonly accepted method of determining the age of VDPV by analysis of VP1-coding sequence alone (11) can produce erroneous estimates. Currently we are preparing the MARSH microarray covering the entire P1 region and propose to use it for more-accurate dating of emerging VDPV.
Recombination in VDPV evolution.
It is well known that vaccine recombinants are generated early after OPV vaccination (6, 10). Recombination is currently considered to be a mechanism of molecular evolution that helps RNA viruses to counter accumulation of adverse mutations that are readily incorporated by the error-prone viral polymerase, as well as to generate sequence variability. Exchange of genomic parts may lead to the emergence of viral particles with genomes that are purged of these mutations and therefore can contribute to maintaining adequate viral fitness despite extremely high mutation rates (9). The link between recombination and the ability of a strain to cause VAPP is still an open question. Intertypic recombinants can be isolated not only from the patients and their contacts (9, 26, 29) but are often excreted by healthy OPV recipients (3, 6, 10). The crossover point of intertypic recombination in genomes of the studied isolates was found within the polymerase-coding region, where recombination was reported very frequently. Generally, crossover points were observed along the entire genome part coding for nonstructural proteins. Recently, type 3 or type 2 recombinants with crossovers in the capsid-coding region near the end of the VP1-coding region were also reported (3, 29).
The ease with which intertypic recombination occurs suggests that intratypic recombination may take place even more frequently. The present study provides us with an example of possible intratypic recombination inside the capsid-coding region of the poliovirus genome. However, close relatedness of recombining partners and the low number of mutations involved makes unequivocal proof of such events difficult, and the alternative explanation is a substantially unequal rate of accumulation of mutations in individual parts of the capsid-coding region. Such inequality has never been convincingly demonstrated, while recombination is a well-known phenomenon. Similarly, the concurrent replication of independent lineages of type 1 poliovirus diverging from a single vaccine predecessor in the organism of VAPP patients was previously demonstrated (16, 18, 22), as was the coexistence of different wild-type polioviruses in healthy children (19). Therefore, we favor the explanation involving the recombination of different lineages of vaccine derivatives (Fig. 3).
The emergence of transmissible VDPV in an adequately vaccinated population.
The VAPP case described in this communication was caused by Sabin 2 derivative as evidenced by properties of the virus isolated on day 3 after the onset of paralysis, as well as by an increase in the serotype-specific antibodies. Since the immunocompromised patient had never received OPV, this is clearly a community acquired VAPP case. There were at least 14 children in the first hospital with ages from 3.5 to 21 months who received OPV 1 to 2 months prior to contact with the AFP patient. Because the stool samples were not collected from those children, it was impossible to trace the true origin of this virus. Following the onset of the disease, the virus rapidly and widely spread in another hospital and was isolated from several contact children, including one who was vaccinated with OPV. The next day after the admission of the VAPP patient the virus was also isolated from hospital sewage. The patient continued to excrete evolving OPV derivatives during at least 78 days. This is the first documented case of spread of revertant poliovirus from the initial polio case to several contacts in well-immunized population and illustrates the plausible mechanism leading to the emergence of virulent VDPV with increased transmissibility.
Paralytic disease in immunodeficient patients following their vaccination with OPV has been recognized for a long time (12, 41), but only during the past decade has the phenomenon received the deserved scrutiny (1, 2, 5, 15, 16, 22, 24, 28, 30). Long-term excretion of iVDPVs poses a potential threat to the lasting success of polio eradication, but there is still no firm evidence of the spread of virus from chronically infected persons to a wider community (11, 20).
Currently, highly diverged OPV derivatives are classified into two major types: the so-called immunodeficient or iVDPVs, observed mainly in developed countries with high OPV coverage, and circulating or cVDPVs, causing polio outbreaks during extensive circulation in populations with poor vaccine coverage and hygiene, crowding, etc. (11, 23). The distinction between these two types of VDPV may be superficial and the conversion of iVDPV to cVDPV under some circumstances cannot be excluded. There are reports of highly evolved VDPVs that do not easily fit the above classification. For instance, such strains were isolated from immunocompetent patients with VAPP (9, 17), a healthy contact of the VAPP case (8), and from sewage without any known source of excretion (4, 32; our unpublished data). The revertant strains described in this paper show that transmissibility can be acquired very early in the process of evolution of vaccine poliovirus. Therefore, dividing VDPVs into circulating and immunocompromised categories, which implicitly suggests that the latter are not transmissible, may not be justified. Furthermore, the strain identified in this study was isolated from a mildly immunocompromised child, but it is conceivable that such strains could emerge in normal vaccine recipients or contacts. We also suggest that at least in countries such as Russia, one of the risk factors for the emergence of transmissible VDPV appears to be children's hospitals and orphanages. Inadequate sanitation as well as close contact between children of different age groups with different vaccination statuses and other diseases potentially weakening the immune system can lead to higher levels of poliovirus transmission and thereby constitute risk factors facilitating the emergence of highly pathogenic vaccine-derived variants.
The findings reported in this paper may have profound implications for the strategy of the polio eradication campaign. First, they suggest that, while OPV may cause very rare VAPP cases, discontinuing OPV use without replacing it with other means of protection against poliomyelitis carries a high risk of the resuming of widespread poliovirus circulation. Second, the proposal to use OPV for emergency response in case it happens after immunization has been stopped appears to be dubious. And finally, it emphasizes the critical importance of surveillance for poliovirus in environments and communities that must continue in the foreseeable future regardless of the immunization policies. All this calls for urgent reevaluation and correction of the current polio eradication strategy.
Acknowledgments
We thank G. Lipskaya, WHO/EURO, and M. Bichurina for their assistance in epidemiological investigation. We express our gratitude to virologists from the Government Center for Sanitary and Epidemiological Surveillance of Saratov for their technical assistance. We would also like to thank Javier Martin and Radu Crainic for providing us with monoclonal antibodies.
This work was supported in part by grants from the World Health Organization, the Defense Advanced Research Project Agency, the National Vaccine Program Office, the INTAS, and by the Russian Foundation for Basic Research.
REFERENCES
- 1.Agol, V. I., G. A. Belov, E. A. Cherkasova, G. V. Gavrilin, M. S. Kolesnikova, L. I. Romanova, and E. A. Tolskaya. 2001. Some problems of molecular biology of poliovirus infection relevant to pathogenesis, viral spread and evolution, p. 43-50. In F. Brown (ed.), Progress in polio eradication: vaccine strategies for the end game, vol. 105. S. Karger, Basel, Switzerland. [PubMed]
- 2.Bellmunt, A., G. May, R. Zell, P. Pring-Akerblom, W. Verhagen, and A. Heim. 1999. Evolution of poliovirus type I during 5.5 years of prolonged enteral replication in an immunodeficient patient. Virology 265:178-184. [DOI] [PubMed] [Google Scholar]
- 3.Blomqvist, S., A. L. Bruu, M. Stenvik, and T. Hovi. 2003. Characterization of a recombinant type 3/type 2 poliovirus isolated from a healthy vaccinee and containing a chimeric capsid protein VP1. J. Gen. Virol. 84:573-580. [DOI] [PubMed] [Google Scholar]
- 4.Blomqvist, S., C. Savolainen, P. Laine, P. Hirttio, E. Lamminsalo, E. Penttila, S. Jorks, M. Roivainen, and T. Hovi. 2004. Characterization of a highly evolved vaccine-derived poliovirus type 3 isolated from sewage in Estonia. J. Virol. 78:4876-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buttinelli, G., V. Donati, S. Fiore, J. Marturano, A. Plebani, P. Balestri, A. R. Soresina, R. Vivarelli, F. Delpeyroux, J. Martin, and L. Fiore. 2003. Nucleotide variation in Sabin type 2 poliovirus from an immunodeficient patient with poliomyelitis. J. Gen. Virol. 84:1215-1221. [DOI] [PubMed] [Google Scholar]
- 6.Cammack, N., A. Phillips, G. Dunn, V. Patel, and P. D. Minor. 1988. Intertypic genomic rearrangements of poliovirus strains in vaccinees. Virology 167:507-514. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2001. Public health dispatch: acute flaccid paralysis associated with circulating vaccine-derived polioviruses—Philippines. 2001. Morb. Mortal. Wkly. Rep. 50:874-875. [PubMed] [Google Scholar]
- 8.Cherkasova, E., M. Laassri, V. Chizhikov, E. Korotkova, E. Dragunsky, V. I. Agol, and K. Chumakov. 2003. Microarray analysis of evolution of RNA viruses: new evidence of circulation of virulent highly divergent vaccine-derived polioviruses. Proc. Natl. Acad. Sci. USA 100:9398-9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherkasova, E. A., E. A. Korotkova, M. L. Yakovenko, O. E. Ivanova, T. P. Eremeeva, K. M. Chumakov, and V. I. Agol. 2002. Long-term circulation of vaccine-derived poliovirus that causes paralytic disease. J. Virol. 76:6791-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuervo, N. S., S. Guillot, N. Romanenkova, M. Combiescu, A. Aubert-Combiescu, M. Seghier, V. Caro, R. Crainic, and F. Delpeyroux. 2001. Genomic features of intertypic recombinant Sabin poliovirus strains excreted by primary vaccinees. J. Virol. 75:5740-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowdle, W. R., E. De Gourville, O. M. Kew, M. A. Pallansch, and D. J. Wood. 2003. Polio eradication: the OPV paradox. Rev. Med. Virol. 13:277-291. [DOI] [PubMed] [Google Scholar]
- 12.Feigin, R. D., M. A. Guggenheim, and S. D. Johnsen. 1971. Vaccine-related paralytic poliomyelitis in an immunodeficient child. J. Pediatr. 79:642-647. [DOI] [PubMed] [Google Scholar]
- 13.Fine, P. E., and I. A. Carneiro. 1999. Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am. J. Epidemiol. 150:1001-1021. [DOI] [PubMed] [Google Scholar]
- 14.Foucar, K., and J. A. Glueken. 1982. Clinical application of immunologic techniques to the diagnosis of lymphoproliferative and immunodeficiency disorders. Lab. Med. 13:403-413. [Google Scholar]
- 15.Friedrich, F. 2000. Molecular evolution of oral poliovirus vaccine strains during multiplication in humans and possible implications for global eradication of poliovirus. Acta Virol. 44:109-117. [PubMed] [Google Scholar]
- 16.Gavrilin, G. V., E. A. Cherkasova, G. Y. Lipskaya, O. M. Kew, and V. I. Agol. 2000. Evolution of circulating wild poliovirus and of vaccine-derived poliovirus in an immunodeficient patient: a unifying model. J. Virol. 74:7381-7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgescu, M. M., J. Balanant, A. Macadam, D. Otelea, M. Combiescu, A. A. Combiescu, R. Crainic, and F. Delpeyroux. 1997. Evolution of the Sabin type 1 poliovirus in humans: characterization of strains isolated from patients with vaccine-associated paralytic poliomyelitis. J. Virol. 71:7758-7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgescu, M. M., J. Balanant, S. Ozden, and R. Crainic. 1997. Random selection: a model for poliovirus infection of the central nervous system. J. Gen. Virol. 78:1819-1828. [DOI] [PubMed] [Google Scholar]
- 19.Hovi, T., N. Lindholm, C. Savolainen, M. Stenvik, and C. Burns. 2004. Evolution of wild-type 1 poliovirus in two healthy siblings excreting the virus over a period of 6 months. J. Gen. Virol. 85:369-377. [DOI] [PubMed] [Google Scholar]
- 20.Hull, H. F. 2001. The future of polio eradication. Lancet Infect. Dis. 1:299-303. [DOI] [PubMed] [Google Scholar]
- 21.Kew, O., V. Morris-Glasgow, M. Landaverde, C. Burns, J. Shaw, Z. Garib, J. Andre, E. Blackman, C. Freeman, J. Jorba, R. Sutter, G. Tambini, L. Venczel, C. Pedreira, F. Laender, H. Shimizu, T. Yoneyama, T. Miyamura, H. van Der Avoort, M. Oberste, D. Kilpatrick, S. Cochi, M. Pallansch, and C. de Quadros. 2002. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 296:356-359. [DOI] [PubMed] [Google Scholar]
- 22.Kew, O. M., R. W. Sutter, B. K. Nottay, M. J. McDonough, D. R. Prevots, L. Quick, and M. A. Pallansch. 1998. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J. Clin. Microbiol. 36:2893-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kew, O. M., P. F. Wright, V. I. Agol, F. Delpeyroux, H. Shimizu, N. Nathanson, and M. A. Pallansch. 2004. Circulating vaccine-derived polioviruses: current state of knowledge. Bull. W. H. O. 82:16-23. [PMC free article] [PubMed] [Google Scholar]
- 24.Khetsuriani, N., D. R. Prevots, L. Quick, M. E. Elder, M. Pallansch, O. Kew, and R. W. Sutter. 2003. Persistence of vaccine-derived polioviruses among immunodeficient persons with vaccine-associated paralytic poliomyelitis. J. Infect. Dis. 188:1845-1852. [DOI] [PubMed] [Google Scholar]
- 25.Korotkova, E. A., R. Park, E. A. Cherkasova, G. Y. Lipskaya, K. M. Chumakov, E. V. Feldman, O. M. Kew, and V. I. Agol. 2003. Retrospective analysis of a local cessation of vaccination against poliomyelitis: a possible scenario for the future. J. Virol. 77:12460-12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipskaya, G. Y., A. R. Muzychenko, O. K. Kutitova, S. V. Maslova, M. Equestre, S. G. Drozdov, R. P. Bercoff, and V. I. Agol. 1991. Frequent isolation of intertypic poliovirus recombinants with serotype 2 specificity from vaccine-associated polio cases. J. Med. Virol. 35:290-296. [DOI] [PubMed] [Google Scholar]
- 27.Macadam, A. J., S. R. Pollard, G. Ferguson, R. Skuce, D. Wood, J. W. Almond, and P. D. Minor. 1993. Genetic basis of attenuation of the Sabin type 2 vaccine strain of poliovirus in primates. Virology 192:18-26. [DOI] [PubMed] [Google Scholar]
- 28.Martin, J., G. Dunn, R. Hull, V. Patel, and P. D. Minor. 2000. Evolution of the Sabin strain of type 3 poliovirus in an immunodeficient patient during the entire 637-day period of virus excretion. J. Virol. 74:3001-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin, J., E. Samoilovich, G. Dunn, A. Lackenby, E. Feldman, A. Heath, E. Svirchevskaya, G. Cooper, M. Yermalovich, and P. D. Minor. 2002. Isolation of an intertypic poliovirus capsid recombinant from a child with vaccine-associated paralytic poliomyelitis. J. Virol. 76:10921-10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minor, P. 2001. Characteristics of poliovirus strains from long-term excretors with primary immunodeficiencies, p. 75-80. In F. Brown (ed.), Progress in polio eradication: vaccine strategies for the end game, vol. 105. S. Karger, Basel, Switzerland. [PubMed]
- 30a.Rezapkin, G., J. Martin, and K. Chumakov. Analysis of antigenic profiles of inactivated poliovirus vaccine and vaccine-derived polioviruses by Block-ELISA method. Biologicals, in press. [DOI] [PubMed]
- 31.Riordan, J. 1962. Isolation of enteroviruses from sewage before and after vaccine administration. Yale J. Biol. Med. 34:512-520. [PMC free article] [PubMed] [Google Scholar]
- 32.Shulman, L. M., Y. Manor, R. Handsher, F. Delpeyroux, M. J. McDonough, T. Halmut, I. Silberstein, J. Alfandari, J. Quay, T. Fisher, J. Robinov, O. M. Kew, R. Crainic, and E. Mendelson. 2000. Molecular and antigenic characterization of a highly evolved derivative of the type 2 oral poliovaccine strain isolated from sewage in Israel. J. Clin. Microbiol. 38:3729-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toyoda, H., M. Kohara, Y. Kataoka, T. Suganuma, T. Omata, N. Imura, and A. Nomoto. 1984. Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J. Mol. Biol. 174:561-585. [DOI] [PubMed] [Google Scholar]
- 34.van der Avoort, H. G., B. P. Hull, T. Hovi, M. A. Pallansch, O. M. Kew, R. Crainic, D. J. Wood, M. N. Mulders, and A. M. van Loon. 1995. Comparative study of five methods for intratypic differentiation of polioviruses. J. Clin. Microbiol. 33:2562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. 1997. Manual for the virologic investigation of poliomyelitis. W.H.O./EPI/GEN/97.1. World Health Organization, Geneva, Switzerland.
- 36.World Health Organization. 1998. Report of the Second Meeting of the Technical Consultation Group for Global Eradication of Poliomyelitis. World Health Organization, Geneva, Switzerland.
- 37.World Health Organization. 2002. Paralytic poliomyelitis in Madagascar. Wkly. Epidemiol. Rec. 77:241-242. [PubMed] [Google Scholar]
- 38.World Health Organization. 2003. Progress towards global eradication of poliomyelitis. Wkly. Epidemiol. Rec. 17:138-144. [PubMed] [Google Scholar]
- 39.Wood, D. J. 2001. The scientific basis for stopping polio immunisation—issues and challenges, p. 69-72. In F. Brown (ed.), Progress in polio eradication: vaccine strategies for the end game, vol. 105. S. Karger, Basel, Switzerland. [PubMed]
- 40.Wood, D. J., R. W. Sutter, and W. R. Dowdle. 2000. Stopping poliovirus vaccination after eradication: issues and challenges. Bull. W. H. O. 78:347-357. [PMC free article] [PubMed] [Google Scholar]
- 41.Wyatt, H. V. 1975. Risk of live poliovirus in immunodeficient children. J. Pediatr. 87:152-153. [DOI] [PubMed] [Google Scholar]
- 42.Yang, C. F., L. De, B. P. Holloway, M. A. Pallansch, and O. M. Kew. 1991. Detection and identification of vaccine-related polioviruses by the polymerase chain reaction. Virus Res. 20:159-179. [DOI] [PubMed] [Google Scholar]
- 43.Yang, C. F., T. Naguib, S. J. Yang, E. Nasr, J. Jorba, N. Ahmed, R. Campagnoli, H. van der Avoort, H. Shimizu, T. Yoneyama, T. Miyamura, M. Pallansch, and O. Kew. 2003. Circulation of endemic type 2 vaccine-derived poliovirus in Egypt from 1983 to 1993. J. Virol. 77:8366-8377. [DOI] [PMC free article] [PubMed] [Google Scholar]