Summary
Efforts to identify pharmaceuticals to treat heritable metabolic liver diseases have been hampered by the lack of models. However, cells with hepatocyte characteristics can be produced from induced pluripotent stem cells (iPSCs). Here we have used hepatocyte–like cells generated from homozygous familial hypercholesterolemia (hoFH) iPSCs to identify drugs that can potentially be repurposed to lower serum LDL-C. We found that cardiac glycosides reduce the production of apolipoprotein B (apoB) from human hepatocytes in culture and the serum of avatar mice harboring humanized livers. The drugs act by increasing the turnover of apoB protein. Analyses of patient medical records revealed that the treatment of patients with cardiac glycosides reduced serum LDL-C levels. These studies highlight the effectiveness of using iPSCs to screen for potential treatments for inborn errors of hepatic metabolism and suggest that cardiac glycosides could provide an approach for reducing hepatocyte production of apoB and treating hypercholesterolemia.
Keywords: induced pluripotent stem cells, drug screen, hypercholesterolemia, hepatocytes, metabolic liver disease
Graphical abstract

Introduction
The Next Generation Genetic Association Studies consortium was developed to provide a functional dimension to the genomic study of cardiovascular disease. The primary goal of this initiative is to exploit the power of cellular reprogramming to generate iPSCs from thousands of patients with defined genetic variations that correlate with cardiovascular disease. To date iPSC lines produced by the consortium are available from genotyped patients with coronary artery disease, platelet aggregation deficiencies, lipid disorders, QT interval and ECG cardiac traits, pulmonary hypertension, insulin resistance, left ventricular hypertrophy, myocardial infarction, and sickle cell anemia (http://www.wicell.org/home/stem-cell-lines/collections/collections.cmsx). Having banks of patient-specific iPSCs provide investigators with cells for functional studies and drug discovery. As proof of feasibility we, therefore, sought to use iPSC–derived hepatocyte–like cells (referred to here as hepatocytes) from a familial hypercholesterolemia patient for a screen to identify pharmaceuticals that could be used to treat hypercholesterolemia in a low-density lipoprotein receptor (LDLR)–independent manner.
Familial hypercholesterolemia is a disease caused primarily by mutations in the LDLR. The disease is characterized by elevated serum low density lipoprotein-cholesterol (LDL-C). In patients with compound heterozygous or homozygous mutations in the LDLR (referred to collectively as hoFH), LDL-C levels reach >500 mg/dL which leads to the formation of xanthomas, severe cardiovascular disease, and early death (Rader et al., 2003). Importantly, although the LDLR is broadly expressed, the disease can be cured by liver transplantation indicating that the hepatic LDLR is the primary regulator of serum LDL-C levels (Alphonse and Jones, 2016). Most drugs that lower serum LDL-C work on the liver through upregulation of LDLR transcription (statins) or stability of the LDLR protein (Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors) (Nissen et al., 2016; Thompson et al., 2003). As a consequence, such drugs are relatively ineffective in patients with hoFH (Cuchel et al., 2014). However, therapies that limit hepatic production of VLDL, the precursor to LDL, are effective in reducing LDL-C in patients with hoFH (Rader and Kastelein, 2014; Davidson, 2009). Lomitapide inhibits the microsomal triglyceride transfer protein (MTTP) which is critical for hepatic VLDL assembly and reduces LDL-C by approximately 40–50%. Mipomersen is an antisense oligonucleotide that targets the mRNA encoding apoB, the essential core protein of VLDL, in the liver and reduces LDL-C by approximately 25% in hoFH patients. Both of these drugs reduce hepatic secretion of VLDL and apoB independently of the LDLR but unfortunately result in increased fat in the liver (Rader and Kastelein, 2014).
To demonstrate the utility of using iPSC–derived hepatocytes as a platform to identify drugs that could be used to treat metabolic liver disease, we used hoFH iPSC–derived hepatocyte–like cells for a screen of a library of existing drugs that could potentially be repurposed (Strittmatter, 2014). Drugs that reduce apoB levels in the media of hoFH hepatocytes should act independently of the LDLR and so we reasoned that success could provide an alternative treatment for hoFH and other forms of difficult-to-treat hypercholesterolemia.
Results
Small molecule screen using HoFH iPSC–derived hepatocyte-like cells
Success in identifying drugs that can be used to target a rare disease relies on the development of screening assays. Unfortunately, primary hepatocytes rapidly de-differentiate upon culture, and given the variability in the genetics of the donors pose challenges for use in high throughput drug discovery (Binda et al., 2003). Recently, technologies have been developed to produce induced pluripotent stem cells (iPSCs) from patients (Takahashi et al., 2007; Yu et al., 2007). Since patient-specific iPSCs can be induced to differentiate into a variety of cell types, including cells with hepatocyte characteristics, investigators can now model heritable human diseases in culture and screen for therapeutics (Lee et al., 2009; Ebert et al., 2009; Rashid et al., 2010; Matsa et al., 2014; Robinton and Daley, 2012; Choi et al., 2013; Szkolnicka et al., 2016; Cameron et al., 2015; Lang et al., 2016; Schwartz et al., 2012; Mallanna et al., 2016). We have previously described the generation of iPSCs from a hoFH patient (Cayo et al., 2012). The patient has a deletion in exon 17 of the maternal allele which is a null mutation, and an A-G transition in exon 17 of the paternal allele, which encodes a receptor that is unable to internalize LDL (Davis et al., 1986). When hepatocyte–like cells are generated from these hoFH iPSCs, they faithfully recapitulate the pathophysiology associated with the liver of hoFH patients (Cayo et al., 2012). In these experiments, hoFH hepatocytes failed to traffic exogenous LDL to endosomes and were unable to increase LDL clearance in response to statin treatment. Moreover, compared to controls, the hoFH iPSC-derived hepatocytes had elevated levels of apoB in the culture medium (Cayo et al., 2012).
The high level of apoB in the medium of hoFH iPSC–derived hepatocytes potentially offers a screenable phenotype for identification of new LDL-C lowering drugs. Any drug capable of reducing LDL-C in the medium of hoFH iPSC–derived hepatocytes must act through a mechanism that is independent of the LDLR. We, therefore, measured apoB in the media by ELISA as our primary assay. A comparison of the ELISA performance on positive and negative control cells confirmed that the assay was appropriate for screening (Z–factor = 0.88) (Fig. 1A). Moreover, the ELISA could distinguish the effect of treating iPSC-derived hepatocytes with vehicle or the protein synthesis inhibitor cycloheximide as a positive control (p=9.7×10−28) (Fig. 1B). We have previously described the production of hepatocyte–like cells from iPSCs using monolayer culture and completely defined conditions (Si-Tayeb et al., 2010a; Mallanna and Duncan, 2013). The procedure is efficient with approximately 80% of cells expressing hepatocyte markers and is highly reproducible with minimal well–to–well variation (Fig 1A). The hoFH iPSCs were differentiated into hepatocyte-like cells in 96–well plates and the levels of apoB measured before and after the application of 2320 small molecules from the SPECTRUM collection drug library (Fig. 1C). The library was selected because it contains ~1300 drugs that have reached clinical trials in the USA, Europe or Japan. Also, the library has ~800 compounds that are natural products. Compounds in the library are structurally diverse and include oxygen heterocycles, diterpenes, sequiterpenes, pentercyclic triterpenes, and alkaloids. Moreover, the library has been previously used to identify drugs for repurposing (Weisman et al., 2006; Kocisko et al., 2003; Fagan et al., 2013; Wang et al., 2010).
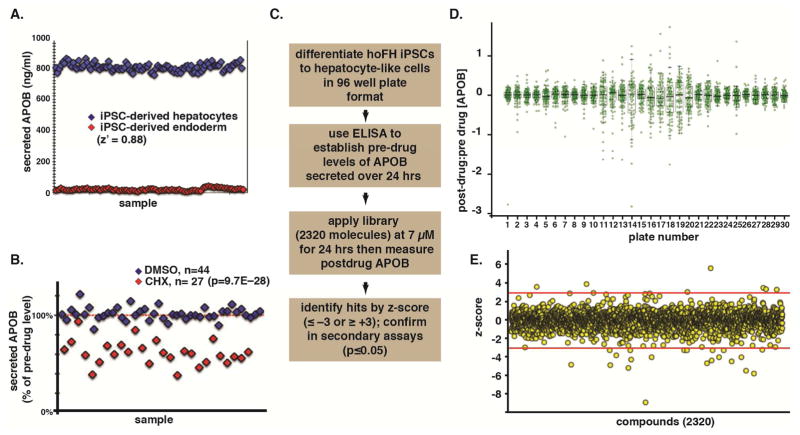
Figure 1. A screen for small molecules that reduce apoB production from hoFH iPSC-derived hepatocyte–like cells.
(A) Graph revealing that an ELISA can efficiently and reliably (z′ = 0.88) distinguish between cells that do (iPSC–derived hepatocytes, blue) and do not (iPSC–derived endoderm, red) secrete apoB. (B) ELISA can also distinguish between the levels of apoB in the medium of iPSC–derived hepatocytes treated with either vehicle (DMSO, blue) or the protein synthesis inhibitor cycloheximide (CHX, red). (C) Schematic overview of the protocol used to identify small molecules that reduce apoB production from hoFH iPSC–derived hepatocyte–like cells. (D) Graph showing the post-drug:pre-drug ratios of apoB identified in the culture medium from the primary screen. Green circles represent individual drugs within the library, and box-and-whisker plots summarize the mean and distributions for each plate (30 plates). (E) Graph showing z-scores (red lines = ± 3) of the effect of 2320 drugs (yellow spheres) on the concentration apoB in the culture medium of treated cells.
The pre– to post–drug treatment ratio of apoB was established for each compound (Fig. 1D) and hits identified by z-score analysis (Fig. 1E) as described by Zhang (Zhang, 2011). Ratios that had a z-score of ≤ −3 (decreased apoB) or ≥ 3 (increased apoB), based on the 3-sigma rule, were considered primary hits. Using these criteria, we found eight drugs that increased and 21 that decreased apoB levels in the culture medium (Table S1). Next, the effect of each drug on apoB levels was compared to vehicle (DMSO) controls (n = 3 biological replicates). Of the 29 drugs identified in the initial screen, 55% were reproducible (p≤0.05), with 13 compounds decreasing the level of apoB (Fig. 2A). Of the compounds that reproducibly decreased apoB secretion, 5 were cardiac glycosides, sharing a similar molecular structure (Fig. 2B). If we reduced the stringency of the z-score to ≤ −2.0 in the primary screen, 7 of 9 cardiac glycosides present in the library were identified (Fig. 2C). We, therefore, tested all 9 of the cardiac glycosides in the SPECTRUM collection in triplicate assays to determine whether they all reduced levels of apoB in the medium of iPSC–derived hepatocytes (Fig. 2A). Remarkably, every cardiac glycoside tested reduced apoB compared to pre-treatment levels and DMSO-treated controls (p≤0.05). Reductions ranged from 29% (Ouabain) to 73% (Gitoxin) (Fig. 2C).
Figure 2. Cardiac glycosides inhibit the concentration of apoB in the medium of cultured hepatic cells.
(A) Box and whisker plot demonstrating that 13 small molecules, including 5 cardiac glycosides (red, asterisks) that were identified as hits in the primary screen, plus 4 additional cardiac glycosides (red, asterisks) present in the library, reproducibly (n=3, p≤0.05) inhibit the levels of apoB measured in the culture medium compared to iPSC–derived hepatocytes treated with vehicle (DMSO, dashed line). (B) Structure of cardiac glycosides present in the library that were found to inhibit apoB production. (C) Table showing the quantitative effect of cardiac glycosides on the levels of apoB secreted by hoFH iPSC-derived hepatocyte–like cells.
We predicted that the ability of cardiac glycosides to lower apoB should not be restricted to hoFH cells. To determine whether the cardiac glycosides also reduced apoB levels in wild-type hepatocytes, we established dose-response curves using HepG2 hepatoma cells (Fig. 3A), primary human hepatocytes (Fig. 3B), and hepatocyte–like cells derived from control (K3) iPSCs (Fig. S1) (Si-Tayeb et al., 2010b). With the exception of strophanthidin, which was less efficient, the cardiac glycosides reduced apoB levels with an EC50 ≤ 20 nM. Based on these data we conclude that cardiac glycosides have an unappreciated ability to lower the levels of apoB in culture medium from both LDLR–deficient and wild-type hepatocytes.
Figure 3. Cardiac glycosides inhibit apoB production at nanomolar concentrations.
Graphs showing 8–point dose response curves generated from the treatment of (A) HepG2 cells or (B) primary human hepatocytes with various cardiac glycosides at 1, 5, 20, 78, 312, 1250, and 5000 nM. See Figure S1 for dose response performed on iPSC–derived hepatocytes.
Cardiac glycosides enhance proteolytic turnover of apoB protein
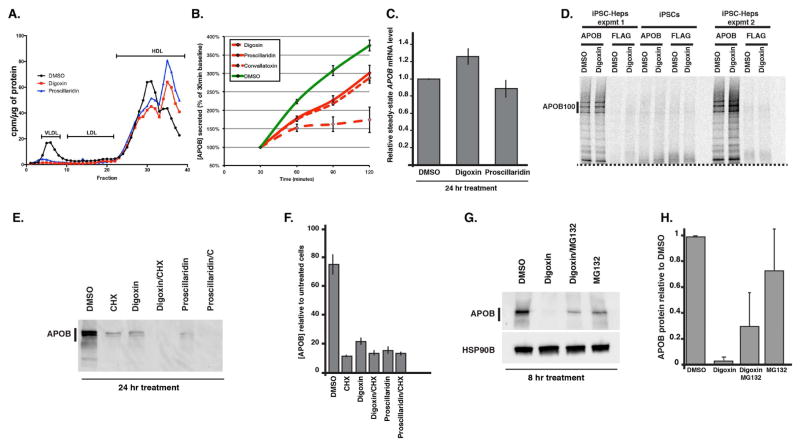
We sought to address the mechanism through which the cardiac glycosides reduced the concentration of apoB in the medium. Since apoB is the central protein component of VLDL and LDL particles, we first determined whether cardiac glycoside treatment resulted in a reduction in VLDL/LDL. Lipoprotein production was measured by [3H]-glycerol labeling of triglycerides followed by FPLC in primary human hepatocytes treated with either DMSO, digoxin or proscillaridin (Fig. 4A). As expected, both digoxin and proscillaridin dramatically reduced VLDL triglyceride levels in the medium of glycoside treated hepatocytes. We next examined the kinetics of apoB accumulation in the culture medium of hoFH iPSC–derived hepatocytes in the presence and absence of digoxin, proscillaridin, or convallatoxin over time. As shown in Fig. 4B, hoFH cells treated with each of the glycosides exhibited a reduction in the concentration of apoB in the medium compared to DMSO within 60 mins of treatment. This rapid response to glycoside administration implied that the effect of the drug was direct rather than an indirect change in cell behavior. Based on this implication, we reasoned that the drug could influence either expression of the APOB gene, apoB protein synthesis, post-translational control of apoB protein, or secretion. Steady-state mRNA levels encoding apoB were determined in wild type (K3) iPSC–derived hepatocytes by RT-qPCR and found to be unaffected by treatment with either digoxin or proscillaridin (Fig. 4C). We next determined whether synthesis of apoB protein was affected by metabolic labeling of nascent proteins followed by immunoprecipitation in the presence or absence of digoxin (Fig. 4D). We first confirmed the specificity of antibodies used to precipitate apoB. As expected, proteins were not precipitated by anti-apoB antibody from lysates of undifferentiated iPSCs that do not express apoB. Similarly, protein was undetected when lysates from iPSC–derived hepatocytes were subjected to precipitation using an anti-FLAG control antibody. When precipitations of apoB were performed on lysates from iPSC–derived hepatocytes labeled with [35S]-Methionine, nascent apoB protein levels were indistinguishable between untreated– and digoxin–treated cells despite the fact that digoxin reduced the level of apoB in the cell medium. Based on these studies we conclude that cardiac glycosides have no effect on expression of apoB or the synthesis of apoB protein and that these drugs must act post-translationally.
Figure 4. Cardiac glycosides reduce steady state cellular levels of apoB protein.
(A) Graph showing the effect of 16 hr treatment with digoxin (310 nM) and proscillaridin (310 nM) compared to DMSO (vehicle) on the production of lipoprotein particles by primary human hepatocytes. Lipoproteins were identified after labeling triglycerides with [3H]-glycerol and separation by FPLC. (B) Graph showing the effect of digoxin, proscillaridin and convallatoxin (50 nM) compared to DMSO on the level of apoB in the medium of iPSC–derived hepatocytes (n=4 biological replicates) over 2 hours of treatment. (C) Bar graph showing the quantification of apoB mRNA by RT-qPCR in iPSC–derived hepatocytes treated with DMSO, digoxin or proscillaridin (n=4 biological replicates). (D) Micrograph showing results of metabolic labeling analyses of apoB. Two sets of iPSC–derived hepatocytes (iPSC-Heps expmt 1 and 2) and undifferentiated iPSCs were labeled for 30 mins with [35S]-Met in the presence of DMSO or digoxin (310 nM). Lysates were subjected to immunoprecipitation using anti-apoB (apoB) and anti-FLAG antibodies. Dashed line indicates the bottom portion of gel that was digitally masked using Adobe Illustrator. (E) Micrograph showing example of immunoblot analysis of iPSC–derived hepatocytes treated for 24 hrs with DMSO, cycloheximide (CHX, 100μM), digoxin (310 nM), and/or proscillaridin (310 nM). (F) Bar graph showing ELISA quantification of apoB in the medium of iPSC–derived hepatocytes treated for 24 hrs with DMSO, cycloheximide (CHX, 100μM), digoxin (310 nM), and/or proscillaridin (310 nM). (G) Immunoblot measuring the level of apoB after treating the cells for 8 hrs with DMSO, digoxin (310 nM), digoxin (310 nM) + MG132 (10 μM), or MG132 (10 μM) alone. (H) Quantification of biological replicates (n=4) of immunoblots on samples treated as in (G).
Post-translational control of apoB-100 protein has been studied in detail and has been shown to be highly regulated and crucial for the formation and secretion of lipoprotein particles (Ginsberg and Fisher, 2009; Sundaram and Yao, 2010). We, therefore, examined the steady–state intracellular levels of apoB protein by immunoblot analyses of iPSC–derived hepatocytes treated for 24 hrs with either DMSO, the protein synthesis inhibitor cycloheximide as a control for protein turnover, digoxin, or proscillaridin. As expected, compared to DMSO–treated control cells cycloheximide resulted in an 8–fold reduction in the cellular level of apoB protein as the intracellular pool of apoB was degraded or secreted (Fig. 4E). Strikingly, treatment of the cells with either digoxin or proscillaridin resulted in a similar reduction in the level of intracellular apoB (Fig. 4E). When cells were treated with both cycloheximide and a cardiac glycoside the reduction in apoB protein level was exacerbated. Using ELISA, we confirmed that digoxin and proscillaridin reduced the level of apoB (p<0.05, n=3 biological replicates) in the media of samples used for immunoblot and RT-qPCR analyses (Fig. 4F).
The observation that intracellular apoB levels were reduced even though synthesis of apoB protein was unaffected by cardiac glycoside treatment implied that the glycosides act by promoting apoB degradation. Degradation of newly synthesized apoB occurs predominantly by the proteasome and regulates lipoprotein production (Fisher and Ginsberg, 2002; Liang, 1998). We therefore addressed whether glycoside–mediated reduction in apoB levels could be rescued by pharmacological inhibition of proteasome activity. Hepatocytes were produced from wild-type iPSCs and treated for 8 hrs with either DMSO, digoxin or digoxin plus the proteasome inhibitor MG132 before measuring apoB by immunoblot (Fig. 4G). Although we observed variation between different experiments in all cases (Fig. 4H, n=4 biological replicates), we found that the addition of MG132 to digoxin–treated hepatocytes increased the level of intracellular apoB. From these data, we conclude that the cardiac glycosides promote the turnover of apoB at least in part through promoting proteasome–mediated degradation.
LDL-C levels are reduced in human patients treated with cardiac glycosides
Cardiac glycosides are used to treat congestive heart failure and atrial fibrillation (Hothi et al., 2013; Ambrosy et al., 2014). Although the use of cardiac glycosides has declined, at its peak digoxin was used to treat 80% of patients with heart failure (Hothi et al., 2013). Despite the clinical use of cardiac glycosides for over 200 years, their ability to reduce human LDL-C levels has not been reported. We, therefore, examined whether cardiac glycosides had LDL-C lowering properties in humans by examining the medical records of patients who had entered the Froedtert Hospital/Medical College of Wisconsin clinics. We first identified a cohort of patients who had historically been prescribed with a cardiac glycoside (n = 5,493). Within this group we also identified patients who had been prescribed an angiotensin-converting-enzyme (ACE) inhibitor, which should have no effect on cholesterol levels, or a statin, which served as a positive control group. Since the time-frame during which a patient was prescribed a particular medication was known, it was possible to retrieve serum LDL-C and serum albumin measurements that had been recorded when patients were either ‘on’ or ‘off’ of drug treatment (Fig. 5). Patients prescribed with each drug were therefore split into cohorts depending on whether they were ‘on’ or ‘off’ of drug when their LDL-C or albumin was measured. An ACE-inhibitor had no significant impact on the serum levels of either LDL-C (‘off drug’ n = 165, ‘on drug’ n = 180, p = 0.44) or albumin (‘off drug’ n = 763, ‘on drug’ n = 1767, p=0.24) (Figs. 5A, B). As expected, treatment with a statin was associated with a reduction in the mean serum LDL-C from 103.6 ± 2.2 mg/dL in ‘off drug’ patients to 89.8 ± 1.9 mg/dL in ‘on drug’ patients (‘off drug’ n = 204, ‘on drug’ n = 221, p<0.0001) while albumin levels were unaffected (‘off drug’ n = 906, ‘on drug’ n = 1865) (Figs. 5A, B). Strikingly, patients prescribed a cardiac glycoside displayed a reduction in serum LDL-C levels that was similar to patients treated with a statin, 103.1 ± 1.8 mg/dL in ‘off drug’ to 94.0 ± 2.3 mg/dL in ‘on drug’ patients (‘off drug’ n = 205, ‘on drug’ n = 324, p = 0.0019), with no effect on serum albumin (‘off drug’ n = 2116, ‘on drug’ n = 770) (Figs. 5A, B).
Fig. 5. Human patients treated with cardiac glycosides have reduced serum LDL-C levels.
(A) Graph showing concentration of LDL in the serum of a cohort of patients (grey circles) when on or off treatment with an angiotensin-converting-enzyme inhibitor (ACE-i) (‘on’ n = 180; ‘off’ n = 165), a statin (‘on’ n = 221; ‘off’ n = 204), or a cardiac glycoside (‘on’ n = 324; ‘off’ n = 205). Black bar shows the mean LDL concentration. (B) Graphs showing the concentration of albumin in the serum of patients (grey circles) when on or off treatment with the indicated drugs. Angiotensin-converting-enzyme inhibitor (ACE-i) (‘on’ n = 1767; ‘off’ n = 763), a statin (‘on’ n = 1865; ‘off’ n = 906), or a cardiac glycoside (‘on’ n = 2116; ‘off’ n = 770). Black bar shows the mean albumin concentration. (C) Graphs showing serum albumin (left panel, n = 87) and LDL (right panel, n = 21) measurements in the same patients before and after treatment with cardiac glycosides. Black bar shows the mean concentration and grey lines indicate the direction of change in each patient when off (blue diamonds) or on (red diamonds) the medication. (D) Graphs showing serum albumin (left panel, n = 715) and LDL-C (right panel, n = 91) measurements in the same patients before and after treatment with statins. Black bar shows the mean concentration and grey lines indicate the direction of change in each patient when off (blue diamonds) or on (red diamonds) the medication.
The above analysis relied on cohorts of patients in which LDL-C measurements were taken either inside (on drug) or outside (off drug) the window of an active prescription. However, the LDL-C measurements of ‘on drug’ and ‘off drug’ cohorts were not necessarily from the same patients. We recognized that definitive human data would require us to retrieve ‘on drug’ and ‘off drug’ LDL-C measurements that could be matched to individuals. This would allow us to determine whether serum LDL-C dropped after a specific patient was given a cardiac glycoside. In searching medical records, we identified patients who had measurements of albumin (n=91) or LDL-C (n=21) taken both within and outside of treatment with a cardiac glycoside (Fig. 5C). A similar cohort of patients treated with a statin was again used as a positive control (Fig. 5D). The serum levels of albumin in these patients were once more unaffected by drug treatment. In contrast, patients displayed a mean serum LDL-C reduction of 31.8 ± 8.6 mg/dL (p=0.0016) during treatment with statins and 25.6 ± 6.3 mg/dL (p = 0.0006) with cardiac glycosides. In 16 of the 21 patients examined, LDL-C levels had dropped substantially following the administration of a cardiac glycoside (Fig. 5C).
Cardiac glycosides reduce LDL–cholesterol levels in humanized avatar mice
Although these retrospective analyses of electronic medical records were convincing, we acknowledged that in contrast to a clinical trial, interpretation of results could be confounded by random variables such as diet, adherence, other drug use, physical condition, etc. We reasoned that it was important to test directly the in vivo effect of the cardiac glycosides in human hepatocytes under controlled conditions. We, therefore, generated avatar mice (Fig. 6A; Fig. S2) in which human hepatocytes replaced the endogenous murine hepatocytes (Azuma et al., 2007; Wilson et al., 2014). Importantly, mice harboring humanized livers have previously been shown to adopt a typical human lipoprotein profile (Ellis et al., 2013; Bissig-Choisat et al., 2015). Such animals, therefore, provide an ideal model in which to test the cholesterol-lowering properties of cardiac glycosides on human hepatocytes in vivo.
Fig. 6. Cardiac glycosides reduce serum levels of human apoB in humanized mice.
(A) Illustrated overview of the procedure for the generation of Fah−/−Rag2−/−Il2gr−/−NOD mice harboring human hepatocytes. (B) Graph showing the ratio of human albumin to human apoB found in the serum of avatar mice harboring hepatocytes from two different donors (donor A red circles, donor B green squares. n=9). (C) Graph showing that treatment of avatar mice (donor A circles, donor B squares) with vehicle (DMSO blue), digoxin (red), or proscillaridin (blue) had no effect on albumin levels. (D) Graph showing the percent change in the concentration of apoB found in the serum of avatar mice (donor A circles, donor B squares) treated with vehicle (DMSO blue), digoxin (red), or proscillaridin (blue). See Figure S2 for characterization of FRGN mice and antibodies. See Figure S3 for data on individual mice.
Avatars were produced using hepatocytes from two donors. Donor A was a 53-year old female and donor B was a 17-year old male. We measured serum apoB and albumin levels by ELISA using human-specific antibodies (Figs. S2, S3) after treatment with DMSO (vehicle), digoxin or proscillaridin. Based on our study of the action of cardiac glycosides on iPSC–derived hepatocytes we expected the response to glycoside treatment to be rapid and so we measured the serum levels of apoB after providing three intraperitoneal injections of glycosides over a 48-hour period. We selected a dose that was 1/8th of the reported LD50 for each drug which equated to 0.5 mg/kg/day for digoxin and 0.6 mg/kg/day for proscillaridin and was within the published range (from 0.1 mg to 2 mg/kg/day). We did not know the relative level of LDL-C of the original hepatocyte donors; however, the ratio of apoB to albumin was approximately 30% higher in the serum of avatars repopulated with hepatocytes from donor A compared to donor B (Fig. 6B). Importantly the levels of human albumin in the serum were unaffected by treatment with digoxin or proscillaridin (Fig. 6C). However, in contrast to DMSO, which had no effect, treatment of the avatar mice with either of the cardiac glycosides significantly reduced serum apoB levels (p≤0.05, n=3 biological replicates) regardless of donor (Fig. 6D; Fig. S3).
Discussion
We have used hepatocyte-like cells derived from hoFH iPSCs in a screen to identify drugs that could potentially be used to treat hypercholesterolemia. Because the hoFH hepatocytes lack a functional LDLR receptor, the effective compounds should act on pathways that are independent of the LDLR. In addition to their potential utility in hoFH patients, such drugs could be useful for lowering LDL-C in patients that cannot reduce their LDL-C sufficiently using existing therapies.
The identification of novel pharmaceuticals, especially for rare diseases, has historically been hampered by the limited availability of cell models that accurately reflect the pathophysiology of the disease (Horvath et al., 2016). Most commonly, cell–based assays have relied heavily on immortalized or cancer cell lines. However, long term culture of such cells results in genetic drift where the cells accumulate genetic rearrangements, aberrant chromosomal copy numbers, and disrupted epigenetic states. Despite these disadvantages, hepatoma cells have been used to identify small molecules with potential therapeutic application. Relevant to the current work, HepG2 cells were recently used to identify small molecules that can increase expression of Tribbles Pseudokinase 1 (TRIB1) (Nagiec et al., 2015). This finding was provocative because genome wide association studies have linked TRIB1 to cardiovascular disease and over-expression of TRIB1 can inhibit secretion of VLDL (Nagiec et al., 2015). Primary cells could potentially circumvent problems associated with genetic drift; however, because each preparation of primary hepatocytes comes from a unique donor, genetic variability amongst donors can significantly impact the reproducibility of screening efforts. Moreover, using primary hepatocytes to model metabolic dysfunctions can be problematic because it is logistically challenging to obtain primary cells from patients with rare diseases. Induced pluripotent stem cells could offer an alternative and robust cell platform for drug discovery (Matsa et al., 2014; Kimbrel and Lanza, 2015). Human iPSCs retain a relatively stable genomic environment, provide an indefatigable source of cells, can be genetically manipulated, and procedures for producing patient–specific iPSCs are relatively straightforward. A large number of protocols have been described that facilitate the production of hepatocyte–like cells from pluripotent stem cells (Cai et al., 2007; Hay et al., 2008; Basma et al., 2009; Song et al., 2009; Touboul et al., 2010; Sullivan et al., 2010; Si-Tayeb et al., 2010a; Asgari et al., 2011). Despite the large number of procedures, in most, if not all, differentiation conditions iPSC–derived hepatocytes do not fully recapitulate liver functions. This caveat is important with regard to drug discovery because a failure to generate fully functional hepatocytes can influence first pass metabolism, can influence drug toxicity profiles (Berger et al., 2015) and in some cases, may prevent the cells from accurately modeling the target disease (Davidson et al., 2015).
Despite caveats, several laboratories have reported the successful generation of iPSCs from patients with a variety of inborn errors of hepatic metabolism and have demonstrated that hepatocytes derived from them can recapitulate the pathophysiology of the disease in culture (Rashid et al., 2010; Cayo et al., 2012; Choi et al., 2013; Tafaleng et al., 2015; Leung et al., 2013; Yi et al., 2012; Li et al., 2015; Zhang et al., 2011). Although the generation of models of hepatic disease using iPSCs have been successful, very few have been translated into platforms for drug screening. Choi and colleagues used iPSCs from a patient with alpha-1 antitrypsin (AAT) deficiency to screen 3131 molecules from The Johns Hopkins’ Drug Library and found five existing drugs that had a capacity to reduce accumulation of misfolded alpha-1 antitrypsin (Choi et al., 2013). In a small-scale screen of five autophagy–inducing drugs, Maetzel and colleagues demonstrated improved autophagic response in hepatocytes generated from Niemann-Pick type C (NPC) iPSCs (Maetzel et al., 2014). Part of the challenge of using iPSC–derived hepatocytes in a drug screening project lies in ensuring that the quality of the differentiations is reproducible, synchronous and efficient. Our use of a relative simple differentiation protocol that relies on cell monolayers and completely defined culture conditions is highly compatible with high throughput analyses. In addition, with the advent of genomic engineering, targeted generation of mutations using CRISPR-CAS 9 within iPSCs is relatively simple (Ding et al., 2013). Exploiting the power of genomic engineering therefore obviates any requirement to access a patient population and so even the rarest of rare diseases can be potentially modeled. This capability is important because it makes the discovery of drugs to treat rare disorders accessible to an academic laboratory.
To the best of our knowledge, there has been no account of a direct effect of cardiac glycosides on lowering LDL-C in humans. However, extract of Nerium oleander, which contain glycosides and polyphenols, has been shown to reduce plasma lipoprotein concentrations in a rat model of induced hyperlipidemia (Gayathri et al., 2011). Studies using cardiac glycosides have also previously been performed on HepG2 hepatoma cells (Campia et al., 2009; Kolkhof et al., 2010). In one study, treatment of HepG2 cells with either digoxin or ouabain increased intracellular cholesterol synthesis by enhancing expression and activity of HMG-CoA reductase through the SREBP2/SCAP complex (Campia et al., 2009). However, when we measured the amount of apoB in the medium of HepG2 cells treated with cardiac glycosides, we found that it dropped substantially for all nine cardiac glycosides tested (Fig. 3A). Similar results were obtained using both primary hepatocytes and iPSC–derived hepatocytes. These findings are not necessarily contradictory because if cardiac glycosides enhance the synthesis of cholesterol by hepatocytes, the degradation of apoB would prevent packaging into lipoprotein particles and the net effect would be a reduction in apoB secretion.
In heart failure and cardiac arrhythmia patients, glycosides act on cardiac myocytes by increasing the contractile force by inhibiting Na+/K+-ATPase activity (Prassas and Diamandis, 2008). Inhibition of the Na+/K+-ATPase disrupts the exchange of sodium and potassium ions across the plasma membrane which indirectly increases intracellular calcium ions to promote contraction (Prassas and Diamandis, 2008). At this time, we do not know definitively whether cardiac glycosides also lower apoB production in hepatocytes by acting on the Na+/K+-ATPase, but experiments are underway to address this hypothesis. We have shown that the downstream mechanism through which cardiac glycosides inhibit VLDL production is by promoting proteasome degradation of the apoB protein. However, the molecular relationship between the Na+/K+-ATPase and intracellular apoB turnover remains speculative and could be multifactorial. The activity of the Na+/K+-ATPase as an ion transporter is well established. However, more recent studies have shown that it also acts as a protein scaffold to support the assembly of a large signaling complex that includes SRC (SRC Proto-Oncogene, Non-Receptor Tyrosine Kinase) and the EGFR (Epidermal Growth Factor Receptor) (Prassas and Diamandis, 2008; Xie and Cai, 2003). Moreover, binding of cardiac glycosides to the Na+/K+-ATPase can activate a number of cellular kinases, including mitogen-activated protein kinases (MAPK) and protein kinase C (PKC) (Xie and Cai, 2003). It is now, therefore, appreciated that cardiac glycosides can influence a broad repertoire of cell processes (Riganti et al., 2011; Newman et al., 2008; Prassas and Diamandis, 2008). Such processes include signal transduction, cell proliferation, glucose metabolism, Ca+ flux and steroid metabolism (Prassas and Diamandis, 2008). It would not be unreasonable to consider that some of these processes could intersect with lipoprotein processing.
Although cardiac glycosides have been used to treat heart failure for over 200 years, some clinicians have concerns over the narrow therapeutic window of the effective dosage and the fact that at high doses these drugs can cause severe arrhythmias and cardiac toxicity. However, the inhibition of Na+/K+-ATPase activity in cardiac myocytes by cardiac glycosides most commonly requires micromolar concentrations (Werdan et al., 1984). In contrast, our results demonstrate that apoB production by hepatocytes is inhibited at nanomolar concentrations of glycosides. These results imply that the therapeutic dose needed to treat hypercholesterolemia by targeting the liver could be lower than that needed to treat heart failure, thereby increasing efficacy and minimizing risk. Optimization of dosage could be determined by using humanized mice and by a controlled trial in patients. Efforts to address this are currently underway. Moreover, researchers have recently generated avatar mice using primary hepatocytes from an LDLR–deficient patient (Bissig-Choisat et al., 2015). Such mice could potentially be used to study the efficacy of cardiac glycosides in treating hoFH livers. Although further experiments are required to determine whether low doses of cardiac glycosides are efficacious, in the event that the optimum therapeutic dose used to lower LDL-C is similar to that used to treat cardiac dysfunction, cardiac toxicity could potentially be avoided by targeting the cardiac glycosides specifically to hepatocytes using small molecule drug conjugates (Wang et al., 2016).
In summary, we believe that our study adds to the potential therapeutic applications of the cardiac glycosides. Specifically, we propose that cardiac glycosides could be used to reduce LDL-C in individuals that are resistant or cannot tolerate statin treatment, which would include hoFH patients. Future study of the mechanisms through which the cardiac glycosides promote apoB turnover may also identify new pathways for the rational design of novel pharmaceuticals. More broadly, the approach that we have described highlights the feasibility of using iPSC-derived hepatocytes to identify treatments for inborn errors of hepatic metabolism in an academic setting. Coupled with the extensive availability of iPSCs from patients generated by the Next Generation Genetic Association Studies consortium, we believe that the relative cost-effectiveness and efficiency of this platform will facilitate an expansion in the discovery of small molecules and biologicals that can be used to treat rare diseases of the liver and cardiovascular system.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for reagents may be directed to, and will be fulfilled by the corresponding author, Dr. Stephen A. Duncan (duncanst@musc.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Pluripotent Stem Cells
The MCW SCRO committee approved all human iPSC work (hSCRO approval #09-005). All iPSCs were produced by us and their characterization has been described elsewhere (Si-Tayeb et al., 2010b; Cayo et al., 2012). JD4 iPSCs were generated from primary dermal fibroblasts (GM02408) from a compound heterozygote familial hypercholesterolemia patient that were provided by the Coriell Institute for Medical Research (Coriell Cell Repositories, Camden, NJ) (Cayo et al., 2012). Control K3 iPSCs were produced from foreskin fibroblasts (CRL2097) obtained from ATCC (Si-Tayeb et al., 2010b).
Humanized FRGN Mice
The MCW IACUC approved all animal experiments. Fah−/− Rag2−/− IL2gr−/− NOD (FRGN) mice were generated by Dr. Markus Grompe (Azuma et al., 2007; Wilson et al., 2014). Breeding colonies of FRGN mice were maintained on water supplemented with 8 mg/L NTBC. To generate avatars, 1 × 106 human primary hepatocytes were introduced into 6–8 week-old male FRGN mice by splenic injection. NTBC was withdrawn from the drinking water and mice were left for 7 days. Mice were then transferred to 8 mg/L NTBC for 3 days. The mice were cycled 7 days off drug followed by 3 days on drug for 2 months. After 2 months of cycling mice were kept without NTBC for around 15 days or until they had lost 15% of body weight at which point they were returned to NTBC for 4 days. This cycle was maintained for the life of the animal. The extent of engraftment was measured by determining the human serum albumin by ELISA, as described (Azuma et al., 2007; Bissig et al., 2010; Ellis et al., 2013). We also confirmed that antibodies used to detect apoB specifically detected the human and not mouse forms of the protein (Fig S2A), levels of human apoB in the serum correlated with the level of human albumin (Fig. S2B), and engraftment and expansion of human hepatocytes recapitulated published results (Azuma et al., 2007; Wilson et al., 2014) by immunostaining liver sections for human albumin, FAH, and human trans Golgi network protein 46 (TGN46) (Fig. S2C). Human hepatocytes for transplantation were obtained either from Thermo Fisher/Life Technologies (donor A, Hu1475) or from Celsis In Vitro Technologies, INC (donor B). Donor A was a 53 yr old, Caucasian female whose cause of death was unknown, occasionally used alcohol (wine, 2 glasses daily) and was an ex-smoker (1 ppd x 30 years, stopped in 2007). Donor B was a 17yr old Caucasian who died from head trauma due to a motor vehicle accident who occasionally used alcohol and dipping tobacco. For drug treatment studies, highly repopulated FRGN mice (>1mg/ml serum human albumin) were maintained using 0.15mg/L NTBC drinking water prior to and during 48-hour drug treatment experiments. Blood samples were collected using 4mm Goldenrod animal lancets (Medipoint, Inc.) and BD Microtainer EDTA-coated plasma collection tubes (Becton Dickinson Vacutainer Systems). Serum samples were collected at times 0, 24, and 48 hours. Mice were weighed daily, and treated with either 0.5 mg/kg digoxin, 0.6 mg/kg proscillaridin or vehicle control (5% DMSO in sterile saline) by i.p. injection at time points 0, 16, and 40 hours. Serum concentrations of human albumin and human apoB were determined using ELISA.
Analysis of Human Patient Medical Records
All medical records were de-identified and considered exempt non-human subjects research. Access to de-identified medical records was approved through an MCW Clinical & Translational Research Data Use Agreement and records were obtained through an honest broker. The Froedtert/MCW Hospital and Clinics Epic Systems electronic medical record was queried using the MCW i2b2 Clinical & Translational Research Informatics Data Warehouse (CTRI-CRDW), and Cohort Discovery Tool. A cohort of patients was identified whose charts included at least one medication order for a cardiac glycoside (n = 5,493). From this cohort of patients, we extracted de-identified medical records data pertinent to our research hypotheses, specifically, serum LDL-C (direct) and serum albumin concentration clinical laboratory results, and medication (cardiac glycosides, statin, and ACE-inhibitor) orders (start/end dates). Laboratory test results (LDL-C, albumin) were flagged as ‘on-drug’ or ‘off-drug’ (cardiac glycoside, statin, and ACE-inhibitor) using the medication orders data (start/end dates) for each individual patient.
If a patient’s records contained multiple laboratory result values within a single on-drug or off-drug window, the multiple values were combined into an average, such that each data point represents a patient’s average laboratory value for the time window. Final cohorts used for analysis are as follows: Patients with LDL-C measurements treated with an ACE-inhibitor (‘on’ n = 180; ‘off’ n = 165), a statin (‘on’ n = 221; ‘off’ n = 204), or a cardiac glycoside (‘on’ n = 324; ‘off’ n = 205). Patients with albumin measurements treated with an ACE-inhibitor (‘on’ n = 1767; ‘off’ n = 763), a statin (‘on’ n = 1865; ‘off’ n = 906), or a cardiac glycoside (‘on’ n = 2116; ‘off’ n = 770). From within the larger cohorts, we identified a series of patients treated with a cardiac glycoside (n=21) or a statin (n=20) with matched LDL-C measurements ‘on drug’ and ‘off drug’ for direct comparison. An identical group was developed for statin (n = 715) or cardiac glycoside (n = 87) treated patients with matched albumin measurements.
Data inclusion/exclusion criteria: For direct LDL-C, any laboratory test results with values below 30 mg/dL were excluded (3 of 1,192 total), along with 4 results that were flagged as ‘error’ within the patients’ charts. For albumin, any tests flagged with ‘error’ were also excluded, as were pathological results <3 or >5.4 g/dL (4,333 of total 59,532 lab results). Statistical significance of all results was determined using Student’s t-tests. Paired data for individual patients on-drug versus off-drug was analyzed using paired t-tests.
METHOD DETAILS
iPS Cell Differentiation
A step-by-step protocol that we used to differentiate human iPSCs into hepatocyte–like cells has been published elsewhere (Mallanna and Duncan, 2013; Si-Tayeb et al., 2010). Specifically, iPSCs were plated as monolayers in 96–well plates. The cells were induced to form endoderm by addition of 100 ng/ml Activin A for 5 days. Endoderm was then converted to hepatic progenitor cells by addition of Bone Morphogenetic Protein 4 (BMP4 20 ng/ml) and Fibroblast Growth Factor 2 (FGF2 10 ng/ml) for a further 5 days. Immature hepatocytes were generated by inclusion of Hepatocyte Growth Factor (HGF 20 ng/ml) and cells were induced to mature by addition of Oncostatin M (OSM 20 ng/ml) each for 5 days.
Drug Screen
HoFH iPSC-JD4 cells were differentiated to hepatocyte–like cells in 96–well plates. A 24 hr pre-drug treatment sample of medium was retained before the addition of drugs from the SPECTRUM collection drug library for 24 hrs at 5 μM. ApoB concentrations were determined for each compound in the small molecule library using a standard curve and four parameter logistic (4PL) regression model in both pre- and post-drug treated samples by ELISA. The pre-drug and post-drug apoB concentrations were combined and expressed as a delta-apoB ratio (post-drug [apoB]:pre-drug [apoB]), and a z-score was generated for each individual compound using the delta-apoB ratio with the standard deviation of the delta-apoB ratios from the parent drug plate (30 drug plates total). Primary hits were validated in secondary replicate experiments (biological replicates n=3), and statistical significance was determined by a Student’s t-test. The SPECTRUM collection drug library was purchased from Microsource Discovery Systems INC. The library consists of 2320 small molecules (http://www.msdiscovery.com/spectrum.html) and has been previously used to identify drugs for repurposing (Weisman et al., 2006; Kocisko et al., 2003; Fagan et al., 2013; Wang et al., 2010).
Enzyme Linked Immunosorbent Assays (ELISA)
A sandwich ELISA to detect human albumin in tissue culture supernatants and mouse sera used a 1/100 dilution of a capture human albumin coating antibody (Bethyl laboratories, A80-129A) and a 1:85,000 dilution of a Horseradish Peroxidase (HRP) conjugated human albumin detection antibody (Bethyl laboratories, A80-129P). Bound antibody was detected using 3,3′,5,5′-tetramethylbenzidine (TMB) and the concentration of albumin in each sample determined by comparing to a standard curve (Bethyl laboratories, RS10-110). Human apoB was detected using a commercial sandwich ELISA (product code: 3715-1H-6; MabTech, Inc) and detected using TMB. The concentration of apoB was determined by comparing to a standard curve using apoB supplied by the manufacturer. This assay has been used to quantify human apoB levels in serum and cell culture medium previously (Aslan et al., 2013; Derwall et al., 2012; Jonker et al., 2010).
Lipoprotein characterization
Primary human hepatocytes were plated on collagen coated dishes in Hepatocyte Thaw Medium (ThermoFisher) for 4–6 hours before medium was changed to William’s E medium (ThermoFisher) with and without DMSO, digoxin (310 nM) or proscillaridin (310 nM). After an overnight incubation, triglycerides were labeled with [3H]-glycerol (10 μCi/ml) conjugated oleate in William’s E medium supplemented with DMSO, digoxin (310 nM) or proscillaridin (310 nM) for 4 hours using standard procedures. The conditioned media was filtered through a .45 um filter to remove cell debris, concentrated on a centrifugal cellulose membrane (EMD Millipore), and fractionated by FPLC.
Metabolic labeling, immunoprecipitation and western blot analyses
iPSC–derived hepatocytes (day 20) and undifferentiated iPSCs were treated with DMSO or digoxin (310 nM) overnight. Cells were then incubated for 30 minutes with methionine free DMEM medium before labeling for 60 mins with [35S]-Met ± digoxin (310 nm). Immunoprecipitation of cell lysates was performed using anti-ApoB (MabTech LDL 17/20) and anti-FLAG M2 antibody (SIGMA). After SDS-PAGE using a 4–15% acrylamide gradient, gels were exposed to a phosphorimager screen and developed using a FLA 9000 Typhoon Storage Phosphorimager (GE Healthcare). For western blots iPSC–derived hepatocytes (day 20) were treated between 8 – 24 hours, depending on the experiment, with DMSO, cycloheximide (100 μM), digoxin (310 nM), proscillaridin (310 nM), MG132 (10 μM), or combinations of these drugs. Protein concentration was determined by BCA assay (Bio-Rad) and 30 μg of protein separated by SDS-PAGE using Any kD™ Mini-protean TGX stain-free™ precast gels (BioRad, CA, #4568123), and transferred to PVDF membranes using the Trans-Blot Turbo™ Transfer System (BioRad, CA, #1704155). Membranes were probed with anti-ApoB (MabTech LDL 17/20) and detected using HRP–conjugated secondary antibodies. Protein levels were calculated using the stain-free Imaging System (Bio-Rad) and were normalized to total protein using Image Lab software (Bio-Rad).
Quantitative Real-Time PCR analysis
RNA was isolated from drug–treated and control iPSC–derived hepatocyte–like cells using the RNeasy mini Kit (Qiagen, #74106). Genomic DNA was removed using the TURBO DNA-free™ Kit (ThermoFisher/Ambion, NY, #AM1907). First strand cDNA was synthesized using M-MLV Reverse Transcriptase (ThermoFisher/Invitrogen, NY, #28025-013). Quantitative real-time PCR was performed on a BioRad CFX384 real-time PCR machine.
QUANTIFICATION AND STATISTICAL ANALYSIS
Figure 1: Data points in panels A, B, D, and E represent independent wells (n=1 well per point), from 96-well cell culture plates. For z′ calculation in panel A, n=107 wells for each of the 2 groups of measurements (iPSC-derived hepatocytes, iPSC-derived endoderm). For panel B, statistical significance was determined using a Student’s t-test. In panel D, each plate is represented by a boxplot and 80 data points representing the 80 drug compounds contained on that plate. Figures and statistics were generated using Microsoft Excel and R.
Figure 2: In panel A, each data point represents the mean and SD (error bars) of 3 independent wells (n=3 wells per point) from 96-well cell culture plates. Statistical significance reported in panel C was determined using a Student’s t-test. Graphs and statistics were generated using GraphPad Prism and Microsoft Excel.
Figure 3: In panel A, each data point represents the mean ± SEM (error bars) of 3 independent wells (n=3 wells per point) from a 96-well cell culture plate. In panel B, each bar represents the mean ± SEM (error bars) of 3 independent wells (n=3 wells per bar) from a 96-well cell culture plate. Graphs were generated using Microsoft Excel.
Figure 4: In panel B, data points represent mean ± SEM (error bars) of 2 independent wells (n=2 wells per point) from a 12-well cell culture plate. Panel C data are from 4 independent wells collected from two independent differentiations and error bars represent SEM. Panel D shows results of metabolic labeling analyses of apoB that is representative of 3 independent differentiations. Panel E is a micrograph showing an immunoblot analysis that is representative of results obtained from 3 independent differentiations of iPSK3 cells. Similar results were obtained from iPSC SV20 cells when measured at 8 hours of treatment. Panel F contains data from three independent differentiations and error bars represent SEM. Panel G immunoblot is representative of 4 independent differentiations and panel H shows quantification of blots with error bars representing SEM. Where appropriate significance was calculated using Student’s t-test.
Figure 5: In panels A&B, each data point represents a single clinical laboratory result (or the mean result value for patients with multiple laboratory results), and horizontal lines represent the mean laboratory result value for the indicated group of data points. Panel A: ACE inhibitor (‘on’ n = 180, ‘off’ n = 165); statin (‘on’ n = 221, ‘off’ n = 204); cardiac glycoside (‘on’ n = 324, ‘off’ n = 205). Panel B: ACE inhibitor (‘on’ n = 1767, ‘off’ n = 763); statin (‘on’ n = 1865, ‘off’ n = 906); cardiac glycoside (‘on’ n = 2116, ‘off’ n = 770). Statistical significance was determined using unpaired Student’s t-tests. In panels C&D, data points represent a single clinical laboratory result (or the mean result value for patients with multiple laboratory results), and are paired such that each ‘on-drug’ data point has a single corresponding ‘off-drug’ point. Horizontal lines represent the mean ± SEM (error bars) laboratory result value for the indicated group of data points. Panel C: n=87 patients/paired albumin measurements, n=21 patients/paired LDL measurements. Panel D: n=715 patients/paired albumin measurements, n=20 patients/paired LDL measurements. Statistical significance was determined using paired Student’s t-tests. Graphs and statistics were generated using GraphPad Prism.
Figure 6: In panels B, C, and D, each data point represents n=1 independent humanized mouse. Horizontal lines represent the mean ± SEM (error bars) of the indicated measurement/ratio for the indicated treatment group. Statistical significance was determined using unpaired Student’s t-tests. Graphs and statistics were generated using GraphPad Prism.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-FLAG M2 | Sigma-Aldrich | F3165 |
| human albumin | Bethyl Laboratories | A80-129A |
| HRP conjugated human albumin | Bethyl Laboratories | A80-129P |
| 3,3′,5,5′-tetramethylbenzidine (TMB) | Bethyl Laboratories | RS10-110 |
| human apoB | MabTech, Inc | 3715-1H-6 |
| anti-human apoB mAbs LDL 17/20 | MabTech, Inc | 3715-3-250 |
| HSP90β | Abcam | ab32568 |
| Goat anti-Mouse IgG | ThermoFisher Scientific | 31430 |
| Goat Anti-Rabbit IgG | ThermoFisher Scientific | 656120 |
| Biological Samples | ||
| pzbFGF BL21 Star E. coli | Teneille Ludwig | {Ludwig et al., 2006, Nat Methods, 3, 637-46} |
| Chemicals, Peptides, and Recombinant Proteins | ||
| B-27® Supplement (50X), serum free | ThermoFisher Scientific | 17504044 |
| B-27® Supplement, minus insulin | ThermoFisher Scientific | A1895601 |
| Activin A Recombinant Human Protein | ThermoFisher Scientific | PHC9563 |
| FGF-Basic (AA 10-155) Recombinant Human Protein | ThermoFisher Scientific | PHG0023 |
| Purified recombinant zebrafish FGF-Basic | Duncan Lab | {Ludwig et al., 2006, Nat Methods, 3, 637-46} |
| BMP4 Recombinant Human Protein | ThermoFisher Scientific | PHC9533 |
| HGF Recombinant Human Protein | ThermoFisher Scientific | PHG0321 |
| Oncostatin M Recombinant Human Protein | ThermoFisher Scientific | PHC5015 |
| Gitoxin | MicroSource Discovery Systems, Inc. | Spectrum Collection; CAS 4562-36-1 |
| Peruvoside | MicroSource Discovery Systems, Inc. | 01501113; CAS 1182-67-2 |
| Strophanthidin | MicroSource Discovery Systems, Inc. | 00100291; CAS 66-28-4 |
| Lanatoside C | MicroSource Discovery Systems, Inc. | 01501205; CAS 7575-22-3 |
| Convallatoxin | MicroSource Discovery Systems, Inc. | 01503994;CAS 508-75-8 |
| Selamectin | MicroSource Discovery Systems, Inc. | Spectrum Collection; CAS 165108-07-6 |
| Hycanthone | MicroSource Discovery Systems, Inc. | Spectrum Collection; 3105-97-3 |
| Donepezil Hydrochloride | MicroSource Discovery Systems, Inc. | Spectrum Collection; 142057-77-0 |
| Digitoxin | MicroSource Discovery Systems, Inc. | 01500246; CAS 71-63-6 |
| Proscillaridin | Santa Cruz Biotechnology | sc-500903; CAS 466-06-8 |
| Digoxin | MicroSource Discovery Systems, Inc. | Spectrum Collection; 20830-75-5 |
| Sulfachlorpyridazine | MicroSource Discovery Systems, Inc. | Spectrum Collection; 80-32-0 |
| Tetroquinone | MicroSource Discovery Systems, Inc. | Spectrum Collection; CAS 319-89-1 |
| Phenylmercuric acetate | MicroSource Discovery Systems, Inc. | Spectrum Collection; CAS 62-38-4 |
| Methscopolamine bromide | MicroSource Discovery Systems, Inc. | Spectrum Collection; CAS 155-41-9 |
| Famciclovir | MicroSource Discovery Systems, Inc. | Spectrum Collection; CAS 104227-87-4 |
| Ouabain Octahydrate | MicroSource Discovery Systems, Inc. | 01500676; CAS 11018-89-6, 630-60-4 |
| Cycloheximide | Sigma-Aldrich | C1988; CAS ‘66-81-9 |
| MG132 | Selleck Chemicals | S2619; CAS 133407-82-6 |
| Dimethyl sulfoxide | Sigma-Aldrich | D8418; CAS 67-68-5 |
| CuRx™ Nitisinone | Yecuris Corporation | 20-0027; CAS 104206-65-7 |
| Critical Commercial Assays | ||
| RNeasy mini Kit | Qiagen | 74106 |
| TURBO DNA-free™ Kit | ThermoFisher/Ambion | AM1907 |
| M-MLV Reverse Transcriptase | ThermoFisher/Invitrogen | 28025-013 |
| TaqMan® Gene Expression | ThermoFisher/Applied Biosystems | 4369016 |
| Power SYBR Green | ThermoFisher/Applied Biosystems | 4367659 |
| Human apoB ELISA development kit (HRP) | MabTech, Inc | 3715-1H-20 |
| Deposited Data – N/A | ||
| Experimental Models: Cell Lines | ||
| HoFH iPSC-JD4 | Stephen Duncan | {Cayo et al., 2012, Hepatology, 56, 2163-71} |
| Wild type iPSC-K3 | Stephen Duncan | {Si-Tayeb et al., 2010, BMC Dev Biol, 10, 81} |
| Wild type iPSC-SV20 | Wenli Yang | {Yang et al., 2015, PLoS One, 10, e0134995} |
| Female Primary Hepatocytes | ThermoFisher/Life Technologies | #Hu1475 |
| Male Primary Hepatocytes | Celsis In Vitro Technologies/Bioreclamation, LLC | #M00995 |
| HepG2 cells | ATCC | #HB-8065 |
| Experimental Models: Organisms/Strains | ||
| Mouse: FRGN; Fah−/−/Rag2−/−/Il2rg−/− NOD | Markus Grompe | {Wilson et al., 2014, Stem Cell Res, 13, 404-12} |
| Recombinant DNA – N/A | ||
| Sequence-Based Reagents | ||
| Primetime Assay apoB 56-FAM/CT GGA TAC C/Zen/G TGT ATG GAA ACT GCT CC/3IABkFQ/ Primer 1-CAT TGC CCT TCC TCG TCT T Primer 2-CCA GAG ACA GAA GAA GCC AAG |
Integrated DNA Technologies | Hs.PT.47.18897448 |
| Primetime Assay rpl13 56-FAM/AGCAGTACC/ZEN/TGTTTAGCCACGATGG/3IABkFQ Primer 1-GCCTTCACAGCGTACGA Primer 2-CGAAGATGGCGGAGGTG |
Integrated DNA Technologies | Hs.PT.58.1788586 |
| Software and Algorithms | ||
| Microsoft Excel for Mac v15.29 | Microsoft | |
| Adobe Illustrator CS6 | Adobe Systems Inc | |
| Adobe Photoshop CS6 | Adobe Systems Inc | |
| Image Lab software | Bio-Rad | 1709691 |
| Graphpad Prism v6 | Graphpad Software, Inc | |
| Other | ||
| Spectrum Collection | Microsource Discovery Systems Inc | http://www.msdiscovery.com/spectrum.html |
| Hepatocyte Thaw Medium | ThermoFisher Scientific | CM7500 |
| Williams’ Medium E | Sigma-Aldrich | W4128 |
| HCM BulletKit (CC-3199 & CC-4182) | Lonza | CC-3198 |
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grants DK55743, DK087377, DK102716 (S.A.D.) and HG006398 (to S. A. D. and D. J. R.) and F30 DK091994 (to M. A. C.). Additional support was received from the Keck Foundation, the Phoebe R. and John D. Lewis Foundation, the Marcus Family, and the Sophia Wolf Quadracci Memorial Fund. Glenn Bushee provided valuable advice for the analyses of electronic medical records that was supported by the Medical College of Wisconsin CTSA Grant 8UL1TR000055 and the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin. Human hepatocytes were provided as a generous gift from Thermo Fisher Scientific, Life Technologies. Lisa Wilson and John Bial from Yecuris Corporation provided expert advice and guidance in repopulation of FRGN mice with human hepatocytes.
Footnotes
Roles of authors
M.C. performed drug screens, cardiac glycoside characterizations, data analyses, contributed to experimental design and was responsible for the analysis of medical records. S.K.M. and F.K.N contributed to the generation of humanized FRGN avatars and apoB analyses. F.D.F., R.J., A.U., and L.B.T. conducted studies on mechanism of glycoside action, and M.D.G., M.C., and P.T. designed and performed protein synthesis studies. M.B. gave technical assistance with high throughput screening. M.G. provided FRGN mice and advice on the production of avatars and experimental design. E. P. and D.J.R. designed and performed triglyceride labeling studies and contributed to data analyses and interpretation and manuscript editing. W.Y. and E.E.M. contributed to the generation of iPSCs. S.A.D. contributed to data analyses, experimental design, and writing of the manuscript.
DATA AND SOFTWARE AVAILABILITY – N/A
ADDITIONAL RESOURCES – N/A
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alphonse PA, Jones PJ. Revisiting Human Cholesterol Synthesis and Absorption: The Reciprocity Paradigm and its Key Regulators. Lipids. 2016;51:519–536. doi: 10.1007/s11745-015-4096-7. [DOI] [PubMed] [Google Scholar]
- Ambrosy AP, Butler J, Ahmed A, Vaduganathan M, van Veldhuisen DJ, Colucci WS, Gheorghiade M. The use of digoxin in patients with worsening chronic heart failure: reconsidering an old drug to reduce hospital admissions. J Am Coll Cardiol. 2014;63:1823–1832. doi: 10.1016/j.jacc.2014.01.051. [DOI] [PubMed] [Google Scholar]
- Asgari S, Moslem M, Bagheri-Lankarani K, Pournasr B, Miryounesi M, Baharvand H. Differentiation and Transplantation of Human Induced Pluripotent Stem Cell-derived Hepatocyte-like Cells. Stem Cell Rev. 2011 doi: 10.1007/s12015-011-9330-y. [DOI] [PubMed] [Google Scholar]
- Aslan I, Kucuksayan E, Aslan M. Effect of insulin analog initiation therapy on LDL/HDL subfraction profile and HDL associated enzymes in type 2 diabetic patients. Lipids Health Dis. 2013;12:54. doi: 10.1186/1476-511X-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basma H, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger DR, Ware BR, Davidson MD, Allsup SR, Khetani SR. Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell-cell interactions in vitro. Hepatology. 2015;61:1370–1381. doi: 10.1002/hep.27621. [DOI] [PubMed] [Google Scholar]
- Binda D, Lasserre-Bigot D, Bonet A, Thomassin M, Come MP, Guinchard C, Bars R, Jacqueson A, Richert L. Time course of cytochromes P450 decline during rat hepatocyte isolation and culture: effect of L-NAME. Toxicol In Vitro. 2003;17:59–67. doi: 10.1016/s0887-2333(02)00118-2. [DOI] [PubMed] [Google Scholar]
- Bissig KD, Wieland SF, Tran P, Isogawa M, Le TT, Chisari FV, Verma IM. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120:924–930. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissig-Choisat B, et al. Development and rescue of human familial hypercholesterolaemia in a xenograft mouse model. Nat Commun. 2015;6:7339. doi: 10.1038/ncomms8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- Cameron K, et al. Recombinant Laminins Drive the Differentiation and Self-Organization of hESC-Derived Hepatocytes. Stem Cell Reports. 2015;5:1250–1262. doi: 10.1016/j.stemcr.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campia I, Gazzano E, Pescarmona G, Ghigo D, Bosia A, Riganti C. Digoxin and ouabain increase the synthesis of cholesterol in human liver cells. Cell Mol Life Sci. 2009;66:1580–1594. doi: 10.1007/s00018-009-9018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayo MA, Cai J, DeLaForest A, Noto FK, Nagaoka M, Clark BS, Collery RF, Si-Tayeb K, Duncan SA. JD induced pluripotent stem cell-derived hepatocytes faithfully recapitulate the pathophysiology of familial hypercholesterolemia. Hepatology. 2012;56:2163–2171. doi: 10.1002/hep.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SM, Kim Y, Shim JS, Park JT, Wang RH, Leach SD, Liu JO, Deng C, Ye Z, Jang YY. Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology. 2013;57:2458–2468. doi: 10.1002/hep.26237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchel M, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35:2146–2157. doi: 10.1093/eurheartj/ehu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MD, Ware BR, Khetani SR. Stem cell-derived liver cells for drug testing and disease modeling. Discov Med. 2015;19:349–358. [PMC free article] [PubMed] [Google Scholar]
- Davidson MH. Novel nonstatin strategies to lower low-density lipoprotein cholesterol. Curr Atheroscler Rep. 2009;11:67–70. doi: 10.1007/s11883-009-0011-0. [DOI] [PubMed] [Google Scholar]
- Davis CG, Lehrman MA, Russell DW, Anderson RG, Brown MS, Goldstein JL. The J.D. mutation in familial hypercholesterolemia: amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell. 1986;45:15–24. doi: 10.1016/0092-8674(86)90533-7. [DOI] [PubMed] [Google Scholar]
- Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, Zapol WM, Bloch KD, Yu PB. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:613–622. doi: 10.1161/ATVBAHA.111.242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Regan SN, Xia Y, Oostrom LA, Cowan CA, Musunuru K. Enhanced Efficiency of Human Pluripotent Stem Cell Genome Editing through Replacing TALENs with CRISPRs. Cell Stem Cell. 2013;12:393–394. doi: 10.1016/j.stem.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FFJ, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EC, et al. Mice with chimeric livers are an improved model for human lipoprotein metabolism. PLoS One. 2013;8:e78550. doi: 10.1371/journal.pone.0078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan RL, Wu M, Chedin F, Brenner C. An ultrasensitive high throughput screen for DNA methyltransferase 1-targeted molecular probes. PLoS One. 2013;8:e78752. doi: 10.1371/journal.pone.0078752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem. 2002;277:17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- Gayathri V, Ananthi S, Chandronitha C, Sangeetha MK, Vasanthi HR. Hypolipidemic potential of flowers of Nerium oleander in high fat diet-fed Sprague Dawley rats. Nat Prod Res. 2011;25:1110–1114. doi: 10.1080/14786419.2010.541883. [DOI] [PubMed] [Google Scholar]
- Ginsberg HN, Fisher EA. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. J Lipid Res. 2009;50(Suppl):S162–6. doi: 10.1194/jlr.R800090-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DC, et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26:894–902. doi: 10.1634/stemcells.2007-0718. [DOI] [PubMed] [Google Scholar]
- Horvath P, et al. Screening out irrelevant cell-based models of disease. Nat Rev Drug Discov. 2016 doi: 10.1038/nrd.2016.175. [DOI] [PubMed] [Google Scholar]
- Hothi SS, Chinnappa S, Tan LB. 200+ years of a misunderstood drug for treating chronic heart failure: digoxin, why and how should we continue using it? Int J Cardiol. 2013;168:645–647. doi: 10.1016/j.ijcard.2012.04.086. [DOI] [PubMed] [Google Scholar]
- Jonker JT, et al. Pioglitazone decreases plasma cholesteryl ester transfer protein mass, associated with a decrease in hepatic triglyceride content, in patients with type 2 diabetes. Diabetes Care. 2010;33:1625–1628. doi: 10.2337/dc09-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrel EA, Lanza R. Current status of pluripotent stem cells: moving the first therapies to the clinic. Nat Rev Drug Discov. 2015;14:681–692. doi: 10.1038/nrd4738. [DOI] [PubMed] [Google Scholar]
- Kocisko DA, Baron GS, Rubenstein R, Chen J, Kuizon S, Caughey B. New inhibitors of scrapie-associated prion protein formation in a library of 2000 drugs and natural products. J Virol. 2003;77:10288–10294. doi: 10.1128/JVI.77.19.10288-10294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolkhof P, Geerts A, Schafer S, Torzewski J. Cardiac glycosides potently inhibit C-reactive protein synthesis in human hepatocytes. Biochem Biophys Res Commun. 2010;394:233–239. doi: 10.1016/j.bbrc.2010.02.177. [DOI] [PubMed] [Google Scholar]
- Lang J, Vera D, Cheng Y, Tang H. Modeling Dengue Virus-Hepatic Cell Interactions Using Human Pluripotent Stem Cell-Derived Hepatocyte-like Cells. Stem Cell Reports. 2016;7:341–354. doi: 10.1016/j.stemcr.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, et al. Induced pluripotent stem cell modeling of multisystemic, hereditary transthyretin amyloidosis. Stem Cell Reports. 2013;1:451–463. doi: 10.1016/j.stemcr.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Guo J, Ying Z, Chen S, Yang L, Chen K, Long Q, Qin D, Pei D, Liu X. Valproic acid-induced hepatotoxicity in alpers syndrome is associated with mitochondrial permeability transition pore opening-dependent apoptotic sensitivity in an induced pluripotent stem cell model. Hepatology. 2015;61:1730–1739. doi: 10.1002/hep.27712. [DOI] [PubMed] [Google Scholar]
- Liang J. Translocation Efficiency, Susceptibility to Proteasomal Degradation, and Lipid Responsiveness of Apolipoprotein B Are Determined by the Presence of beta Sheet Domains. Journal of Biological Chemistry. 1998;273:35216–35221. doi: 10.1074/jbc.273.52.35216. [DOI] [PubMed] [Google Scholar]
- Maetzel D, Sarkar S, Wang H, Abi-Mosleh L, Xu P, Cheng AW, Gao Q, Mitalipova M, Jaenisch R. Genetic and chemical correction of cholesterol accumulation and impaired autophagy in hepatic and neural cells derived from Niemann-Pick Type C patient-specific iPS cells. Stem Cell Reports. 2014;2:866–880. doi: 10.1016/j.stemcr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallanna SK, Cayo MA, Twaroski K, Gundry RL, Duncan SA. Mapping the Cell-Surface N-Glycoproteome of Human Hepatocytes Reveals Markers for Selecting a Homogeneous Population of iPSC-Derived Hepatocytes. Stem Cell Reports. 2016 doi: 10.1016/j.stemcr.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallanna SK, Duncan SA. Differentiation of hepatocytes from pluripotent stem cells. Curr Protoc Stem Cell Biol. 2013;26(Unit 1G.4) doi: 10.1002/9780470151808.sc01g04s26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsa E, Burridge PW, Wu JC. Human stem cells for modeling heart disease and for drug discovery. Sci Transl Med. 2014;6:239ps6. doi: 10.1126/scitranslmed.3008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiec MM, et al. Modulators of hepatic lipoprotein metabolism identified in a search for small-molecule inducers of tribbles pseudokinase 1 expression. PLoS One. 2015;10:e0120295. doi: 10.1371/journal.pone.0120295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RA, Yang P, Pawlus AD, Block KI. Cardiac glycosides as novel cancer therapeutic agents. Mol Interv. 2008;8:36–49. doi: 10.1124/mi.8.1.8. [DOI] [PubMed] [Google Scholar]
- Nissen SE, et al. Comparison of PCSK9 Inhibitor Evolocumab vs Ezetimibe in Statin-Intolerant Patients: Design of the Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin-Intolerant Subjects 3 (GAUSS-3) Trial. Clin Cardiol. 2016;39:137–144. doi: 10.1002/clc.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 2008;7:926–935. doi: 10.1038/nrd2682. [DOI] [PubMed] [Google Scholar]
- Rader DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. J Clin Invest. 2003;111:1795–1803. doi: 10.1172/JCI18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader DJ, Kastelein JJ. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation. 2014;129:1022–1032. doi: 10.1161/CIRCULATIONAHA.113.001292. [DOI] [PubMed] [Google Scholar]
- Rashid ST, et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 2010;120:3127–3136. doi: 10.1172/JCI43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganti C, Campia I, Kopecka J, Gazzano E, Doublier S, Aldieri E, Bosia A, Ghigo D. Pleiotropic effects of cardioactive glycosides. Curr Med Chem. 2011;18:872–885. doi: 10.2174/092986711794927685. [DOI] [PubMed] [Google Scholar]
- Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RE, Trehan K, Andrus L, Sheahan TP, Ploss A, Duncan SA, Rice CM, Bhatia SN. Modeling hepatitis C virus infection using human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:2544–2548. doi: 10.1073/pnas.1121400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010a;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Tayeb K, Noto FK, Sepac A, Sedlic F, Bosnjak ZJ, Lough JW, Duncan SA. Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC Dev Biol. 2010b;10:81. doi: 10.1186/1471-213X-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- Strittmatter SM. Overcoming Drug Development Bottlenecks With Repurposing: Old drugs learn new tricks. Nat Med. 2014;20:590–591. doi: 10.1038/nm.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GJ, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51:329–335. doi: 10.1002/hep.23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram M, Yao Z. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr Metab (Lond) 2010;7:35. doi: 10.1186/1743-7075-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkolnicka D, Lucendo-Villarin B, Moore JK, Simpson KJ, Forbes SJ, Hay DC. Reducing Hepatocyte Injury and Necrosis in Response to Paracetamol Using Noncoding RNAs. Stem Cells Transl Med. 2016;5:764–772. doi: 10.5966/sctm.2015-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafaleng EN, et al. Induced pluripotent stem cells model personalized variati. 2015;62:147–157. doi: 10.1002/hep.27753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- Touboul T, et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 2010;51:1754–1765. doi: 10.1002/hep.23506. [DOI] [PubMed] [Google Scholar]
- Wang C, Tao W, Wang Y, Bikow J, Lu B, Keating A, Verma S, Parker TG, Han R, Wen XY. Rosuvastatin, identified from a zebrafish chemical genetic screen for antiangiogenic compounds, suppresses the growth of prostate cancer. Eur Urol. 2010;58:418–426. doi: 10.1016/j.eururo.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cheetham AG, Angacian G, Su H, Xie L, Cui H. Peptide-drug conjugates as effective prodrug strategies for targeted delivery. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman JL, Liou AP, Shelat AA, Cohen FE, Guy RK, DeRisi JL. Searching for new antimalarial therapeutics amongst known drugs. Chem Biol Drug Des. 2006;67:409–416. doi: 10.1111/j.1747-0285.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K, Wagenknecht B, Zwissler B, Brown L, Krawietz W, Erdmann E. Cardiac glycoside receptors in cultured heart cells--I. Characterization of one single class of high affinity receptors in heart muscle cells from chick embryos. Biochem Pharmacol. 1984;33:55–70. doi: 10.1016/0006-2952(84)90370-8. [DOI] [PubMed] [Google Scholar]
- Wilson EM, Bial J, Tarlow B, Bial G, Jensen B, Greiner DL, Brehm MA, Grompe M. Extensive double humanization of both liver and hematopoiesis in FRGN mice. Stem Cell Res. 2014;13:404–412. doi: 10.1016/j.scr.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Cai T. Na+-K+--ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3:157–168. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- Yi F, Qu J, Li M, Suzuki K, Kim NY, Liu GH, Belmonte JC. Establishment of hepatic and neural differentiation platforms of Wilson’s disease specific induced pluripotent stem cells. Protein Cell. 2012;3:855–863. doi: 10.1007/s13238-012-2064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang S, et al. Rescue of ATP7B function in hepatocyte-like cells from Wilson’s disease induced pluripotent stem cells using gene therapy or the chaperone drug curcumin. Hum Mol Genet. 2011;20:3176–3187. doi: 10.1093/hmg/ddr223. [DOI] [PubMed] [Google Scholar]
- Zhang XD. Illustration of SSMD, z score, SSMD*, z* score, and t statistic for hit selection in RNAi high-throughput screens. J Biomol Screen. 2011;16:775–785. doi: 10.1177/1087057111405851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.