Abstract
Prostate cancer is the most common non-cutaneous malignancy in men. It is generally considered a cancer of the elderly, and the median age of presentation is 68 years. However 10% of new diagnoses in the USA occur in men aged ≤ 55 years. This may be due to more prevalent screening nowadays, and may also reflect the diagnosis of an increasingly recognized but underappreciated entity, i.e. early-onset prostate cancer. Patients with early onset prostate cancer pose unique challenges. Current data suggest that early-onset prostate cancer is a distinct phenotype—from both an etiological and clinical perspective— that deserves further attention. We present a case of a 28-year-old man who presented with lower urinary tract symptoms and was diagnosed with advanced stage prostate cancer.
Key Words: Prostate, Prostate cancer, Urology
Background
Prostate cancer is most common malignancy in men, with presentation in elderly males. Recent studies show increase in incidence of prostate cancer in young males. Prostate cancer in younger age group is generally undifferentiated and associated with poor prognosis. Our case represents one of the youngest reported cases of prostate cancer.
Our case demonstrates that prostate cancer is not only “disease of old age”, as believed earlier, and should be kept as differential when treating young males for lower urinary tract symptoms.
Case Report
A 28-year-old man presented with lower abdominal pain, weak urine stream, nocturia and urinary hesitancy for 3 weeks. Symptoms had gradually become worse to the point where he could no longer void without straining and had 1 episode of acute urinary retention. He also complained of lower back ache and pressure for the past 4 days. He denied fever and other constitutional symptoms. He did not endorse burning during micturition, changes in bowel habitus, erectile dysfunction or painful erection and had not noticed any blood in urine or semen. No history of urinary tract infection, nephrolithiasis, malignancy or neurological disease was reported. The patient had no known medical conditions.
On exam, he was afebrile with pulse rate 82/min, blood pressure 128/76 mmHg, respiratory rate 16/min. General physical and systemic examination was unremarkable. Digital rectal exam revealed a hard, non-tender, multinodular prostate with irregular surface.
Investigations
Laboratory analysis revealed white blood cell count 8.3 × 109/l, hemoglobin 13.2 g/dl, platelet count 214 × 109/l. Urinalysis, urine culture, electrolytes including fasting and post prandial blood sugar, serum calcium and alkaline phosphatase were unremarkable. Serum creatinine was 0.8 mg/dl and prostate specific antigen (PSA) level was 5.85 ng/ml (normal PSA 0–4 ng/ml).
Pelvic ultrasonography demonstrated a heterogeneous prostatic mass with enlarged aortocaval and left internal iliac lymph nodes each. Magnetic resonance imaging of the abdomen and pelvis revealed an enlarged multi-lobulated prostate with marked diffusion restriction, and extra capsular extension into the bilateral seminal vesicles and the bladder neck (fig. 1). There were also multiple enlarged pelvic lymph nodes. Abdominopelvic viscera appeared normal.
Fig. 1.
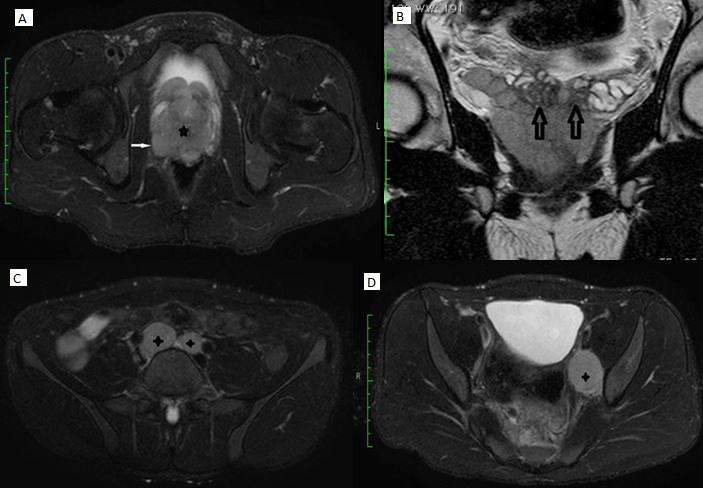
Radiographic findings (MRI). A Large T2 hypo intense mass (*) almost replacing the prostate gland with extra capsular invasion (arrow); B T2 coronal images show invasion of mass into bilateral seminal vesicles (arrows); C Enlarged bilateral common iliac lymph nodes (+); D Enlarged left internal iliac lymph node (+).
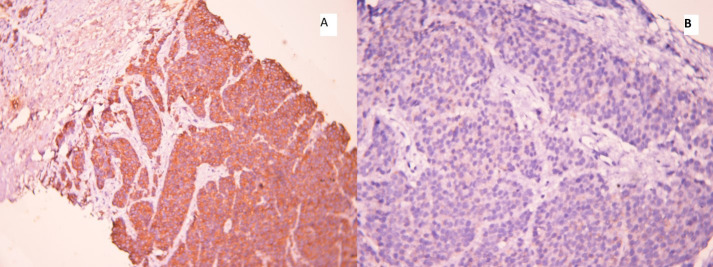
A transrectal ultrasound guided biopsy of the prostate demonstrated adenocarcinoma of prostate with Gleason score 4 + 5. Immunohistochemistry confirmed the diagnosis of poorly differentiated adenocarcinoma of prostate with sections showing focal PSA positivity along with strong pan-cytokeratin, AMACR and synaptophysin positivity (fig. 2). Ki 67 index was elevated to 40%. Sections were negative for CK 7, CK 20, TTF1, MIC 2 and myogenin. The patient refused genetic testing.
Fig. 2.
Histopathological features. Immunohistochemistry showing strong pan-cytokeratin (A) and AMACR (B) positivity.
Differential Diagnosis
The patient's presentation: lower abdominal pain, weak urinary stream and nocturia; points towards urinary tract infection which was ruled out by unremarkable urine analysis and sterile urine culture report. Another diagnosis which comes to mind is diabetes mellitus which was ruled out by normal fasting and post prandial blood sugar levels.
Nephrolithiasis is a very important differential as the patient presents with lower urinary tract symptoms and if the calculus is in the bladder or the urethra, it can cause poor urinary stream. Our patient's urinary and serum calcium and phosphate levels were unremarkable and pelvic ultrasonography showed no hydroureteronephrosis or calculus in ureters, bladder or urethra.
With a history of lower urinary tract symptoms, urethral stricture is also a possibility but there was no history of any trauma or previous catheterization. This makes traumatic stricture an unlikely diagnosis. No recurrent or previous episodes of urinary tract infections or burning micturition rules out urethral stricture as a diagnosis.
Prostatitis can present as urinary tract infection, but on digital rectal examination prostate was non-tender and also urine analysis was unremarkable and had no leukocytes or any microorganism. Also transrectal ultrasonography didn't reveal any signs of prostatitis.
Digital rectal examination revealed nodular hard prostate which raised the suspicion of prostate cancer which was then confirmed by transrectal ultrasonography and biopsy.
Treatment
In view of the extra capsular extension and lymph node spread, androgen deprivation therapy (ADT) was offered to the patient who chose bilateral orchidectomy over chemical/hormonal androgen deprivation. Semen preservation was performed and bilateral orchidectomy performed without incident. He received daily adjuvant hormonal chemotherapy with bicalutamide after surgery.
Outcome and Follow-up
Follow-up at 12-months after presentation showed no urological complaints, with a PSA level of 0.45 ng/ml. He is continuing to receive bicalutamide treatment.
Discussion
To the best of our knowledge, the literature on prostate cancer contains < 30 reported cases of prostate cancer among men ≤ 40 years of age, with an incidence of 0.8–1.1% [1,2,3,4,5].
PSA-based screening has induced an important age migration effect in which the incidence of prostate cancer has increased in men of lower age group.
Early onset prostate cancer (diagnosed in men < 55 years of age) is considered a different clinical entity from prostate cancer diagnosed at an older age. A number of large population based studies have demonstrated poor survival among patients < 50 years of age with advanced prostate cancer or unknown stage disease as compared to older patients [6,7,8,9,10,11,12,13]. PSA level is lower in these patients due to poorly differentiated adenocarcinoma of prostate. So in poorly differentiated carcinoma, PSA level is not indicative of prostate cancer.
Patients diagnosed with high grade tumors (Gleason score of 8–10) at ages 35–44 are also at higher odds of succumbing to prostate cancer as compared to patients aged 65–74 years. Moreover a strong genetic component has been associated with early onset prostate cancer.
Lange et al. [14] reported that men with early onset prostate cancer are more likely to have a greater number of genetic variants, which are associated with an increased risk of prostate cancer, as compared to older patients. Although a majority of patients with early onset prostate cancer are diagnosed with moderately differentiated disease, the management of patients with early onset prostate cancer poses unique clinical challenges.
Cases with organ limited prostate cancer may benefit from prostatectomy but same is not the case in locally advanced or metastatic prostate cancer. Patients who are diagnosed with prostate cancer with associated lymph node involvement are broadly categorized into 2 subgroups: patients who have detectable lymph nodes on imaging (dN+) and patients who have no detectable lymph node involvement on imaging but have node positive disease at time of surgery (pN+). According to the 2013 National
Comprehensive Cancer Network guidelines [15], patients with dN+ disease should be managed with ADT or RT-ADT.
On the other hand, patients with lymph node involvement detected at radical prostatectomy are offered observation, ADT or RT-ADT. ADT can be achieved medically by hormonal drugs or surgically by orchidectomy. Our patient had locally advanced disease with imaging identifying local LN spread and surgery was not conducted. He was appropriately managed with ADT as per his wishes.
Learning Points/Take Home Messages
With changing environmental factors and PSA screening, a larger number and proportion of young males with prostate cancer are coming into attention.
Most young patients with prostate cancer have moderately differentiated, organ confined disease.
Given the otherwise longer life expectancy in younger patients, treatment should be initiated promptly rather than using the wait and watch method generally used in older age group males.
There is more risk of treatment related adverse effects in the younger population for the same reason.
References
- 1.Yamamoto S, Senzaki A, Yamagiwa K, Tanaka T, Oda T. Prostatic carcinoma in a young adult: a case report. Hinyokika Kiyo. 1990;36:617–622. [PubMed] [Google Scholar]
- 2.Roberts JT, Essenhigh DM. Adenocarcinoma of prostate in 40-year-old body-builder. Lancet. 1986;2(8509):742. doi: 10.1016/s0140-6736(86)90251-5. [DOI] [PubMed] [Google Scholar]
- 3.Davis BE, Weigel JW. Adenocarcinoma of the prostate discovered in 2 young patients following total prostatovesiculectomy for refractory prostatitis. J Urol. 1990;144:744–745. doi: 10.1016/s0022-5347(17)39573-3. [DOI] [PubMed] [Google Scholar]
- 4.Ruska KM, Partin AW, Epstein JI, Kahane H. Adenocarcinoma of the prostate in men younger than 40 years of age: diagnosis and treatment with emphasis on radical prostatectomy findings. Urology. 1999;53:1179–1183. doi: 10.1016/s0090-4295(99)00020-5. [DOI] [PubMed] [Google Scholar]
- 5.D'Aprile M, Santini D, Di Cosimo S, Gravante G, Vincenzi B, Spoto S, Costantino S, Rabitti C, Tonini G. Atypical case of metastatic undifferentiated prostate carcinoma in a 36 years old man: clinical report and literature review. Clin Ter. 2000;151:371–374. [PubMed] [Google Scholar]
- 6.Gronberg H, Damber JE, Jonsson H, Lenner P. Patient age as a prognostic factor in prostate cancer. J Urol. 1994;152:892–895. doi: 10.1016/s0022-5347(17)32601-0. [DOI] [PubMed] [Google Scholar]
- 7.Harrison GS. The prognosis of prostatic cancer in the younger man. Br J Urol. 1983;55:315–320. doi: 10.1111/j.1464-410x.1983.tb03307.x. [DOI] [PubMed] [Google Scholar]
- 8.Huben R, Natarajan N, Pontes E, Mettlin C, Smart CR, Murphy GP. Carcinoma of prostate in men less than fifty years old. Data from American College of Surgeons' National Survey. Urology. 1982;20:585–588. doi: 10.1016/0090-4295(82)90304-1. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DE, Lanieri JP, Jr, Ayala AG. Prostatic adenocarcinoma occurring in men under 50 years of age. J Surg Oncol. 1972;4:207–216. doi: 10.1002/jso.2930040305. [DOI] [PubMed] [Google Scholar]
- 10.Lin DW, Porter M, Montgomery B. Treatment and survival outcomes in young men diagnosed with prostate cancer: a population-based cohort study. Cancer. 2009;115:2863–2871. doi: 10.1002/cncr.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merrill RM, Bird JS. Effect of young age on prostate cancer survival: a population based assessment (United States) Cancer Causes Control. 2002;13:435–443. doi: 10.1023/a:1015764507609. [DOI] [PubMed] [Google Scholar]
- 12.Tjaden HB, Culp DA, Flocks RH. Clinical adenocarcinoma of the prostate in patients under 50 years of age. J Urol. 1965;93:618–621. doi: 10.1016/S0022-5347(17)63840-0. [DOI] [PubMed] [Google Scholar]
- 13.Wilson JM, Kemp IW, Stein GJ. Cancer of the prostate. Do younger men have a poorer survival rate? Br J Urol. 1984;56:391–396. doi: 10.1111/j.1464-410x.1984.tb05828.x. [DOI] [PubMed] [Google Scholar]
- 14.Lange EM, Salinas CA, Zuhlke KA, Ray AM, Wang Y, Lu Y, Ho LA, Luo J, Cooney KA. Early onset prostate cancer has a significant genetic component. Prostate. 2012;72:147–156. doi: 10.1002/pros.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitin T, Blute M, Lee R, Efstathiou J. Management of lymph node-positive prostate cancer: the role of surgery and radiation therapy. Oncology (Williston Park) 2013;27:647–655. [PubMed] [Google Scholar]