Abstract
The proteins that compose a herpesvirus virion are thought to contain the functional information required for de novo infection, as well as virion assembly and egress. To investigate functional roles of Kaposi's sarcoma-associated herpesvirus (KSHV) virion proteins in viral productive replication and de novo infection, we attempted to identify virion proteins from purified KSHV by a proteomic approach. Extracellular KSHV virions were purified from phorbol-12-tetradecanoate-13-acetate-induced BCBL-1 cells through double-gradient ultracentrifugation, and their component proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Thirty prominent protein bands were excised and subjected to high-performance liquid chromatography ion trap mass spectrometric analysis. This study led to the identification of 24 virion-associated proteins. These include five capsid proteins, eight envelope glycoproteins, six tegument proteins, and five proteins whose locations in the virions have not yet been defined. Putative tegument proteins encoded by open reading frame 21 (ORF21), ORF33, and ORF45 were characterized and found to be resistant to protease digestion when purified virions were treated with trypsin, confirming that they are located within the virion particles. The ORF64-encoded large tegument protein was found to be associated with capsid but sensitive to protease treatment, suggesting its unique structure and array in KSHV virions. In addition, cellular β-actin and class II myosin heavy chain type A were found inside KSHV virions and associated with tegument-capsid structure. Identification of KSHV virion proteins makes it possible to study the functional roles of these virion proteins in KSHV replication and pathogenicity.
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV), also known as human herpesvirus 8, is a human DNA tumor virus and the etiological agent of KS. KSHV is also associated with two lymphoproliferative disorders, primary effusion lymphoma and multicentric Castleman's disease (reviewed in reference 21). On the basis of phylogenetic analysis, this virus has been classified as a member of the Gammaherpesvirinae subfamily and the Rhadinovirus genus and is closely related to herpesvirus saimiri of squirrel monkeys and Epstein-Barr virus (EBV) (22).
As a gammaherpesvirus, KSHV has two modes of infection: latency and lytic replication. In latently infected cells that contain a limited number of viral genomes, no infectious virus is produced. Only a few viral genes are expressed during latency, and these are referred to as latent genes. Latency can be interrupted, and KSHV switches to the lytic life cycle by spontaneous or induced reactivation, which leads to the expression of most or all of the viral genes and production of infectious virion particles (20, 29). In KS lesions, most of the spindle cells of endothelial origin were found to be latently infected with KSHV and a small percentage of the cells was undergoing spontaneous lytic replication (35, 36, 44). Increasing evidence suggests that the small percentage of viral lytic replication plays great roles in viral pathogenicity. First, unlike latent infection with most other DNA tumor virus, latent infection with KSHV does not provide the infected cells a growth advantage and latency alone is not sufficient to sustain KS tumorigenesis (13, 41). Studies have shown that the lytic replication cycle directly contributes to viral tumorigenesis by spreading viruses to target cells and providing paracrine regulation for KS development (9). Furthermore, a recent study by Grundhoff and Ganem (13) suggested a new role for lytic replication in sustaining the population of latently infected cells that otherwise are quickly lost by segregation of latent viral episomes as spindle cells divide. This observation indicated that KSHV lytic replication and constant primary infection of fresh cells are crucial for viral tumorigenicity and pathogenesis. However, little is known about the late phase of KSHV lytic replication, including virion particle formation and egress. In addition, release of infectious virions will lead to de novo infection of fresh cells but the early events of de novo infection largely remain unknown.
Study of herpesvirus virion proteins will help in understanding the processes and mechanisms of virion particle production and primary infection. A herpesvirus virion contains more than 30 virus-encoded proteins that are assembled into four morphologically distinct components of the virion: the inner nucleoprotein core, which contains the double-stranded viral DNA genome; the icosadelahedral capsid shell, which encloses the viral DNA core; the lipid envelope, which bears various membrane glycoproteins on the surface; and the electron-dense material between the capsid and the envelope, which is defined as tegument (reviewed in references 30 and 31). The nucleocapsid of KSHV has been investigated, and five capsid proteins have been identified. They are open reading frame 25 (ORF25)-encoded major capsid protein (MCP), ORF62-encoded triplex component I (TRI-1), ORF26-encoded TRI-2, ORF65-encoded small capsid protein (also designated small capsomer-interacting protein or SCIP), and the ORF17.5-encoded scaffolding or assembly protein (SCAF) (24, 43). Except for SCIP, the capsid proteins are in general conserved in structure and function among all herpesviruses. However, the smallest capsid protein SCIPs have the least sequence homology among herpesvirus family members and have been implicated in virus specificity during infection (24, 39). Inspection of the sequence of the KSHV genome revealed seven ORFs that encode potential glycoproteins, i.e., ORF8 (gB [glycoprotein B]), ORF K8.1, ORF22 (gH), ORF39 (gM), ORF47 (gL), ORF53 (gN), and ORF68 (25, 33). gB was found to interact with cellular receptor integrin α3β1 and mediate virus entry through an endocytosis process (2, 3). ORF K8.1 encodes an envelope glycoprotein of two forms, K8.1A of 228 amino acids and K8.1B of 167 amino acids. K8.1A was shown to interact with host cells through binding to heparan sulfate-like moieties (10, 42). The tegument is a complex and poorly characterized layer of proteins. Some of these tegument proteins are believed to be released into infected cells following entry of the virus into the cells. In this manner, tegument proteins may exert important regulatory function immediately after entry. Some play roles in the KSHV virion assembly, envelopment, or budding process. Thus, it is impossible to fully understand KSHV virion formation and de novo infection without investigating structures and functions of KSHV tegument proteins.
To learn more about KSHV primary infection, virion formation, and egress, we decided to identify protein components that are associated with KSHV virions. Here we report our efforts to purify KSHV virion particles and identify viral proteins that are associated with purified virions by a proteomic approach. This study led to the identification of 24 KSHV virion-associated viral proteins and some cellular proteins. Experiments that confirmed the association of some of these proteins with the virions and their localization in the virions are also presented.
MATERIALS AND METHODS
Purification of KSHV virions.
BCBL-1, a latently KSHV-infected primary effusion lymphoma cell line, was maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum. For virus production, BCBL-1 cells (0.5 × 106/ml) were induced with 20 ng of phorbol-12-tetradecanoate-13-acetate (TPA) per ml and 0.5 μM ionomycin (Sigma, St. Louis, Mo.) for 5 to 7 days. The medium was collected and cleared by centrifugation at 4,000 × g for 30 min and then at 8,000 × g for 15 min to remove cells and cell debris. The supernatant was filtered through 0.45-μm-pore-size filters. Virions were pelleted at 27,000 rpm for 1 h through a 5% sucrose cushion (5 ml) in a Beckman SW28 rotor and resuspended in 1× phosphate-buffered saline (PBS) plus 0.1% bacitracin (Sigma) in 1/100 of the original volume. The concentrated virus particles were centrifuged through a 20 to 35% Nycodenz (Sigma) step gradient at 24,000 rpm for 2 h. The virus band at the gradient junction was collected. The virions were then diluted with 1× PBS and pelleted at 27,000 rpm for 1 h. The pellets were resuspended in 1× PBS and further purified through a 20 to 35% continuous Nycodenz gradient.
Mass spectrometric analysis.
Purified virions were resolved on a 3 to 8% Tris-acetate NuPAGE gel and a 12% Bis-Tris NuPAGE gel (Invitrogen, Carlsbad, Calif.) and stained with colloidal Coomassie G-250 (Invitrogen). Prominent protein bands were excised and subjected to trypsin digestion. A portion of the peptide digest was injected onto a nanocapillary reverse-phase high-performance liquid chromatograph coupled to a nanoelectrospray ionization source of an ion trap mass spectrometer (ThermoFinnigan LCQ). Mass spectrometry measures peptide masses and then fragments individual peptides to produce micro-liquid chromatography ion trap mass spectrometry (LC-MS/MS) spectra of fragments that reflect the peptide sequence. The LC-MS/MS spectra were run against a sequence database with the program SEQUEST. Mass spectrometry was carried out in the protein microchemistry mass spectrometry facility at the Wistar Institute.
Antibodies and Western blotting.
Monoclonal antibody against ORF45 was generated by using baculovirus-synthesized ORF45 as an antigen. Mouse polyclonal antibodies against the ORF11, ORF21 (thymidine kinase [TK]), ORF33, and ORF64 proteins were generated by using bacterially produced recombinant protein as antigens. A rabbit polyclonal antibody to K8.1 was obtained from Jae Jung at the New England Regional Primate Research Center. A rabbit antibody to ORF50 was from Don Ganem at the University of California at San Francisco. An anti-LANA rabbit antibody was from Rolf Renne at Case Western Reserve University. Rabbit antibodies against gB and monoclonal hybridoma supernatant against K8.1A and -B were obtained from Bala Chandran at the University of Kansas Medical Center. A monoclonal antibody against β-actin was purchased from Sigma, and a rabbit polyclonal antibody against nonmuscle myosin II heavy chain isoform A was purchased from Covance (Denver, Pa.). Whole-cell extract equivalent to 0.1 × 106 to 0.2 × 106 cells (∼0.5 ml of BCBL-1 culture) and purified virion lysate equivalent to 5 ml of induced BCBL-1 culture medium were resolved on 4 to 12% Bis-Tris NuPAGE gels (Invitrogen) and transferred to Hybond enhanced chemiluminescence nitrocellulose membranes (Amersham, Amersham, United Kingdom). The membranes were blocked in 5% dried milk in 1× PBS plus 0.2% Tween 20 (PBST) and then incubated with diluted primary antibodies for 2 h at room temperature or 4°C overnight. Anti-rabbit or anti-mouse immunoglobulin G antibody conjugated to horseradish peroxidase (Amersham) was used as the secondary antibody. The enhanced chemiluminescence system (Amersham) was used for detection of antibody-antigen complexes.
Trypsin treatment of purified virions.
Purified virions (10 μg of total protein) were treated with trypsin (4 μg/ml; Promega, Madison, Wis.) in 100 μl of buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM CaCl2) at 37°C for 1 h. Trypsin digestions were terminated by adding phenylmethylsulfonyl fluoride (PMSF) to a concentration of 0.5 mM and 1/100 volume of protease inhibitors (Sigma). In parallel, Triton X-100 was added to a final of concentration of 1% to remove the viral envelope and expose the tegumented capsid to the protease. Samples were analyzed by Western blotting.
Detergent treatment of purified virions.
Double-gradient-purified virions were treated with 1% Triton X-100 plus 0.5% deoxycholate (DOC) for 30 min at 37°C. The reaction mixture was separated into two fractions, supernatant and pelleted tegument-nucleocapsid, by centrifugation at 100,000 × g for 1 h. Equal amounts of protein from the supernatant and pellet were analyzed by Western blotting.
RESULTS AND DISCUSSION
Purification of extracellular KSHV particles from infected cells.
The major hurdle to KSHV virion study was the difficulty in purification of virion particles with both quality and quantity. That was largely due to the lack of a permissive cell system capable of supporting productive replication of the virus. In a recent study on a virion protein encoded by KSHV ORF45, we purified KSHV virion particles from cell culture medium released from TPA-induced BCBL-1 cells. We obtained a small amount of virion particles with satisfactory purity (46). In the present study, we used the same procedure to purify extracellular KSHV particles for identification of the entire repertoire of KSHV virion proteins. In brief, extracellular KSHV virions were purified from induced BCBL-1 cells through double-gradient ultracentrifugation (see Materials and Methods). The virus purification scheme was monitored by the loss of the precursor to KSHV gB, which is known to be associated with the cell membrane. Only the mature form of this protein was found in virions (1). Purified virions and BCBL-1 lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a nitrocellulose membrane, and immunoblotted with anti-gB antibody, as well as control antibodies against various KSHV proteins. As previously reported, gB was initially synthesized as a 110-kDa precursor molecule and then processed to 75- and 54-kDa mature forms that are present in the virion envelope (1, 4). In our preparation, the 110-kDa precursor was only detected in the induced BCBL-1 cell lysate but not in purified virions. The purified virions contained only mature forms of gB with molecular masses of 75 and 54 kDa, indicating that the purified virions were free of cellular membrane contamination (data not shown, but for a similar image, see Fig. 2F).
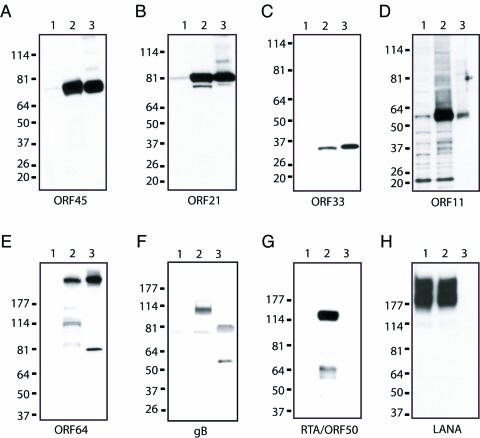
FIG. 2.
Association of KSHV putative virion proteins with purified virions. Uninduced and TPA-induced BCBL-1 cell extracts (lanes 1 and 2) and gradient-purified virions (lane 3) were resolved by SDS-4 to 12% PAGE, transferred onto nitrocellulose membranes, and immunoblotted with mouse monoclonal antibody against ORF45 (A), mouse polyclonal antibody against ORF21 (TK) (B), mouse polyclonal antibody against ORF33 (C), mouse polyclonal antibody against ORF11 (D), mouse polyclonal antibody against ORF64 (E), rabbit polyclonal immunoglobulin G against gB (F), rabbit polyclonal antibody against RTA/ORF50 (G), and rabbit polyclonal antibody against LANA (H). The values on the left are molecular sizes in kilodaltons.
Identification of proteins associated with purified KSHV virions.
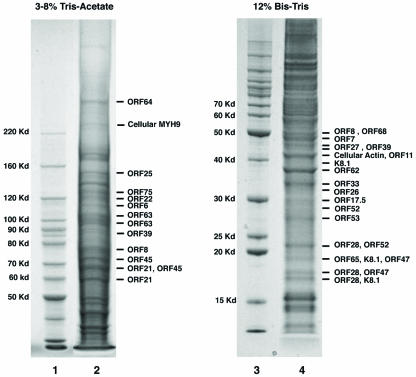
SDS-PAGE analysis of aliquots of purified virions (4 to 12% Bis-Tris SDS-PAGE, silver staining) revealed 30 to 40 protein bands. We decided to determine the protein identity of each of the prominent bands in the gel by mass spectrometric analysis. To gain better resolution, the virion lysate was resolved on two SDS-PAGE gels for larger and smaller proteins, respectively. A 3 to 8% Tris-acetate gel was used to resolve 50-kDa and larger proteins, and a 12% Bis-Tris gel was used to separate 50-kDa and smaller proteins (Fig. 1). Both gels were stained with colloidal Coomassie blue G-250. Thirty prominent protein bands were excised from these two gels and subjected to in-gel trypsin digestion. A portion of each peptide digest was injected onto a nanocapillary reverse-phase high-performance liquid chromatograph coupled to a nanoelectrospray ionization source of an ion trap mass spectrometer (ThermoFinnigan LCQ). Mass spectrometry measures peptide masses and then fragments individual peptides to produce LC-MS/MS spectra of fragments that reflect the peptide sequence. The MS/MS spectra were run against a nonredundant database, as well as a customized KSHV database, with the program SEQUEST. Of the 30 protein bands excised from the gels, 26 contained peptides positively identifying proteins encoded by the KSHV genome (Fig. 1). The protein identities and the sequences of corresponding peptides that were identified by MS/MS are listed in Table 1. In general, if more than three peptide sequences in a database entry have been matched by MS/MS spectra, the protein identity is considered to be at a high confidence level. However, for some small proteins (especially small glycoproteins), we only found one or two matched peptides. If these peptides were found with good cross-correlation scores (Xcorr > 2.5) (>2.5) and spectra, we also included them as putative virion components. By this standard, 24 viral proteins were assigned as putative KSHV virion-associated proteins and listed in Table 2.
FIG. 1.
Protein composition of gradient-purified KSHV virions. Purified virion lysate was resolved on 3 to 8% Tris-acetate (lane 2) and 12% Bis-Tris NuPAGE (lane 4) gels with molecular markers (lanes 1 and 3) and stained with colloidal Coomassie G-250. Prominent bands were excised, digested in gel with trypsin, and subjected to LC-MS/MS analysis. The resultant MS/MS spectra were run against a sequence database with the SEQUEST program. Matched KSHV or cellular proteins are indicated at the right of each gel.
TABLE 1.
KSHV virion protein tryptic peptides identified by mass spectrometry
| Gene | Protein | Size (amino acids) | Number(s) and sequence(s) of corresponding peptide(s) |
|---|---|---|---|
| ORF6 | VP6 or SSB | 1,133 | 4 peptides: 555-565, TLIVDIPSFVK; 716-727, AMLMVPKTIKIK; 913-925, LRPIITVPLVVNK; 963-971, NNVSSMLRK |
| ORF7 | VP7 | 695 | 4 peptides: 388-401, LSKLLAAGQLNLGK; 402-429, CSTESCQSEARRQLVGGGKPEEVLRDAK; 430-446, HRQELYLQKVARDGFKK; 613-623, ELVLSVSLYNR |
| ORF8 | gB (75 kDa) | 440 | 3 peptides: 59-66, SVDFYQFR; 79-89, FNLEQTCPDTK; 218-230, TTVNCEIVDMIAR |
| ORF8 | gB (54 kDa) | 405 | 10 peptides: 441-467, SASTAAAGGGGSTDNLSYTQLQFAYDK; 470-481, DGINQVLEELSR; 490-499, DNLMWYELSK; 500-512, INPTSVMTAIYGR; 575-588, NEIILTNNQVETCK; 589-598, DTCEHYFITR; 639-648, AIELYSSAEK; 650-662, LASSVFDLETMFR; 789-802, NILLGMHQQEER; 829-845, GYKPLTQSLDISPETGE |
| ORF11 | VP11 | 407 | 6 peptides: 43-48, VPLLR; 67-78, AYPNFTFDNTHR; 80-97, QQTETYTAFYAFGDQNNK; 199-217, SRLAKPFARLSAETTEECR, 275-285, HYIPVIYSGPK; 380-388, TIELPGGVK |
| ORF17.5 | VP17.5 or SCAF | 282 | 2 peptides: 31-45 SAFLSMLQSSIDGMK; 139-148, NIAEIQSELK |
| ORF21 | VP21 or TK | 580 | 15 peptides: 61-74, TSYIYDVPTVPTSK; 110-125, LSATDDDSGDYAPMDR; 126-132, FAFQSPR; 151-168, PADASMGDVGWADLQGLK; 197-205, DGGFAFSPR; 217-243, QILSNPAIKPR; 252-266, NVYLLYLEGVMGVGK; 267-281, STLVNAVCGILPQER; 282-294, VTSFPEPMVYWTR; 328-333, FSLPFR; 342-354, MMQPWNVGGGSGR; 396-414, ATEGDVVAILTLSSAESLR; 422-433, KNDGTVEQNYIR; 423-433, NDGTVEQNYIR; 558-569, AINWPALESQSK |
| ORF22 | gH | 730 | 7 peptides: 148-157, LVLGDIFASK; 170-180, VFYPMIVMAVK; 234-245, GHATYDELTFAR; 249-257, YALVAILPK; 487-497, FSKPDSLNIYR; 517-527, AEAPQSSALTR; 661-669, VQTNLFLDK |
| ORF25 | VP25 or MCP | 1,376 | 37 peptides: 25-33, ESAADGLFK; 34-41, SFQLLLGK; 113-118, QYIVMK; 144-157, ETPLDFTEYAGAIK; 158-172, TITSALQFGMDALER; 173-182, GLVDTVLAVK; 185-193, HAPPVFILK; 194-203, TLGDPVYSER; 260-284, GVSTYTTASGQQVALETTDSVMR; 310-321, GANLVTAVSYGR; 325-332, NFEQFMAR; 333-347, IVDHPNALPSVEGDK; 348-359, AALADGHDEIQR; 373-381, FVAIESLQR; 421-437, GVESPAIQSTETWVVNK; 438-451, NNVPLCFGYQNALK; 458-484, MHNPTQSAQALNQAFPDPDGGHGYGLR; 485-, YEQTPNMNLFR; 496-504, TFHQYYMGK; 505-515, NVAFVPDVAQK; 516-530, ALVTTEDLLHPTSHR; 742-753, IGDQNYDNPQNR; 754-760, ATFINLR; 763-777, MEDLVNNLVNIYQTR; 786-804, HVLDVAPLDENDYNPVLEK; 861-869, DILQAGDIR; 877-893, VLCTSFLTCPFVTQAAR; 898-911, RDPAQSFFATHEYGK; 899-911, DPAQSFATHEYGK; 973-992, NAVVFNVPSNLMAEYEEWHK; 993-1018, SPVAAYAASCQATPGAISAMVSMHQK; 1032-1042, MHPGFAMTVVR; 1057-1070, ASTSMFVGLPSVVR; 1201-1217, AACVVSCDAYSNESAER; 1233-1242, STNNPWASQR; 1243-1255, GSLGDVLYNITFR; 1281-1292, GLYTLVNEYSAR |
| ORF26 | VP26 or TRI-2 | 305 | 3 peptides: 27-38, IGSVLPLGDCHR; 235-251, HPLTEVFEGVVPDEVTR; 252-265, IDLDQLSVPDDITR |
| ORF27 | VP27 | 290 | 4 peptides: 29-47, TTVYLPDTEPWVVETDATK; 48-60, DAFLSDGIVDMAR; 65-76, GALPSNSHNGLR; 238-247, DYGLFISQPR |
| ORF28 | 102 | 2 peptides 65-73, ATVAYQVLR; 74-96, TLGPQAGSHAPPTVGIATQEPYR | |
| ORF33 | VP33 | 334 | 14 peptides: 15-29, ECIWTVNPMSGDHIK; 67-97, VMGPCVAVGINGEMIMYVVSQCVSVRPVPGR; 98-116, DGMALIYFGQFLEEASGLR; 117-126, FPYIAPPPSR; 135-145, QELVHTSQVVR; 146-159, RGDLTNCTMGLEFR; 147-159, GDLTNCTMGLEFR; 217-223, GHVNVFR; 224-241, GYCSAQSPGLSNICPCIK; 257-270, NFLGLLFDPIVQSR; 276-303, ITSHPTPTHVENVLTGVLDDGTLVPSSK; 304-310, APWVLLR; 311-317, MSDYFSR; 318-324, LLIYECK |
| ORF39 | gM | 399 | 2 peptides: 350-363, TPTVHQKPPPLPAK; 372-379, DISTPAPR |
| ORF45 | VP45 | 407 | 7 peptides: 32-47, FIFPPPPLSSLPGFGR; 142-149, PVAVVAGR; 208-228, NSVPGTQSSPYSDPDEGPSWR; 208-231, NSVPGTQSSPYSDPDEGPSWRPLR; 266-288, HFSHQPPSSEEDGEDQGEVLSQR; 289-297, IGLMDVGQK; 356-381, GHLPTQSPSTSAHSISSGSTTTAGSR |
| ORF47 | gL | 167 | 1 peptide: 91-101, LGSWASQENLR |
| K8.1 | gp35/37 | 228 | 1 peptide: 125-133, DAHYNAEIR |
| ORF52 | VP52 | 131 | 3 peptides: 10-20, KDLTMEDLTAK; 52-68, EAQLTATVGALSAAAAK; 98-117, IDVCMSDGGTAKPPPGANNR |
| ORF53 | gN | 110 | 1 peptide: 103-110, FVDEVVHA |
| ORF62 | VP62 or TRI-1 | 331 | 2 peptides: 14-25, QVLGLLPPPTHR; 40-50, DLLSKYAASTR |
| ORF63 | VP63 | 927 | 13 peptides: 26-33, LLMEFQLR; 75-92, AAYFLEPPSSIDPLEAAR; 129-141, LLAHYADQIAGFK; 202-218, EGLLLPSGIPSEEVLAK; 219-231, TLVTEHHELFVSR; 232-245, SNSTETAVTMPVSK; 430-439, HPGISDIPLR; 480-492, TTWGGAVPANLAR; 493-512, DIDTGPNTQHISSTPPPTLK; 715-724, TIQTIEQATR; 826-844, DTTEAFLQSLAQPVVQGQR; 865-881, INPQFTDAQANIPPSIK; 903-913, SAFEAPDDELR |
| ORF64 | VP64 | 2,635 | 10 peptides: 98-115, IFQSPEFYGLIGQDAAIR; 526-538, LGTAQPIPVIVDR; 669-682, ETSMAELIETITAR; 876-890, NAVDQILTDAEGLLK; 1294-1321, LSHALQSGDLQQATVGTPLELPATEYAR; 1367-1385, TLIPHPDAIVADGLPAFLK; 1447-1464, PLSLQDPVGFEGIIYDK; 1469-1486, ESYETGLEGLSWLEQTIK; 2195-2210, LLQSQVSATWSDIFSR; 2603-2610, ITLISFIR |
| ORF65 | VP65 or SCIP | 170 | 4 peptides: 6-14, VRDPVIQER; 15-27, LDHDYAHHPLVAR; 28-45, MNTLDQGNMSQAEYLVQK; 89-105, VSAASAYDAGTFTVPSR |
| ORF68 | 545 | 4 peptides: 389-406, ALQTLQCEVMGHIENNVK; 457-465, VVSEDVLFR; 514-521, TTSLDIIR; 532-545, LDLAHPSQTSHLYA | |
| ORF75 | VP75 | 1,296 | 20 peptides: 139-153, ITQTLLEPHPPQFIR; 154-178, AFTQNTDLVPYEGLEVPEGPQPVAR; 216-222, YFVIPGR; 301-321, GVQQAEMLGGAGVPTLGGFLK; 325-345, TIATTPGNALAVCSISTTTSK; 353-364, MIPQQTVVCLGR; 365-379, FEPTDGPDTYPNLYR; 401-409, GPIFSGLNR; 419-426, HLQALAPR; 427-435, TGLELFVSK; 499-509, LCACQITVLGR; 630-647, VPDTVEAITPSMANLLHK; 648-654, DFETWVK; 655-667, ALPQELLPVPAWR; 1127-1141, SESSPYTYGPTPPQR; 1153-1168, LYDSHWLNIQIPQNTK; 1177-1192, GTVLPSWAQGEYLGVR; 1193-1203, YEQDALEYILR; 1206-1224, GEITLYHGNAADETLPAR; 1229-1245, NPTGNSTVAGLTSSDGR |
TABLE 2.
KSHV virion proteins identified by mass spectrometry
| Category and gene | Protein | Size (amino acids:) | Homolog in:
|
Function | ||
|---|---|---|---|---|---|---|
| HSV-1 | HCMV | EBV | ||||
| Capsid proteins | ||||||
| ORF17.5 | VP17.5 | 282 | UL26.5 | UL80 | BVRF2 | SCAF |
| ORF25 | VP25 | 1,376 | UL19 | UL86 | BCLF1 | MCP |
| ORF26 | VP26 | 305 | UL18 | UL85 | BDLF1 | TRI-2 |
| ORF62 | VP62 | 331 | UL38 | UL46 | BORF1 | TRI-1 |
| ORF65 | VP65 | 170 | UL35 | UL48/49 | BFRF3 | SCIP |
| Envelope proteins | ||||||
| ORF8 | gB | 845 | UL27 | UL55 | BALF4 | gB |
| K8.1 | gp35/37 | 228 | Glycoprotein | |||
| ORF22 | gH | 730 | UL22 | UL75 | BXLF2 | gH |
| ORF28 | 102 | BDLF3 | Homologous to EBV gp150 | |||
| ORF39 | gM | 399 | UL10 | UL100 | BBRF3 | gM |
| ORF47 | gL | 167 | UL1 | UL115 | BKRF2 | gL |
| ORF53 | gN | 110 | UL49.5 | UL73 | BLRF1 | gN |
| ORF68 | 545 | UL32 | UL52 | BFLF1 | Glycoprotein | |
| Tegument proteins | ||||||
| ORF21 | VP21 | 580 | UL23 | BXLR1 | TK | |
| ORF33 | VP33 | 334 | UL16 | UL94 | BGLF2 | |
| ORF45 | VP45 | 407 | BKRF4 | IRF-7 binding protein | ||
| ORF63 | VP63 | 927 | BOLF1 | Tegument protein | ||
| ORF64 | VP64 | 2,635 | UL36 | UL48 | BPLF1 | Tegument protein |
| ORF75 | VP75 | 1,296 | BNRF1 | Tegument protein | ||
| Other (uncategorized) | ||||||
| ORF6 | VP6 | 1,133 | UL29 | UL57 | BALF2 | Single-stranded DNA binding protein |
| ORF7 | VP7 | 695 | UL28 | UL56 | BALF3 | Transport protein |
| ORF11 | VP11 | 407 | Raji LF2 | Homologous to EBV LF2 | ||
| ORF27 | VP27 | 290 | BDLF2 | |||
| ORF52 | VP52 | 131 | BLRF2 | |||
Antibodies against some of the virion proteins were raised and used to examine whether these proteins are indeed associated with purified virions. Proteins from purified virions and BCBL-1 cells were resolved on SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with these antibodies, as well as control antibodies against various KSHV proteins. The ORF11, ORF21 (TK), ORF33, ORF45, and ORF64 proteins were easily detected in TPA-induced BCBL-1 cells and in the purified virion lysate (Fig. 2, lanes 2 and 3) but not in uninduced BCBL-1 cells (lane 1). KSHV envelope protein gB (Fig. 2F) was also detected in the virion preparation as expected. In contrast, immediate-early protein RTA/ORF50 (Fig. 2G) and latent protein LANA (Fig. 2H) were not detected in the virion preparation.
Protease sensitivities of KSHV virion-associated proteins.
The availability of antibodies against ORF45, ORF21, ORF33, and ORF64, respectively, enabled us to determine whether their proteins are incorporated into the virus particles or nonspecifically adhere to the outside of virions. The purified virions were treated by trypsin digestion in the absence or presence of Triton X-100. If any of these proteins is present within the virion, the protein should be protected from trypsin digestion by the virion envelope but can be degraded when the virion envelope is dissolved by detergent treatment. Contaminating proteins that are nonspecifically copurified with KSHV virions, as well as viral envelope proteins, should be degraded by protease treatment regardless the presence of detergents. To determine the fate of the putative virion proteins in these treatments, trypsin-treated virions were lysed and analyzed by Western blotting. As shown in Fig. 3, ORF21, ORF33, and ORF45 were resistant to trypsin digestion in the absence of detergent but were degraded only when Triton X-100 was present (Fig. 3, lanes 1 and 2), suggesting that these three proteins are located within virions. However, as a control, glycoprotein K8.1 was sensitive to trypsin treatment both in the presence (Fig. 3, lanes 2) and in the absence (lane 1) of detergent. ORF64 was also found to be sensitive to trypsin digestion, and the protein can be degraded in the presence of trypsin and in the absence of detergent (Fig. 3, lanes 1 and 2).
FIG. 3.
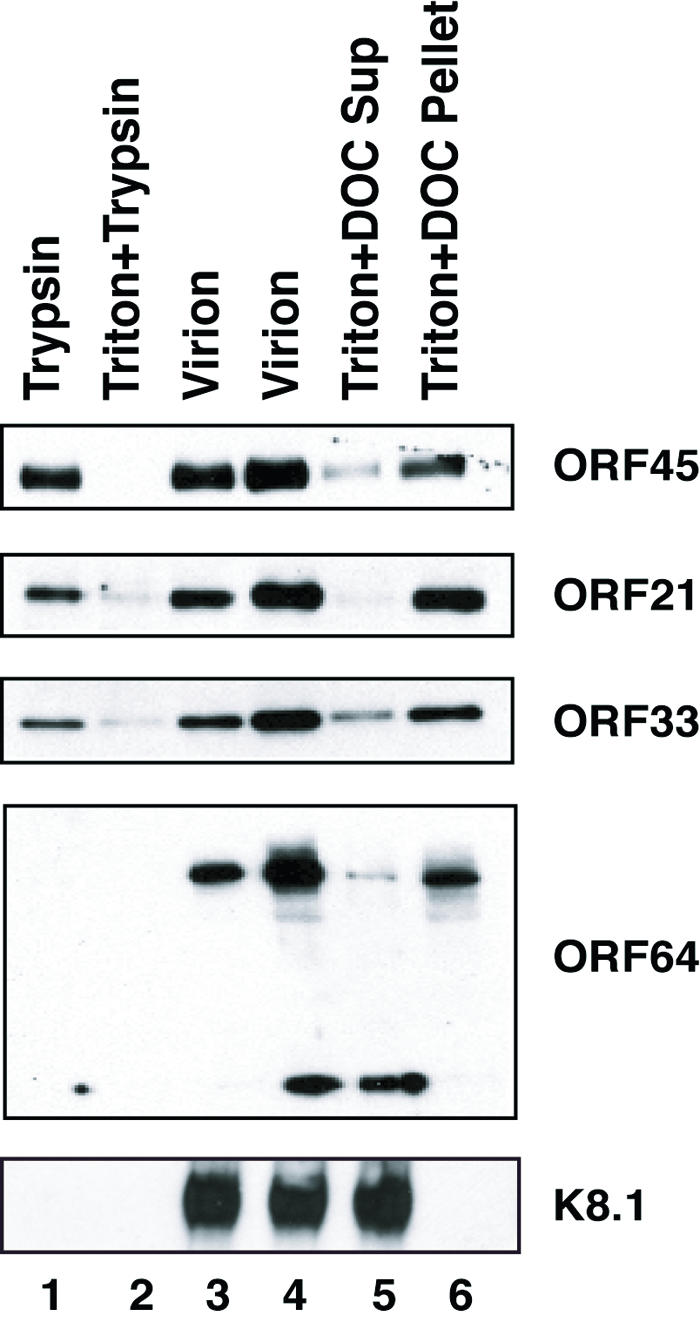
Effect of trypsin digestion and detergent treatment on the association of putative tegument proteins with purified virions. Purified virions were treated with trypsin either in the absence (lane 1) or in the presence (lane 2) of 1% Triton X-100 for 1 h at 37°C or left untreated (lane 3). The proteolysis reactions were terminated by addition of 0.5 mM PMSF and 1/100 volume of protease inhibitor. The samples were analyzed by Western blotting with antibodies as indicated. In addition, virions were left untreated (lane 4) or treated with 1% Triton X-100 plus 0.5% DOC for 30 min and then centrifuged at 100,000 × g for 1 h. The supernatant (Sup) (lane 5) and pelleted tegument-capsid (lane 6) were analyzed by Western blotting.
Association of tegument proteins ORF21, ORF33, ORF45, and ORF64 with capsid.
We asked where the proteins encoded by ORF21, ORF33, ORF45, and ORF64 are located in virions. Purified virions were treated with 1% Triton X-100 plus 0.5% DOC and then subjected to high-speed centrifugation. Both the supernatant and pellet were analyzed by immunoblotting. The ORF21, ORF33, and ORF45 proteins showed similar patterns in that most of them were detected in the pellet with capsid proteins, but small amounts of these proteins were released into the supernatant fraction (Fig. 3, lanes 5 and 6). As a control, glycoprotein K8.1 was released from the virions into the supernatant after the detergent treatments (Fig. 3, lane 5) and was not detected in the pellet (lane 6). These observations suggest that ORF21, ORF33, and ORF45 are neither membrane components nor proteins adhering to the surface of virions. Instead, these three proteins remained associated with the pelleted tegument-capsid structure. However, their association with the viral capsid was breakable by harsher detergent treatment. Increasing evidence suggests that the herpesvirus tegument is an organized structure built through specific protein-protein interaction and associated with the capsid (19, 45). Thus, ORF21, ORF33, and ORF45 are surely components of the KSHV tegumented capsid. Given that the KSHV nucleocapsid components and structure have been determined and studied (24, 43) and ORF21, ORF33, and ORF45 were not among the nucleocapsid components, we conclude that ORF21, ORF33, and ORF45 are tegument proteins.
Anti-ORF64 antibodies, both mouse and rabbit polyclonal, reacted with two species of protein in virions, full-length ORF64 of 290 kDa and a protein of ∼80 kDa (Fig. 2E). The 80-kDa protein is likely truncated ORF64 of the C-terminal region because the antibodies that reacted with it were raised with a 400-amino-acid fragment of ORF64 near its C terminus. Full-length ORF64 was associated with the capsid. In contrast, the 80-kDa truncated fragment was not found in the tegumented capsid pellet (Fig. 3). In addition, the 80-kDa fragment and full-length ORF64 were accessible to digestion when whole virions were treated with trypsin (Fig. 3). According to these observations, we speculate that ORF64 is a tegument protein that is associated with the capsid at one end (possibly the N terminus) and attached to the envelope at the other end (the C terminus).
Capsid proteins.
Of the 24 virion proteins that are listed in Table 2, 5 have been known as KSHV capsid proteins (24). They include 153-kDa MCP (ORF25), 36-kDa TRI-1 (ORF62), 34-kDa TRI-2 (ORF26), 19-kDa SCIP (ORF65), and 29-kDa SCAF (ORF17.5). All five of these proteins were detected in the bands of their expected molecular masses.
Envelope glycoproteins.
The KSHV genome encodes a number of glycoproteins. Some have been experimentally shown to be virion components. They include ORF8 (gB), ORF22 (gH), ORF39 (gM), ORF47 (gL), ORF53 (gN), ORF68, and K8.1A and -B (1, 4, 10, 15, 23, 28, 33). All seven of these glycoproteins were positively identified in the virions by this mass spectrometric analysis. gH was identified with high confidence in a band of 120 kDa. The molecular mass of the protein was higher than predicted (81 kDa), probably because of glycosylation of the protein. The gB, gM, gL, gN, and ORF68 proteins were identified in bands with the predicted or previously reported molecular masses (1, 15, 23, 33).
K8.1 expresses multiple glycoproteins ranging from 26 to 72 kDa, representing different degrees of glycosylation. The mature form(s) of K8.1 on virions is a highly glycosylated protein of 68 to 72 kDa (47). Our mass spectrometric analysis detected only one peptide of K8.1 but in several positions in the gel. Given that K8.1 is a short protein but heavily glycosylated, we think that the failure to detect more peptides of the protein in this mass spectrometric analysis was attributed to the heavy glycosylation and small size of the protein. On the basis of this consideration, together with the fact that K8.1 was detected in the virion envelope by Western blotting (Fig. 3), K8.1 is included in the list of KSHV virion proteins that were identified in this study (Table 2).
ORF28 encodes a small protein of 102 amino acids. Its counterpart (or positional homologue) in EBV, BDLF3, is known to encode a glycoprotein, gp150 (16, 26). Although the sequence homology between ORF28 of KSHV and BDLF3 of EBV is limited (22), both proteins have a feature of type I membrane proteins as predicted with the PHD prediction program (32). However, the predicted extracellular domain of KSHV ORF28 is much shorter than that of EBV BDLF3. On the basis of its structural similarity to EBV BDLF3, KSHV ORF28 is listed in the glycoprotein-envelope protein category in Table 2. The ORF28 analogue of murine gammaherpesvirus 68 was also found to be a virion protein (7).
Tegument proteins.
The tegument lies between the nucleocapsid and the envelope. Little is known about its structure and function. In structure, some believe that the tegument is an amorphous layer of protein (14); others have shown that it is an at least partly ordered structure (45). In function, some of the tegument proteins are released into infected cells following viral entry and exert important regulatory functions immediately after entry; some play roles in virion assembly, envelopment, and egress. In accordance to the sequence homology with the tegument proteins of other well-characterized herpesviruses, five KSHV ORFs were predicted to encode tegument proteins. They are ORF19, ORF63, ORF64, ORF67, and ORF75 (33). Three of these assigned tegument proteins, namely, ORF63, ORF64, and ORF75, were identified in this proteomic study. In addition, several other proteins, such as ORF21 (TK), ORF33, and ORF45, were found in the virion and apparently located in the tegument region because they were resistant to trypsin treatment and associated with capsid (Fig. 3). These putative tegument proteins are listed in Table 2 under the category of tegument proteins. Our analysis did not pick up ORF19- and ORF67-encoded proteins probably because these are either absent in virions or present at a relatively low level so that their protein bands were missed.
The ORF64 gene product is the largest protein (290 kDa) encoded by the KSHV genome. ORF64 analogues are found in all subfamilies of Herpesviridae, including alphaherpesviruses (e.g., herpes simplex virus type 1 [HSV-1] UL36) and betaherpesviruses (e.g., cytomegalovirus UL48). The structure and function of the HSV-1 UL36 gene product have been characterized. HSV-1 UL36 was found to be tightly associated with the nucleocapsid via interaction with the VP5 protein (18). An electron cryomicroscopic study visualized filamentous and convoluted material approximately 200 Å long and 40 Å thick extending from the surface of the pentons of the HSV-1 capsid. This density extends from the interface between the upper domains of adjacent VP5 (major capsid protein) subunits in the penton. It is very likely that the visualized filamentous material was the UL36 protein (45). HSV-1 UL36 is essential for the HSV-1 life cycle, and at least two functions of UL36 have been demonstrated. (i) UL36 is known to be required for release of the viral DNA genome into the nuclei of host cells during de novo viral infection (6), and (ii) a null mutation of UL36 resulted in accumulation of unenveloped DNA-filled capsids in the cytoplasm, suggesting a role for UL36 in envelopment and maturation of virions (12). Like its homologues in alphaherpesviruses, KSHV ORF64 is tightly associated with the capsid (Fig. 3). However, our trypsin treatment assay revealed peculiar behavior of ORF64 in that it could be degraded when intact virion particles were treated with trypsin. We speculate that filamentous ORF64 protein may bind to the capsid with one end and attach to the envelope with the other end. Thus, ORF64 may play a role in positioning the DNA-filled capsid at the cell membrane by interacting with the membrane (or a membrane protein) where virion maturation and egress occur.
ORF75/FGARAT, a protein only seen in gammaherpesviruses, was found in our mass spectrometric analysis. The ORF75 analogues of herpesvirus saimiri and murine gammaherpesvirus 68 have been shown to be virion proteins (7, 8).
Viral TK in KSHV virions.
KSHV-encoded TK (or ORF21) was identified in KSHV virions and likely located in the tegument region. This is the first case in which a herpesvirus TK was found in virions. Some other herpesviruses, such as HSV-1 and EBV, also encode TKs, and some have been intensively studied. No evidence indicates that TK is present in HSV-1 virions. Studies with TK-defective mutant HSV-1 showed that the viral TK is not simply required for HSV infection in vivo but is critical for efficient reactivation of the virus from the latent state in mouse ganglions (11, 17, 38). HSV-1 TK phosphorylates thymidine and deoxycytidine in latently infected nonreplicating cells (neurons) that do not express detectable levels of cellular TK (reviewed in reference 37). The presence of TK in KSHV virions raises a series of intriguing questions. Why is KSHV TK present in virions? Does KSHV de novo infection require TK activity? What is the functional role of KSHV TK in the viral life cycle? We speculate that TK may play an addition role in the KSHV life cycle that may not be required for the HSV-1 life cycle.
Uncategorized virion proteins.
ORF52, a small protein of 21 kDa, is conserved among gammaherpesviruses. Its homologues in EBV (34) and murine gammaherpesvirus 68 (7) were reported previously. Several other proteins were also found in purified KSHV virions and are listed in Table 2 as uncategorized virion proteins. They include ORF6, ORF7, ORF11, and ORF27. The localization of these proteins in a virion awaits the development of experimental reagents (such as specific antibodies) and further characterization.
Cellular proteins in KSHV virions.
Some cellular proteins were detected in the virion preparation by mass spectrometric analysis (Table 3). Most of these cellular proteins are abundant species in cells. Although we cannot rule out the possibility that the presence of some of these cellular proteins in the virion preparation was the result of simple contamination with cellular components, we think that it is more likely that these cellular proteins were specifically or nonspecifically packaged into virions. First, the most common contamination source in purified virions is membranous vesicles that cosediment with virions in gradient ultracentrifugation. Our data including elimination of precursors of viral gB and glycoprotein K8.1 in purified virions suggested that the virion preparation used for mass spectrometric analysis can be considered free of cellular membrane contamination (1, 47). Second, cellular proteins could contaminate virion preparations by simply adhering to the virion exterior. As shown below, we have examined the localization of two virion-associated cellular proteins, namely, nonmuscle β-actin and class II myosin heavy chain, in virions by treating purified virions with trypsin and found that both proteins were resistant to trypsin digestion. This result suggests that at least these two cellular proteins are localized within virions rather than adhering to the virion exterior. Third, nonvirion or cellular viral proteins such as the LANA, K8, and ORF50 proteins were not detected in the virion preparation by either Western blotting or mass spectrometry.
TABLE 3.
Selected cellular proteins associated with KSHV virionsa
| SwissProt identification code | Cellular protein | Size (amino acids) | MS spectrumb | Coverage (%)c |
|---|---|---|---|---|
| gi:46397333 | β-Actin, nonmuscle | 376 | 10 | 33.6 |
| gi:6306978 | Annexin A2 | 339 | 8 | 28.6 |
| gi:1139621 | Annexin A6 (annexin VI) | 673 | 16 | 30 |
| gi:21361181 | ATPase, Na/K-transporting α1 | 1,023 | 13 | 16 |
| gi:10863927 | Cyclophilin A | 165 | 5 | 33.9 |
| gi:4503571 | Enolase-1 | 434 | 6 | 20.7 |
| gi:4503483 | Eukaryotic translation elongation factor 2 | 858 | 14 | 17.5 |
| gi:31645 | Glyceraldehyde-3-phosphate dehydrogenase | 335 | 7 | 34.6 |
| gi:2495339 | Heat shock protein 70 | 640 | 10 | 21 |
| gi:6016267 | Heat shock protein 90 | 733 | 18 | 26.7 |
| gi:4504965 | L-plastin (lymphocyte cytosolic protein 1) | 627 | 12 | 25 |
| gi:12667788 | Myosin, nonmuscle heavy polypeptide 9 | 1,960 | 26 | 17 |
| gi:4505763 | Phosphoglycerate kinase | 417 | 13 | 47 |
| gi:33286418 | Pyruvate kinase 3 (isoform 1) | 531 | 26 | 51 |
| gi:125604 | Pyruvate kinase M2 | 531 | 6 | 15.1 |
| gi:13027392 | RAB7, member of ras oncogene family | 207 | 8 | 44 |
| gi:135717 | Thrombospondin 1 | 1,170 | 29 | 30.6 |
| gi:29135265 | Transferrin | 704 | 15 | 29 |
| gi:30584583 | Tyrosine-3-monooxygenase | 256 | 11 | 52 |
| gi:2136692 | 14-3-3 protein zeta chain | 245 | 6 | 26 |
| gi:2507205 | Unknowm (RIKEN cDNA 3110065L21) | 1,675 | 28 | 21.9 |
Criteria for selection of cellular proteins listed: more than eight unique peptides identified by LC-MS/MS or >15% coverage of protein by the peptides.
Number of polypeptides detected from this protein by LC-MS/MS.
Percent coverage of protein by peptides detected by LC-MS/MS.
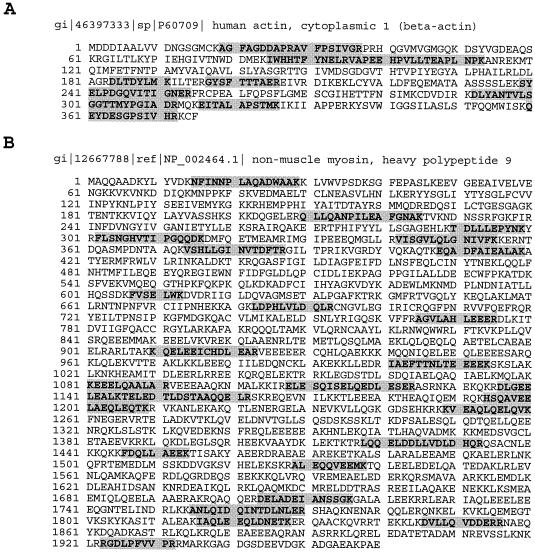
Of these virion-associated cellular proteins, two have been further characterized. An actin was identified in a protein band migrating at approximately 43 kDa. Ten tryptic peptides derived from this protein were identified with high levels of confidence (Fig. 4A). In mammalian cells, six major isoforms of actin were identified. The amino acid sequences of these isoforms are nearly identical, varying primarily within the N-terminal 20 amino residues. They are classified into two groups: muscle and nonmuscle actins. Muscle actins consist of α-skeletal, α-cardiac, and γ-smooth muscle actins, which are found mostly in muscle cells, while α-smooth muscle actin is found in both muscle and nonmuscle cells. β- and γ-nonmuscle actins are found in a wide variety of nonmuscle cell types (reviewed in reference 27). The alignment of 10 peptide sequences derived by mass spectrometric analysis with human actin sequences showed that the virion-associated actin is either β- or γ-nonmuscle actin. These two isoforms differ only in the first 10 amino acids, and we were not able to distinguish them on the basis of our mass spectrometric data. However, an antibody specific to β-actin was found to react with the 43-kDa protein in KSHV virions (Fig. 5), suggesting that the actin present in KSHV virions is nonmuscle β-actin.
FIG. 4.
Matches of the peptides identified by MS/MS from 43- and 240-kDa protein bands, respectively, with human nonmuscle β-actin and class II myosin heavy chain type A. The peptides identified by MS/MS from each band and their respective sequences (shaded regions) are shown superimposed on the amino acid sequences of the β-actin (A) and myosin (B) proteins, respectively.
FIG. 5.
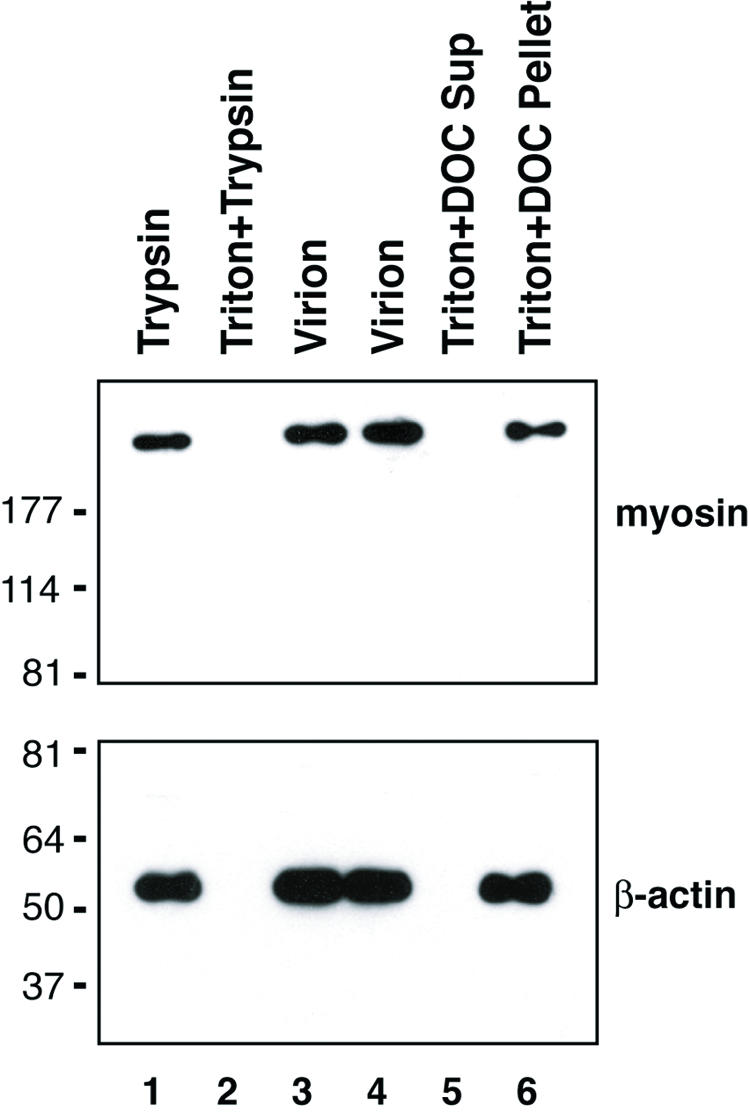
Effect of trypsin digestion and detergent treatment on association of cellular cytoskeletal proteins with purified KSHV virions. Purified virions were treated with trypsin either in the absence (lane 1) or in the presence (lane 2) of 1% Triton X-100 for 1 h at 37°C or left untreated (lane 3). The proteolysis reactions were terminated by addition of 0.5 mM PMSF and 1/100 volume of protease inhibitor. The samples were analyzed by Western blotting with antibodies against β-actin and class II myosin heavy peptide A, respectively. In addition, virions were left untreated (lane 4) or treated with 1% Triton X-100 plus 0.5% DOC for 30 min and then centrifuged at 100,000 × g for 1 h. The supernatant (Sup) (lane 5) and pelleted tegument-capsid (lane 6) were analyzed by Western blotting. The values on the left are molecular sizes in kilodaltons.
Another KSHV-associated cellular protein is nonmuscle myosin heavy polypeptide 9 (also designated class II myosin heavy chain type A). Twenty-seven peptides were identified with high confidence and matched to the 226-kDa myosin (Fig. 4B).
To examine whether the nonmuscle β-actin and myosin were packaged inside the virions or were contaminating components adhering to the virion exterior, the sensitivities of these two proteins to trypsin digestion and detergent treatment were investigated. The results showed that β-actin and myosin displayed similar patterns upon trypsin digestion and detergent treatment. Both molecules were resistant to trypsin in the absence of detergent but became accessible to digestion when Triton X-100 was present (Fig. 5, lanes 1 and 2), suggesting that these two cellular proteins are located within virions. When purified virions were treated with 1% Triton X-100 plus 0.5% DOC and then subjected to high-speed centrifugation, both β-actin and myosin were detected in the pellet with capsid and tegument proteins (Fig. 5, lanes 5 and 6). On the basis of these data, we believe that the β-actin and myosin proteins are integral components of the viral tegument.
Actin filamentous structure has been seen in HSV-1 virions in an electron cryomicroscopic study (14). An unconventional actin was found in human cytomegalovirus virions (5). Although speculative, it is conceivable that actins found in herpesvirus virions are involved in transport of viral particles from the nucleus to the cytoplasmic membrane (5). Myosins are a superfamily of actin-based motor proteins with a growing number of attributed functions. Nonmuscle myosin II has been shown to physically interact with HSV-1 tegument protein VP22 and play a role in viral egress (40). The presence of actin and myosin in KSHV virions has led to the suggestion that the myosin-actin cytoskeleton is involved in intracellular capsid transport and assembly and egress of virions.
Nomenclature of KSHV virion-associated proteins.
We propose a nomenclature for the proteins contained in KSHV virions. In this nomenclature, KSHV virion proteins are designated by the prefix VP (for virion protein) and numbered in the order of their ORFs in the KSHV genome (Table 2). For example, the virion protein encoded by ORF45 is named VP45 and the ORF64-specified virion protein is called VP64. Of these virion proteins, five capsid proteins have been named on the basis of their structural roles in the capsid (24). They are MCP, encoded by ORF25; TRI-1 and TRI-2 (the two putative triplex components), encoded by ORF62 and ORF26, respectively; SCIP, encoded by ORF65; and SCAF, encoded by ORF17.5. We suggest continuing to use the functional names of these capsid proteins (MCP, TRI-1, etc.) as the primary names but including them in the VP nomenclature for integrity of the nomenclature.
The nomenclature for glycoproteins appears to be unified among well-characterized herpesviruses, including HSV-1, cytomegalovirus, varicella-zoster virus, and KSHV. Glycoproteins of most herpesviruses, including KSHV, are named in accordance with the nomenclature established for HSV-1 (gB, gM, etc.). This system has been widely used and accepted in the field of herpesvirology. This nomenclature reflects the analogues of glycoproteins in other herpesviruses. Thus, glycoproteins are not included in the VP nomenclature.
Acknowledgments
We thank Kaye Speicher and Thomas Beer of the Wistar Institute proteomics facility for superb mass spectrometric analyses. We thank Jae Jung at New England Regional Primate Research Center for K8.1 antibody, Don Ganem at the University of California at San Francisco for the ORF50 antibody, Bala Chandran at the University of Kansas Medical Center for the polyclonal antibody against gB and the monoclonal hybridoma supernatant against K8.1A and -B, and Rolf Renne at Case Western Reserve University for the LANA antibody.
This work was supported by National Institutes of Health grant CA86839 to Y.Y.
REFERENCES
- 1.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235-249. [DOI] [PubMed] [Google Scholar]
- 2.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 3.Akula, S. M., N. P. Pramod, N.-S. Walia, F.-Z. Wang, B. Fegley, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J. Virol. 77:7978-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baghian, A., M. Luftig, J. B. Black, Y. X. Meng, C. P. Pau, T. Voss, P. E. Pellett, and K. G. Kousoulas. 2000. Glycoprotein B of human herpesvirus 8 is a component of the virion in a cleaved form composed of amino- and carboxyl-terminal fragments. Virology 269:18-25. [DOI] [PubMed] [Google Scholar]
- 5.Baldick, C. J., Jr., and T. Shenk. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 70:6097-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batterson, W., D. Furlong, and B. Roizman. 1983. Molecular genetics of herpes simplex virus. VIII. Further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J. Virol. 45:397-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bortz, E., J. P. Whitelegge, Q. Jia, Z. H. Zhou, J. P. Stewart, T.-T. Wu, and R. Sun. 2003. Identification of proteins associated with murine gammaherpesvirus 68 virions. J. Virol. 77:13425-13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron, K. R., T. Stamminger, M. Craxton, W. Bodemer, R. W. Honess, and B. Fleckenstein. 1987. The 160,000-Mr virion protein encoded at the right end of the herpesvirus saimiri genome is homologous to the 140,000-Mr membrane antigen encoded at the left end of the Epstein-Barr virus genome. J. Virol. 61:2063-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cesarman E, E. A. Mesri, and M. C. Gershengorn. 2000. Viral G protein-coupled receptor and Kaposi's sarcoma: a model of paracrine neoplasia? J. Exp. Med. 191:417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandran, B., C. Bloomer, S. R. Chan, L. Zhu, E. Goldstein, and R. Horvat. 1998. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology 249:140-149. [DOI] [PubMed] [Google Scholar]
- 11.Coen, D. M., M. Kosz-Vnenchak, J. G. Jacobson, D. A. Leib, C. L. Bogard, P. A. Schaffer, K. L. Tyler, and D. M. Knipe. 1989. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. USA 86:4736-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai, P. 2000. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J. Virol. 74:11608-11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundhoff, A., and D. Ganem. 2004. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Investig. 113:124-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunewald, K., P. Desai, D. C. Winkler, J. B. Heymann, D. M. Belnap, W. Baumeister, A. C. Steven. 2003. Three-dimensional structure of herpes simplex virus from cryo-electron tomography. Science 302:1396-1398. [DOI] [PubMed] [Google Scholar]
- 15.Koyano, S., E. C. Mar, F. R. Stamey, and N. Inoue. 2003. Glycoproteins M and N of human herpesvirus 8 form a complex and inhibit cell fusion. J. Gen. Virol. 84:1485-1491. [DOI] [PubMed] [Google Scholar]
- 16.Kurilla, M. G., T. Heineman, L. C. Davenport, E. Kieff, and L. M. Hutt-Fletcher. 1995. A novel Epstein-Barr virus glycoprotein gp150 expressed from the BDLF3 open reading frame. Virology 209:108-121. [DOI] [PubMed] [Google Scholar]
- 17.Leist, T. P., R. M. Sandri-Goldin, and J. G. Stevens. 1989. Latent infections in spinal ganglia with thymidine kinase-deficient herpes simplex virus. J. Virol. 63:4976-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNabb, D., and R. J. Courtney. 1992. Characterization of the large tegument protein (ICP1/2) of herpes simplex virus type 1. Virology 190:221-232. [DOI] [PubMed] [Google Scholar]
- 19.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, G., L. Heston, E. Grogan, L. Gradoville, M. Rigsby, R. Sun, D. Shedd, V. M. Kushnaryov, S. Grossberg, and Y. Chang. 1997. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 71:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore, P. S., and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus, p. 2803-2833. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 22.Moore, P. S., S. J. Gao, G. Dominguez, E. Cesarman, O. Lungu, D. M. Knowles, R. Garber, P. E. Pellett, D. J. McGeoch, and Y. Chang. 1996. Primary characterization of a herpesvirus agent associated with Kaposi's sarcoma. J. Virol. 70:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naranatt, P. P., S. M. Akula., and B. Chandran. 2002. Characterization of gamma2-human herpesvirus-8 glycoproteins gH and gL. Arch. Virol. 147:1349-1370. [DOI] [PubMed] [Google Scholar]
- 24.Nealon, K., W. W. Newcomb, T. R. Pray, C. S. Craik, J. C. Brown, and D. H. Kedes. 2001. Lytic replication of Kaposi's sarcoma-associated herpesvirus results in the formation of multiple capsid species: isolation and molecular characterization of A, B, and C capsids from a gammaherpesvirus. J. Virol. 75:2866-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neipel, F., J. C. Albrecht, and B. Fleckenstein. 1997. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J. Virol. 71:4187-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nolan, L. A., and A. J. Morgan. 1995. The Epstein-Barr virus open reading frame BDLF3 codes for a 100-150 kDa glycoprotein. J. Gen. Virol. 76:1381-1392. [DOI] [PubMed] [Google Scholar]
- 27.Pollard, T. D., and J. A. Cooper. 1986. Actin and actin binding proteins. A critical evaluation of mechanisms and functions. Annu. Rev. Biochem. 55:987-1035. [DOI] [PubMed] [Google Scholar]
- 28.Raab, M.-S., J.-C. Albrecht, A. Birkmann, D. Lang, B. Fleckenstein, and F. Neipel. 1998. The immunogenic glycoprotein gp35-37 of human herpesvirus 8 is encoded by open reading frame K8.1. J. Virol. 72:6725-6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 30.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 31.Roizman, B., and P. E. Pellett. 2001. The family Herpesviridae: a brief introduction, p. 2381-2398. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 32.Rost, B. 1996. PHD: predicting one-dimensional protein structure by profile-based neural networks. Methods Enzymol. 266:525-539. [DOI] [PubMed] [Google Scholar]
- 33.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serio, T. R., A. Angeloni, J. L. Kolman, L. Gradoville, R. Sun, D. A. Katz, W. Van Grunsven, J. Middeldorp, and G. Miller. 1996. Two 21-kilodalton components of the Epstein-Barr virus capsid antigen complex and their relationship to ZEBRA-associated protein p21 (ZAP21). J. Virol. 70:8047-8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun, R., S.-F. Lin, K. Staskus, L. Gradoville, E. Grogan, A. Haase, and G. Miller. 1999. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J. Virol. 73:2232-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tenser, R. B. 1994. The role of herpes simplex virus thymidine kinase expression in pathogenesis and latency, p. 68-86. In Y. Becker and G. Darai (ed.), Pathogenicity of human herpes simplex viruses due to specific pathogenicity genes. Springer-Verlag, Berlin, Germany.
- 38.Tenser, R. B., K. A. Hay, and W. A. Edris. 1989. Latency-associated transcript but not reactivatable virus is present in sensory ganglion neurons after inoculation of thymidine kinase-negative mutants of herpes simplex virus type 1. J. Virol. 63:2861-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trus, B. L., J. B. Heymann, K. Nealon, N. Cheng, W. W. Newcomb, J. C. Brown, D. H. Kedes, and A. C. Steven. 2001. Capsid structure of Kaposi's sarcoma-associated herpesvirus, a gammaherpesvirus, compared to those of an alphaherpesvirus, herpes simplex virus type 1, and a betaherpesvirus, cytomegalovirus. J. Virol. 75:2879-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Leeuwen, H., G. Elliott, and P. O'Hare. 2002. Evidence of a role for nonmuscle myosin II in herpes simplex virus type 1 egress. J. Virol. 76:3471-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, C. Y., and B. Sugden. 2004. New viruses shake old paradigms. J. Clin. Investig. 113:21-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, F. Z., S. M. Akula, N. P. Pramod, L. Zeng, and B. Chandran. 2001. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J. Virol. 75:7517-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu, L., P. Lo, X. Yu, J. K. Stoops, B. Forghani, and Z. H. Zhou. 2000. Three-dimensional structure of the human herpesvirus 8 capsid. J. Virol. 74:9646-9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhong, W., H. Wang, B. Herndier, and D. Ganem. 1996. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 93:6641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou, Z., D. Chen, J. Jakana, F. J. Rixon, and W. Chiu. 1999. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J. Virol. 73:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu, F. X., and Y. Yuan. 2003. The ORF45 protein of Kaposi's sarcoma-associated herpesvirus is associated with purified virions. J. Virol. 277:4221-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu, L., V. Puri, and B. Chandran. 1999. Characterization of human herpesvirus-8 K8.1A and -B glycoproteins by monoclonal antibodies. Virology 262:237-249. [DOI] [PubMed] [Google Scholar]