Abstract
Colorectal cancer (CRC) is the third most common malignant disease in the United States (U.S.). Almost two-thirds of CRC survivors are living 5 years following diagnosis. The prevalence of CRC survivors is likely to increase dramatically over the coming decades with further advances in early detection and treatment and the aging and growth of the U.S. population. Survivors are at risk for a CRC recurrence, a new primary CRC, other cancers, as well as both short and long-term adverse effects of the CRC and the modalities used to treat it. CRC survivors may also have psychological, reproductive, genetic, social, and employment concerns following treatment. Communication and coordination of care between the treating oncologist and the primary care clinician is critical to effectively and efficiently manage the long-term care of CRC survivors. The following guidelines are intended to assist primary care clinicians in delivering risk-based health care for CRC survivors who have completed active therapy.
Keywords: colorectal cancer, survivorship, clinical care, follow-up, guidelines, primary care, quality of life, survivorship care plan, long-term effects, late effects, care coordination
INTRODUCTION
Over the past two decades, increasing attention has been given to understanding the long-term and late effects experienced by cancer survivors as a result of their cancer diagnosis or treatment.1–4 Long-term (side) effects caused by cancer or its treatment that are present during treatment and may persist for months or years may be physical or psychosocial in nature. In contrast, late effects of the cancer or cancer therapy may occur months or even years after a cancer diagnosis and again may include second cancers, physical problems, or psychosocial issues. Along the cancer continuum, there are at least three distinct phases of cancer survivorship: from diagnosis to the end of initial treatment, the transition from treatment to extended survival, and long-term survival.5 While clinical practice guidelines exist for diagnosis and treatment, there are few evidence-based clinical care guidelines for posttreatment care. The ever increasing number of cancer survivors living posttreatment poses a challenge to oncology and primary care clinicians to provide ongoing optimal clinical follow-up care.6 To meet this demand, it is important to equip primary care clinicians with the necessary resources to recognize and manage the health risks and maximize quality of life (QoL) of cancer survivors. The National Comprehensive Cancer Network (NCCN) has developed consensus-based guidelines for treatment of patients with colon and rectal cancers, and which also include some recommendations regarding follow-up care after completion of treatment.7,8 As well, the NCCN has developed survivorship care guidelines addressing long-term or late occurring psychosocial and physical problems and preventive health.9 In addition, the American Society of Clinical Oncology (ASCO) clinical practice guidelines for cancer survivorship care focus on prevention and management of symptoms experienced by survivors of many types of cancer. To date, ASCO has released three evidence-based cancer survivor care guidelines, focused on fatigue, anxiety and depression, and neuropathy.10–12 ASCO has also updated their fertility preservation guideline13 and offers a provisional clinical opinion on the integration of palliative care into oncology care.14
This CRC survivorship care guideline builds on previous guidelines by providing primary care clinicians with recommendations for providing comprehensive care for CRC survivors. These guidelines provide guidance on 1) methods to identify and manage the potential physical and psychosocial long-term and late effects of CRC and its treatment; 2) surveillance for recurrence and screening for second primary cancers; 3) health promotion; and 4) how to enhance communication between the oncology team and primary care clinicians. The goal of these guidelines is to optimize the care delivered for cancer survivors, and to help improve the overall health and QoL of CRC survivors.
Gaps in posttreatment cancer survivorship resources and clinical follow-up care were identified through the work of the National Cancer Survivorship Resource Center (The Survivorship Center; cancer.org/survivorshipcenter).15 Aims of The Survivorship Center are to help survivors achieve optimal health and QoL and increase awareness of posttreatment survivorship as a public health issue. To this end, The Survivorship Center convened a group of experts to review existing literature and clinical practices to develop comprehensive clinical follow-up care guidelines for CRC survivors, specifically those who are stage I–III, with no evidence of disease.
BACKGROUND
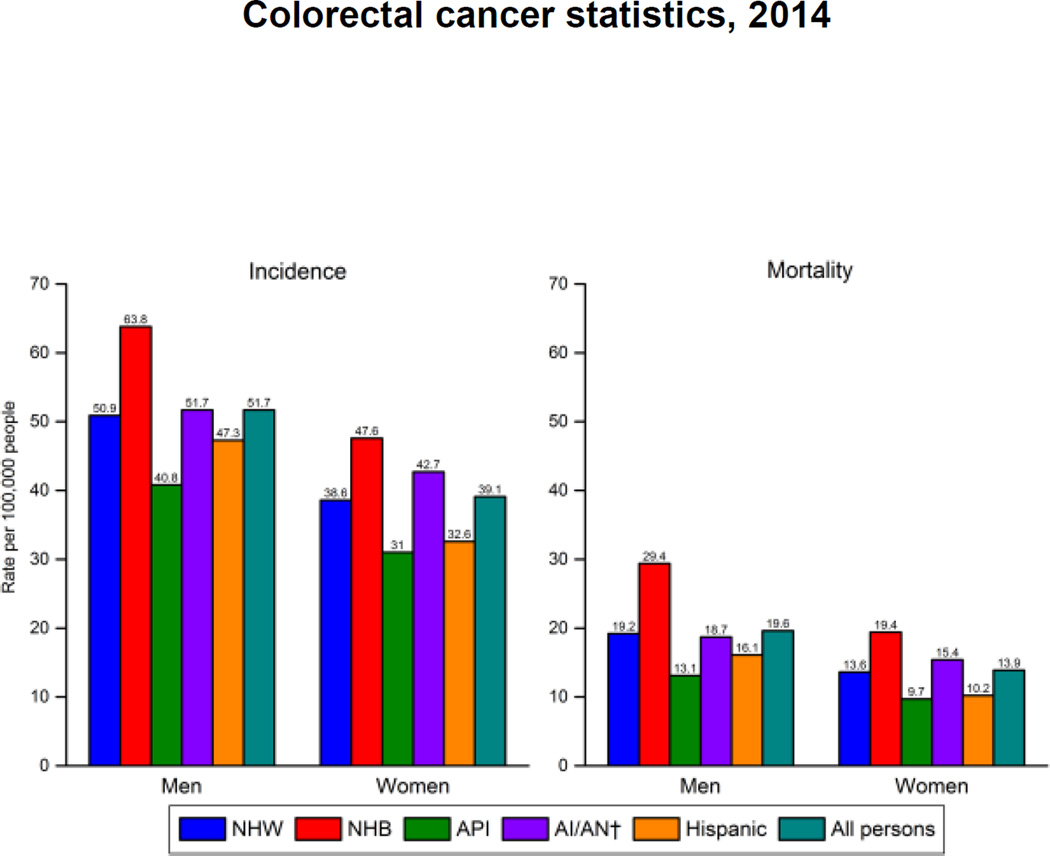
Approximately 132,700 individuals will be diagnosed with CRC in the US in 2015.16 The incidence of CRC has declined over the past 20 years, in large part due to increased screening and removal of precancerous polyps. The rate of decline in incidence is greater among non-Hispanic white males than among African American males and similar between non-Hispanic white and African American females.16 Other racial and ethnic groups have lower incidence rates than these two populations.17 Approximately 49,700 patients will die from CRC in 2015.16 Mortality rates are highest among African American males and approximately 50% higher than the second highest group non-Hispanic white and American Indian / Alaska Native males. Among females, mortality rates are significantly higher for African Americans, followed by American Indians / Alaska Natives and non-Hispanic whites.17 (Figure 1: Colorectal Cancer Incidence and Mortality Rates* by Race/Ethnicity and Sex, United States, 2006–2010)18
Figure 1. Colorectal Cancer Incidence and Mortality Rates* by Race/Ethnicity and Sex, United States, 2006–2010.
Description:
Colorectal cancer incidence and mortality rates by race/ethnicity and sex during 2006 through 2010.
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117.
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Mortality-All COD, Aggregated With State, Total US (1969–2010) <Katrina/Rita Population Adjustment>. Bethesda, MD: National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch; 2013. Released April 2013; underlying mortality data provided by National Center for Health Statistics, 2013.
Colorectal cancer survivors comprise about 9% of the nearly 15 million cancer survivors alive in the U.S., making it the second and third most common cancer site among male and female cancer survivors, respectively.19 The majority of CRC survivors are age 60 or older.16 The overall health and QoL experienced by survivors is, in part, influenced by the stage at diagnosis and the types and duration of therapy. Only 40% of CRC is diagnosed at a local stage (stages I & II), whereas 36% of cancers are diagnosed at a regional stage, involving the regional lymph nodes (stage III) and 20% are diagnosed at a distant stage when distant metastases have occurred (stage IV).17 The type of treatment will vary depending on the stage at diagnosis, but the most common treatment is surgery, with additional therapy including systemic chemotherapy and radiation therapy (the latter is employed much more often in rectal cancer than in colon cancer) given either in the neoadjuvant or adjuvant setting. Potential physical long-term and late effects affecting CRC survivors include chronic peripheral neuropathy, infertility, secondary cancers, and bowel dysfunction. Survivors may also experience psychosocial issues such as distress, depression, anxiety, body image, sexual dysfunction and intimacy concerns, as well as financial issues resulting from workforce displacement and/or costs of treatment.20
METHODS
Literature Review
To develop the ACS CRC Survivorship Care Guidelines, The Survivorship Center staff conducted an initial review of relevant literature and reviewed publically available U.S. and international clinical practice guidelines. The original literature search was conducted in the fall of 2011 using PubMed, identifying articles published between 2000 and 2011 using combinations of the following key words and phrases: cancer survivor, colon cancer, rectal cancer, colorectal cancer, chemotherapy, cognitive dysfunction, depression, distress management, fecal incontinence, follow-up care, genetic counseling and testing, guidance, guidelines, hand and foot syndrome, health promotion, late effects, late sequelae, long-term effects, meta-analysis, monitoring, neuropathy, pain management, palliative care, post-treatment, primary care physician, psychosocial, radiation, recurrence, screening, second cancer, sexual dysfunction, surgery, surveillance, survivor, survivorship, symptom management, systematic review, and treatment complications. Studies were excluded that a) reported on studies of childhood cancer, b) reported on qualitative studies, c) were published in languages other than English, and c) specifically addressed metastatic (stage IV) CRC (due to the likelihood that these survivors participate in ongoing treatment and do not fall into the “long-term / extended survivorship” phases).
In January and February 2012, the initial literature search was supplemented by an environmental scan of publically available U.S. and international clinical practice guidelines and reports relevant to the clinical management of CRC patients and survivors, regardless of intended readership. Surveillance guidelines specific to CRC from the National Comprehensive Cancer Network (NCCN) and national and international sources relevant to the impact of CRC and interventions for long-term and late effects were reviewed. Sources included: ACS, American College of Gastroenterology, American Gastroenterological Association, American Psychosocial Oncology Society, ASCO, American Society of Colon and Rectal Surgeons, American Society for Gastrointestinal Endoscopy, American Society for Radiation Oncology, Canadian Association of Psychosocial Oncology, Institute of Medicine, National Cancer Institute, NCCN, National Guidelines Clearinghouse, MD Anderson Clinical Tools and Resources Colon and Rectal Cancer Survivorship algorithm, Oncology Nursing Society, Society of American Gastrointestinal and Endoscopic Surgeons, Society of Gastroenterology Nurses and Associates, Inc., and the Society of Surgical Oncology.
From May 2012 through June 2014, the CRC guideline was put on hold as The Survivorship Center directed it efforts to writing the ACS Prostate Cancer Survivorship Care Guidelines manuscript.21 In September 2014, The Survivorship Center reconvened the CRC Survivorship Care Guidelines expert workgroup to update the literature review, review the levels of evidence according to previously published methods, and consider any revisions. A small writing group was convened to complete the guidelines manuscript.
Due to the time lapse, in September 2014, an updated literature search was conducted. Search terms included cancer survivor + review or meta-analysis or systematic review + guidelines or guidance paired with colorectal cancer; colorectal cancer survivor or colorectal cancer patient post-treatment + (symptom management, late effects, long-term effects, psychosocial care, palliative care, health promotion, surveillance, screening for new cancers, self-management, guidelines or guidance, follow up or follow-up, side effects + chemotherapy, side effects + radiation, side effects + surgery, treatment complications, genetic counseling and testing, survivor or patient interventions, provider interventions, provider education, and barriers). Literature identified included: guidelines / guidance developed by other organizations (e.g. NCCN follow-up recommendations, ASCO follow-up recommendations), specific medical centers (e.g. MD Anderson), or available from other countries (e.g. Australian Cancer Survivorship Centre); recent meta-analyses and review papers (since 2004 following publication of the National Action Plan for Cancer Survivorship); and individual studies, with the highest priority given to papers that met the following criteria: peer reviewed publication in English since 2004, unless a seminal paper prior to this date still carries the most weight, including randomized controlled trials, prospective studies, and well-conducted population-based case-control studies; large studies of more than 200 cancer cases analyzed, and high quality assessment of covariates and analytic methods: analyses controlled for important confounders (e.g. pre-existing comorbid conditions).
A total of 226 articles (available as a literature review summary table supplement to this manuscript) met the inclusion criteria for the literature review and were used to create the guidelines.
Literature Synthesis and Workgroup Recommendations
In May 2012, The Survivorship Center staff integrated evidence from the initial literature review to develop an initial draft of CRC survivorship care guidelines that was reviewed by the expert workgroup. The Survivorship Center Steering Committee and staff, and ACS leadership nominated experts practicing in either primary care or surgical/oncological settings that care for CRC survivors. Workgroup members were selected based on their expertise in at least one of the following domains: gastroenterology, health services, medical oncology, oncology nursing, preventive medicine, primary care, public health, and surgical oncology. The expert workgroup consisted of 9 initial members who were emailed a structured 13-question survey about the accuracy and relevance of the draft guidelines document (Appendix A). Written responses were compiled and distributed in advance of a conference call to discuss the feedback and reach consensus on conflicting recommendations.
Led by Khaled El-Shami, MD, PhD, The GW Medical Faculty Associates, a Hematologist/Oncologist with board certification in Medical Oncology and Internal Medicine, the workgroup participated in a webinar discussion of the existing evidence base as well as themes and discrepancies from the comments. Based on written and verbal feedback, The Survivorship Center staff revised the draft guidelines. The Survivorship Center staff sought additional evidence and clinical expertise to support practice-based recommendations and explore issues identified by the expert workgroup members that were not identified by the literature review. Based on a combination of published evidence and practice-based experience, The Survivorship Center staff drafted clinical follow-up care recommendations to be considered for inclusion in the guidelines. This revised draft of guidelines recommendations was presented to the American Cancer Society Mission Outcomes Committee, Chief Medical Officer, and National Board of Directors for review and were approved in May 2012.
In September 2014, the initial literature review was updated using the search terms outlined in the previous section.21 Workgroup members were asked to consider the following criteria as they synthesized their findings from the published literature:
level of evidence: I – Meta analyses of randomized controlled trials (RCTs), IA – RCT of CRC survivors; IB – RCT based on cancer survivors across multiple sites, IC – RCT not based on cancer survivors, but on general population experiencing a specific long-term or late effect (e.g. managing urinary incontinence, erectile dysfunction, etc.); IIA – non-RCT based on CRC survivors, IIB – non-RCT based on cancer survivors across multiple sites, IIC – non-RCT not based on cancer survivors, but on general population experiencing a specific long-term or late effect; III – case study; 0 – expert opinion, observation, clinical practice, literature review, or pilot study.
consistency across studies, including across study designs (separating results by study design when presenting the evidence);
dose-response when presenting long-term or late effects associated with chemo- or radiation therapy;
race / ethnicity differences in diagnosis and treatment that may impact the risk of long-term or late effects; and
second primary cancers for which CRC survivors are at high risk due to cancer treatment exposure, genetic factors, lifestyle behaviors, etc.
While new articles were added to the literature review, there was no change in the guidelines. In May 2015, the guidelines manuscript was sent to internal and external experts for final review and comment prior to submission for publication. The process of guideline development was aligned with the ACS process for creating cancer screening guidelines, and a comparison of this methodology has been previously published.21 In December of 2011,22 changes were put into effect to ensure the ACS process was in alignment with the new Institute of Medicine standards for how guidelines should be developed.23 According to the ACS process, every five years these guidelines will be updated as new research is available to support revision.
GUIDELINES FOR THE PRIMARY CARE MANAGEMENT OF COLORECTAL CANCER SURVIVORS
Each of the essential components of comprehensive cancer survivorship care are discussed in the following sections: Surveillance for Colorectal Cancer Recurrence and Screening for Second Primary Cancers, Assessment and Management of Physical and Psychosocial Effects of Colorectal Cancer and Treatment, Routine Health Promotion Needs, and Coordination of Care Among Specialists and Primary Care Clinicians.1
SURVEILLANCE FOR COLORECTAL CANCER RECURRENCE
Surveillance for CRC recurrence is applicable to survivors who have completed primary treatment for stage I, II and III cancer and are without evidence of disease. The goal of surveillance is to detect recurrent or metachronous (e.g. new primary) disease early thereby improving long-term outcomes through timely intervention.
While these guidelines can be extrapolated to surveillance strategies for patients with stage I disease or patients with resected metastatic (stage IV) CRC without evidence of disease there are little to no data to inform these recommendations.
The ASCO clinical practice guideline endorsement of the Cancer Care Ontario Guidelines on Follow-up Care, Surveillance Protocol, and Secondary Prevention Measures for Survivors of Colorectal Cancer emphasized that if a patient is not a candidate for surgery or systemic therapy because of severe comorbid conditions, then surveillance tests should not be performed.24 Testing should only be performed in patients in whom the results will change treatment decisions. We endorse this ASCO recommendation.
Recommendation 1: Clinical follow-up care provided to CRC survivors should be individualized based on the specific diagnosis and treatment protocol. Level of Evidence = 2A
The guiding principle of surveillance is that it should be based on assessment of a patient’s risk of recurrence, in the context of functional status and patient preferences. Factors associated with a high risk of recurrence include poorly differentiated histology (exclusive of those cancers that are microsatellite instability-high [MSI-H]), lymphatic or vascular invasion, bowel obstruction, having had fewer than 12 lymph nodes examined, perineural invasion, localized perforation, and close, indeterminate, or positive resection margins.
In addition, unless there is a family history or a known genetic syndrome, CRC survivors are at average risk for other cancers and it is recommended that primary care clinicians screen for second primary cancers, as they would in the general population.7,8
Recommendation 2: CRC survivors should receive surveillance colonoscopy according to a schedule based on based on risk. Level of Evidence = 2A
The survivorship timeline (time zero) starts at the time of resection (or time of diagnosis if resection is not part of index treatment). Testing intervals are based on the assumption that treatment is not ongoing and that no evidence of recurrence or metastatic disease was found at the end of treatment. The literature is not definitive with regard to how often surveillance for recurrent disease should be conducted and, to a lesser extent, which modalities to employ for surveillance. In the U.S., there are surveillance guidelines from the NCCN and ASCO. These recommendations differ slightly as a result of differences in results of included clinical trials25 used to form guideline recommendations. Results from trials do not give a consistent answer to questions about an optimal surveillance program, and importantly, do not provide definitive evidence on outcomes related to early detection of recurrent disease or second primary tumors.
For survivors of colon and rectal cancers, NCCN recommends the following surveillance schedule which we endorse (see Table 1).7 For survivors of stage I cancers, colonoscopy is recommended 1 year after resection unless no preoperative colonoscopy occurred due to emergent presentation, in which case colonoscopy is recommended 3–6 months after surgery. If no abnormalities are detected, repeat colonoscopy is then recommended at 3 years and then every 5 years thereafter.
Table 1.
Surveillance Guidelines for Colorectal Cancer Recurrence and Screening and Early Detection of Second Primary Cancers (Stage I–III)
| Guideline (Level of Evidence for this table is 2A*) |
|---|
1–2 Years Post-treatment*:
|
3–5 Years Post-treatment*:
|
5+ Years Post-treatment*:
|
NOT Recommended:
|
Optimal timing unknown:
|
H & P indicates history and physical; CEA, carcinoembryonic antigen; NED, no evidence of disease; CBC, complete blood count; HNPCC, hereditary nonpolyposis colorectal cancer; FAP, familial adenomatous polyposis.
Level of evidence: I, meta analyses of randomized controlled trials (RCTs); IA, RCT of colorectal cancer survivors; IB, RCT based on cancer survivors across multiple sites; IC, RCT not based on cancer survivors, but on general population experiencing a specific long-term or late effect (e.g., chronic diarrhea, sexual dysfunction, etc.); IIA, non-RCT based on colorectal cancer survivors; IIB, non-RCT based on cancer survivors across multiple sites; IIC, non-RCT not based on cancer survivors but on general population experiencing a specific long-term or late effect (e.g., chronic diarrhea, sexual dysfunction, etc.); III, case study; 0, expert opinion, observation, clinical practice, literature review, or pilot study.
National Comprehensive Cancer Network rating indicates that “based upon lower-level evidence, there is uniform consensus that the intervention is appropriate.”
Generally, ASCO agrees with the NCCN recommendations, but does not recommend the colonoscopy at 3 years. Rather, ASCO recommends colonoscopy every 5 years after the initial post-therapy colonoscopy. Detection of adenomatous polyps during surveillance will necessitate more frequent follow-up.
Recommendation 3: CRC survivors should receive a history and physical every 3–6 months in the first 2 years and every 6 months in years 3–5, and annually after 5 years. Level of Evidence = 2A
For survivors of stage II and III cancers, for the first 2 years, physicians should take the patient’s history and conduct a physical examination as an opportunity to identify symptoms, offer counseling, and coordinate posttreatment care.
Recommendation 4: Carcinoembryonic antigen (CEA) testing should be conducted every 3–6 months for the first 2 years and every 6 months for years 3–5 for those with T2 or greater lesions. CEA is not recommended after 5 years. Level of Evidence = 2A
For the first 2 years carcinoembryonic antigen (CEA) testing is recommended every 3–6 months. Over the next 3 years, CEA testing is recommended every 6 months for T2 or greater lesions when the potential exists for further therapeutic intervention of recurrent disease. After 5 years, routine CEA is not monitored.
Recommendation 5: Chest/abdominal/pelvic CT should be performed every 12 months (stages I-III) or every 3–6 months (stage IV, NED) for up to 5 years. PET-CT is not recommended and routine CT is not recommended after 5 years. Level of Evidence = 2A
In addition, for stage III cancer, annual chest/abdomen/pelvis CT scans are recommended for up to 5 years. After 5 years, routine CT scans are not recommended. Routine use of PET/CT is not recommended in this setting.
In contrast to the NCCN recommendations, ASCO recommends CT scans of the abdomen and chest annually for only 3 years for CRC survivors. For rectal cancer survivors, a pelvic CT scan is also recommended, and the oncologist’s judgment should be used to determine the frequency of pelvic scans based on recurrence risk in patients (typically every 6–12 months for 2–3 years, then annually until 3–5 years from surgery). PET scans are not recommended as an acceptable substitution.
Recommendation 6: Survivors of stage IV with no evidence of disease following treatment should receive CT scans of the chest/abdomen/pelvis every 3–6 months in the first 2 years and then every 6–12 months in years 3–5. Level of Evidence = 2A
For survivors of stage IV cancer with no evidence of disease following treatment, similar surveillance practices are recommended except that the interval between CT scans should be shorter. CT scans of the chest/abdomen/pelvis are recommended every 3–6 months in the first 2 years and then every 6–12 months in the next 3 years. As with other stages, routine CT scans beyond 5 years or routine PET-CT scans at any interval are not recommended.
Recommendation 7: Rectal cancer survivors who undergo low anterior resection should receive proctoscopy every 6 months for 5 years. Level of Evidence = 2A
Specific to rectal cancer only, the NCCN recommends that proctoscopy be considered every 6 months for 5 years for patients who undergo low anterior resection. The NCCN guidelines also recommend that patients undergo limited endoscopic evaluation of the rectal anastomosis to identify local recurrence, but optimal timing for surveillance is currently unknown.
The American Society of Clinical Oncology endorsed the Cancer Care Ontario Clinical Practice Guideline on surveillance protocols24 for patients with stage II and III CRC. In the guideline, shorter intervals of follow-up are recommended for patients at higher risk of recurrence (e.g. stage IIIc, genetic syndromes, and CEA fluctuations). A medical history, physical examination, and CEA testing should be performed every 3–6 months for 5 years. A shorter interval is considered earlier in the surveillance period since 80% of recurrences occur in the first two to 2.5 years in patients with a high risk of recurrence. The ASCO Panel noted the principles of conditional survival estimates which are based on time already survived after diagnosis and treatment. Taking survival time into account allows for improved accuracy of prognostication. For CRC, there are very high conditional survival rates at 4–5 years after treatment, lending evidence to support “stop dates” for surveillance protocols, especially since disease-specific survival is very good after 3 years without clinical, serologic, or radiologic evidence of disease recurrence.26
In contrast to the NCCN recommendations, ASCO recommends CT scans of the abdomen and chest annually for only 3 years for CRC survivors. For rectal cancer survivors, a pelvic CT scan is also recommended, and the oncologist’s judgment should be used to determine the frequency of pelvic scans based on recurrence risk in patients (typically every 6–12 months for 2–3 years then annually until 3–5 years from surgery). PET scans are not considered an acceptable substitution.
SCREENING FOR SECOND PRIMARY CANCERS
Recommendation 8: CRC survivors should receive age- and sex-appropriate screening for patients with an average risk, except for female CRC survivors with Lynch Syndrome (see Recommendation 9) Level of Evidence = 2A
Screening for other malignancies, such as breast, cervical, prostate, or lung cancer, should be continued for CRC survivors according to age, gender, and risk factor criteria as per ACS guidelines.27 In addition, some CRC survivors have an elevated risk of second primary cancers due to genetic factors, and therefore should undergo a more intensive regimen of screening. Table 2 summarizes the ACS screening recommendations for each of these cancers among average-risk individuals.27
Table 2.
ACS Recommendations for the Early Detection of Cancer in Average-Risk, Asymptomatic Individuals
| CANCER SITE |
POPULATION | TEST OR PROCEDURE |
FREQUENCY |
|---|---|---|---|
| Breast | Women ages ≥20 y |
BSE | It is acceptable for women to choose not to do BSE or to do BSE regularly (monthly) or irregularly. Beginning in their early 20s, women should be told about the benefits and limitations of BSE. Whether a woman ever performs BSE, the importance of prompt reporting of any new breast symptoms to a health professional should be emphasized. Women who choose to do BSE should receive instruction and have their technique reviewed on the occasion of a periodic health examination. |
| CBE | For women in their 20s and 30s, it is recommended that CBE be part of a periodic health examination, preferably at least every 3 y. Asymptomatic women aged ≥40 y should continue to receive a CBE as part of a periodic health examination, preferably annually. |
||
| Mammography | Begin annual mammography at age 40 y.a | ||
| Cervix | Women, aged 21–65 y |
Pap test and HPV DNA test |
Cervical cancer screening should begin at age 21 y. For women aged 21–29 y, screening should be done every 3 y with conventional or liquid-based Pap tests. For women aged 30–65 y, screening should be done every 5 y with both the HPV test and the Pap test (preferred), or every 3 y with the Pap test alone (acceptable). Women aged >65 y who have had ≥3 consecutive negative Pap tests or ≥2 consecutive negative HPV and Pap tests within the last 10 y, with the most recent test occurring within the last 5 y, and women who have had a total hysterectomy should stop cervical cancer screening if they no longer have a cervix and are without a history of CIN2 or a more severe diagnosis in the past 20 y or cervical cancer ever. Women at any age should not be screened annually by any screening method. |
| Colorectal | Men and women, ages ≥50 y |
FOBT with at least 50% test sensitivity for cancer, or FIT with at least 50% test sensitivity for cancer, or |
Annual, starting at age 50 y. Testing at home with adherence to manufacturer's recommendation for collection techniques and number of samples is recommended. FOBT with the single stool sample collected on the clinician's fingertip during a DRE in the health care setting is not recommended. Guaiac-based toilet bowl FOBT tests also are not recommended. In comparison with guaiac-based tests for the detection of occult blood, immunochemical tests are more patient-friendly, and are likely to be equal or better in sensitivity and specificity. There is no justification for repeating FOBT in response to an initial positive finding. |
| Stool DNA test,b or |
Interval uncertain, starting at age 50 y. | ||
| FSIG, or | Every 5 y, starting at age 50 y. FSIG can be performed alone, or consideration can be given to combining FSIG performed every 5 y with a highly sensitive guaiac-based FOBT or FIT performed annually. |
||
| DCBE or | Every 5 y, starting at age 50 y. | ||
| Colonoscopy | Every 10 y, starting at age 50 y. | ||
| CT colonography |
Every 5 y, starting at age 50 y. | ||
| Endometrial | Women, at menopause |
At the time of menopause, women at average risk should be informed about the risks and symptoms of endometrial cancer and strongly encouraged to report any unexpected bleeding or spotting to their physicians. |
|
| Lung | Current or former smokers aged 55–74 y in good health with at least a 30 pack-y history |
LDCT | Clinicians with access to high-volume, high-quality lung cancer screening and treatment centers should initiate a discussion about lung cancer screening with apparently healthy patients aged 55–74 y who have at least a 30 pack-y smoking history, and who currently smoke or have quit within the past 15 y. A process of informed and shared decision-making with a clinician related to the potential benefits, limitations, and harms associated with screening for lung cancer with LDCT should occur before any decision is made to initiate lung cancer screening. Smoking cessation counseling remains a high priority for clinical attention in discussions with current smokers, who should be informed of their continuing risk of lung cancer. Screening should not be viewed as an alternative to smoking cessation. |
| Prostate | Men, aged ≥50 y | DRE and PSA | Men who have at least a 10-y life expectancy should have an opportunity to make an informed decision with their health care provider about whether to be screened for prostate cancer, after receiving information about the potential benefits, risks, and uncertainties associated with prostate cancer screening. Prostate cancer screening should not occur without an informed decision-making process. |
| Cancer- related checkup |
Men and women, aged ≥20 y |
On the occasion of a periodic health examination, the cancer-related checkup should include examination for cancers of the thyroid, testicles, ovaries, lymph nodes, oral cavity, and skin, as well as health counseling about tobacco, sun exposure, diet and nutrition, risk factors, sexual practices, and environmental and occupational exposures. |
ACS indicates American Cancer Society; BSE, breast self-examination; CBE, clinical breast examination; Pap, Papanicolaou; HPV, human papillomavirus; FOBT, fecal occult blood test; FIT, fecal immunochemical test; DRE, digital rectal examination; FSIG, flexible sigmoidoscopy; DCBE, double-contrast barium enema; CT, computed tomography; LDCT, low-dose helical CT; PSA, prostate-specific antigen.
Beginning at age 40 y, annual CBE should ideally be performed prior to mammography.
The stool DNA test approved for colorectal cancer screening in 2008 is no longer commercially available. New stool DNA tests are presently undergoing evaluation and may become available at some future time.
Patients should not undergo cancer screening without first having a discussion with their primary care clinician about the risks, benefits, and limitations of the particular screening modalities and implications of positive screening tests. This is as true for cancer survivors as for the general population. In considering the benefits of screening, primary care clinicians and patients should consider the patient’s overall health and life expectancy, and whether any patient characteristics place the patient at elevated risk for a specific cancer type.
When possible, primary care clinicians should take the opportunity to acknowledge to patients when professional society recommendations disagree. Such discordance is most notable in the cases of breast and prostate cancer screening recommendations. While ACS currently recommends annual mammography beginning at age 40,28 updated guidelines are expected to be released later this year. The U.S. Preventive Services Task Force (USPSTF) provides a far more conservative recommendation of beginning biennial mammography at age 50 and does not support teaching breast self-examination at the time of this writing. Given an average age of CRC diagnosis of 68,17 it is likely that mammographic screening will be indicated for a substantial portion of female survivors regardless of the guideline followed. For men, USPSTF recommends against routine prostate cancer screening. ACS suggests that patients and their primary care clinicians make the decision as to whether to screen based on an adequate understanding of the harms (overdiagnosis, overtreatment, false positive tests, complications of testing and treatment), benefits (decreased likelihood of late-stage diagnosis of prostate cancer), and uncertainties of screening.29
Women with Lynch Syndrome
Recommendation 9: Female CRC survivors with Lynch Syndrome should receive annual endometrial sampling and transvaginal ultrasound. Level of Evidence = 2A
Women with Lynch Syndrome, also known as hereditary non-polyposis colorectal cancer (HNPCC), constitute a group with a clearly elevated risk for subsequent cancer diagnoses. These women have a 27% to 71% lifetime risk of endometrial cancer—greater than that of CRC—and a 3% to 14% lifetime risk of ovarian cancer.30,31 Therefore, based on expert opinion, ACS suggests that women who are confirmed carriers of a Lynch Syndrome mutation or who are likely carriers based on mutation status or incidence patterns of family members begin screening with annual endometrial biopsy at age 35.27
Regardless of HNPCC or CRC status, endometrial sampling has a sensitivity of 99.6% in postmenopausal women and 91% in premenopausal women for detection of endometrial carcinoma32 and is minimally invasive. Transvaginal ultrasound (TVUS) alone is not a reliable screen for endometrial cancer in premenopausal women given highly variable endometrial thickness during the menstrual cycle. Its sensitivity in asymptomatic postmenopausal women is approximately 83%,33 considerably lower than that of biopsy in this group, though it is also thought to be useful for detection of ovarian neoplasms. Evidence does support the effectiveness of prophylactic hysterectomy and oophorectomy as a means of prevention for both endometrial and ovarian cancer in women with HNPCC.34 For early detection of ovarian cancer, a 1994 NIH consensus panel recommended at least annual rectovaginal examination, CA-125 assessment, and TVUS in women with HNPCC and certain other cancer syndromes until age 35, at which time they advocated bilateral oophorectomy.35 This recommendation was based on the significantly increased risk of ovarian cancer in these patients. Finally, endometrial biopsy should be performed in any woman with Lynch Syndrome who reports irregular or postmenopausal vaginal bleeding.36
ASSESSMENT AND MANAGEMENT OF PHYSICAL AND PSYCHOSOCIAL LONG-TERM AND LATE EFFECTS OF COLORECTAL CANCER AND ITS TREATMENT
The risk of physical long-term and late effects following therapy for CRC is associated with several factors, including: a) type of primary tumor, b) type of chemotherapy, c) duration and dose of treatment(s) (increasing cumulative dose and duration of therapy increases the potential risk), and d) age of patient during treatment. Commonly used chemotherapy and biotherapy agents used to treat CRC include 5-fluorouracil (5-FU), oxaliplatin, and capecitabine. These drugs have been administered to patients in different combinations and at varying dosages and lengths of time, which may relate to the possible long-term and late effects. Primary care clinicians should refer to the patient’s cancer treatment summary for the specific drugs and doses. Table 3 lists potential physical and psychosocial long-term and late effects associated with surgery, radiation, and chemotherapy, which are described in the rest of this section.
Table 3.
Summary of Potential Long-term and Late Effects of Colorectal Cancer and Its Treatment.
| Treatment Type | Long-Term Effects | Late Effects |
|---|---|---|
| Surgery |
|
|
| Pelvic Radiation |
|
|
| Chemotherapy |
|
|
| General Psychosocial Long-term and Late Effects | ||
| ||
Bowel/Gastrointestinal Issues
Recommendation 10: Primary care clinicians should ask CRC survivors about whether they are experiencing diarrhea, rectal bleeding, rectal incontinence or other bowel dysfunction and treat symptoms similar to those in the general population. Level of Evidence = III
Chronic diarrhea, i.e. diarrhea lasting longer than four weeks, which limits activities and negatively impacts QoL, is one of the most common long-term conditions, affecting almost half of CRC survivors.37 Among patients who undergo low anterior resection (LAR) for rectal cancer, and other lower surgical anastomoses, bowel dysfunction is common including increased stool frequency, bowel incontinence and perianal irritation, decreased stool and flatus discrimination, and more incomplete evacuations.38,39 Rates of bowel problems are significantly increased in rectal cancer survivors treated with pelvic radiation, regardless of whether it was administered preoperatively or postoperatively.40,41
Empirical support to guide optimal management of bowel problems is limited (level III). However, anti-diarrheal medications such as Loperamide (Imodium) or diphenoxylate/atropine (Lomotil) are common first-line treatment for chronic diarrhea after radiation therapy. Dietary adjustments, especially elimination of raw vegetables, can be of benefit.42 Low-fat diets, probiotic supplementation, and elemental diets also may be beneficial among patients treated with pelvic radiation.43 Persistent symptoms may necessitate referral to gastroenterology. Options for treatment of fecal incontinence include medical therapy such as bulking agents or antidiarrheal medications to reduce stool frequency and improve stool consistency, biofeedback therapy to improve control of the pelvic floor and abdominal wall musculature, and surgery.
Cardiovascular Effects
Recommendation 11: Monitor CRC survivors who are obese or who have had prior coronary artery disease and received 5-fluorouracil or capecitabine for cardiovascular disease. Level of Evidence = 0
The risk of cardiovascular morbidity does not appear to be increased in long-term CRC survivors. In a large British cohort study, Khan and colleagues did not observe an excess risk of heart failure or coronary artery disease among CRC survivors.44 Nevertheless, there are some important aspects regarding the cardiovascular system in CRC survivors that should be noted. It has long been recognized that 5-fluorouracil can induce acute endothelial dysfunction, generally manifested as chest pain but rarely resulting in an acute myocardial infarction45,46 Therapy with capecitabine, a metabolite of 5-fluorouracil, has also rarely resulted in acute myocardial infarction.47 Individuals with pre-existing coronary artery disease are at increased risk for this acute toxicity.45–47 Fortunately, once therapy is complete, there does not appear to be any lasting cardiovascular risk attributable to these two anti-metabolite agents. To date, there has not been convincing evidence, beyond occasional case reports, of acute or long-term cardiotoxicity associated with oxaliplatin therapy.
While adjuvant therapy for CRC appears to have a relatively low risk for acute or chronic cardiotoxicity, there are indirect pathways within a subset of CRC survivors which may hasten the progression of cardiovascular disease (CVD). Obesity and sedentary lifestyles are associated with an increased risk of CRC.48,49 Thus, it should not be surprising that in a large population-based cohort study, Hawkes and colleagues found that CVD was diagnosed by 36 months after the cancer diagnosis in 16% of survivors without known pre-existing disease. The primary risk factor for developing hypertension, diabetes, and ischemic heart disease was obesity at the time of CRC diagnosis and persistent sedentary lifestyles.50 In a recent study, Cramer et al reported that CRC survivors, regardless of whether they were treated with adjuvant therapy or not, had substantially reduced exercise capacity. This theme of diminished exercise capacity and cardiorespiratory fitness is common across cancer groups and is a key catalyst, when combined with pre-existing obesity and lifelong sedentary behaviors, in the development of CVD.51 Thus, it is imperative that primary care clinicians counsel CRC survivors regarding the well-studied adverse impact of obesity and sedentary behaviors and the critical need for modifications in what often have been lifelong habits.
Cognitive Function
Recommendation 12: Screen for cognitive problems, and assess depression and anxiety that may worsen cognition and refer for treatment. Level of Evidence = 0
Patients have reported changes in cognitive function attributed to cancer treatment with chemotherapy for over 20 years, though the mechanism is still not well understood.52 The majority of studies focus on breast cancer patients, so there is a paucity of data on other cancers, however, a national cross-sectional study looked at self-reported memory problems and found that patients who had undergone treatment for cancer were 40% more likely to report memory problems than those without cancer, regardless of the type of cancer or treatment.53 In a prospective, population-based cohort of CRC survivors, chemotherapy was associated with worsening cognitive function, particularly for individuals under age 70.54
The symptoms reported by patients complaining of cognitive decline vary but may include decreased executive functioning skills, slower processing time or reaction response, diminished organizational skills, loss of language or math skills, and/or difficulty with concentration or attention. These often translate into lower health-related QoL scores, especially as patients transition back to work.55 These symptoms can be difficult to interpret clinically as there is often discordance between the subjective complaints of memory loss and objective testing. Memory impairment may be confounded by physical symptoms associated with treatment such as fatigue or pain as well as mental health concerns (stress, anxiety or depression). The NCCN guidelines on survivorship care suggest screening for treatable causes that may worsen cognitive impairment such as depression and anxiety, though data are lacking for evidence-based recommendations regarding routine screening for cognitive decline in this population.
For patients that report a change in memory or cognitive function, there are a few tools including the Mini Mental Status Exam (MMSE) or the Functional Assessment of Cancer Therapy-Cognitive (FACT-Cog) that may be used for screening. A caveat of these screening tools is that they are not sensitive at determining deficits in executive functioning, so they may underestimate cognitive decline.55 For positive screens, the next step would be a referral for formal neurocognitive testing. Neurocognitive testing can quantify and define specific problems that may impact activities of daily living or their work which can be helpful for patients to understand.
Unfortunately, there are no proven treatments for cognitive impairment related to cancer treatment; however, referral for cognitive rehabilitation strategies, e.g. those used for patients after strokes may be helpful and studies testing the effects of physical activity on cognition are ongoing.
Dental / Oral
Recommendation 13: Ask CRC survivors if they are experiencing symptoms of mucositis, loss of taste or dry mouth and treat similar to population with average risk. Level of Evidence = 0
In a prospective cohort study of CRC survivors, loss of taste and dry mouth were found to be significant late effects in patients who had received chemotherapy as measured by QoL scores 5 years posttreatment.54 Dry mouth can lead to tooth decay, mouth sores or gum disease. Empirical support for recommendations is lacking; however, good oral hygiene (brushing teeth with fluoride containing toothpaste, flossing regularly, etc.) can prevent these complications but if the symptoms are severe, referral to a dentist is recommended for further evaluation and management.
Distress / Depression / Anxiety
Recommendation 14: Screen CRC survivors for psychosocial distress, depression and anxiety using a validated screening tool; special attention should be paid to survivors with a stoma, and those who report sexual dysfunction. Level of Evidence = I/0 (psychosocial screen), IIA (stoma)
Recommendation 15: Refer patients to the appropriate mental health professionals or resources in the community as indicated. In addition, follow-up with the survivor to assess adherence and ensure that the need was met, identify potential barriers, and seek alternative approaches as needed. Level of Evidence = I
Where appropriate, these guidelines leverage the ASCO guideline adaptation of a Pan-Canadian Practice Guideline on Screening, Assessment, and Care of Psychosocial Distress (Depression, Anxiety) in Adults With Cancer.11
Many cancer survivors report ongoing difficulties in recovery and returning to ‘normal’ following treatment.16,17,19 Some survivors of cancer experience fear of recurrence,56 contributing to significant mental health problems for which they already have an increased risk, including distress, depression, and anxiety.57,58 Prevalence estimates for anxiety, depression, and distress in cancer survivors are widely variable, the result of inconsistency in the use of measurement tools and differences in methodological approaches, such as the choice of comparators from the general population. However, among cancer survivors generally, the estimated prevalence of anxiety and depression is 17.9% and 11.6%, respectively.59 Among CRC survivors specifically, an estimated 24% report depression scores on a standard screening tool high enough to warrant evaluation for clinical depression.60 Furthermore, 8% of CRC survivors experience distress severe enough to require follow-up.60
Studies suggest that CRC patients and survivors fitted with stoma devices report higher levels of depression and anxiety, poorer social functioning, more problems with body image, and more side effects from chemotherapy, compared to those without a stoma. For example, a prospective study of 249 CRC patients assessed at 3, 6, 12, and 24 months, reported poorer QoL in stoma patients, who demonstrated significantly greater impairments on sexual functioning and diminished capacity to perform roles.61 These problems were most pronounced among male CRC survivors with a stoma. The timing of the stoma procedure was an important factor; patients whose stoma was made during the primary procedure fared better than patients whose stoma was made some time after the initial operation.61 Thus, it is recommended that primary care clinicians pay particular attention to those CRC survivors with a stoma, especially those whose stoma was made later in the treatment trajectory and male survivors, who may experience significantly greater impairments in functioning and overall QoL.
In order to provide timely and appropriate support for their patients with a history of CRC, primary care clinicians should be familiar with the mental health concerns they may experience, the tools to screen for and assess these problems, and the resources at their disposal to care for their patients. Primary mental health issues revolve around fear of recurrence,56 distress, depression, and anxiety. The NCCN defines distress as “a multi-factorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms and its treatment.”62 A well-known tool for initial screening is the distress thermometer ((Figure 2: NCCN Distress Thermometer and Problem List, Figure (DIS-A), from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management V.2.2014)) which is similar to the rating scale used to measure pain: 0 (no distress) to 10 (extreme distress). A score of four or higher63 suggests a level of distress that has clinical significance. Additionally, a 38-item Problem List (Figure 2) asks patients to identify their problems in five categories: practical, family, emotional, spiritual/religious, and physical. These tools are available from the NCCN Guidelines for Distress Management (http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf).62 Similarly, the Survivor Unmet Needs Survey (SUNS) and the Short-Form Survivor Unmet Needs Survey (SF-SUNS) can be utilized to distinguish between problems which survivors experience and problems which they desire help in managing across a range of life areas, including financial concerns, information and access and continuity of care.64,65
Figure 2. NCCN Distress Thermometer and Problem List, Figure (DIS-A), from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management V.2.2014.
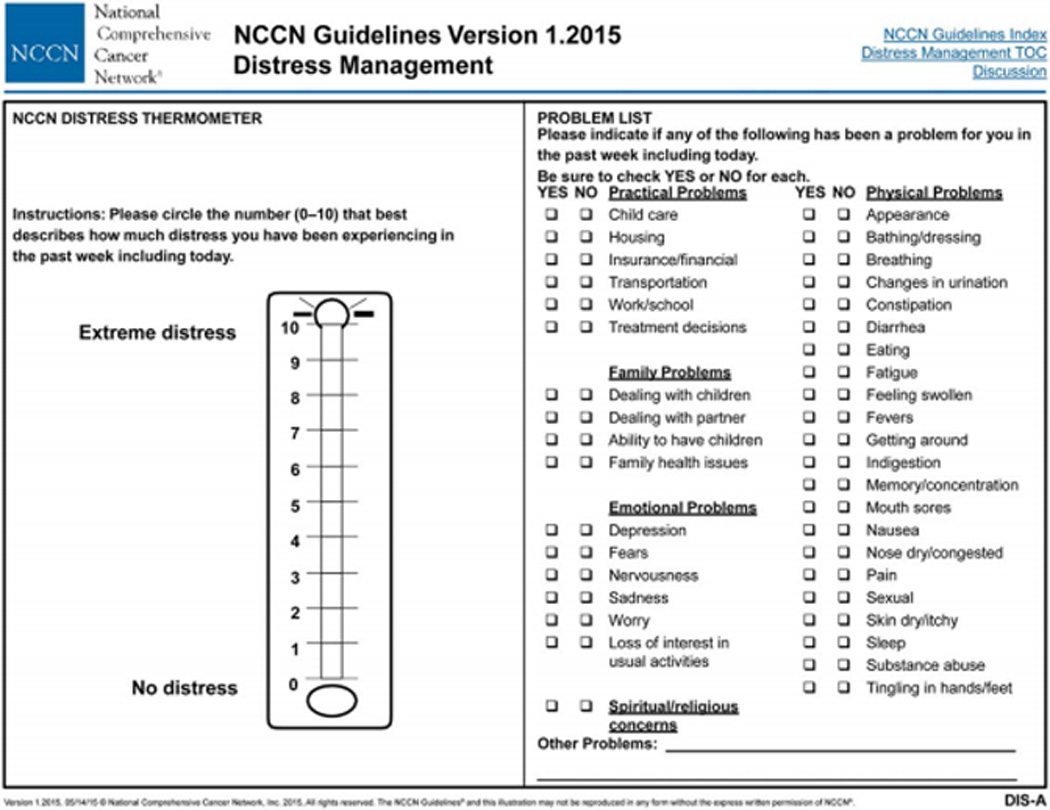
Description:
NCCN Distress Thermometer and Problem List, Figure (DIS-A), from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management. The NCCN Distress Management Panel developed the Distress Thermometer, a now well-known tool for initial screening, which is similar to the successful rating scaled used to measure pain: 0 (no distress) to 10 (extreme distress). The DT serves as a rough initial single-item question screen, which identifies distress coming from any source, even if unrelated to cancer. The receptionist can give it to the patient in the waiting room. The screening tool developed by the NCCN Distress Management Panel includes a 39-item Problem List, which is on the same page with the DT. The Problem List asks patients to identify their problems in five different categories: practical, family, emotional, spiritual/religious, and physical. The panel notes that the Problem List may be modified to fit the needs of the local population.
- Reproduced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management (V.2.2014). © 2014 National Comprehensive Cancer Network, Inc. Available at: NCCN.org. Accessed (April 27, 2015). To view the most recent and complete version of the NCCN Guidelines®, go on-line to NCCN. org.
Depression is a mood disorder that causes a persistent feeling of sadness and loss of interest while anxiety is an intense, excessive, and persistent worry and fear about everyday situations.66–68 Both depression and anxiety can initially be screened using a variety of instruments. One commonly used measure is the validated Hospital Anxiety Depression Scale (HADS). The HADS is a 14-item self-report instrument that consists of two distinct scales, one for depression and one for anxiety, each scored from 0 to 3, with a final score between 0 and 21. A score of 9 or higher on either scale suggests a level of depression or anxiety that has clinical significance.69
Another validated instrument that may be used to screen for depression in cancer survivors is the CES-D Scale (http://www.chcr.brown.edu/pcoc/cesdscale.pdf). This scale is 20 questions; each scored 0 to 3, concerning emotions and feelings over the past week.70,71 A score of 16 or higher suggests a level of depression that has clinical significance.72 This tool identifies significantly more clinical cases than the HADS in similar populations, including both true cases and false positives, with more variable results.72
Treatment of anxiety and depression is effective in people with cancer, therefore if a patient has a clinically significant score on any of the previously discussed instruments, it is recommended that primary care clinicians refer and/or connect patients to the appropriate psychosocial oncology specialists, mental health professionals and/or resources in the community.7 After referring to the appropriate resource(s), primary care clinicians should follow-up with patients to check their adherence. If a patient has difficulties adhering to recommendations, primary care clinicians should work to help identify these challenges and find a way for the patient to overcome these obstacles before discussing alternative interventions to help the patient comply.11 The American Psychosocial Oncology Society (www.apos.org) can help primary clinicians identify these resources. The efficacy of psychosocial support for patients including those with CRC is supported by one RCT showing a survival benefit for those who received these services.73 Other evidence for psychosocial interventions comes from observational studies linking poor emotional well-being and survival.74 Exercise has also been shown to improve well-being in cancer survivors, as documented in a Cochrane review.75
Fatigue
Cancer-related fatigue (CRF) is a potential long-term effect of chemotherapy that is prevalent in cancer survivors and often causes significant disruption in functioning and QoL.24 NCCN defines fatigue as, “a distressing, persistent, subjective sense of physical, emotional, and /or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.”76 Fatigue is reported by patients more frequently than any other symptom during the course of cancer and its treatment77–81 and is often the most severe and most bothersome symptom reported due to its persistence and interference with daily activities.82–84 In a multicenter study of cancer survivors (patients with complete remission or no evidence of disease, and not currently receiving treatment), researchers observed a 23% prevalence of fatigue in short-term (≤5 years; n=117) and 43% in long-term (≥to 5 years; n=23) CRC survivors. 27% of CRC survivors reported moderate to severe fatigue.77 29% of cancer survivors (for all four cancer types combined) reported moderate/severe fatigue that was associated with poor performance status and a history of depression. Gender was not found to be a significant factor among CRC survivors.77
Recommendation 16: Assess with a validated fatigue instrument, recommend physical activity similar to that which is recommended for the general population, and refer to specialists for psychosocial support or rehabilitation as indicated. Level of Evidence = I
The high prevalence of moderate to severe CRF in survivors warrants routine screening, assessment, and management of patient-reported fatigue. ASCO recommends that clinicians should screen every patient for the presence of CRF and gauge its severity periodically throughout long-term survivorship.24 If present, fatigue should be assessed quantitatively on a 0 to 10 scale (0=no fatigue and 10=worst fatigue imaginable); those patients with a severity of more than 4 should be further evaluated by a history and physical examination.24 For patients who report moderate to severe fatigue, comprehensive assessment should be conducted, and medical and treatable contributing factors addressed.
Cancer-related fatigue typically has several different contributing factors in any one patient. Primary care clinicians should work with patients and caregivers to improve assessment and identify management strategies. Managing CRF includes consistent, reliable screening and assessment using a validated instrument and patient-report; treatment of comorbidities that may be a contributing factor; and multimodal and individually tailored interventions (e.g. exercise, psychoeducational and self-management strategies, efforts to manage concurrent symptoms and improve sleep quality, medications and complementary therapies) to improve patient-reported symptoms.19,76,85,86 Patient-report is important to fatigue assessment.76,87 Fatigue management should be initiated when patients rate their fatigue as moderate or severe. A single screening question may be efficient to quickly screen for fatigue in clinical practice to identify patients who may benefit from further multifactorial evaluation.88 Various patient self-report measures of CRF are available.85 Institutional tools exist such as the M.D. Anderson Symptom Inventory (MDASI), a well-validated multi-symptom assessment tool that utilizes a numeric scale of 1 to 10 to rate patient-reported fatigue severity and symptom interference with functioning77,89,90 National tools including the Brief Fatigue Inventory for rapid assessment of fatigue severity,82 FACT G-7 (Figure 3: Functional Assessment of Cancer Therapy – General – (7 item version; be used with patients of any tumor type) ((FACT G-7 (Version 4))) a rapid version of the Functional Assessment of Cancer Therapy-General (FACT-G) for monitoring symptoms and concerns,78 the FSI to assess intensity, frequency, and disruptive impact on QoL,91 the MFSI-SF to assess multidimensional manifestation of fatigue,92 and the FACT-C (Figure 4: FACT-C) used to assess HRQOL (combines the FACT-G assessment with additional CRC-specific measurement).93,94
Figure 3. Functional Assessment of Cancer Therapy – General – (7 item version; be used with patients of any tumor type) ((FACT G-7 (Version 4)).
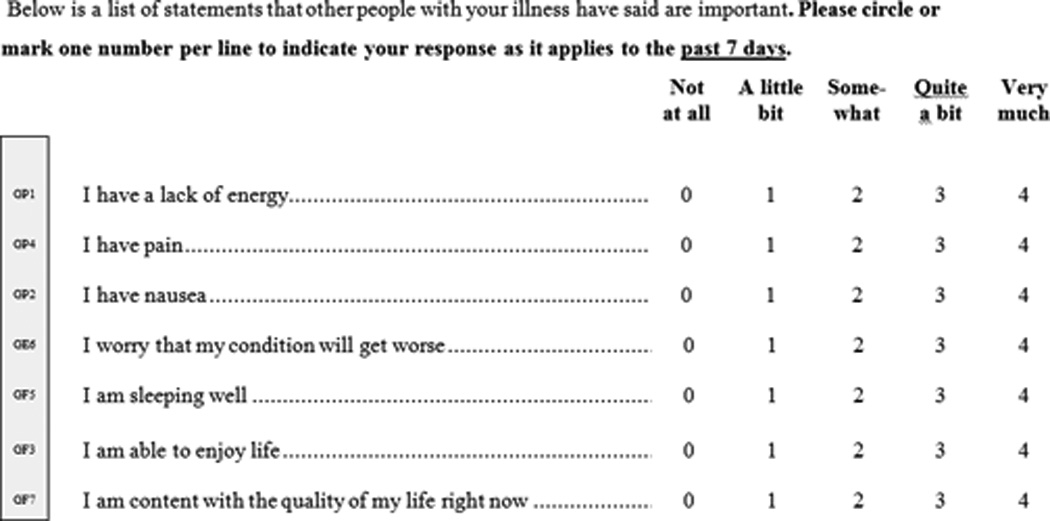
Description:
General measure for functional assessment of cancer therapy to be used with patients of any tumor type.
- Cella DF, Tulsky DS, Gray G, Sarafian B, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3), 570–579. http://www.facit.org/FACITOrg/Questionnaires.
Figure 4. FACT-C.
Description:
Cancer specific measure for functional assessment of cancer therapy to be used with patients with colorectal cancer.
- Cella DF, Tulsky DS, Gray G, Sarafian B, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3), 570–579. http://www.facit.org/FACITOrg/Questionnaires.
In terms of management strategies, evidence indicates that physical activity interventions, psychosocial interventions, and mind-body interventions may reduce CRF in posttreatment patients. There is limited evidence for use of psychostimulants in the management of fatigue in patients who are disease-free after active treatment.
Numerous RCTs and meta-analyses document that physical activity improves aerobic capacity, prevents muscle loss and deconditioning, and it may produce favorable effects on sleep, mood, body composition, and the immune system and cytokine milieu, while promoting self-efficacy (evidence level I).75,95–97 Primary care clinicians should counsel survivors to engage in regular physical activity, avoid inactivity and return to normal daily activities as soon as possible following diagnosis.
For chronic CRF, primary care clinicians should refer survivors to rehabilitative specialists to address lingering fatigue, and provide supportive care recommendations.
General supportive care recommendations for patients with fatigue include optimizing nutritional status and preventing weight loss, balancing rest with physical activity, and attention-restoring activities such as exposure to natural environments and pleasant distractions like music.76
Neuropathy
Chemotherapy-induced peripheral neuropathy (C-IPN) is a potential long-term effect of neurotoxicity caused by chemotherapeutic agents. Chemotherapy-induced peripheral neuropathy can lead to permanent symptoms and disability in upwards of 40% of cancer survivors negatively affecting QoL.98 Oxaliplatin is commonly considered standard therapy in CRC adjuvant chemotherapy regimens. Oxaliplatin-induced peripheral neuropathy (O-IPN) is common among survivors one or more years posttreatment.99–103 Cumulative oxaliplatin-induced peripheral neuropathy is reported to be partially reversible in approximately 80% of patients and completely resolves in approximately 40% at 6 to 8 months posttreatment. Chronic cumulative O-IPN persists posttreatment and severe O-IPN resolves approximately 13 weeks posttreatment in most patients.99,104 Signs and symptoms may continue to develop and worsen for an additional 2 to 6 months posttreatment, also known as “coasting.”12,105 Sensory nerve dysfunction is most common. The large sensory nerves are affected, leading to symptoms of paresthesias, such as “pins and needles” or tingling, numbness, pressure, cold, and warmth that are experienced in the absence of a stimulus; dysesthesias or distortion of sensory perception resulting in an abnormal and unpleasant sensation, and numbness in the hands and feet.106–108 Clinical exam may uncover impairment in perception of touch, vibration, and proprioception. Nerve endings in the hands and feet are usually affected earliest by neurotoxicity in a symmetrical, length-dependent manner affecting the longest nerve fibers in the body first. Disabling symptoms like sensory ataxia, pain and severe numbness can interfere with functional ability and QoL.
Recommendation 17: Assess with Total Neuropathy Score or other validated tool for CRC survivors who received oxaliplatin and refer to rehabilitation and pain management specialists as indicated. Level of Evidence = 0
Pre-existing factors that may increase patient risk for developing O-IPN include pre-existing neuropathy, alcoholism and diabetes mellitus.99,100,109 Higher cumulative drug dose is a possible indicator for developing long-term O-IPN. A descriptive study reported that persistent grades 2 and 3 O-IPN was more common in patients who received a cumulative dose of more than 900 mg/m2 suggesting influence of oxaliplatin administration on long-term O-IPN.99,110
Currently no standardized assessment tool or questionnaire for O-IPN has been used in studies of O-IPN. The National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) or “common toxicity criteria” (toxicity graded as mild- Grade 1, moderate-Grade 2, severe-Grade 3)108 has been applied more widely; in addition to the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Oxaliplatin-Specific Neurotoxicity questionnaire (FACT/GOG-Ntx), a reliable and valid instrument for assessing the impact of neuropathy on health-related QoL; a neuropathic symptom questionnaire; and neurophysiological examinations (e.g. nerve conduction studies).99,107,111 Use of the Total Neuropathy Score (TNSc) (Figure 5: Total Neuropathy Score) which does not require specialized equipment or training may be more suitable for clinical practice.
Figure 5. Total Neuropathy Score.
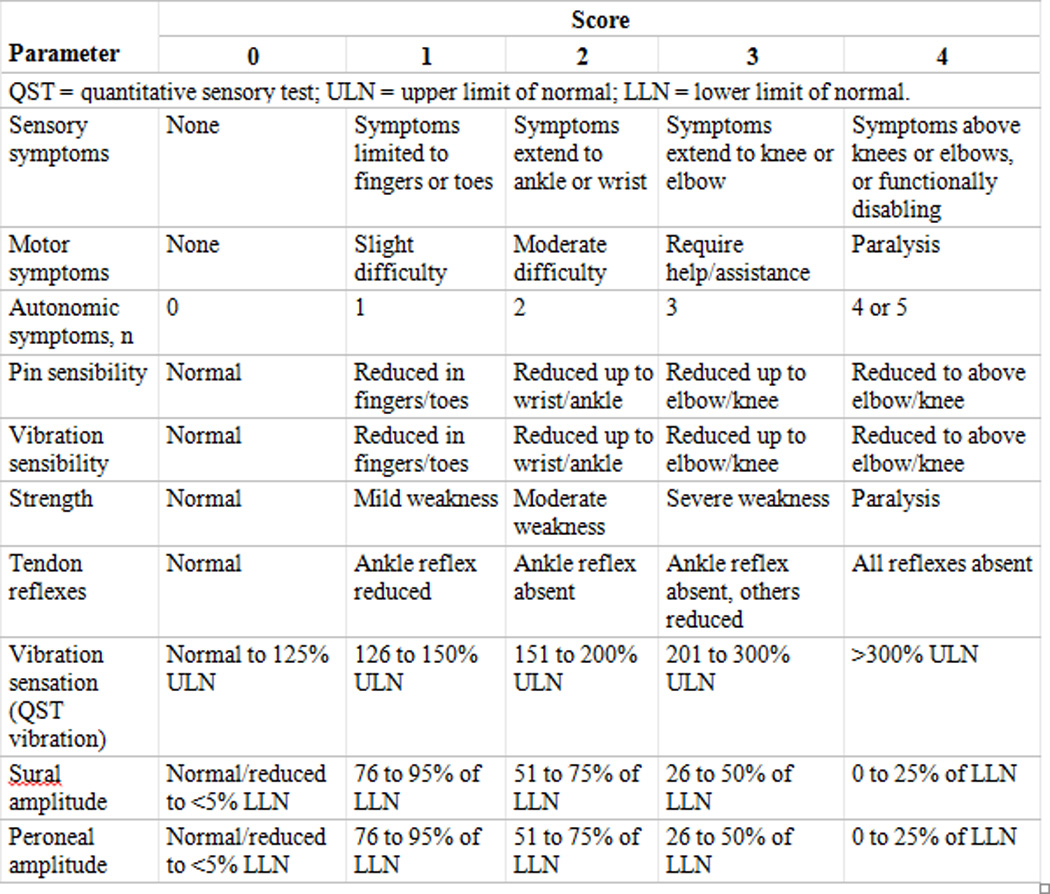
Description:
The total neuropathy score is a validated measure of peripheral nerve function.
- DR Cornblath, Chaudhry V, Carter K, et al. Total neuropathy score: validation and reliability study. Neurology. 1999;53(8):1660.
At present strong evidence for standard therapy or neuroprotective strategy (e.g. topical agents, antidepressants, and or antiepileptics) for O-IPN is lacking. Prevention is key to preventing O-IPN by identifying patients who may be at increased risk of developing severe or persistent forms of O-IPN.99
Although C-IPN trials are inconclusive regarding tricyclic antidepressants (such as nortriptyline), gabapentin, and a compounded topical gel containing baclofen, amitriptyline HCL, and ketamine, these agents may be offered on the basis of data supporting their utility in other neuropathic pain conditions given the limited other C-IPN treatment options.12
To treat existing C-IPN, the best available data support a moderate recommendation for treatment with duloxetine. The effect of duloxetine was studied in a randomized, placebo-controlled, cross-over trial of 231 patients with C-IPN. Patients received 30 mg of duloxetine or placebo for the first week and 60 mg of duloxetine or placebo for 4 more weeks. Patients who received duloxetine reported a significant decrease in average pain compared with those who received placebo (P = .003). In addition to a decrease in pain, data from the trial also supported that duloxetine decreased numbness and tingling symptoms.12,112
Primary care clinicians should refer survivors for rehabilitative medicine treatments including physical therapy and pain management as needed. For disabling chronic C-IPN, primary care clinicians should refer survivors to neurology or to occupational and physical therapy.113
Ostomy / Stoma
Recommendation 18: For CRC survivors with a stoma, monitor for sexual dysfunction, distress, depression, anxiety and QoL. Refer to specialists for support as indicated. Level of Evidence = I
Colon cancer survivors are less likely than rectal cancer survivors to need a permanent stoma. To better understand the long-term impacts of ostomies on CRC survivors, McMullen et al. conducted a mixed methods study of health-related QoL in CRC survivors who were at least 5 years posttreatment and had permanent ostomies.114 The qualitative study explored themes in written responses to open-ended survey questions related to physical, psychological, social, and spiritual domains. There were 178 responses to the question, “Many people have shared stories about their lives with an ostomy. Please share with us the greatest challenge you have encountered in having an ostomy.” Six themes and various subthemes emerged from the content analysis. One of the challenges CRC survivors face relates to caring for the ostomy and appliances. These challenges include routine ostomy care, achieving bowel regularity, issues with leakage, gas, and odor, and skin irritations at the ostomy site. Examples of dealing with the ostomy and appliances include finding the right equipment, equipment failures, and dietary changes and adaptations. Many of these issues can be addressed by a trained ostomy therapist. Patients with an ostomy may benefit from additional psychosocial support to adjust to and live with an ostomy appliance. The efficacy of psychosocial intervention including patient education is supported by numerous RCTs and a systematic review (level I) documenting positive effects on stoma-related knowledge, health-related quality of life, and cost-reduction.115
Pain
Recommendation 19: Monitor patients who received pelvic irradiation for chronic proctitis and manage symptoms as indicated. Level of Evidence = I
Chronic pain is one of the uncommon but important sequelae of CRC and its treatment. The most important risk factor for development of chronic pain is pelvic irradiation resulting in chronic proctitis. Chronic pain is known to contribute to functional limitation and negatively impacts the QoL in CRC survivors. While there are no specific guidelines to managing pain in the context of CRC survivorship, interventions with pharmacotherapy including the use of opioid analgesics,116 utilization of pain management services, if available, and incorporation of behavioral interventions/physical activity and/or rehabilitation/physical therapy have demonstrated efficacy in pain control in systematic reviews in other cancers or pain syndromes (evidence level I).75,117
Sexual Function / Fertility
Recommendation 20: Primary care clinicians should address sexual function when managing CRC survivors. For CRC survivors of childbearing age, who experience infertility due to treatment, refer for psychosocial support. Level of Evidence = 0, IA (oral phosphodiesterase-5 inhibitors in men), IC (vaginal moisturizers and lubricants for women)
Colorectal cancer is fairly uncommon during the reproductive years. The incidence of CRC is 3.3 and 3.8 per 100,000 persons for females and males, respectively, in the U.S. between the ages of 15 and 39 years.17 As of 2008, it was estimated that there were about 27,000 CRC survivors in the U.S. who were age 44 or younger.118 Reflecting the increasing incidence of CRC as individuals age, over half of this estimate was survivors between 40 and 44 years of age.118,119 Given the relatively small number of CRC survivors treated during their reproductive years, there have consequently been few studies evaluating gonadal function and infertility following therapy. The primary therapy associated with infertility in women with rectal cancer is pelvic radiotherapy.120 Even with contemporary approaches to minimize the radiation exposure to normal surrounding tissues, the ovaries often receive substantial doses unless they are surgically transposed prior to radiation.121 With a diminishing primordial follicle pool in women in their 30s and 40s, the doses of radiation necessary to induce acute ovarian failure is lower than for women treated with pelvic radiation as a child or adolescent. In men, despite shielding of the testes, the dose of radiation is often enough to damage the germinal epithelium and cause azoospermia.122 In the treatment of other cancer types, 5-fluorouracil has not been shown to cause infertility in women or men. Oxaliplatin is moderately gonadotoxic.120 In a woman whose primordial follicle pool is diminished by age, treatment with oxaliplatin may induce ovarian failure and premature menopause, thereby causing infertility.123 Fertility rates in males do not appear to be substantially affected, though this remains an understudied area.
While infertility affects a relatively small percent of CRC survivors, sexual dysfunction is a problem that spans across the age spectrum. In general, sexual dysfunction is prevalent following treatment for CRC, particularly among rectal cancer survivors. Study in this area is quite complex. A substantial proportion of individuals are diagnosed with CRC at an age when sexual activity is beginning to wane. Thus, to interpret prevalence data or a change in sexual activity, it is important to have a similarly aged non-cancer population. Adding to the complexity of studies, surgical and radiation techniques have evolved, often aimed at reducing long-term outcomes such as sexual dysfunction while providing adequate local control of the tumor. For example, in the mid-1980s, total mesorectal excision (TME) was introduced as a surgical technique for resecting rectal cancer, with a goal of preserving autonomic nerve function and preventing urologic problems and sexual dysfunction. Thus, there are multiple subgroups of CRC survivors, depending upon tumor location, surgical technique, having an ostomy, and the use of preoperative radiotherapy. Further complicating the study of sexual function in CRC survivors is the fact that key outcomes, and definitions of function, are different between males and females, and often different from one study to another. Needless to say, the number of adequately powered prospective studies with a non-cancer comparison population is low. Nevertheless, there are several key findings regarding sexual function that have been consistently reported across studies and should be addressed in evaluating a CRC survivor.
Even with contemporary surgical approaches intent on sparing autonomic nerve function, which is important for erectile function in males, the rectal cancer size and location often precludes full preservation of nerve function. In addition, radiotherapy is a frequent method of local tumor control for rectal cancer (but not for colon cancer). Thus, in males, sexual dysfunction is more common among rectal cancer survivors than following radiotherapy for colon cancer.124,125 In a large population-based study of CRC survivors who were 12 to 36 months following their diagnosis, 25% of rectal cancer survivors reported difficulties with sexual matters; 11% of colon cancer reported difficulties.126 Den Oudsten and colleagues surveyed 1359 CRC survivors who were a mean age of 70 years at time of study and about 4 years since their initial diagnosis.127 A higher proportion of male rectal cancer survivors reported erectile dysfunction (54%) than the normative (non-cancer) population (27%). Similarly, male rectal cancer survivors frequently reported ejaculatory problems (68%). Despite these problems, there was no difference in sexual enjoyment between male rectal cancer survivors and men in the normative population. Moreover, male CRC survivors were fairly similar to the normative population with respect to erectile dysfunction, ejaculatory problems, and sexual enjoyment. In a well-designed prospective study of 990 patients diagnosed with rectal cancer at a mean age of 64 years and randomized to TME with or without preoperative radiotherapy, Lange et al reported several interesting findings.128 Among men, 20.8% were not sexually active at the time of their cancer diagnosis. Of the men who were sexually active at time of cancer diagnosis, 28.5% were no longer active by two years after radiotherapy. Postoperative erectile dysfunction and ejaculatory problems developed or worsened in 79.8% and 72.2% of men, respectively. Unfortunately, there was not a non-cancer comparison population, so it is difficult to know how much normal aging influenced these changes. In multivariate models, anastomotic leakage and excessive perioperative blood loss (perhaps a proxy for surgical nerve damage) were associated with worsening function. While radiotherapy was not independently associated with sexual dysfunction, the interval from radiotherapy to last evaluation was likely too short to determine the additive effect of radiation.
Female CRC survivors, regardless of whether the cancer was in the colon or rectum, are substantially more likely to report sexual dysfunction, including dyspareunia, than women in the normative population.124–127 While vaginal dryness appears to occur with similar frequency among colon and rectal cancer survivors, dyspareunia is more common in those treated for a rectal cancer.127 In the aforementioned prospective study by Lange and colleagues, only 51.7% of female CRC patients were sexually active at time of cancer diagnosis.128 Of those who were sexually active, 18.4% were no longer active by two years after the cancer diagnosis. Dyspareunia and vaginal dryness developed or worsened over time in 59.1% and 56.6% of the women, respectively. A temporary or definitive stoma was the only factor in multivariate analysis that was associated with worsening of either outcome. While radiotherapy was independently associated with general sexual dysfunction in women, it was not associated with the development of dyspareunia or vaginal dryness.
Multiple studies have shown a strong correlation between sexual dysfunction and psychosocial distress.129–132 Thus, primary care clinicians should address sexual function when managing CRC survivors. Some therapies are available for men and women experiencing symptoms or signs of sexual dysfunction. In men, particularly those treated with pelvic radiotherapy, Leydig cell dysfunction should be evaluated and testosterone replacement initiated if indicated. The efficacy of oral phosphodiesterase-5 inhibitors for male survivors experiencing erectile dysfunction has been shown in one RCT.133 Women with vaginal dryness may benefit from the use of vaginal moisturizers and water or silicone-based lubricants during intercourse, as recommended by the International Menopause Society for postmenopausal women without a cancer history.134 If available, referral for counseling and / or sexual health programs may be beneficial.124,125,132,135
Urinary Bladder Issues
Recommendation 21: Screen CRC survivors for urinary incontinence and retention and manage as you would a patient of average risk of urinary dysfunction. Level of Evidence = IC
Urinary complications are common after treatment for CRC. Both surgery and radiation can affect the bladder and cause symptoms such as urinary incontinence or retention that affect QoL scores. Interestingly, the type of surgery (open v. laparoscopic) does not seem to make a difference in the Global Rating QoL scores.136 However, long term urinary complications were slightly more frequent in patients who underwent ostomies v. anastomosis: urinary retention (ostomy 6%, anastomosis 1.3%) and urinary incontinence (ostomy 2.1%, anastomosis 0.0%).137
Functional voiding disturbances such as stress, urge or overflow incontinence have all been reported postoperatively and may be deemed long-term effects. Urinary retention occurs when there is injury to the pelvic nerves during mobilization of the rectum. Newer surgical techniques have made this much less common and fortunately this is often transient and does not progress as a long-term effect when recognized and treated early in the postoperative period. For patients with prolonged urinary retention after the surgery, referral to an urologist for urodynamic studies should be done to elucidate the diagnosis. Patients with hypocontractile bladders may require clean intermittent catheterization, while patients with adherence abnormalities may respond well to medical therapy with anticholinergic medications.138
Stress and urge urinary incontinence are more common with prevalence exceeding 50% up to five years postoperatively.139 However, this is difficult to interpret as background rates of incontinence in the general population are also high. Evidence-based treatment guidelines are lacking for post-surgical incontinence in CRC survivors; thus, recommendations are based on interventions used in the general population. Kegel exercises can be helpful for stress incontinence due to pelvic floor dysfunction, but pelvic floor strengthening may be limited if denervation occurred during surgery.140 Other conservative therapies such as dietary modification (limiting caffeine and fluid intake) or medications may also be useful. Anticholinergic drugs are effective in stress incontinence and antimuscarinic drugs are used for urge or mixed incontinence.
Pelvic radiation used for adjunctive therapy for CRC can lead to fibrosis of the bladder wall, weakening of the pelvic floor muscles and thinning of the lining of the bladder. Urinary incontinence, frequency, urgency, dysuria or hematuria are manifestations of radiation therapy for CRC that may persist after treatment ends in a small number of patients. There is no good evidence on which to base treatment guidelines, therefore management is based on expert opinion and is aimed at controlling symptoms. For urgency and frequency, avoiding foods that irritate the bladder (citrus/tomatoes/caffeine) may be beneficial. Kegel exercises or bladder retraining are useful for incontinence. Persistent hematuria after radiation is rare, thus a urology referral for cystoscopy may be warranted to look for other causes of hematuria.
HEALTH PROMOTION
Recommendation 22: It is recommended that primary care clinicians provide routine general medical care and health promotion recommendations, and continue to treat patients’ chronic conditions, recognizing that cancer treatments worsen the severity of many underlying chronic conditions. Level of Evidence = 0, III (weight); 0, IB (physical activity); 0 (nutrition); 0, III (tobacco cessation); 0 (alcohol use)
Health promotion recommendations for CRC survivors are provided in Table 5. Where appropriate, these guidelines leverage the ACS Nutrition and Physical Activity Guidelines for Cancer Survivors.141
Table 5.
Health Promotion Guidelines
| Guideline | Level of Evidencea |
|---|---|
Counsel survivors to achieve and maintain a healthy weight.
|
0, III |
Counsel survivors to engage in regular physical activity.
|
0, IB |
Counsel survivors to achieve a dietary pattern that is high in vegetables, fruits, and whole grains.
|
0 |
| Counsel survivors to avoid tobacco products or offer cessation counseling and/or refer survivors to cessation counseling and resources. |
0, III |
Counsel survivors to avoid or limit alcohol consumption.
|
0 |
| Refer survivors with chronic bowel problems or surgery that affects normal nutrient absorption to a registered dietitian to modify their diets to accommodate these changes and maintain optimal health. |
0 |
Level of evidence: I, meta analyses of randomized controlled trials (RCTs); IA, RCT of colorectal cancer survivors; IB, RCT based on cancer survivors across multiple sites; IC, RCT not based on cancer survivors, but on general population experiencing a specific long-term or late effect (e.g., chronic diarrhea, sexual dysfunction, etc.); IIA, non-RCT based on colorectal cancer survivors; IIB, non-RCT based on cancer survivors across multiple sites; IIC, non-RCT not based on cancer survivors but on general population experiencing a specific long-term or late effect (e.g., chronic diarrhea, sexual dysfunction, etc.); III, case study; 0, expert opinion, observation, clinical practice, literature review, or pilot study
Information
Colorectal cancer survivor and caregiver information needs should be routinely assessed and information about the late effects of CRC treatment, as well as information on risk reduction and health promotion, should be provided.
There are no completed large randomized trials directly assessing the impact of obesity, physical activity, specific dietary patterns or tobacco use on CRC progression or mortality. There is, however, a growing body of prospective and observational data supporting associations between these factors and outcomes in CRC survivors.
Obesity
Obesity is at epidemic proportions in the U.S. and obesity rates of 17% to 35% have been reported in trials of adjuvant chemotherapy in patients with colon cancer.142 Increasing evidence indicates that being overweight or obese increases the risk of CRC recurrence and of being diagnosed with other obesity-related cancers. A number of these studies also suggest that obesity reduces the likelihood of disease-free and overall survival.143–145 Colorectal cancer survivors should consume a well-balanced diet and weight management should be considered a priority standard of care. Individuals who recently faced a cancer diagnosis are often motivated to live a healthier lifestyle, particularly if the changes are linked to a higher likelihood of avoiding a recurrence. For CRC survivors who are overweight or obese, primary care clinicians should encourage increased physical activity and healthier eating, focusing on lower total calorie intake. Referral to weight loss programs and frequent follow-up by the primary care clinician are sensible interventions.
Physical Activity
Evidence suggests that increased physical activity levels are associated with better physical functioning, reduced fatigue, increases oxygen consumption and better patient-reported QoL.141 A small number of studies also suggest that CRC survivors who increased their physical activity from pre- to post-diagnosis may decrease their total mortality risk.146 Colorectal cancer survivors may experience substantial benefits from increasing exercise, affecting multiple domains of well-being. Many cancer survivors may not feel ready to engage in the recommended exercise level of 150 minutes per week. Thus, attention should be given to helping patients gradually increase their activity levels with the goal of exercising 150 minutes per week.
Nutrition
A number of dietary patterns including higher intake of red meat and processed meat, refined grains, and sugary desserts have been associated with a statistically significant increase in CRC recurrence and poorer overall survival.147,148 Colorectal cancer survivors should follow nutritional guidelines to reduce their risk of a second primary cancer, as well as reducing their risk for other chronic conditions such as CVD. Following a diet that is high in vegetables, fruits and whole grains is ideal and diets should have low amounts of saturated fats, as well as appropriate dietary fiber.
Additional diet and nutritional recommendations for CRC survivors based on these ACS guidelines include CRC survivors with chronic bowel problems or surgery that affects normal nutrient absorption should be referred to a registered dietitian to modify their diets to accommodate the changes and maintain optimal health (as described under Bowel/Gastrointestinal Issues in the Long-Term and Late Effects section of this paper), and ensure a sufficient Vitamin D status and consume recommended levels of calcium.149,150
Smoking Cessation
Studies have indicated that smokers who smoked prior to a diagnosis of CRC as well as those who smoke after diagnosis are nearly twice as likely to die as a result of their cancer, and have more than double the risk of overall mortality compared to non-smokers.151,152 In spite of these risks a recent survey found that nearly 10% of cancer survivors continued to smoke more than 9 years after their diagnosis.153
Tobacco cessation is an important part of posttreatment care of cancer patients. Primary care clinicians should counsel CRC survivors to avoid tobacco and offer cessation when appropriate.
In summary, it is recommended that primary care clinicians follow ACS Guidelines on Nutrition and Physical Activity for Prevention and Early Detection of Cancer and Nutrition and Physical Activity for Cancer Survivors to inform counseling on routine health promotion.
CARE COORINDATION AND PRACTICE IMPLICATIONS
Recommendation 23: Initiate and maintain direct communication with all specialists involved in your patient’s oncology care and symptom management. Request a treatment summary and follow-up care plan to guide coordination of follow-up care posttreatment. Level of Evidence = 0, III (treatment summary and survivorship care plan); 0, IA (care coordination for chronic conditions); 0 (psychosocial referral); 0 (rehabilitation referral); 0, I (follow-up care regimen)
The clinical follow-up care planning process and coordination among care providers are essential to ensure all health needs of the cancer survivor are met. It is recommended that the primary care clinician initiate and maintain direct communication among oncology and specialty providers regarding clinical follow-up care. This communication should clearly specify roles of clinicians related to clinical follow-up care. Patients completing primary treatment should be provided with a comprehensive treatment summary and clinical follow-up care plan (survivorship care plan or SCP) from the primary treating specialist(s) who coordinated the oncology treatment. While the use of these tools has been endorsed, there are few high quality studies of SCPs. Some have reported that survivor satisfaction with, and self-reported understanding of, their SCP were very high. One study found that breast cancer survivors with SCPs were better able to correctly identify the clinician responsible for their follow-up care, and another suggested a reduction of unmet needs among patients with SCPs. Health professionals have cited the time required to develop SCPs (1–4 hours) as a significant barrier to their implementation and utilization.154,155
One of the central challenges confronting all health care clinicians dedicated to improving care for cancer survivors is the widespread lack of well-defined smoothly functioning inter-disciplinary care teams. Many primary care clinicians have not focused specifically on what it means to serve as the leader or coordinator of a cancer survivor’s clinical team. Others believe they do not have the expertise to serve in this role. The need for this type of coordination has been widely accepted and is supported by available evidence, even though that evidence base is immature. Primary care clinicians must now realize that it is the standard of care for call cancer patients to have a treatment summary and a survivorship care plan. Accordingly, primary care clinicians should initiate and maintain direct communication among patient, specialty providers, and primary care clinicians regarding clinical follow-up care, clearly specify roles of clinicians related to clinical follow-up care, and proactively contact the oncology specialist to obtain a treatment summary and clinical follow-up care plan. The emergence of new payment models, such as accountable care organizations, may facilitate development of this new higher level of coordination.
LIMITATIONS
A significant limitation of this review is the limited evidence-base to provide clear and specific recommendations for the prevention and management of long-term and late effects. Lack of clinical trials is a limitation of the current state-of-the-science for survivorship and a limitation of the recommendations for management indicated in the tables. There are few prospective, randomized control trials testing interventions among CRC survivors. The majority of the citations characterizing the risk and magnitude of risk of late effects and management recommendations rely predominantly on case-control studies with fewer than 500 participants and reviews that combine studies with varying outcome measures. There were several cohort studies that used population-based data to estimate the risk of late effects.
Other limitations include lack of patient/consumer participation in the guideline process; lack of a radiation oncologist on the expert workgroup; and reliance on previous guidelines for surveillance. Additionally, the literature review was not managed by a clinical epidemiologist due to limited resources. The literature review and environmental scan were conducted by project staff. An ACS librarian and the ACS Principal Investigator for The Survivorship Center were consulted for supplemental literature searches. Furthermore, the guidelines did not result directly from the development of specific clinical questions asked prior to the literature review; and recommendations for inclusion were not systematically evaluated through an instrument such as the Rigor of Development subscale of the Appraisal for Guidelines for Research and Evaluation (AGREE II).
Management recommendations are based on current evidence in the literature, but most evidence is not sufficient to warrant a strong recommendation. Rather, recommendations should be seen as possible management strategies given the current limited evidence base.
SUMMARY
Due to the potential significant impacts of cancer and its treatment on CRC survivor health and QoL, it is imperative that cancer survivors receive high-quality, comprehensive, coordinated clinical follow-up care. This care should focus on both the physical and psychosocial impacts and take into consideration the individual’s treatment and needs. Historically, the focus of clinical follow-up care has been on screening and surveillance for recurrence and new cancers, but it is now clear that it should also entail detection and management of the long-term and late impacts. Moreover, cancer survivors need to be counseled on health promotion strategies to help minimize or mitigate these impacts. One key recommendation made here and elsewhere is that survivors and primary care clinicians receive a survivorship care plan, which includes a concise summary of treatment as well as a clinical follow-up care plan. This tool facilitates a discussion with the patient and all clinicians and presents an opportunity to improve care coordination by clarifying roles.
Despite gaps in the evidence base regarding critical components of clinical follow-up care, enough evidence exists to provide consensus-based guidelines to improve posttreatment care until additional evidence can be generated. This guideline on clinical follow-up care for CRC survivors is geared toward the diverse group of primary care clinicians who provide much-needed care for a wide variety of patients, some of whom may be CRC survivors.
In addition to this article, tools and resources are available to assist primary care clinicians in implementing the recommendations. The CA Journal offers the CA Patient Page, a tool to help patients understand how to use the guidelines to talk to their doctor about care coordination, healthy behaviors, surveillance and screening, and symptom management. Primary care clinicians can access the free CA Patient Page for CRC survivors or download it at (insert URL). The Survivorship Center offers The GW Cancer Institute’s Cancer Survivorship E-Learning Series for Primary Care Providers (The E-Learning Series), a free, innovative online continuing education program to educate primary care providers about how to better understand and care for survivors in the primary care setting. Continuing education credits (CEs) are available at no cost to physicians, nurse practitioners, nurses and physician assistants for each 1-hour module. Learn more about The E-learning Series at cancersurvivorshipcentereducation.org.
Supplementary Material
Table 4.
Guidelines for the Assessment and Management of Physical and Psychosocial Long-Term and Late Effects
| Guideline | Level of Evidencea |
|---|---|
Bowel/Gastrointestinal Issues
|
III |
Cognitive Function
|
0 |
Dental/Oral
|
0 |
Distress / Depression / Anxiety
|
I |
Fatigue
|
I |
Neuropathy
|
0 |
Ostomy/Stoma Issues
|
I |
Pain
|
I |
Sexual Functioning/Fertility
|
0, IA (oral phosphodiesterase-5 inhibitors in men), IC (vaginal moisturizers and lubricants for women) |
| Urinary/Bladder Post-Surgical
Radiation
|
IC |
Level of evidence: I, meta analyses of randomized controlled trials (RCTs); IA, RCT of colorectal cancer survivors; IB, RCT based on cancer survivors across multiple sites; IC, RCT not based on cancer survivors, but on general population experiencing a specific long-term or late effect (e.g., chronic diarrhea, sexual dysfunction, etc.); IIA, non-RCT based on colorectal cancer survivors; IIB, non-RCT based on cancer survivors across multiple sites; IIC, non-RCT not based on cancer survivors but on general population experiencing a specific long-term or late effect (e.g., chronic diarrhea, sexual dysfunction, etc.); III, case study; 0, expert opinion, observation, clinical practice, literature review, or pilot study.
Table 6.
Care Coordination Guidelines
| Guideline | Level of Evidencea |
|---|---|
|
0, III |
|
0, IA |
|
I |
|
0 |
|
0, I |
FAP indicates familial adenomatous polyposis.
Level of evidence: I, meta analyses of randomized controlled trials (RCTs); IA, RCT of colorectal cancer survivors; IB, RCT based on cancer survivors across multiple sites; IC, RCT not based on cancer survivors, but on general population experiencing a specific long-term or late effect (e.g., chronic diarrhea, sexual dysfunction, etc.); IIA, non-RCT based on colorectal cancer survivors; IIB, non-RCT based on cancer survivors across multiple sites; IIC, non-RCT not based on cancer survivors but on general population experiencing a specific long-term or late effect (e.g., chronic diarrhea, sexual dysfunction, etc.); III, case study; 0, expert opinion, observation, clinical practice, literature review, or pilot study
Acknowledgments
In addition to the authors of the current article, the American Cancer Society would like to thank the following members of the Colorectal Cancer Survivorship Care Guidelines Expert Workgroup for their contribution to the development of the Colorectal Cancer Survivorship Care Guidelines:
Corry Chapman, MD, Catholic Charities, Washington, DC
Zana Correa, NP, BC, Memorial Sloan Kettering Cancer Center, New York, NY
Sarah Huff, RN, BSN, OCN, David Lee Cancer Center, Charleston Area Medical Center, Charleston, WV
Paul J. Limburg, MD, MPH, Mayo Clinic, Rochester, MN
Jeffrey A. Meyerhardt, MD, MPH, Dana-Farber Cancer Institute, Boston, MA
Fran Zandstra, RN, MBA, OCN, MD Anderson Cancer Center, Houston, TX
Robert A. Smith, PhD, American Cancer Society, Atlanta, GA
The American Cancer Society Colorectal Cancer Survivorship Care Guidelines Expert Workgroup would like to thank the following individuals for their helpful review and comments on this article:
Otis Brawley, MD, FACP, American Cancer Society, Atlanta, GA
Lewis E. Foxhall, MD, The University of Texas MD Anderson Cancer Center, Houston, TX
Ted Gansler, MD, MPH, MBA, American Cancer Society, Atlanta, GA
Michael Jefford, MD, PhD, MPH, Peter MacCallum Cancer Centre, Melbourne, Australia
Thomas K. Oliver, American Society of Clinical Oncology, Alexandria, VA
Steven R. Patierno, PhD, Duke Cancer Institute, Duke University Medical Center, Durham, NC
Marcus Plescia, MD, MPH, Mecklenburg County Health Department, Charlotte, NC
Richard Wender, MD, American Cancer Society, Atlanta, GA
Funding Acknowledgement:
This journal article was supported, in part, by Cooperative Agreement #5U55DP003054 from the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
No industry funding was used to support this work.
Author Disclosures:
Jennifer Bretsch reports cooperative agreement funding from the American Cancer Society/Centers for Disease Control and Prevention for the National Cancer Survivorship Research Center project. Rachel Cannady reports cooperative agreement funding from the American Cancer Society/Centers for Disease Control and Prevention for the National Cancer Survivorship Resource Center project. Rebecca L. Cowens-Alvarado reports cooperative agreement funding from the American Cancer Society/Centers for Disease Control and Prevention for the National Cancer Survivorship Resource Center project. Nicole L. Erb reports cooperative agreement funding from the American Cancer Society/Centers for Disease Control and Prevention for the National Cancer Survivorship Resource Center project. Paul Limburg reports royalties from Exact Sciences and medical advisory board fees from Everyday Health Media, LLC, paid to Mayo Clinic, outside the submitted work. Mandi L. Pratt-Chapman reports cooperative agreement funding from the American Cancer Society/Centers for Disease Control and Prevention for the National Cancer Survivorship Resource Center project; as well as a grant from Genentech, a consulting fee from Pfizer, and event sponsorships from Amgen, Genentech and Takeda Oncology, outside the submitted work. Johnie Rose reports grants from Genomic Health, Inc., outside the submitted work. Anne Willis reports cooperative agreement funding from the American Cancer Society/Centers for Disease Control and Prevention for the National Cancer Survivorship Resource Center project; as well as a grant from Genentech and event sponsorships from Amgen, Takeda Oncology and Genentech, outside the submitted work. Sandra L. Wong reports grants from the American Cancer Society (Research Scholar Grant RSG-12-269-01-CPHS) and the Agency for Healthcare Research and Quality (K08HS020937-01), outside the submitted work.
AMERICAN CANCER SOCIETY 2012–2015 COLORECTAL CANCER SURVIVORSHIP CARE GUIDELINES EXPERT WORKGROUP
Volunteer Members: April Barbour, MD, MPH, FACP (George Washington University, Washington, DC); Jennifer Bretsch, MS, CPHQ (American Society of Clinical Oncology, Alexandria, VA); Corry Chapman, MD (Catholic Charities, Washington, DC); Zana Correa, NP, BC (Memorial Sloan Kettering Cancer Center, New York, NY); Khaled El-Shami, MD, PhD (George Washington University, Washington, DC); Sarah Huff, RS, BSN, OCN (David Lee Cancer Center, Charleston Area Medical Center, Charleston, WV); Paul J. Limburg, MD, MPH (Mayo Clinic, Rochester, MN); Jeffrey A. Meyerhardt, MD, MPH (Dana-Farber Cancer Institute, Boston, MA); Kevin C. Oeffinger, MD (Memorial Sloan Kettering Cancer Center, New York, NY); Johnie Rose, MD, PhD (Case Western Reserve University School of Medicine/Case Comprehensive Cancer Center, Cleveland, OH); Sandra L. Wong, MD, MS, (University of Michigan, Ann Arbor, MI); and Fran Zandstra, RN, MBA, OCN (MD Anderson Cancer Center, Houston, TX). The George Washington University Cancer Institute staff members: Mandi L. Pratt-Chapman, MA (Washington, DC); and Anne Willis, MA (Washington, DC). American Cancer Society staff members: Durado D. Brooks, MD, MPH (Atlanta, GA); Rachel Cannady, BS (Atlanta, GA); Rebecca L. Cowens-Alvarado, MPH (Atlanta, GA); Nicole L. Erb, BA (Atlanta, GA); Katherine Sharpe, MTS (Atlanta, GA); Robert A. Smith, PhD (Atlanta, GA); Kevin Stein, PhD (Atlanta, GA)
Contributor Information
Khaled El-Shami, George Washington University, Washington, DC.
Kevin C. Oeffinger, Memorial Sloan Kettering Cancer Center, New York, NY.
Nicole L. Erb, American Cancer Society, Atlanta, GA.
Anne Willis, The George Washington University Cancer Institute, Washington, DC.
Jennifer Bretsch, American Society of Clinical Oncology, Alexandria, VA.
Mandi L. Pratt-Chapman, The George Washington University Cancer Institute, Washington, DC.
Rachel Cannady, American Cancer Society, Atlanta, GA.
Sandra L. Wong, University of Michigan, Ann Arbor, MI.
Johnie Rose, Case Western Reserve University School of Medicine/Case Comprehensive Cancer Center, Cleveland, OH.
April Barbour, George Washington University, Washington, DC.
Kevin Stein, American Cancer Society, Atlanta, GA.
Katherine Sharpe, American Cancer Society, Atlanta, GA.
Durado D. Brooks, American Cancer Society, Atlanta, GA.
Rebecca L. Cowens-Alvarado, American Cancer Society, Atlanta, GA.
References
- 1.Institute of Medicine. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 2.President’s Cancer Panel 2003–2004 Annual Report. Living beyond cancer: finding a new balance. 2004 [Google Scholar]
- 3.LIVESTRONG. A national action plan for cancer survivorship: advancing public health strategies. 2004 [Google Scholar]
- 4.Institute of Medicine. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 5.Mullan F. Seasons of survival: reflections of a physician with cancer. N Engl J Med. 1985;313(4):270–273. doi: 10.1056/NEJM198507253130421. [DOI] [PubMed] [Google Scholar]
- 6.McCabe MS, Bhatia S, Oeffinger KC, et al. American Society of Clinical Oncology statement: achieving high-quality cancer survivorship care. J Clin Oncol. 2013;31(5):631–640. doi: 10.1200/JCO.2012.46.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network I. NCCN clinical practice guidelines in oncology: colon cancer, version 2.2015. doi: 10.6004/jnccn.2009.0056. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network I. NCCN clinical practice guidelines in oncology: rectal cancer, version 2.2015. doi: 10.6004/jnccn.2009.0057. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network I. NCCN clinical practice guidelines in oncology: survivorship, version 1.2015. doi: 10.6004/jnccn.2017.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32(17):1840–1850. doi: 10.1200/JCO.2013.53.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605–1619. doi: 10.1200/JCO.2013.52.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 13.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 15.Cowens-Alvarado R, Sharpe K, Pratt-Chapman M, et al. Advancing survivorship care through the National Cancer Survivorship Resource Center: developing American Cancer Society guidelines for primary care providers. CA Cancer J Clin. 2013;63(3):147–150. doi: 10.3322/caac.21183. [DOI] [PubMed] [Google Scholar]
- 16.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 17.Howlader NNA, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012 [Google Scholar]
- 18.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 19.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 20.K M. Excellent care for cancer survivors: a guide to fully meet their needs in medical offices and in the community. 2011 [Google Scholar]
- 21.Skolarus TA, Wolf AM, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64(4):225–249. doi: 10.3322/caac.21234. [DOI] [PubMed] [Google Scholar]
- 22.Brawley O, Byers T, Chen A, et al. New American Cancer Society process for creating trustworthy cancer screening guidelines. JAMA. 2011;306(22):2495–2499. doi: 10.1001/jama.2011.1800. [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 24.Meyerhardt JA, Mangu PB, Flynn PJ, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31(35):4465–4470. doi: 10.1200/JCO.2013.50.7442. [DOI] [PubMed] [Google Scholar]
- 25.Rose J, Augestad KM, Cooper GS. Colorectal cancer surveillance: what's new and what's next. World J Gastroenterol. 2014;20(8):1887–1897. doi: 10.3748/wjg.v20.i8.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sargent DJ, Patiyil S, Yothers G, et al. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol. 2007;25(29):4569–4574. doi: 10.1200/JCO.2006.10.4323. [DOI] [PubMed] [Google Scholar]
- 27.Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2015: a review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2015;65(1):30–54. doi: 10.3322/caac.21261. [DOI] [PubMed] [Google Scholar]
- 28.Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin. 2003;53(3):141–169. doi: 10.3322/canjclin.53.3.141. [DOI] [PubMed] [Google Scholar]
- 29.Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 30.Koornstra JJ, Mourits MJ, Sijmons RH, Leliveld AM, Hollema H, Kleibeuker JH. Management of extracolonic tumours in patients with Lynch syndrome. Lancet Oncol. 2009;10(4):400–408. doi: 10.1016/S1470-2045(09)70041-5. [DOI] [PubMed] [Google Scholar]
- 31.Barrow E, Robinson L, Alduaij W, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin. Genet. 2009;75(2):141–149. doi: 10.1111/j.1399-0004.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 32.Dijkhuizen FP, Mol BW, Brolmann HA, Heintz AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer. 2000;89(8):1765–1772. [PubMed] [Google Scholar]
- 33.Breijer MC, Peeters JA, Opmeer BC, et al. Capacity of endometrial thickness measurement to diagnose endometrial carcinoma in asymptomatic postmenopausal women: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2012;40(6):621–629. doi: 10.1002/uog.12306. [DOI] [PubMed] [Google Scholar]
- 34.Schmeler KM, Lynch HT, Chen LM, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354(3):261–269. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 35.National Institutes of Health. NIH Consensus Development Program. Ovarian cancer: screening, treatment, and followup. 1994 [Google Scholar]
- 36.Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA. 2006;296(12):1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 37.Ramsey SD, Berry K, Moinpour C, Giedzinska A, Andersen MR. Quality of life in long term survivors of colorectal cancer. Amer J Gastroenterol. 2002;97(5):1228–1234. doi: 10.1111/j.1572-0241.2002.05694.x. [DOI] [PubMed] [Google Scholar]
- 38.Emmertsen KJ, Laurberg S Rectal Cancer Function Study G. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. The Br J Surg. 2013;100(10):1377–1387. doi: 10.1002/bjs.9223. [DOI] [PubMed] [Google Scholar]
- 39.Guren MG, Eriksen MT, Wiig JN, et al. Quality of life and functional outcome following anterior or abdominoperineal resection for rectal cancer. Eur J Surg Oncol. 2005;31(7):735–742. doi: 10.1016/j.ejso.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Braendengen M, Tveit KM, Bruheim K, Cvancarova M, Berglund A, Glimelius B. Late patient-reported toxicity after preoperative radiotherapy or chemoradiotherapy in nonresectable rectal cancer: results from a randomized Phase III study. Int J Radiat Oncol Biol Phys. 2011;81(4):1017–1024. doi: 10.1016/j.ijrobp.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Lange MM, Martz JE, Ramdeen B, et al. Long-term results of rectal cancer surgery with a systematical operative approach. Ann Surg Oncol. 2013;20(6):1806–1815. doi: 10.1245/s10434-012-2832-2. [DOI] [PubMed] [Google Scholar]
- 42.Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol. 2005;23(25):6199–6206. doi: 10.1200/JCO.2005.14.779. [DOI] [PubMed] [Google Scholar]
- 43.Gami BHK, Blake P, et al. How patients manage gastrointestinal symptoms after pelvic radiotherapy. Aliment Pharmacol Ther. 2003;18(10):987–994. doi: 10.1046/j.1365-2036.2003.01760.x. [DOI] [PubMed] [Google Scholar]
- 44.Khan NF, Mant D, Carpenter L, Forman D, Rose PW. Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: a database study. Brit J Cancer. 2011;105(Suppl 1):S29–S37. doi: 10.1038/bjc.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jensen SA, Sorensen JB. 5-fluorouracil-based therapy induces endovascular injury having potential significance to development of clinically overt cardiotoxicity. Cancer Chemother Pharmacol. 2012;69(1):57–64. doi: 10.1007/s00280-011-1669-x. [DOI] [PubMed] [Google Scholar]
- 46.Polk A, Vistisen K, Vaage-Nilsen M, Nielsen DL. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol. 2014;15:47. doi: 10.1186/2050-6511-15-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polk A, Vaage-Nilsen M, Vistisen K, Nielsen DL. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev. 2013;39(8):974–984. doi: 10.1016/j.ctrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Martinez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon cancer in women. Nurses' Health Study Research Group. J Natl Cancer Inst. 1997;89(13):948–955. doi: 10.1093/jnci/89.13.948. [DOI] [PubMed] [Google Scholar]
- 49.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122(5):327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 50.Hawkes AL, Lynch BM, Owen N, Aitken JF. Lifestyle factors associated concurrently and prospectively with co-morbid cardiovascular disease in a population-based cohort of colorectal cancer survivors. Eur J Cancer. 2011;47(2):267–276. doi: 10.1016/j.ejca.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50(15):1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 52.Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65(2):123–138. doi: 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jean-Pierre P, Winters PC, Ahles TA, et al. Prevalence of self-reported memory problems in adult cancer survivors: a national cross-sectional study. J Clin Oncol. 2012;8(1):30–34. doi: 10.1200/JOP.2011.000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jansen L, Hoffmeister M, Chang-Claude J, Koch M, Brenner H, Arndt V. Age-specific administration of chemotherapy and long-term quality of life in stage II and III colorectal cancer patients: a population-based prospective cohort. Oncologist. 2011;16(12):1741–1751. doi: 10.1634/theoncologist.2011-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tannock IF, Ahles TA, Ganz PA, Van Dam FS. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol. 2004;22(11):2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 56.Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surv. 2013;7(3):300–322. doi: 10.1007/s11764-013-0272-z. [DOI] [PubMed] [Google Scholar]
- 57.Boyes AW, Girgis A, Zucca AC, Lecathelinais C. Anxiety and depression among long-term survivors of cancer in Australia: results of a population-based survey. Comment. Med J Aust. 2009;191(5):295. doi: 10.5694/j.1326-5377.2009.tb02801.x. [DOI] [PubMed] [Google Scholar]
- 58.Hoffman KE, McCarthy EP, Recklitis CJ, Ng AK. Psychological distress in long-term survivors of adult-onset cancer: results from a national survey. Arch Int Med. 2009;169(14):1274–1281. doi: 10.1001/archinternmed.2009.179. [DOI] [PubMed] [Google Scholar]
- 59.Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14(8):721–732. doi: 10.1016/S1470-2045(13)70244-4. [DOI] [PubMed] [Google Scholar]
- 60.Denlinger CS, Barsevick AM. The challenges of colorectal cancer survivorship. J Natl Compr Cancer Netw. 2009;7(8):883–893. doi: 10.6004/jnccn.2009.0058. quiz 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ross L, Abild-Nielsen AG, Thomsen BL, Karlsen RV, Boesen EH, Johansen C. Quality of life of Danish colorectal cancer patients with and without a stoma. Support Care Cancer. 2007;15(5):505–513. doi: 10.1007/s00520-006-0177-8. [DOI] [PubMed] [Google Scholar]
- 62.National Comprehensive Cancer Network I. NCCN clinical practice guidelines: distress management, version 2.2014. doi: 10.6004/jnccn.2019.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel D, Sharpe L, Thewes B, Bell ML, Clarke S. Using the Distress Thermometer and Hospital Anxiety and Depression Scale to screen for psychosocial morbidity in patients diagnosed with colorectal cancer. J Affect Disord. 2011;131(1–3):412–416. doi: 10.1016/j.jad.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 64.Campbell HS, Sanson-Fisher R, Turner D, Hayward L, Wang XS, Taylor-Brown J. Psychometric properties of cancer survivors' unmet needs survey. Support Care Cancer. 2010;19(2):221–230. doi: 10.1007/s00520-009-0806-0. [DOI] [PubMed] [Google Scholar]
- 65.Campbell HS, Hall AE, Sanson-Fisher RW, Barker D, Turner D, Taylor-Brown J. Development and validation of the Short-Form Survivor Unmet Needs Survey (SF-SUNS) Support Care Cancer. 2014;22(4):1071–1079. doi: 10.1007/s00520-013-2061-7. [DOI] [PubMed] [Google Scholar]
- 66.Mayo Clinic. Depression (major depressive disorder) Diseases and Conditions. 2014 [Google Scholar]
- 67.Mayo Clinic. Anxiety. Diseases and Conditions. 2014 [Google Scholar]
- 68.Medeiros M, Oshima CT, Forones NM. Depression and anxiety in colorectal cancer patients. J Gastrointest Cancer. 2010;41(3):179–184. doi: 10.1007/s12029-010-9132-5. [DOI] [PubMed] [Google Scholar]
- 69.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 70.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12(2):277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 71.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 72.Krebber AM, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121–130. doi: 10.1002/pon.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuchler T, Bestmann B, Rappat S, Henne-Bruns D, Wood-Dauphinee S. Impact of psychotherapeutic support for patients with gastrointestinal cancer undergoing surgery: 10-year survival results of a randomized trial. J Clin Oncol. 2007;25(19):2702–2708. doi: 10.1200/JCO.2006.08.2883. [DOI] [PubMed] [Google Scholar]
- 74.Sharma A, Walker LG, Monson JR. Baseline quality of life factors predict long term survival after elective resection for colorectal cancer. Intl J Surg Oncol. 2013;2013:269510. doi: 10.1155/2013/269510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mishra SISR, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8 doi: 10.1002/14651858.CD007566.pub2. CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.National Comprehensive Cancer Network I. NCCN clinical practice guidelines in oncology: cancer-related fatigue, version 2.2015 [Google Scholar]
- 77.Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120(3):425–432. doi: 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yanez B, Pearman T, Lis CG, Beaumont JL, Cella D. The FACT-G7: a rapid version of the functional assessment of cancer therapy-general (FACT-G) for monitoring symptoms and concerns in oncology practice and research. Ann Oncol. 2013;24(4):1073–1078. doi: 10.1093/annonc/mds539. [DOI] [PubMed] [Google Scholar]
- 79.Wang XS, Cleeland CS, Mendoza TR, et al. Impact of cultural and linguistic factors on symptom reporting by patients with cancer. J Natl Cancer Inst. 2010;102(10):732–738. doi: 10.1093/jnci/djq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minton O, Strasser F, Radbruch L, Stone P. Identification of factors associated with fatigue in advanced cancer: a subset analysis of the European palliative care research collaborative computerized symptom assessment data set. J Pain Symptom Manage. 2012;43(2):226–235. doi: 10.1016/j.jpainsymman.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 81.Kuhnt S, Ernst J, Singer S, et al. Fatigue in cancer survivors--prevalence and correlates. Onkologie. 2009 Jun;32(6):312–317. doi: 10.1159/000215943. [DOI] [PubMed] [Google Scholar]
- 82.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 83.Servaes P, Gielissen MF, Verhagen S, Bleijenberg G. The course of severe fatigue in disease-free breast cancer patients: a longitudinal study. Psychooncology. 2007;16(9):787–795. doi: 10.1002/pon.1120. [DOI] [PubMed] [Google Scholar]
- 84.Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30(30):3687–3696. doi: 10.1200/JCO.2012.41.7238. [DOI] [PubMed] [Google Scholar]
- 85.Mitchell SA. Cancer-related fatigue: state of the science. PM & R. 2010;2(5):364–383. doi: 10.1016/j.pmrj.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 86.Mitchell SA, Beck SL, Hood LE, Moore K, Tanner ER. Putting evidence into practice: evidence-based interventions for fatigue during and following cancer and its treatment. Clin J Oncol Nurs. 2007;11(1):99–113. doi: 10.1188/07.CJON.99-113. [DOI] [PubMed] [Google Scholar]
- 87.Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol. 2013;20(3):e233–e246. doi: 10.3747/co.20.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Butt Z, Wagner LI, Beaumont JL, et al. Use of a single-item screening tool to detect clinically significant fatigue, pain, distress, and anorexia in ambulatory cancer practice. J Pain Symptom Manage. 2008;35(1):20–30. doi: 10.1016/j.jpainsymman.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 89.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 90.JanJan NAWX, Mendoza T, et al. Utility of the M.D. Anderson Symptom Assessment Inventory (MDASI) for symptom evaluation during chemoradiation in patients with gastrointestinal malignancies. J Clin Oncol. 2007;25((18): suppl 6613) [Google Scholar]
- 91.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 1998 May;7(4):301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 92.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer practice. 1998 May-Jun;6(3):143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 93.Chambers SK, Meng X, Youl P, Aitken J, Dunn J, Baade P. A five-year prospective study of quality of life after colorectal cancer. Qual Life Res. 2012;21(9):1551–1564. doi: 10.1007/s11136-011-0067-5. [DOI] [PubMed] [Google Scholar]
- 94.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 95.Klika RJ, Callahan KE, Drum SN. Individualized 12-week exercise training programs enhance aerobic capacity of cancer survivors. Physician Sportsmed. 2009;37(3):68–77. doi: 10.3810/psm.2009.10.1731. [DOI] [PubMed] [Google Scholar]
- 96.Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: a systematic review. Cancer Treat Rev. 2010;36(2):185–194. doi: 10.1016/j.ctrv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 97.Speed-Andrews AE, Courneya KS. Effects of exercise on quality of life and prognosis in cancer survivors. Curr Sports Med Rep. 2009;8(4):176–181. doi: 10.1249/JSR.0b013e3181ae98f3. [DOI] [PubMed] [Google Scholar]
- 98.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44(11):1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 99.Beijers AJ, Mols F, Vreugdenhil G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer. 2014;22(7):1999–2007. doi: 10.1007/s00520-014-2242-z. [DOI] [PubMed] [Google Scholar]
- 100.Krishnan AV, Goldstein D, Friedlander M, Kiernan MC. Oxaliplatin-induced neurotoxicity and the development of neuropathy. Muscle Nerve. 2005;32(1):51–60. doi: 10.1002/mus.20340. [DOI] [PubMed] [Google Scholar]
- 101.Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer. 2004;12(9):619–625. doi: 10.1007/s00520-004-0657-7. [DOI] [PubMed] [Google Scholar]
- 102.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 103.Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol. 2013;31(21):2699–2707. doi: 10.1200/JCO.2013.49.1514. [DOI] [PubMed] [Google Scholar]
- 104.Grothey A. Oxaliplatin-safety profile: neurotoxicity. Semin Oncol Nurs. 2003;30(4 Suppl 15):5–13. doi: 10.1016/s0093-7754(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 105.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oral Biol. 2012;82(1):51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 106.Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol Nurs. 2006;33(1):15–49. doi: 10.1053/j.seminoncol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 107.Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63(6):419–437. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 108.U.S. Department of Health and Human Services NIoH, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. 2010 [Google Scholar]
- 109.Brouwers EE, Huitema AD, Boogerd W, Beijnen JH, Schellens JH. Persistent neuropathy after treatment with cisplatin and oxaliplatin. Acta Oncol. 2009;48(6):832–841. doi: 10.1080/02841860902806609. [DOI] [PubMed] [Google Scholar]
- 110.Vatandoust S, Joshi R, Pittman KB, et al. A descriptive study of persistent oxaliplatin-induced peripheral neuropathy in patients with colorectal cancer. Support Care Cancer. 2014;22(2):513–518. doi: 10.1007/s00520-013-2004-3. [DOI] [PubMed] [Google Scholar]
- 111.Land SR, Kopec JA, Cecchini RS, et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol. 2007;25(16):2205–2211. doi: 10.1200/JCO.2006.08.6652. [DOI] [PubMed] [Google Scholar]
- 112.Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Choi M, Craft B, Geraci SA. Surveillance and monitoring of adult cancer survivors. Am J Med. 2011;124(7):598–601. doi: 10.1016/j.amjmed.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 114.McMullen CK, Hornbrook MC, Grant M, et al. The greatest challenges reported by long-term colorectal cancer survivors with stomas. J Support Oncol. 2008;6(4):175–182. [PubMed] [Google Scholar]
- 115.Danielsen AK, Burcharth J, Rosenberg J. Patient education has a positive effect in patients with a stoma: a systematic review. Colorectal Dis. 2013;15(6):e276–e283. doi: 10.1111/codi.12197. [DOI] [PubMed] [Google Scholar]
- 116.Schmidt-Hansen M, Bennett MI, Arnold S, Bromham N, Hilgart JS. Oxycodone for cancer-related pain. Cochrane Database Syst Rev. 2015;2 doi: 10.1002/14651858.CD003870.pub5. CD003870. [DOI] [PubMed] [Google Scholar]
- 117.Negrini S, Imperio G, Villafane JH, Negrini F, Zaina F. Systematic reviews of physical and rehabilitation medicine Cochrane contents. Part 1. Disabilities due to spinal disorders and pain syndromes in adults. Eur J Phys Rehab Med. 2013;49(4):597–609. [PubMed] [Google Scholar]
- 118.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20(10):1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.SEER. Table 1.23. U.S. Complete Prevalence Counts, Invasive Cancers Only. 2012 Jan 1; By Age at Prevalence. [Google Scholar]
- 120.Spanos CP, Mamopoulos A, Tsapas A, Syrakos T, Kiskinis D. Female fertility and colorectal cancer. Int J Colorectal Dis. 2008;23(8):735–743. doi: 10.1007/s00384-008-0483-3. [DOI] [PubMed] [Google Scholar]
- 121.Elizur SE, Tulandi T, Meterissian S, Huang JY, Levin D, Tan SL. Fertility preservation for young women with rectal cancer--a combined approach from one referral center. J Gastrointest Surg. 2009;13(6):1111–1115. doi: 10.1007/s11605-009-0829-3. [DOI] [PubMed] [Google Scholar]
- 122.Hermann RM, Henkel K, Christiansen H, et al. Testicular dose and hormonal changes after radiotherapy of rectal cancer. Radiother Oncol. 2005;75(1):83–88. doi: 10.1016/j.radonc.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 123.Cercek A, Siegel CL, Capanu M, Reidy-Lagunes D, Saltz LB. Incidence of chemotherapy-induced amenorrhea in premenopausal women treated with adjuvant FOLFOX for colorectal cancer. Clin Colorectal. 2013;12(3):163–167. doi: 10.1016/j.clcc.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 124.Ho VP, Lee Y, Stein SL, Temple LK. Sexual function after treatment for rectal cancer: a review. Dis Colon Rectum. 2011;54(1):113–125. doi: 10.1007/DCR.0b013e3181fb7b82. [DOI] [PubMed] [Google Scholar]
- 125.Donovan KA, Thompson LM, Hoffe SE. Sexual function in colorectal cancer survivors. Cancer Control. 2010;17(1):44–51. doi: 10.1177/107327481001700106. [DOI] [PubMed] [Google Scholar]
- 126.Downing A, Morris EJ, Richards M, et al. Health-related quality of life after colorectal cancer in England: a patient-reported outcomes study of individuals 12 to 36 months after diagnosis. J Clin Oncol. 2015;33(6):616–624. doi: 10.1200/JCO.2014.56.6539. [DOI] [PubMed] [Google Scholar]
- 127.Den Oudsten BL, Traa MJ, Thong MS, et al. Higher prevalence of sexual dysfunction in colon and rectal cancer survivors compared with the normative population: a population-based study. Eur J Cancer. 2012;48(17):3161–3170. doi: 10.1016/j.ejca.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 128.Lange MM, Marijnen CA, Maas CP, et al. Risk factors for sexual dysfunction after rectal cancer treatment. Eur J Cancer. 2009;45(9):1578–1588. doi: 10.1016/j.ejca.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 129.Philip EJ, Nelson C, Temple L, et al. Psychological correlates of sexual dysfunction in female rectal and anal cancer survivors: analysis of baseline intervention data. J Sex Med. 2013;10(10):2539–2548. doi: 10.1111/jsm.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Traa MJ, Orsini RG, Den Oudsten BL, et al. Measuring the health-related quality of life and sexual functioning of patients with rectal cancer: does type of treatment matter? Int J Cancer. 2014;134(4):979–987. doi: 10.1002/ijc.28430. [DOI] [PubMed] [Google Scholar]
- 131.Ball M, Nelson CJ, Shuk E, et al. Men's experience with sexual dysfunction post-rectal cancer treatment: a qualitative study. J Cancer Educ. 2013;28(3):494–502. doi: 10.1007/s13187-013-0492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carter J, Stabile C, Seidel B, et al. Baseline characteristics and concerns of female cancer patients/survivors seeking treatment at a Female Sexual Medicine Program. Support Care Cancer. 2015 doi: 10.1007/s00520-014-2573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park SY, Choi GS, Park JS, Kim HJ, Park JA, Choi JI. Efficacy and safety of udenafil for the treatment of erectile dysfunction after total mesorectal excision of rectal cancer: a randomized, double-blind, placebo-controlled trial. Surgery. 2015;157(1):64–71. doi: 10.1016/j.surg.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 134.Sturdee DW, Panay N International Menopause Society Writing G. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric. 2010;13(6):509–522. doi: 10.3109/13697137.2010.522875. [DOI] [PubMed] [Google Scholar]
- 135.Averyt JC, Nishimoto PW. Addressing sexual dysfunction in colorectal cancer survivorship care. J Gastrointest Oncol. 2014;5(5):388–394. doi: 10.3978/j.issn.2078-6891.2014.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Korolija D, Sauerland S, Wood-Dauphinee S, et al. Evaluation of quality of life after laparoscopic surgery: evidence-based guidelines of the European Association for Endoscopic Surgery. Surg Endosc. 2004;18(6):879–897. doi: 10.1007/s00464-003-9263-x. [DOI] [PubMed] [Google Scholar]
- 137.Liu L, Herrinton LJ, Hornbrook MC, Wendel CS, Grant M, Krouse RS. Early and late complications among long-term colorectal cancer survivors with ostomy or anastomosis. Dis Colon Rectum. 2010;53(2):200–212. doi: 10.1007/DCR.0b013e3181bdc408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Delacroix SE, Jr, Winters JC. Voiding dysfunction after pelvic colorectal surgery. Clin Colon Rectal Surg. 2010;23(2):119–127. doi: 10.1055/s-0030-1254299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Panjari M, Bell RJ, Burney S, Bell S, McMurrick PJ, Davis SR. Sexual function, incontinence, and wellbeing in women after rectal cancer--a review of the evidence. J Sex Med. 2012;9(11):2749–2758. doi: 10.1111/j.1743-6109.2012.02894.x. [DOI] [PubMed] [Google Scholar]
- 140.Lange MM, van de Velde CJ. Faecal and urinary incontinence after multimodality treatment of rectal cancer. PLoS Med. 2008;5(10):e202. doi: 10.1371/journal.pmed.0050202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 142.Sinicrope FA, Foster NR, Yothers G, et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer. 2013;119(8):1528–1536. doi: 10.1002/cncr.27938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gibson TM, Park Y, Robien K, et al. Body mass index and risk of second obesity-associated cancers after colorectal cancer: a pooled analysis of prospective cohort studies. J Clin Oncol. 2014;32(35):4004–4011. doi: 10.1200/JCO.2014.56.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98(22):1647–1654. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 145.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26(25):4109–4115. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25(7):1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 147.Meyerhardt JA, Sato K, Niedzwiecki D, et al. Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Natl Cancer Inst. 2012;104(22):1702–1711. doi: 10.1093/jnci/djs399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298(7):754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 149.U.S. Department of Health & Human Services. National Institutes of Health. Office of Dietary Supplements. Vitamin D. Fact Sheet for Health Professionals. 2014 [Google Scholar]
- 150.U.S. Department of Health & Human Services. National Institutes of Health. Office of Dietary Supplements. Calcium. Dietary Supplement Fact Sheet. 2013 [Google Scholar]
- 151.Yang B, Jacobs EJ, Gapstur SM, Stevens V, Campbell PT. Active smoking and mortality among colorectal cancer survivors: the Cancer Prevention Study II nutrition cohort. J Clin Oncol. 2015;33(8):885–893. doi: 10.1200/JCO.2014.58.3831. [DOI] [PubMed] [Google Scholar]
- 152.Zhu Y, Yang SR, Wang PP, et al. Influence of pre-diagnostic cigarette smoking on colorectal cancer survival: overall and by tumour molecular phenotype. Br J Cancer. 2014;110(5):1359–1366. doi: 10.1038/bjc.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Westmaas JL, Alcaraz KI, Berg CJ, Stein KD. Prevalence and correlates of smoking and cessation-related behavior among survivors of ten cancers: findings from a nationwide survey nine years after diagnosis. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1783–1792. doi: 10.1158/1055-9965.EPI-14-0046. [DOI] [PubMed] [Google Scholar]
- 154.Mayer DK, Birken SA, Check DK, Chen RC. Summing it up: an integrative review of studies of cancer survivorship care plans (2006–2013) Cancer. 2015;121(7):978–996. doi: 10.1002/cncr.28884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brennan ME, Gormally JF, Butow P, Boyle FM, Spillane AJ. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014;111(10):1899–1908. doi: 10.1038/bjc.2014.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.