Abstract
Type 1 diabetes acceleration in nonobese diabetic (NOD) mice through coxsackievirus B4 (CVB4) infection requires a preexisting critical mass of autoreactive T cells in pancreatic islets, and in the absence of this insulitic threshold, CVB4 infection leads to long-term disease protection. To understand this acceleration and protection process, we challenged 8- and 12-week-old NOD mice containing a disruption in interleukin-4 (IL-4) or gamma interferon (IFN-γ) genes (NOD IL-4−/− and NOD IFN-γ−/−, respectively) with a diabetogenic, pancreatropic Edwards strain of CVB4. The elimination of IL-4 did not alter the rate of insulitis or diabetes development in NOD mice, while the elimination of IFN-γ delayed these events several weeks. CVB4 infection in 8-week-old mice only significantly accelerated the onset of diabetes in a subset of standard, but not IL-4- or IFN-γ-deficient, NOD mice. Long-term diabetes protection was established in standard NOD mice as well as in the NOD IFN-γ−/− mice that did not rapidly develop disease following CVB4 infection at 8 weeks of age. When mice were infected at 12 weeks of age, the onset of diabetes was accelerated in NOD IL-4−/− mice, while neither acceleration nor long-term protection was elicited in NOD IFN-γ−/− mice. No differences were observed in the kinetics of CVB4 clearance in pancreases from NOD, NOD IL-4−/−, and NOD IFN-γ−/− mice. Collectively, these results suggest that at the insulitis threshold at which CVB4 infection can first accelerate the onset of diabetes in NOD mice, IL-4 as well as IFN-γ contributes to this pathogenic process. The protective mechanism against diabetes elicited in NOD mice infected with CVB4 prior to the development of a critical threshold level of insulitis requires neither IL-4 nor IFN-γ.
Investigators have long believed that the pathogenesis of autoimmune type 1 diabetes is influenced in some fashion by the interplay between genetics and environment (2, 3). While much progress has occurred with respect to identifying genes both within and outside the major histocompatibility complex (MHC) that contribute to susceptibility or resistance to type 1 diabetes, advances in characterizing environmental agents that are unequivocally associated with disease pathogenesis have been slow (1, 17). Since the late 1950s, much of the research directed at uncovering environmental agents associated with type 1 diabetes has focused on the role of group B coxsackieviruses (CVB) (10, 11, 16). While most investigations of CVB and type 1 diabetes have involved humans, previous studies have shown the effectiveness of nonobese diabetic (NOD) mice as a model to investigate the viral events related to disease pathogenesis (13, 22, 27). Our own such investigations have indicated that the infection of NOD mice with the Edwards strain of coxsackievirus B4 (CVB4) can either accelerate type 1 diabetes or provide long-term disease protection, depending on the preexisting mass of autoreactive T cells within the pancreatic islets (22).
The role that cytokines play in the pathogenesis of type 1 diabetes has also become the object of intense investigations over the last decade (20). In both NOD mice and humans, the regulatory cytokines interleukin-4 (IL-4) and gamma interferon (IFN-γ) have been proposed to protect against or promote diabetes development, respectively (8, 9, 20). Viral infections may induce imbalances in these cytokines that possibly alter the development of type 1 diabetes in susceptible individuals. As a result, the goal of this study was to investigate the effect that CVB4 infection has on the progression of type 1 diabetes in NOD mice that are genetically deficient in either IL-4 or IFN-γ.
MATERIALS AND METHODS
Animals.
The following three strains of female mice were used for experiments: NOD/Lt, NOD IL-4−/− (official designation, NOD-IL-4tm1Cgn), and NOD IFN-γ−/− (official designation, NOD-Ifngtm1Ts). The last two stocks were established by congenically transferring a functionally inactivated IL-4 or IFN-γ gene into the NOD background (23). Both stocks are homozygous for linkage markers delineating the NOD variant of all known genetic loci contributing to diabetes susceptibility or resistance (24). After brother-sister mating at the Jackson Laboratory (Bar Harbor, Maine), experimental mice were shipped to the University of Florida Animal Resources Unit, where they were maintained in the Infectious Disease Control Facility in a specific-pathogen-free environment. All procedures were performed under the appropriate University of Florida Institute for Animal Care and Use Committee approval.
Treatment.
Randomly selected groups of mice were established based on the type of treatment administered (i.e., a saline control or the pancreatropic CVB4 Edwards strain) as well as the age of the mice at treatment (i.e., either 8 or 12 weeks old). CVB4-infected mice were challenged by intraperitoneal injection with 5 × 105 PFU of the virus in 200 μl of saline, while control animals were injected with the saline vehicle alone (22). After injections, blood glucose levels were monitored weekly by the use of Chemstrips (Boehringer Mannheim, Indianapolis, Ind.), with consecutive values of >240 mg/dl on two occasions of >24 h apart being considered diagnostic of type 1 diabetes.
Assessment of pancreatic viral titer.
After mice were euthanized, whole pancreases from mice challenged in accordance with the viral treatment protocol were harvested, weighed, placed into phosphate-buffered saline, minced, sonicated, and subjected to three freeze-thaw cycles and then low-speed centrifugation. Tenfold serial dilutions of the cleared lysates were made in phosphate-buffered saline. Two-hundred-microliter aliquots were adsorbed onto 35-mm-diameter wells of confluent BSC40 cells (American Type Culture Collection, Rockville, Md.). Following overlay with 1% methylcellulose medium and incubation for 72 h (37°C), the overlay was removed, and the monolayers were fixed with methanol-oxaloacetate and stained with crystal violet. Plaques were counted, and titers were calculated as follows: (number of plaques/volume of inoculate)/dilution factor.
Assessment of insulitis development.
Pancreases from mice assessed for insulitis development were fixed in Bouin's solution and sectioned at three nonoverlapping levels. Granulated β cells were stained with aldehyde fuchsin, and leukocytes were stained with a hematoxylin-and-eosin counterstain. Islets (at least 20 per mouse) were individually scored as follows: 0, no lesions; 1, peri-insular leukocytic aggregates, usually periductal infiltrates; 2, <25% islet destruction; 3, >25% islet destruction; and 4, complete islet destruction. An insulitis score for each mouse was obtained by dividing the total score for each pancreas by the number of islets examined. Data are presented as mean insulitis scores ± standard errors of the means for the indicated experimental groups.
Analyses of cell types comprising insulitic lesions.
Pancreatic islets from 8- and 12-week-old NOD, NOD IL-4−/−, and NOD IFN-γ−/− female mice were isolated and dispersed into single cell suspensions as previously described (12, 19). For all experimental groups, two pools of islet cells were prepared from three mice of each strain and age. The proportion of total leukocytes within each islet cell pool was then assessed by flow cytometry (FACSCalibur; BD Biosciences, San Jose, Calif.) with a CD45.1-specific monoclonal antibody (A20-1.7) conjugated to a green fluorescein isothiocyanate tag. The proportions of CD4 and CD8 T cells among CD45-positive leukocytes were assessed with the antibodies GK1.5 and 53-6.72, respectively, conjugated to a blue fluorescent allophycocyanin tag. An allophycocyanin-conjugated CD11b-specific monoclonal antibody (M1/70) was used to determine the proportions of macrophages/dendritic cells (DC) among CD45-positive leukocytes. Proportions of B cells among CD45-positive leukocytes were assessed by use of the B220-specific monoclonal antibody RA3-6B2 conjugated to a red fluorescent phycoerythrin tag. All analyses were restricted to cells that were shown to be viable by their exclusion of propidium iodide.
Statistics.
Kaplan-Meier life-table analysis (GraphPad Prism, San Diego, Calif.) was used to evaluate statistical significance for diabetes frequencies, with Mann-Whitney testing utilized for comparisons of insulitis scores. P values of <0.05 were considered significant.
RESULTS
Kinetics of insulitis and diabetes in NOD, NOD IL-4−/−, and NOD IFN-γ−/− mice.
Alterations in cytokine balances may account for why a CVB infection prior to or after the development of a critical threshold level of insulitis retards or accelerates diabetes, respectively, in NOD mice. As a means of testing this hypothesis, we set out to determine whether infection with the Edwards CVB strain differentially accelerates or inhibits the onset of diabetes in IL-4- or IFN-γ-deficient NOD mice (NOD IL-4−/− and NOD IFN-γ−/−, respectively) after they have developed various levels of insulitis. We had previously determined that at approximately 8 weeks of age, the majority of standard NOD female mice had developed the threshold level of insulitis required for a subsequent CVB4 infection to accelerate the onset of diabetes (22). Thus, we initially determined when the NOD IL-4−/− and NOD IFN-γ−/− stocks developed levels of insulitis that were quantitatively and qualitatively similar to those of 8-week-old standard NOD females.
The rates of spontaneous diabetes development are identical for NOD and NOD IL-4−/− mice (23). For this reason, it was not surprising that standard and IL-4-deficient NOD female mice were characterized by equivalent levels of insulitis at 8 weeks of age (Table 1). By 12 weeks of age, the levels of insulitis had increased equivalently in both standard and IL-4-deficient NOD female mice. While not differing in its final frequency, diabetes begins to develop approximately 4 weeks later in NOD IFN-γ−/− mice than in standard NOD mice (23). This was reflected by lower levels of insulitis at both 8 and 12 weeks of age in NOD IFN-γ−/− mice than in standard NOD female mice (Table 1). However, the levels of insulitis in 12-week-old NOD IFN-γ−/− and 8-wk-old standard NOD female mice did not statistically differ.
TABLE 1.
Insulitis levels in NOD and cytokine-disrupted NOD female mice at 8 and 12 weeks of age
| Strain | Insulitis levela (n) at week:
|
|
|---|---|---|
| 8 | 12 | |
| NOD | 1.60 ± 0.25 (12) | 2.35 ± 0.44 (8)c |
| NOD IL-4−/− | 1.29 ± 0.10 (4) | 2.48 ± 0.46 (4)c |
| NOD IFN-γ−/− | 0.33 ± 0.06 (5)b | 1.66 ± 0.41 (11) |
Values are mean insulitis scores ± standard errors of the means.
Significantly lower (P < 0.05 by Mann-Whitney analysis) than the levels in age-matched standard NOD and NOD IL-4−/− females.
Significantly higher (P < 0.05 by Mann-Whitney analysis) than the levels in strain-matched females at 8 weeks of age.
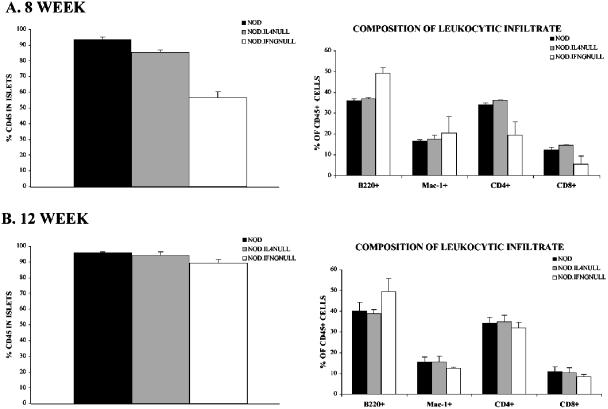
We next compared the qualitative compositions of islet-infiltrating leukocytes at 8 and 12 weeks of age for NOD, NOD IL-4−/−, and NOD IFN-γ−/− female mice by flow cytometry. In agreement with the histological analyses, there were equivalent proportions of leukocytes (identified by CD45 expression) in the pancreatic islets of 8-week-old NOD and NOD IL-4−/− mice (Fig. 1A, left panel). Also in agreement with the histological analyses was the finding that for 8-week-old mice, there were significantly fewer leukocytes in the islets of NOD IFN-γ−/− females than in those of either standard NOD or NOD IL-4−/− mice. Among the CD45-positive leukocytes in islets from 8-week-old NOD and NOD IL-4−/− mice, the proportions of CD4 T cells, CD8 T cells, and macrophages/DC did not differ (Fig. 1A, right panel). The proportions of CD4 and CD8 T cells among CD45-positive leukocytes in the islets of 8-week-old NOD IFN-γ−/− females were also reduced compared to the proportions in NOD and NOD IL-4−/− mice, but this difference was not statistically significant. In mice at 8 weeks of age, the proportion of B cells among islet-infiltrating CD45-positive leukocytes from NOD IFN-γ−/− females was somewhat higher than that in either NOD or NOD IL-4−/− mice. The significance, if any, of this finding is unknown. While histological analyses indicated progressively higher levels of pancreatic β-cell destruction in mice between 8 and 12 weeks of age, there was no significant change in the proportions of CD45-positive leukocytes in the islets of NOD and NOD IL-4−/− female mice (compare the left panels of Fig. 1A and B). However, by 12 weeks of age, the proportion of CD45-positive leukocytes in the islets of NOD IFN-γ−/− females was equivalent to that in the islets of NOD and NOD IL-4−/− mice (Fig. 1B, left panel). Among the islet-infiltrating CD45-positive leukocytes from 12-week-old NOD, NOD IL-4−/−, and NOD IFN-γ−/− females, there were no differences in the proportions of CD4 T cells, CD8 T cells, and macrophages/DC (Fig. 1B, right panel). As observed in 8-week-old mice, the proportion of B cells among islet-infiltrating leukocytes of 12-week-old NOD IFN-γ−/− females was somewhat higher than that in NOD and NOD IL-4−/− mice. Hence, in terms of quantitative and qualitative levels of insulitis, 8-week-old NOD and NOD IL-4−/− females are equivalent to 12-week-old NOD IFN-γ−/− females. Given these findings, we chose to challenge 8- and 12-week-old female NOD, NOD IL-4−/−, and NOD IFN-γ−/− mice with CVB4 (5 × 105 PFU intraperitoneally).
FIG. 1.
Degrees of insulitis and inflammatory cell compositions in nonvirally infected NOD, NOD IL-4−/−, and NOD IFN-γ−/− mice. The insulitic infiltrate of 12-week-old NOD IFN-γ−/− females was quantitatively and qualitatively equivalent to those of NOD and NOD IL-4−/− mice at 8 weeks of age. At 8 (A) and 12 (B) weeks of age, two pools of dispersed islet cells (generated from three mice each) were prepared from NOD (black bars), NOD IL-4−/− (gray bars), and NOD IFN-γ−/− (white bars) females. Data in the left panels represent the mean proportions ± standard deviations of CD45-expressing leukocytes within the two islet cell pools prepared from each type of donor. The right panels depict the mean proportions ± standard deviations of CD4 T cells, CD8 T cells, B cells, and macrophages/DC among the CD45-positive leukocytes within the two islet cell pools of each donor type.
Type 1 diabetes is accelerated in NOD mice infected with CVB at 8 weeks of age, but not in NOD IL-4−/− mice.
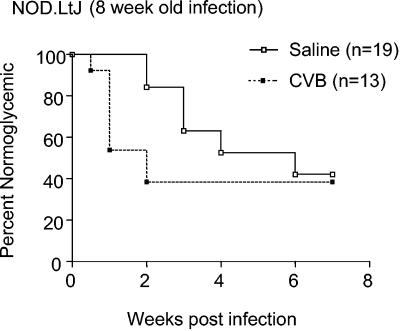
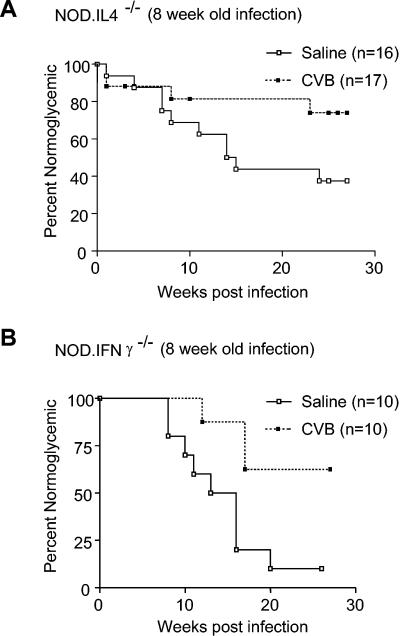
Similar to what we observed in a previous study (22), type 1 diabetes was accelerated (i.e., diagnosed within 10 days) in 50% of NOD females that were challenged with CVB4 at 8 weeks of age compared to saline-treated controls (Fig. 2) (P = 0.01 for onset of diabetes within 2 weeks of viral challenge). Also as previously observed, those NOD females that did not rapidly develop diabetes after a CVB4 challenge at 8 weeks of age remained disease-free. In contrast, despite initial levels of insulitis that were quantitatively and qualitatively similar to those in standard NOD mice (Table 1; Fig. 1), diabetes was not accelerated in NOD IL-4−/− females that were challenged with CVB4 at 8 weeks of age (Fig. 3). Indeed, in contrast to the case for saline-treated controls, there was a trend towards disease protection in NOD IL-4−/− females that were challenged with CVB4 at 8 weeks of age (Fig. 3) (P = 0.06). Specifically, over the 25-week follow-up period, diabetes developed in only 23% (4 of 17) of the CVB4-challenged NOD IL-4−/− mice, compared to 63% (10 of 16) of the saline-treated controls. Hence, IL-4 is not crucial to the mechanism by which CVB4 infection retards rather than accelerates diabetes in NOD mice that have not yet developed a critical threshold level of insulitis. However, these results suggest that IL-4 plays a role in the mechanism by which CVB4 infection accelerates diabetes in NOD mice that have already developed the levels of insulitis found in half or more of 8-week-old females.
FIG. 2.
Effect of a CVB4 challenge on development of diabetes in NOD mice. A challenge of NOD mice with the CVB4 Edwards strain differentially accelerates or inhibits the onset of diabetes in NOD mice. As observed previously (22), a challenge of female NOD mice at 8 weeks of age can either induce a rapid onset of overt diabetes (i.e., within 2 weeks) or impart long-term protection. Kaplan-Meier life-table analysis was utilized to compare the rates of diabetes for saline-treated and CVB4-challenged animals. P = 0.01 for the onset of diabetes within 2 weeks of viral challenge.
FIG. 3.
Effect of CVB4 challenge on development of diabetes in cytokine-disrupted NOD mice. (A) Compared to saline-treated controls, there was a trend towards disease protection in NOD IL-4−/− females challenged with CVB4 at 8 weeks of age. Kaplan-Meier life-table analysis was utilized to compare the rates of diabetes for saline-treated and CVB4-infected animals. P = 0.06 for the difference in the rates of diabetes development throughout the study. (B) Acceleration of type 1 diabetes relative to saline-treated controls was not observed for NOD IFN-γ−/− mice challenged with CVB4 at 8 weeks of age. Kaplan-Meier life-table analysis was utilized to compare the rates of diabetes for saline-treated and CVB4-challenged animals. P = 0.01 for the difference in the rates of diabetes development throughout the study.
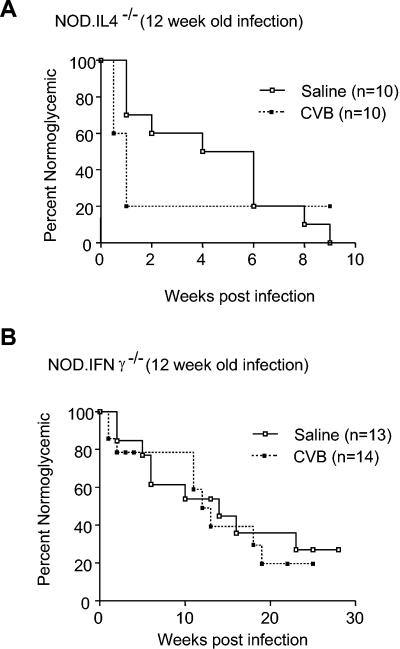
IL-4 is not required for acceleration of diabetes in 12-week-old CVB4-infected mice.
When preclinical β-cell destruction becomes more advanced, it appears that IL-4 production is no longer required for the mechanisms by which a CVB4 infection accelerates the progression to overt diabetes. This conclusion was supported by the finding that the progression to overt diabetes was markedly accelerated in NOD IL-4−/− females that were challenged with CVB4 at 12 weeks of age compared to saline-treated controls (Fig. 4) (P = 0.03 for onset of diabetes within 2 weeks of viral infection). By 10 days postinfection, 8 of 10 CVB4-challenged mice, versus 3 of 10 saline-treated control mice, had developed overt diabetes. Indeed, an additional 6 weeks were required for an equivalent level of disease to develop in saline-injected NOD IL-4−/− control mice.
FIG. 4.
Effect of CVB4 challenge on development of diabetes in cytokine-disrupted NOD mice. (A) Overt diabetes was markedly accelerated in NOD IL-4−/− females challenged with CVB4 at 12 weeks of age compared to saline-treated controls. Kaplan-Meier life-table analysis was utilized to compare the rates of diabetes for saline-treated and CVB4-challenged animals. P = 0.03 for the onset of diabetes within 2 weeks of viral challenge. (B) NOD IFN-γ−/− females infected with CVB4 at 12 weeks of age developed diabetes at the same rate as saline-treated controls. Kaplan-Meier life-table analysis was utilized to compare the rates of diabetes for saline-treated and CVB4-challenged animals. The P value was not significant for the difference in the rates of diabetes development throughout the study.
A threshold level of insulitis is required and IFN-γ is critical for CVB4 acceleration of diabetes.
No acceleration of type 1 diabetes relative to saline-treated controls was observed for NOD IFN-γ−/− mice that were challenged with CVB4 at 8 weeks of age (Fig. 3). In fact, NOD IFN-γ−/− mice that were challenged with CVB4 at 8 weeks of age exhibited a significantly retarded rate of diabetes development compared to saline-treated controls (P = 0.01). This may have been the result of 8-week-old NOD IFN-γ−/− females not yet attaining the threshold level of insulitis required to enable a subsequent CVB4 infection to accelerate, rather than retard, the progression to overt diabetes. As shown in Table 1 and Fig. 1, by 12 weeks of age NOD IFN-γ−/− females developed levels of insulitis that were quantitatively and qualitatively equivalent to those in 8-week-old standard NOD females and which, in the latter case, were sufficient to permit a CVB4 infection to accelerate the onset of diabetes. Hence, we hypothesized that a CVB4 infection at 12 weeks of age would accelerate the development of diabetes in NOD IFN-γ−/− females. However, NOD IFN-γ−/− females that were challenged with CVB4 at 12 weeks of age developed diabetes at the same rate as saline-treated controls (Fig. 4). Collectively, these data indicate that IFN-γ production is critical for the mechanism by which a CVB4 infection accelerates the progression to overt diabetes in NOD mice that have already developed a prerequisite level of insulitis. In contrast, IFN-γ production is not critical for the mechanism by which a CVB4 infection retards diabetes development in NOD mice that have not previously developed a critical threshold level of insulitis.
Pancreatic viral clearance is similar in NOD mice and mice with disrupted cytokines.
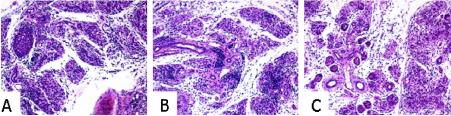
As part of this study, we also addressed the issue of whether the environment of cytokine disruption in NOD mice influenced the degree of pancreatic inflammation caused by CVB4 infection as well as the rate at which the virus was cleared from the pancreas in these animals. A qualitative histological assessment of pancreases at 3, 7, and 14 days post-CVB4 challenge (10- to 11-week-old animals were challenged) revealed that this virus imparted dramatic pancreatic inflammation in all three strains. Specifically, while pancreatic inflammation was present in samples collected on day 3, by 7 days after infection (Fig. 5) marked acinar damage was present in NOD, NOD IL-4−/−, and NOD IFN-γ−/− mice. At later stages (day 14), acinar destruction was complete, with tissue replacement by cells of an apparent adipose lineage. However, the principle question was the quantitative rate at which the virus remained in the pancreases of CVB4-challenged animals. Specifically, viral titers from whole pancreases of infected animals were evaluated by a plaque assay (Table 2). These studies indicated that for all three stocks, viral loads were equivalently high at 3 days postchallenge, but with virtually complete clearance observed by day 7.
FIG. 5.
Representative images from pancreases of NOD and cytokine-disrupted NOD mice 7 days after CVB4 infection. Animals (n = 3/strain) were 10 to 11 weeks old at the time of CVB4 infection. The slides were processed by routine methods and stained with hematoxylin and eosin. Intensive acinar infiltration and destruction were present in NOD (A), NOD IL-4−/− (B), and NOD IFN-γ−/− (C) mice. The images are representatives from a single animal of each strain. Magnification, ×20.
TABLE 2.
Pancreatic viral titers in animals challenged with CVB4
| Strain | Viral titer in pancreasa
|
||
|---|---|---|---|
| 72 h | 7 day | 14 day | |
| NOD | 6.7 × 105 | 0 | 0 |
| NOD IL-4−/− | 8.5 × 105 | 35 | 0 |
| NOD IFN-γ−/− | 4.5 × 105 | 40 | 0 |
Values are presented as means from two animals for each time point at the postchallenge time indicated.
DISCUSSION
Among the earliest evidence associating CVB infections with type 1 diabetes were serological studies reporting elevated levels of neutralizing antibodies directed towards CVB4 in recently diagnosed patients compared to healthy controls, an observation that has been confirmed by dozens of studies across a wide range of populations (5-7, 15, 18, 21). Additional evidence linking CVB infections with type 1 diabetes appeared in 1979 when Yoon and colleagues reported the isolation of a diabetogenic variant of CVB from a 10-year-old boy at the time of disease onset which, upon subsequent inoculation into mice, induced hyperglycemia, beta-cell necrosis, and insulitis (30). Subsequent studies failed to replicate the isolation of CVB strains with the exact same characteristics. However, pancreatropic CVB4 variants such as the so-called Edwards strain have aided investigators seeking to understand the association between the group B coxsackieviruses and type 1 diabetes. The CVB4 Edwards strain was originally isolated in 1958 from the myocardial tissue of an infant (Edwards) with generalized coxsackievirus infection and inflammation of the pancreas. This strain of CVB4 causes extensive necrosis of the acinar cells of the exocrine pancreas, with little (if any) destruction of endocrine cells, ducts, and vessels.
In 1998, Horwitz et al. (13) reported that a CVB4 infection accelerated diabetes development in a stock of NOD mice transgenically expressing the BDC2.5 T-cell receptor (TCR), which is known to recognize some pancreatic β-cell autoantigen other than glutamic acid decarboxylase. These results ran contrary to an earlier hypothesis that CVB infection might contribute to diabetes development by serving as a molecular mimic for glutamic acid decarboxylase (26). Instead, Horwitz et al. proposed that islets in a pancreatic environment where exocrine inflammation has been induced by a CVB infection undergo subclinical levels of damage and release normally sequestered antigens that subsequently trigger autoreactive diabetogenic T-cell responses. However, this hypothesis could not explain why, in the study by Horwitz et al. (13), a CVB infection could induce diabetes in a TCR transgenic NOD stock in which virtually all T cells were of the single BDC2.5 specificity, but not in standard 6-week-old NOD females, which harbor a wide array of β-cell-autoreactive T-cell clonotypes. We subsequently reasoned that this may be due to the fact that insulitis develops more rapidly in the BDC2.5 TCR transgenic NOD stock than in standard NOD mice. Indeed, we too found that diabetes development was not accelerated in NOD females infected with CVB4 at 6 weeks of age (22). In contrast, when 8-week-old NOD females who had developed significantly higher levels of insulitis were challenged with CVB4, a significantly accelerated progression to overt diabetes resulted. Hence, the inflammatory mediators produced in response to CVB4 infection of the exocrine pancreas can provide a bystander activation effect that accelerates diabetes development, but this can only occur after the prior accumulation of a critical threshold level of insulitic β-cell-autoreactive T cells. Interestingly, our earlier study also paradoxically indicated that CVB4 infection retards rather than accelerates diabetes in NOD mice that have not yet developed a critical threshold level of insulitis. Hence, the timing of CVB infections, rather than their simple presence or absence, as well as the composition (e.g., lymphocytic phenotype or cytokine profile) of cells within the insulitis lesion, is likely an important factor in determining whether diabetes development is accelerated or retarded. This notion was recently confirmed (in part) by Tracy et al. (27), who reported that the inoculation of 4-to 8-week-old NOD mice with any of nine different CVB3 strains significantly reduced the incidence of diabetes 2- to 10-fold over a 10-month period. Furthermore, those studies indicated that more protection against the age-related development of diabetes was conferred by the more pathogenic CVB3 strains than by the less virulent or avirulent strains.
A widely held hypothesis is that in both humans and NOD mice, the autoimmune processes leading to the development of type 1 diabetes are promoted or inhibited, respectively, by the regulatory cytokines IFN-γ and IL-4 (8, 9, 20). Depending on timing, a CVB infection can alter the balance of IFN-γ or IL-4 production in the pancreas or elsewhere, leading to a significantly divergent outcome in the initial progression towards diabetes. To test this hypothesis, we determined whether CVB4 infection differentially accelerated or inhibited the onset of diabetes in NOD IL-4−/− and NOD IFN-γ−/− mice after they had developed various levels of insulitis.
Compared to the case for standard NOD mice, there is about a 4-week delay in the spontaneous development of diabetes in the NOD IFN-γ−/− stock (23). The basis for this delay is likely explained by our findings that NOD IFN-γ−/− females do not develop, until 12 weeks of age, insulitis lesions that contain similar numbers and types of immune cells as those seen in standard NOD females at 8 weeks of age (Table 1; Fig. 1). While the levels and cellular compositions of the insulitic infiltrates in 8-week-old NOD and 12-week-old NOD IFN-γ−/− females were equivalent, CVB4 infection only accelerated diabetes development in the former strain (i.e., NOD mice). This finding indicates that it is not just the numerical level and cellular composition of a preexisting insulitic infiltrate that determine whether a subsequent CVB4 infection accelerates the progression to overt diabetes, but also whether these cells can produce or respond to IFN-γ. When younger, 8-week-old NOD IFN-γ−/− females with lower levels of insulitis than those present at 12 weeks of age were challenged with CVB4, diabetes resistance was induced. These results indicate that while IFN-γ contributes to the mechanism by which CVB4 infection accelerates the onset of diabetes in NOD mice that have already developed the necessary level of insulitis, this cytokine is not part of the process by which an infection prior to this time results in disease resistance.
The mechanistic basis by which IFN-γ production is required for a CVB infection to trigger a rapid progression to overt diabetes in NOD mice that have already developed a prerequisite level of insulitis will be the subject of future studies. However, we feel that it is more likely that in this CVB4 challenge paradigm, IFN-γ contributes to the acceleration of diabetes by exerting effects on immune cells rather than by being directly cytotoxic to pancreatic β cells. This hypothesis is based on a previous report that the specific expression of a dominant-negative form of the IFN-γ receptor in pancreatic β cells had no effect on the rate of diabetes development in NOD mice (25).
We previously found that the genetic elimination of IL-4 does not alter the kinetics of diabetes development in NOD mice (23). Thus, we were not surprised to find in the present study that insulitis development was quantitatively and qualitatively similar in standard and IL-4-deficient NOD mice (Table 1). However, diabetes development was only accelerated in standard NOD, and not NOD IL-4−/−, females after a challenge with CVB4 at 8 weeks of age. One interpretation of these results is that when insulitis is present at the levels found in 8-week-old NOD female mice, the IL-4 induced during CVB4-induced inflammation actually has a positive effect on accelerating diabetogenic autoimmune responses. Insulitis levels quantitatively increased from 8 to 12 weeks of age in NOD IL-4−/− females, and a CVB4 challenge at the latter time resulted in a significantly accelerated rate of diabetes development. This may indicate that when insulitis is present at the higher levels found in 12-week-old than in 8-week-old NOD females, a subsequent CVB4 challenge no longer must induce IL-4 production in order to accelerate diabetes development. However, it should be noted that the subsequent rate of diabetes development in the particular cohort of NOD IL-4−/− females that were treated with saline at 8 weeks of age was somewhat slower than what is usually observed. Hence, it is also possible that the failure of CVB4 challenge at 8 weeks of age to accelerate diabetes development in this particular group of NOD IL-4−/− females was due to the fact that they had not yet developed the prerequisite level of insulitis.
We previously found that at 8 weeks of age, the intra-islet levels of IFN-γ and IL-10 in NOD IL-4−/− females are, respectively, no different or significantly lower than the levels in standard NOD mice (23). Thus, the ability of a CVB4 infection at 8 weeks of age to trigger a rapid progression to overt diabetes in standard NOD, but not NOD IL-4−/−, females is unlikely to be due to differential intra-islet levels of IFN-γ, which was found in the present study to play an essential pathogenic role in this process. On the other hand, IL-10, which is generally thought to exert systemic immunosuppressive effects, has been found to paradoxically promote diabetes development when expressed locally in the islets of NOD mice, perhaps by enhancing the activity of β-cell-autoreactive CD8 T cells (4). Hence, it is possible that the ability of a CVB4 infection initiated at 8 weeks of age to trigger rapid progression to overt diabetes in standard NOD, but not NOD IL-4−/−, females is due to the lower intra-islet levels of IL-10 in the latter strain.
It was essential to assess the potential impact of the absence of a cytokine (i.e., IL-4 or IFN-γ) on viral growth in the pancreas and to determine whether variations in this parameter may have impacted our interpretations. While our previous studies of NOD and NOD scid mice (22) inferred that this would not represent a significant issue (i.e., viral replication was similar in these two stocks), we could not definitively exclude this possibility. However, our analyses over a 14-day period revealed similar patterns of viral clearance between NOD, NOD IL-4−/−, and NOD IFN-γ−/− mice.
In addition to the role that cytokines may play to influence the degree of pancreatic destruction after CVB infection, other host factors have recently been investigated. A report by Yap et al. (29) indicated that differences in the diabetogenic capacity of various CVB4 substrains in SJL mice were not due to their tropism for islet cells but to the degree of damage inflicted on the exocrine pancreas and the resulting capacity for regeneration of both acinar and islet tissues by the host. These results suggested that the key to CVB-induced damage resides in the exocrine tissue and the prevention of islet neogenesis rather than in direct effects on existing islets. Horwitz et al. (14) demonstrated that CVB4 infection of pancreatic beta cells does not directly cause beta-cell death but rather that the phagocytosis of beta cells by macrophages follows infection. Through additional studies of adoptive transfer, these authors suggested that macrophages represent the initiating pathogenic cell type during virus-mediated diabetes. Finally, to examine yet another facet by which the host response may influence the diabetogenic capacity of viruses, Wen and colleagues (28) compared a viral mimic with other microbial components derived from bacteria for the ability to trigger diabetes development in C57BL/6-rat insulin promoter-B7.1 mice. Interestingly, only the viral mimic induced the development of diabetes in that model system. Further mechanistic studies suggested that diabetes is induced in part by a combination of direct recognition of the viral-like stimulus in pancreatic islets through the expression of Toll-like receptor 3, which mediates innate immune responses. These studies support the potential role for the host's innate immune response and the differential expression of Toll-like receptors in the process of virally triggered diabetogenesis. Taken collectively with our studies indicating the role for cytokines in such processes, it appears that various components of the host's immune response, as well as aspects related to the pancreatic environment (both endocrine and exocrine), influence the capacity of viruses to impart beta-cell death and overt hyperglycemia.
In summary, these studies suggest a paradoxical role for cytokines wherein the induction of IL-4 as well as IFN-γ is a necessary cofactor that allows a CVB4 infection to trigger an accelerated onset of diabetes in NOD mice that have already developed a critical threshold level of insulitis. However, when even higher levels of insulitis have accumulated, IL-4 may no longer be required for a subsequent CVB4 infection to accelerate diabetes development. IFN-γ also is not necessary for the process by which a CVB4 infection retards rather than accelerates diabetes in NOD mice that have not yet developed a critical threshold level of insulitis. There has been a considerable ongoing effort to determine if the progression to overt diabetes can be inhibited in individuals who have already developed detectable levels of β-cell autoimmunity by protocols that promote or inhibit, respectively, the production of IL-4 and IFN-γ. However, our present data indicate that great caution should be taken with such protocols, since enhancing the ability of individuals with preexisting β-cell autoimmunity to produce IL-4 may actually increase their chances of developing overt diabetes following a subsequent naturally acquired CVB infection or inflammatory insult.
Acknowledgments
These studies were supported in part by funds from the National Institutes of Health and the Juvenile Diabetes Research Foundation.
REFERENCES
- 1.Abiru, N., L. Yu, M. Redondo, and G. Eisenbarth. 2000. Modification of the environment is not the most efficient way to prevent type 1 diabetes. Diabetes Technol. Ther. 2:609-616. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson, M., and G. Eisenbarth. 2001. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 358:221-229. [DOI] [PubMed] [Google Scholar]
- 3.Bach, J. F. 1994. Predictive medicine in autoimmune diseases: from the identification of genetic predisposition and environmental influence to precocious immunotherapy. Clin. Immunol. Immunopathol. 72:56-61. [DOI] [PubMed] [Google Scholar]
- 4.Balasa, B., J. D. Davies, J. Lee, A. Good, B. T. Yeung, and N. Sarvetnick. 1998. IL-10 impacts autoimmune diabetes via a CD8+ T cell pathway circumventing the requirement for CD4+ T and B lymphocytes. J. Immunol. 161:4420-4427. [PubMed] [Google Scholar]
- 5.Caggana, M., N. P. Chan, and A. Ramsingh. 1993. Identification of a single amino acid residue in the capsid protein VP1 of coxsackievirus B4 that determines the virulent phenotype. J. Virol. 67:4797-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman, N. M., A. I. Ramsingh, and S. Tracy. 1997. Genetics of coxsackievirus virulence. Curr. Top. Microbiol. Immunol. 223:227-258. [DOI] [PubMed] [Google Scholar]
- 7.Crowell, R. L., and B. J. Landau. 1997. A short history and introductory background on the coxsackieviruses of group B. Curr. Top. Microbiol. Immunol. 223:1-11. [DOI] [PubMed] [Google Scholar]
- 8.Delovitch, T. L., and B. Singh. 1997. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity 7:727-738. [DOI] [PubMed] [Google Scholar]
- 9.Falcone, M., and N. Sarvetnick. 1999. Cytokines that regulate autoimmune responses. Curr. Opin. Immunol. 11:670-676. [DOI] [PubMed] [Google Scholar]
- 10.Fohlman, J., and G. Friman. 1993. Is juvenile diabetes a viral disease? Ann. Med. 25:569-574. [PubMed] [Google Scholar]
- 11.Gamble, D. R., M. L. Kinsley, M. G. Fitzgerald, R. Bolton, and K. W. Taylor. 1969. Viral antibodies in diabetes mellitus. Br. Med. J. 3:627-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerling, I. C., D. V. Serreze, S. W. Christianson, and E. H. Leiter. 1992. Intrathymic islet cell transplantation reduces beta-cell autoimmunity and prevents diabetes in NOD/Lt mice. Diabetes 41:1672-1676. [DOI] [PubMed] [Google Scholar]
- 13.Horwitz, M. S., L. M. Bradley, J. Harbertson, T. Krahl, J. Lee, and N. Sarvetnick. 1998. Diabetes induced by coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat. Med. 4:781-785. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz, M. S., A. Ilic, C. Fine, B. Balasa, and N. Sarvetnick. 2004. Coxsackieviral-mediated diabetes: induction requires antigen-presenting cells and is accompanied by phagocytosis of beta cells. Clin. Immunol. 110:134-144. [DOI] [PubMed] [Google Scholar]
- 15.Huber, S. A., C. J. Gauntt, and P. Sakkinen. 1999. Enteroviruses and myocarditis: viral pathogenesis through replication, cytokine induction, and immunopathogenicity. Adv. Virus Res. 51:35-72. [DOI] [PubMed] [Google Scholar]
- 16.Hyoty, H., M. Hiltunen, and M. Lannrot. 1998. Enterovirus infections and insulin dependent diabetes mellitus—evidence for causality. Clin. Diagn. Virol. 9:77-84. [DOI] [PubMed] [Google Scholar]
- 17.Jaeckel, E., M. Manns, and M. Von Herrath. 2002. Viruses and diabetes. Ann. N. Y. Acad. Sci. 958:7-25. [DOI] [PubMed] [Google Scholar]
- 18.Maugh, T. H. 1975. Diabetes—epidemiology suggests a viral connection. Science 188:347-351. [DOI] [PubMed] [Google Scholar]
- 19.Pierce, M. A., H. D. Chapman, C. M. Post, A. Svetlanov, S. Efrat, M. Horwitz, and D. V. Serreze. 2003. Adenovirus early region 3 (E3) anti-apoptotic 10.4K, 14.5K, and 14.7K genes decrease the incidence of autoimmune diabetes in NOD mice. Diabetes 52:1119-1127. [DOI] [PubMed] [Google Scholar]
- 20.Rabinovitch, A. 1998. An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab. Rev. 14:129-151. [DOI] [PubMed] [Google Scholar]
- 21.Ramsingh, A. L., W. T. Lee, D. N. Collins, and L. E. Armstrong. 1997. Differential recruitment of B and T cells in coxsackievirus B4-induced pancreatitis is influenced by a capsid protein. J. Virol. 71:8690-8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serreze, D., E. Ottendorfer, C. Gauntt, T. M. Ellis, and M. A. Atkinson. 2000. Acceleration of insulin dependent diabetes mellitus by a coxsackievirus infection requires a preexisting critical mass of autoreactive T cells in pancreatic islets. Diabetes 49:708-711. [DOI] [PubMed] [Google Scholar]
- 23.Serreze, D. V., H. D. Chapman, C. M. Post, E. A. Johnson, W. L. Suarez-Pinzon, and A. Rabinovitch. 2001. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J. Immunol. 166:1352-1359. [DOI] [PubMed] [Google Scholar]
- 24.Serreze, D. V., H. D. Chapman, D. S. Varnum, M. S. Hanson, P. C. Reifsnyder, S. D. Richard, S. A. Fleming. E. H. Leiter, and L. D. Shultz. 1996. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu(null) mice. J. Exp. Med. 184:2049-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas, H. E., J. L. Parker, R. D. Schreiber, and T. W. H. Kay. 1998. IFNγ action on pancreatic β cells causes MHC class I upregulation but not diabetes. J. Clin. Investig. 102:1249-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian, J., P. V. Lehmann, and D. L. Kaufman. 1994. T cell cross-reactivity between coxsackievirus and glutamate decarboxylase is associated with a murine diabetes susceptibility allele. J. Exp. Med. 180:1979-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tracy, S., K. M. Drescher, N. M. Chapman, K. S. Kim, S. D. Carson, S. Pirruccello, P. H. Lane, J. R. Romero, and J. S. Leser. 2002. Toward testing the hypothesis that group B coxsackieviruses (CBV) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J. Virol. 76:12097-12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen, L., J. Peng, Z. Li, and F. S. Wong. 2004. The effect of innate immunity on autoimmune diabetes and the expression of Toll-like receptors on pancreatic islets. J. Immunol. 172:3173-3180. [DOI] [PubMed] [Google Scholar]
- 29.Yap, I. S., G. Giddings, E. Pocock, and J. K. Chantler. 2003. Lack of islet neogenesis plays a key role in beta-cell depletion in mice infected with a diabetogenic variant of coxsackievirus B4. J. Gen. Virol. 84:3051-3068. [DOI] [PubMed] [Google Scholar]
- 30.Yoon, J. W., M. Austin, T. Onodera, and A. L. Notkins. 1979. Virus-induced diabetes-mellitus-isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N. Engl. J. Med. 300:1173-1179. [DOI] [PubMed] [Google Scholar]