Abstract
Background
Moringa oleifera is a tropical tree often used as a herbal medicine, including by people who test positive for HIV. Since herbal constituents may interact with drugs via inhibition of metabolizing enzymes, we investigated the effects of extracts of M. oleifera on the CYP3A4-mediated 6ß-hydroxylation of testosterone.
Methods
Methanolic and aqueous leaf and root of extracts of M. oleifera with concentrations between 0.01 and 10 mg/ml were incubated with testosterone and mixed-sex human liver microsomes in the presence of NADPH. Metabolite concentrations were determined by HPLC. The cytotoxicity of the extracts was tested with HepG2 cells using the MTT formazan assay.
Results
Significant CYP3A4 inhibitory effects were found, with IC50 values of 0.5 and 2.5 mg/ml for leaf-methanol and leaf-water extracts, respectively. Root extracts were less active. Cytotoxicity was observed only with the leaf-water extract (IC50 = 6 mg/ml).
Conclusions
Further investigation is warranted to elucidate the potential of M. oleifera for clinically significant interactions with antiretroviral and other drugs.
Keywords: Moringa oleifera, CYP3A4, herb-drug interaction
Introduction
When herbs are taken concomitantly with drugs, there is a potential for clinically significant interactions. Herbs contain a mixture of naturally occurring phytochemicals which may be substrates for enzymes or transporters that act on drugs, potentially inhibiting the drugs’ metabolism or transport. Alternatively, these constituents may interact with nuclear receptors to induce production of enzymes or transporters [1,2,3]. These processes can result in altered drug absorption, distribution, metabolism and/or elimination. Toxicity or sub-therapeutic drug concentrations, pathogen resistance and treatment failure are possible outcomes.
Herbal therapies are widely used, particularly in developing countries [4]. The risk of interaction increases with the number of concomitantly administered drugs/herbs. People on antiretroviral therapy are at very high risk because of the multitude of drugs associated with highly active antiretroviral therapy (HAART) and treatment of opportunistic infections, and the frequent use of herbal medicines by these patients (Monera T G., 2008. Moringa oleifera supplementation by HIV positive people). However, little is yet known about most herbs and their specific interactions with conventional medicines Herbal supplements are not regulated as conventional drugs in most countries and do not require rigorous clinical trials before registration. Further, studies of prescription drugs rarely consider interactions with herbs.
Moringa oleifera is a tree of the Moringaceae family widely cultivated throughout the tropics and subtropics whose leaves, seed pods, seeds, seed oil, roots, bark, flowers, and sap are commonly consumed as foods and in traditional medicines. Its use as an inexpensive component in foods, nutritional supplements, or medicines by patients with HIV/AIDS has been advocated by several African governments and is common [5].
An evaluation of the potential of the most widely used African herbs to interact with antiretroviral drugs may be helpful in improving the clinical outcome of patients on HAART. It would enable risk identification and assessment, and if necessary, execution of risk reduction strategies such as dosage time or dosage regimen adjustments. In this study, we investigated the effects of the crude methanol and water extracts of Moringa oleifera leaf and root on the metabolic activity of CYP3A4, the major drug-metabolizing CYP isoform in vitro. We also examined the cytotoxicity of these extracts.
Materials and Methods
Chemicals
Mixed-sex human liver microsomes were purchased from Celsis In Vitro Technologies (Baltimore, MD, USA). NADPH, testosterone and 6β-hydroxy-testosterone were obtained from Sigma-Aldrich (St Louis, MO, USA). Ketoconazole was obtained from U.S. Pharmacopeia (Rockville, MD, USA). Moringa root and leaf powder samples were obtained from Waterfalls Nursery in Harare. NADPH regenerating system reagents were obtained from BD Biosciences (San Jose, CA, USA). High performance liquid chromatography-grade methanol was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Paracetamol and an MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) cell toxicity assay (TOX-1) kit were obtained from Sigma-Aldrich (St. Louis, MO, USA). Cell culture media were obtained from the UCSF Cell Culture Facility.
Herbal Extraction
Extracts of dried Moringa oleifera leaf and root powders were prepared by sonication. The powders were first ground into fine powder with a mortar and pestle. A portion of the fine powder was suspended in 7–8 ml of solvent at a concentration of 100 mg/ml. The mixtures were sonicated for a total of 5 minutes using a Thermo Fisher Scientific (Waltham, MA) model 550 Sonic Dismembrator equipped with a Misonix (Farmingdale, NY) 20 kHz model CL4 Ultrasonic Converter. Sonication was conducted for intervals of 30 seconds, followed by immersion of the samples in ice water to cool them back to room temperature. Samples were next stirred for 2 hours in the dark at room temperature. The mixtures were then centrifuged at 10,000 × g for 5 minutes and the supernatant solutions re-centrifuged in microcentrifuge tubes at 13000 × g for 5 minutes. The pellets were discarded, and the pH of aqueous extracts was adjusted to 7.4 using dilute NaOH. The samples were stored at −80°C. All extract concentrations are based on the initial concentration of suspended plant powder during the sonication step.
Human Liver Microsomes Assay
The CYP3A4 inhibitory properties of crude water extracts of Moringa root and leaf were tested with human liver microsomes. Microsomal incubations were conducted in a total volume of 700 μl in reaction mixtures containing 50 mM sodium/potassium phosphate (pH 7.4), 0.4 mg/ml microsomal protein, 100 μM testosterone, and 1 mM NADPH. When methanol extracts were used, the aliquot of extract was evaporated under N2 and redissolved in reaction buffer with vortexing and sonicating. It was confirmed that the pH of the reaction mixture was unaffected by the extract before proceeding. Prior to addition of NADPH, the other reaction mixture components were pre-incubated at 37°C for 5 minutes. Reaction was initiated by the addition of NADPH or an NADPH-regenerating system and was performed at 37°C in a shaking water bath. Aliquots (100 μl) were removed 0, 10, 20, 40 and 60 minutes after the start of the reaction, mixed with 50 μl of cold methanol and placed on dry ice to stop the reaction, then centrifuged at 13000 × g for 5 minutes, with the supernatant collected for analysis.
HPLC Analysis
HPLC analysis was conducted on 25 μl aliquots of microsomal digest samples with an Agilent 1100 HPLC system, using a Phenomenex (Torrance, CA) Prodigy 5 micron ODS(3) 250 × 2 mm column maintained at 40°C mobile phase pumped at 0.25 ml/min. Analyte elution was monitored by absorbance at 254 nm. Retentions were 4.7 minutes for 6β-hydroxy-testosterone and 10.5 minutes for testosterone.
Calculations
The inhibitory effect of a Moringa oleifera extract is given as an IC50 value, which was calculated using nonlinear regression (KaleidaGraph, Synergy Software, Reading, PA USA) to fit the data to either the Hill equation:
or to a related equation which may give better fits for more steeply sloping data:
where x = inhibitor concentration, c = x value giving 50% inhibition = IC50, and d = “Hill slope”.
Cytotoxicity Assay
Human hepatocellular carcinoma (HepG2) cells were obtained from the American Type Culture Collection (Rockville, MD), and grown in flat-bottomed 75 cm2 flasks in a standard cell culture incubator at 37°C in an atmosphere of 5% CO2. The cells were cultured in Eagle’s minimum essential medium with Earle’s BSS, containing 2 mM L-glutamine, 1.5 g/l sodium bicarbonate, 5.5 mM glucose, 0.1 mM nonessential amino acids mixture, and 1.0 mM sodium pyruvate, supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin and 100 ug/ml streptomycin. The culture medium was changed every 2–3 days and cells were subcultured every 4–5 days. Samples of medium containing test agents (8 mg/ml of plant extract or 50 mM paracetamol as a positive control) were prepared and filter-sterilized. In the case of methanolic plant extracts, the extract was first evaporated under a stream of N2, and then dissolved in medium through sonication and vortexing.
For the experiment, the cells were seeded into a 96-well plate at a density of 1.0 × 105 cells (in 0.1 ml medium)/well, and grown for 24 hours. The medium was then replaced with medium containing the test agents (a maximum of 8% of the medium by volume). Serial dilutions of medium (1:2 dilutions for plant extracts, and 1:3 dilutions for paracetamol) were made in rows of wells. After 48 hours, the medium was replaced with 20 μl of a new culture medium (Hank’s BSS without phenol red containing 5% heat-inactivated fetal bovine serum, 0.1 mM nonessential amino acids mixture, 1.0 mM sodium pyruvate, 100 U/ml penicillin and 100 ug/ml streptomycin) plus 10 μl of MTT reagent per well, and incubation was continued for 3 hours at 37°C with 5% CO2. The MTT formazan formed by dehydrogenases of living cells (Vistica et al., 1991) was solubilised by adding 100 μl of acidified (0.1 M HCl) isopropanol containing 10% Triton X-100 per well and incubating 1 hour at 37°C with shaking. Dissolution of all formazan crystals was confirmed by inspection of the wells under a microscope. A plate reader was used to measure absorbance at 570 nm (formazan) and 690 nm [6] The experiment was done in triplicate.
Results
The methanol and water extracts of M. oleifera leaf and root inhibited the 6β-hydroxylation of testosterone by CYP3A4 present in human liver microsomes. Activity decreased with increasing inhibitor concentration (Figures 1 and 2). The estimated IC50 values are 0.5 and 2.5 mg/ml for methanol and water leaf extracts, respectively. Under the same conditions, 2.5 μM of the positive control ketoconazole inhibited CYP3A4 97.6%. The inhibition from the root extracts was too low for determination of IC50 values (> 10 mg/ml).
Figure 1. Inhibitory effect of methanolic extracts of Moringa oleifera leaf and root on the CYP3A-mediated 6β-hydroxylation of testosterone.
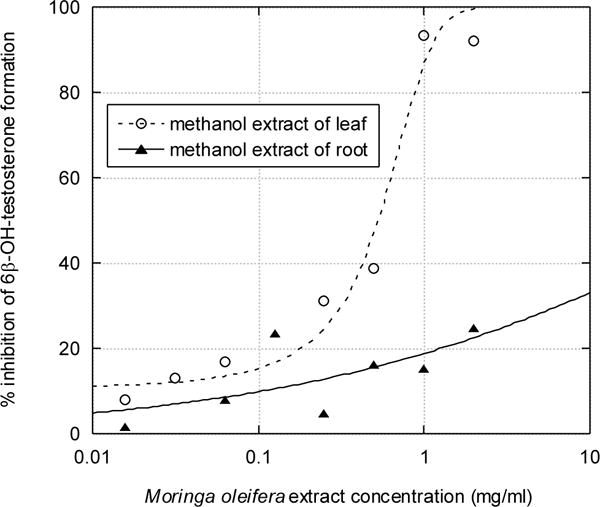
As described in Materials and Methods, 100 μM of testosterone was incubated with 0.4 mg/ml of human liver microsomes for 60 minutes, and metabolite formation was measured by HPLC. The inhibition vs. time data was fitted to equation (1), for root extract, or equation (2), for leaf extract, using nonlinear regression; this gives an IC50 value of 0.5 mg/ml for methanolic leaf extract.
Figure 2. Inhibitory effect of aqueous extracts of Moringa oleifera leaf and root on the CYP3A-mediated 6β-hydroxylation of testosterone by human liver microsomes.
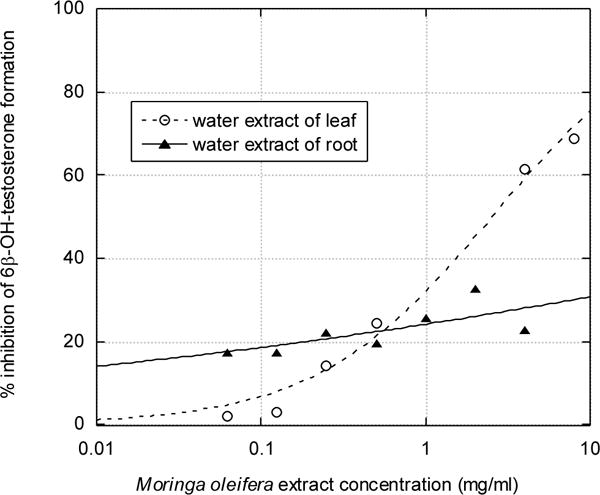
As described in Materials and Methods, 100 μM of testosterone was incubated with 0.4 mg/ml of human liver microsomes for 60 minutes, and metabolite formation was measured by HPLC. The inhibition vs. time data was fitted to equation (1) using nonlinear regression, giving an IC50 value of 2.5 mg/ml for aqueous leaf extract.
In the HepG2 cell viability assay, only the water extract of M. oleifera leaf displayed activity, with an IC50 of 6 mg/ml. The other extracts showed no toxicity up to 8 mg/ml. The IC50 of the positive control, paracetamol, was 12 mM (1.8 mg/ml) (Figure 3).
Figure 3. Cytotoxicity of methanolic and aqueous extracts of Moringa oleifera leaf and root on HepG2 human hepatocellular carcinoma cells as measured by the MTT assay.
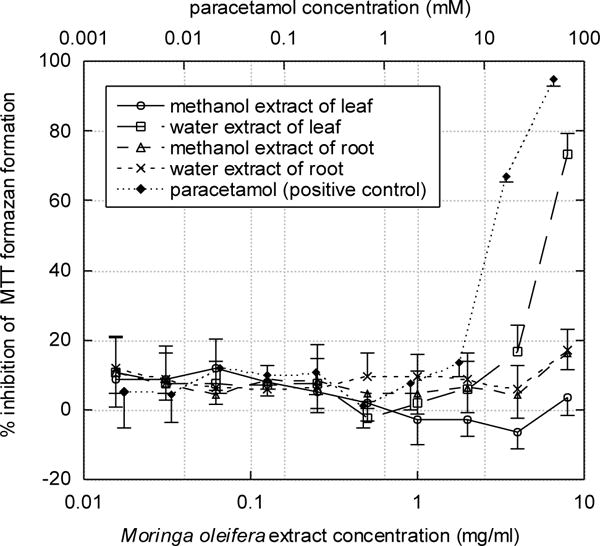
As described in Materials and Methods, HepG2 cells were exposed to Moringa extracts at the concentrations shown for 48 hours, then exposed to MTT for 3 hours, and the formation of formazan was measured. IC50 values for the aqueous leaf extract and the positive control paracetamol (obtained by fitting the data to equation (1) using nonlinear regression) were 6 mg/ml and 12 mM, respectively. Standard deviations for triplicate determinations are indicated.
Discussion
The leaf extracts of M. oleifera exhibited in vitro CYP3A4 inhibitory activity, and in one case some cytotoxicity. Extracts of other plants such as Sutherlandia and grapefruit juice have been found to also inhibit CYP3A4 activity[7]. The difference in CYP3A4 inhibition between the leaf extracts and the root extracts was probably due to the presence of different constituents and/or different amounts of constituents in different plant parts. Even though M. oleifera appears not to contain significant amounts of any potent inhibitor (observed IC50 values ≥ 0.5 mg/ml), it might still result in clinically significant inhibition of drugs that are metabolized via CYP3A4. Several drugs, such as the macrolide antibiotic erythromycin, have been shown to exhibit clinically relevant drug interactions even with relatively high IC50 values [8]In addition, the possibility of synergistic or antagonistic effects can become a factor when more than one inhibitory agent is present in a system [9].
These conclusions should, however, be viewed in light of the limitations of this assay. The concentrations used are high and unlikely to be reached in plasma after oral administration of the herb (although interactions could potentially be mediated by gut CYP3A4 in the absence of efficient absorption into the bloodstream). Another limitation is that the microsomal system bypasses hepatic uptake and efflux transporters which play important roles in drug disposition and metabolism. The interplay of transporters and enzymes, both in the liver and in the gut, must be considered in evaluating potential drug interactions [10].
The human CYP3A4 inhibitory activity exhibited in vitro makes Moringa a potential risk for herb/ARV interactions. In vitro data alone, however, are insufficient to conclude whether concomitant administration of Moringa oleifera with antiretroviral drugs to HIV/AIDS patients would result in a clinically significant interaction. Further in vitro and/or in vivo, and if warranted, clinical investigation of the effect of M. oleifera on CYP3A4 activity should be done to assess this possibility.
Acknowledgments
Liz Corbett, Tropical and Infectious Diseases, London School of Hygiene and Tropical Medicine, Biomedical Research and Training Institute and Peter Mason, Biomedical Research and Training institute, Harare, Zimbabwe for their comments and critical assessment of the manuscript. Part of this work was made possible through a fellowship received by Tsitsi Grace Monera from the Forgarty International Centre, National Institutes of Health (NIH-USA) through the International Clinical, Operational and Health Services and Training Award (ICOHRTA) Programme”.
Footnotes
Conflict of interest: No conflict of interest is declared.
References
- 1.Fugh-Berman A. Herb–drug interactions. Lancet. 2000;355(9198):134–138. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 2.Fugh-Berman A, Ernst E. Herb-drug interactions: review and assessment of report reliability. Br J Clin Pharmacol. 2001;52(5):587–595. doi: 10.1046/j.0306-5251.2001.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs. 2001;61(15):2163–2175. doi: 10.2165/00003495-200161150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Kim HS. Do not put too much value on conventional medicines. J Ethnopharmacol. 2005;100(1–2):37–9. doi: 10.1016/j.jep.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Anwar F, Latif S, Ashraf M. Moringa oleifera: A Food Plant with Multiple Medicinal Uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 6.Vistica DT, Skehan P, Scudiero D, Monks A, Pittman A, Boyd MR. Tetrazolium-based assays for cellular viability: a critical examination of selected parameters affecting formazan production. Cancer Res. 1991;51:2515–2520. [PubMed] [Google Scholar]
- 7.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47(2):331–385. [PubMed] [Google Scholar]
- 8.Benet LZ, Cummins CL, Wu C-Y. Transporter-enzyme interactions: implications for predicting drug-drug interactions from in vitro data. Curr Drug Metab. 2003;4:393–398. doi: 10.2174/1389200033489389. [DOI] [PubMed] [Google Scholar]
- 9.Mills E, et al. Impact of African herbal medicines on antiretroviral metabolism. AIDS. 2005;19:95–97. doi: 10.1097/00002030-200501030-00013. [DOI] [PubMed] [Google Scholar]
- 10.Guo LQ, Taniguchi M, Xiao YQ, Baba K, Ohta T, Yamazoe Y. Inhibitory effect of natural furanocoumarins on human microsomal cytochrome P450 3A activity. Jpn J Pharmacol. 2000;82(2):122–129. doi: 10.1254/jjp.82.122. [DOI] [PubMed] [Google Scholar]
- 11.Echizen H, Kawasaki H, Chiba K, Tani M, Ishizaki T. A potent inhibitory effect of erythromycin and other macrolide antibiotics on the mono-N-dealkylation metabolism of disopyramide with human liver microsomes. J Pharmacol Exp Ther. 1993;264(3):1425–1431. [PubMed] [Google Scholar]


