Abstract
The Cardiovascular Disease in Women Committee of the American College of Cardiology, in conjunction with interested parties (from the National Heart, Lung, and Blood Institute, American Heart Association, European Society of Cardiology), convened a working group to develop a consensus on the syndrome of myocardial ischemia with no obstructive coronary arteries (INOCA). In general, these patients have elevated risk for a cardiovascular event (including acute coronary syndrome, heart failure hospitalization, stroke, and repeated cardiovascular procedures) vs reference subjects, and appear to be at higher risk for development of heart failure with preserved ejection fraction (HFpEF). A subgroup of these patients also has coronary microvascular dysfunction (CMD) and evidence of inflammation. This document provides a summary of findings and recommendations toward the development of an integrated approach for identifying and managing patients with INOCA, and outlining knowledge gaps in the area. Working group members critically reviewed available literature and current practices for risk assessment and state-of-the-science techniques in multiple areas, with a focus on next steps needed to develop evidence-based therapies. This report presents highlights of this working group review and a summary of suggested research directions to advance this field in the next decade.
Keywords: Ischemia with no obstructed coronary arteries (INOCA), coronary microvascular dysfunction (CMD)
Definition and Terminology
Patients presenting with the syndrome of symptoms and signs suggesting ischemic heart disease (IHD) but found to have no obstructed coronary arteries (INOCA) are increasingly recognized.1–4 Specifically, these patients most often have symptoms suspected to be due to ischemia, prompting coronary angiography, yet no obstructive coronary artery disease (CAD), i.e. ≥50% diameter stenosis, is found. Considerable evidence now documents that this syndrome is associated with a prognosis that is clearly not benign, yet no clinical practice management guidelines exist for these patients. While there is likely overlap between INOCA and myocardial infarction with no obstructive coronary arteries (MINOCA) which appears to be increasingly described5–7, the primary need and focus is on non-MI syndromes. To summarize current knowledge and provide next steps for developing evidence-based management strategies for INOCA, a “Think Tank” was convened at the American College of Cardiology (ACC) Heart House, Washington D.C., May 17, 2016. This report summarizes those presentations and discussions.
Prevalence, Costs and Prognostic Significance
The ACC-National Cardiovascular Data Registry (ACC-NCDR) and National Heart, Lung and Blood Institute–sponsored Women’s Ischemic Syndrome Evaluation (WISE) databases suggest that at least 3–4 million women and men with signs/symptoms suggestive of myocardial ischemia have no obstructive CAD.8, 9 Such individuals incur health-care costs and disabilities similar to many with obstructive CAD, in part due to angina and heart failure (HF) hospitalizations and repeated testing.8, 10 These cost burdens related to hospitalizations and repeated angiography are confirmed by a European consecutive-patient registry.11
Cardiovascular disease (CVD) is the leading cause of death in Americans. Although more women than men die annually from CVD, predominantly IHD12, women presenting with symptoms and signs of myocardial ischemia are more likely to have no obstructive CAD on coronary angiography compared with men in the setting of both chronic and acute coronary syndromes (ACS).4, 8, 13, 14 Such patients are often reassured but offered no specific management, yet data now document a heightened risk of adverse CVD outcomes compared with age and sex-matched reference subjects.2, 10, 15
Almost two-thirds of women undergoing clinically indicated coronary angiography for suspected IHD in the original cohort of the WISE had INOCA.8, 13 During follow-up they had an intermediate risk for major adverse cardiac event (MACE) (death, non-fatal myocardial infarction [MI], non-fatal stroke, and HF hospitalization) rate exceeding 2.5% yearly by 5 years, as well as elevated rates of readmission, repeat angiography triggered by symptom burden.10, 16 At 10 years, CVD death or MI occurred in 6.7% of those with “no evident angiographic CAD”, and in 12.8% among those with non-obstructive CAD.17 Of note, women with INOCA are ~4 times more likely than men to be readmitted within 180 days for ACS/chest pain.16 Large, consecutive-case registry reports have replicated this heightened risk for adverse prognosis and extended the findings to men.2, 3, 18 Given the increased economic role women play in society, it is imperative to many stakeholders (Health and Human Services, Department of Labor, Department of Defense) that we understand and manage this epidemic to avoid direct and indirect economic burden (missed work).
Predictors of Adverse Outcomes
Older age, hypertension, diabetes, and smoking have been associated with increased mortality, while sex, hyperlipidemia, family history of premature CAD, or pre-test CAD likelihood have not.19 Risk-adjusted analyses found that “non-obstructive” CAD conferred increased mortality risk vs. that of patients with “no evident” CAD.19
Chest pain persisting at 1-year follow-up predicted MACE among those with INOCA in the WISE.20 Measures of non-obstructive CAD extent and severity (e.g., WISE CAD severity score, number of vessels involved, etc.) also appear important in prognosis, but these measures are not well developed.2, 3, 17 A large cohort undergoing coronary computed tomographic angiography (CCTA), with patients propensity-matched for age, CAD risk factors, and “angina typicality”, observed elevated death/MI rates in those with “non-obstructive CAD” vs “normal” angiograms.21
Pathophysiology
Mechanisms contributing to INOCA appear multifactorial and may operate alone or in combination.22, 23 Although these may include hypertension, severe aortic stenosis, severe anemia, type II MI, shunts, certain drugs, HF or cardiogenic shock, Prinzmetal variant angina (coronary spasm), myocardial diseases (e.g. myocarditis, etc.), congenital heart disease, coronary anomalies, myocardial bridging and other causes in an occasional patient, underlying mechanisms and appropriate diagnostic and management strategies in these settings are usually apparent. Accordingly, the remainder of this document will focus on clinical situations where the pathophysiologic mechanism for INOCA remains unclear after initial evaluation.
Mechanisms of Coronary Flow Regulation
Coronary blood flow is closely linked with metabolite production, which modulates vascular smooth muscle tone. Voltage-gated potassium channels (Kv 1.5) are critical in coupling myocardial blood flow to myocardial metabolism. Table 1 summarizes intrinsic mechanisms of coronary blood flow regulation.
Table 1.
Intrinsic mechanisms of coronary artery vasoreactivity
| Factor | Arteries (Endothelial Dysfunction) | Arterioles (Endothelial Dysfunction) |
|---|---|---|
| Serotonin | Constricts | Dilates (Constricts) |
| Vasopressin | Dilates (±Constricts) | Constricts |
| Endothelin | Constricts | Constricts |
| Thromboxane | Constricts | Constricts |
| Acetylcholine | Dilates (Constricts) | Dilates (±Constricts) |
| Adenosine | Dilates | Potent Dilator |
| Nitric Oxide | Dilates | Dilates |
| H2O2 | Dilates | Potent Dilator |
| Norepinephrine | Constricts | No Direct Effect |
Coronary Microvascular Dysfunction (CMD)
One proposed mechanism contributing to INOCA is CMD24, defined as epicardial and/or microvascular endothelial and/or non-endothelial dysfunction that limits myocardial perfusion, most often detected as reduced coronary flow reserve (CFR). CMD may occur in the absence of obstructive CAD and myocardial diseases, in myocardial diseases, in obstructive CAD, or may be iatrogenic.24 Coronary vasomotor dysfunction, even without flow-limiting stenosis, identifies patients at risk for cardiac death.25–27 There is a distribution of risk across the CFR range from those with angiographic obstructive disease, to those with “diffuse non-obstructive atherosclerosis”, to those with “normal appearing angiograms”, to those with only coronary microvacular dysfunction. There is limited correlation between anatomic CAD severity and functional Impairment, as reflected in the CFR.28 Patients with low CFR, independent of angiographic severity of obstructive disease, have increased risk for adverse outcomes. For example, diabetic patients without obstructive CAD but with impaired CFR experienced cardiac death rates similar to those for nondiabetic patients with CAD.29 Prospective studies are needed to assess modifiability of CFR to therapy and its ability to reclassify patients at varying risk across the anatomic spectrum of CAD.
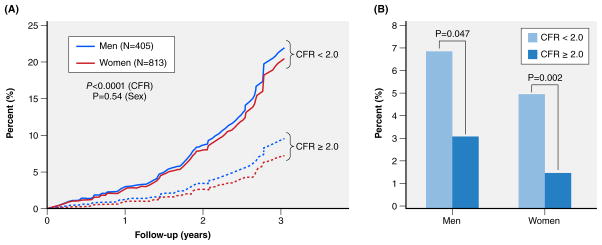
Coronary reactivity testing, usually with adenosine or an analog, is required for diagnosis of CMD. In the WISE, a CFR, defined as an invasive Doppler time-averaged peak hyperemic coronary flow velocity/resting flow velocity <2.32 best predicted adverse outcomes in INOCA women, with a 5-year MACE rate of 27%, vs. 9.3% for those with a CFR ≥2.32 (p=0.01).30 Importantly, CFR was a continuous predictor of MACE, similar to blood pressure and LDL-C, rather than having a step-like threshold for “normal vs abnormal values”. Similar findings (Table 2) have been observed in other studies that include noninvasive CFR velocity measurements by transthoracic echo Doppler or absolute measurements (in ml/min/gm myocardium) by positron emission tomography (PET).26, 31, 32 The latter study provides the most definitive data on CMD and adverse outcome risk among INOCA patients.32 In those with CFRPET <2.0, the MACE (cardiac death, MI, late-revascularization, or HF-hospitalization) rate was increased at 3 years vs those with higher CFRs. A CFR of <2 was associated with 7.8% and 5.6% annualized MACE among symptomatic men and women without obstructive CAD vs 3.3% and 1.7%, respectively, for those with CFR ≥2.0.32 Interestingly, while women comprise ~70% of the INOCA population41, increased risk associated with limited CFR does not appear different for women vs. men (Figure 1). A recent publication demonstrates the excess cardiovascular risk in women relative to men is associated with severely impaired coronary flow reserve, not with obstructive CAD42.
Table 2.
Natural History Studies of Patients with Coronary Microvascular Dysfunction
| Author Year | N | Population | Method | Outcome measure | Follow-up | CMD Outcome Predictor | |
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||
| Pepine (WISE) 201030 | 189 | Women, angina/ischemia most with nonobst CAD | Intracoronary Ado-CFR Doppler flow wire | Death, nonfatal MI, nonfatal stroke, HF hospitalization | 5.4 yrs. (mean) | Yes | Yes |
| Balazs (SZEGED) 201131 | 45 | Women, angina/ischemia, no obstructive CAD | Vasodilator CFR, Doppler /TEE, TTE | Death, CV hospitalization | 102±26 mos. 113 median | Yes | Yes |
| Murthy 201432 | 1218 813 W 405 M |
No obstructive CAD (excluded by CTA or PET) | Stress Perfusion Imaging (PET) | CV death, MI, late revasc. (>90 ds) or HF-hospitalization | 3 years | Yes | Yes |
| Britten 200433 | 120 | Post PCI/mild CAD | IC Papaverine or Ado-CFR Doppler flow wire | Cardiac death, ACS, revasc., stroke | 6.5±3 yrs. (14–125 mos.) | Yes | Yes |
| Schindler 200634 | 72 | CAD risk factors without flow-limiting stenosis | CPT-MBF increase with 13N-NH3 PET | CV death, ACS, MI, PCI/CABG, stroke, PTA | 66±8 mos. | Yes | No |
| Rigo 200735 | 86 | CAD, LAD 51–75% stenosis | Vasodilator LAD CFR, Doppler /TTE | Non-fatal MI | 30 mos. 14 median | Yes | Yes |
| Nemes 200836 | 397 | Hospitalized, angina, mostly severe CAD, TEE for AA | Vasodilator LAD CFR, Doppler /TEE | CV death, HF. thrombosis | 41±12 mos. | Yes | Yes |
| Herzog 200937 | 229 | Suspect CAD/66% had severe CAD | Vasodilator CFR with 13N-NH3 PET | CV death, nonfatal MI, hospitalization, PCI/CABG | 5.5±2.1 yrs. | Yes | Yes |
| Tio 200938 | 344 | Severe CAD, not revasc., LV systolic dysfunction | Vasodilator CFR with 13N-NH3 PET | Cardiac death | 85 mos. (1–138 mos) | Yes | Yes |
| Cortigiani 201039 | 1,660 | Chest pain, Normal DSE | Vasodilator LAD CFR, Doppler /TTE | Death, MI, revasac. | 19 mo. median | Yes | Yes |
| Ziadi 201140 | 677 | Most had severe CAD | Vasodilator CFR with 82Rb PET | CV death, nonfatal MI | 387 ds. (375–416 ds) | Yes | Yes |
Abbreviations: CAD = coronary artery disease, CFR = coronary flow reserve, CMD = coronary microvascular dysfunction, CV = cardiovascular, DSE = dobutamine stress echo, HF = heart failure, MI = myocardial infarction, PCI = percutaneous coronary intervention, TEE = transesophageal echocardiography.
Figure 1.
Sex effects on CMD measured by PET and Adverse Outcomes in Symptomatic Patients without Obstructive CAD. Data obtained from Murthy et al 32
Age, Sex and Other Risk Variables
Conditions associated with increased risk for CMD appear similar to those for obstructive CAD and include traditional atherosclerosis risk factors like aging, hypertension, diabetes mellitus, and dyslipidemia.43 Aging (see below) leads to increased arterial wall stiffness, medial thickening, and lumen enlargement, resulting in increased pulse pressure and hypertrophy of arteries leading to endothelial dysfunction, dysregulation of ventricular-aortic coupling, and subendocardial hypoperfusion, contributing to CMD.44 Hypertension is associated with remodeling of small arteries, including coronary arteries,45 and leads to arteriolar constriction and reduced microvascular density.46 CMD may also be associated with diabetes47 as chronic hyperglycemia reduces endothelial-dependent and -independent coronary vasodilator capacity.48, 49 Hypercholesterolemia may lead to CMD50, but higher HDL-cholesterol and lower triglyceride levels are associated with higher microvascular flow.
Although CMD is most prevalent in midlife women, WISE data do not support a role for estrogen deficiency.51 However, traditional CAD risk factors explained <20% of the variation in CMD in the WISE cohort.51 Traditional risk factors are not always present in CMD, and novel risk markers like those associated with inflammation may contribute.51, 52 There is a correlation between high sensitivity C-reactive protein (hsCRP) and number of ischemic episodes during ambulatory ECG monitoring.53 CRP is increased among subjects with microvascular angina vs. controls, further supporting a possible role of inflammation and endothelial dysfunction in causing CMD.54 Patients with increased hsCRP have an attenuated rise in CBF in response to acetylcholine (Ach).55, 56 Systemic lupus erythematosus is frequently associated with angina and CMD,57 while prior breast cancer chemotherapy may also be associated with CMD.58 CFR is reduced among patients with “normal” or “minimally diseased” coronary arteries and either systemic lupus erythematosus or rheumatoid arthritis, and prolonged systemic inflammation may also contribute to premature CAD in these patients.59
Conduit Vessel Stiffness
Aortic pulse wave velocity (aPWV) with other vessel stiffness indices explained >50% of CFR variance in a WISE substudy.60 Arterial stiffness is known to predict cardiovascular events beyond traditional risk factors. When arterial stiffness indices were assessed by magnetic resonance imaging (MRI), ultrasound, and tonometry in asymptomatic subjects from the community,61 peripheral and central pulse pressure, augmentation index, carotid-femoral PWV, and aortic arch PWV all increased with age, but ascending aortic strain and distensibility decreased with age. The best markers of subclinical large artery stiffening were aortic arch distensibility in younger individuals and aortic arch PWV after age 50.61
Atherosclerosis
The pathophysiology of atherosclerosis has shifted from a lipid storage disease with large lipid pool thin fibrous cap atheroma (e.g. “rupture vulnerable” plaque) and flow-limiting plaque resulting in vessel occlusion to a more chronic inflammatory process interrupted by periods of minor plaque rupture, erosion and distal embolism.62 The initial phase begins early in life, as oxidant stress related to various risk conditions (genetic predisposition, elevated blood pressure, diabetes, LDL-cholesterol, environmental factors such as tobacco, etc.) activates endothelial cells and probably vascular smooth muscle cells.62 Bone marrow–derived inflammatory cells (monocytes) join endothelial and smooth muscle cells of the artery wall to initiate and perpetuate a less intensive, chronic inflammatory response leading to endothelial and vascular smooth muscle dysfunction. Multiple adhesion molecules for leukocytes, chemo-attractant cytokines, and activators of leukocyte function actively participate in the atherogenic process. This process is systemic with variable endothelial and vascular smooth muscle dysfunction in all vessels (large and small) and plaque growth, within large and medium-sized arteries, leading to different clinical manifestations of ischemia depending on the acuity of the process and organ(s) involved.62 Evidence linking microvascular and inflammatory responses to risk factors indicates that oxidative stress, reduced nitric-oxide (NO)-bioavailability, and endothelial activation are common early features of coronary microvascular responses to atherosclerosis risk factors.63
Almost all INOCA patients with chronic angina studied by intravascular ultrasound (IVUS) to date have some coronary atherosclerosis.22, 64 Given sampling limitations of IVUS as used in these reports, those findings strongly suggest that atherosclerosis is a key mediator of the syndrome. In support of this hypothesis, a greater burden of risk factors is associated with more atherosclerosis, concealed by “compensatory” positive remodeling, yielding diffuse non-obstructive CAD.64 Plaque rupture by IVUS was not observed in INOCA patients from two series of patients with chronic angina. 5, 22, 64 Two single-center reports of non-obstructive CAD presenting with ACS suggest that plaque rupture is observed in the minority: 38% of 50 women6 and 37% of men and women.5 The former study found that plaque ulceration was also frequent, in addition to late gadolinium enhancement with an ischemic pattern of injury.6 The latter study found plaque ruptures frequently appeared in more voluminous plaques with large plaque burden and positive remodeling.5
Non-Obstructive CAD
Prior work evaluating patients suspected to have myocardial ischemia found ~40% of women and 8% of men had “non-significant CAD” (30%–49% stenosis).14 The Coronary Artery Surgery Study (CASS) registry reported 39% of women and 11% of men with angina had “normal coronary arteries.”65 However, CASS lacked an angiographic core lab, and in a sample retrospectively reviewed variation in interpretations of proximal lesions was unacceptably high.66 Furthermore, it is unclear how many patients were retrospectively entered, enhancing “survival bias” to limit adverse outcome estimates.67
More recent analyses have linked angiographic measures of extent of non-obstructive CAD with increased risk for adverse outcomes. These include number of major vessels involved with non-obstructive CAD,3 WISE-CAD Severity Score,17 and TIMI-frame counts.68 In a European consecutive case registry of patients undergoing clinically-indicated coronary angiography, the prevalence of non-obstructive CAD was 65% among women and 32% in men.2 The ACC-NCDR prospective registry of patients undergoing clinically indicated invasive coronary angiography found that the prevalence of non-obstructive CAD was 51% in women and 32% in men.9 Diffuse non-obstructive CAD is increasingly recognized with the more widespread use of FFR which may be of use to assess long moderate lesions. Measures from CCTA are also evolving. Except for the variables cited above, the other variables have not been studied in detail, and limited positive findings lack replication in other large cohorts.
Blood Pressure
Thickened and stiffened microvessels have poor auto regulatory capacity, allowing transmission of increased blood pressure to the microvessels.24 Along with hydraulic factors like blood pressure level, intramural factors such as impaired coronary microvascular density and impaired myocardial perfusion likely contribute to INOCA.
Lipids
Dyslipidemia contributes to, and statin treatment improves, coronary endothelial dysfunction.69 Additional contributing roles include myocardial ischemia-related steatosis which appears to be mechanistically linked with impairments in ventricular relaxation in women with CMD evidenced by magnetic resonance spectroscopy.70 Specifically, women with CMD had higher myocardial triglyceride (TG) content (0.83 ± 0.12% vs. 0.43 ± 0.06%; P = 0.025) and lower diastolic circumferential strain rate (168 ± 12 vs. 217 ± 15%/s; P = 0.012), with myocardial TG content correlating inversely with diastolic circumferential strain rate (r = −0.779; P = 0.002), 70 suggesting that CMD triggers a metabolic shift away from free fatty acids, resulting in ectopic fat deposition in cardiomyocytes. Mitochondria functions including ROS signaling, apoptosis, steroid synthesis, hormonal signaling (mtER), and sexual dimorphism in the expression of mitochondria-related genes may be involved.71
Obesity, Metabolic Syndrome and Diabetes
Obesity-related hypertension, cardiomyocyte hypertrophy, and impaired cardiac vascular adaptation to metabolic needs are well documented in obesity. Decreased serum adiponectin levels and impaired CFR occur in women with “normal” epicardial coronary arteries.72 Insulin-resistance is strongly associated with both microvascular and macrovascular coronary disorders, and confers high risk for CVD morbidity and mortality (women > men).4, 73 Coronary microvascular abnormalities are highly prevalent and worsen with progressive glucose intolerance with or without obstructive CAD, and non-obstructive CAD is highly prevalent in those with diabetes.34, 74, 75
Cardiac Autonomic Nervous System (ANS)
Abnormal cardiac adrenergic nerve function is well documented in INOCA patients.76–78 Among patients experiencing mental stress-induced angina, exposure to cold triggers angina, rest angina, and/or early morning angina. There is close interplay between the ANS and endothelium, whereby β-adrenergic receptor activation of vascular smooth muscle cells (VSMCs) induces vasodilation, α-adrenergic receptor activation induces vasoconstriction, and muscarinic receptor activation induces vasoconstriction.79, 80
An abnormal vascular response to Ach may be a sign of defective bioavailable nitric oxide, prostacyclin, or excess endothelium-derived hyperpolarizing factor release, or it could be indicative of increased smooth muscle cell sensitivity to muscarinic stimulation or excessive release of endothelium-derived contracting factor, a finding in HF.81, 82 Sympathetically-mediated effects of mental stress on the coronary microcirculation may also be deleterious.83 For example, CMD after percutaneous coronary intervention is due to sympathetically mediated vasoconstriction and may be prevented or attenuated by oral pretreatment with an α1-adrenergic antagonist.84 Normally, increased sympathetic activity dilates coronary resistance vessels to increase myocardial blood flow, modulated at least partially by endothelium.81
Platelet Dysfunction or Other Coagulopathy
Platelet reactivity, in response to collagen/adenosine diphosphate (ADP) stimulation, decreases after exercise in patients with angina, positive exercise tests, and “smooth coronary arteries” (e.g. INOCA).85 Flow cytometry measures at rest and exercise86 in INOCA patients demonstrated that increases in platelet receptor expression and leukocyte–platelet aggregate formation (to ADP) were consistently lower after exercise than before. These findings agree with and expand upon prior work demonstrating lower whole blood platelet reactivity to collagen/ADP in INOCA patients after exercise, in contrast with absence of change in controls and an increase in CAD patients.13, 14 Changes in platelet receptor expression and leukocyte-platelet aggregate formation have been reported following exercise in INOCA,86 and adenosine has been shown to inhibit ADP- and thrombin-induced monocyte-platelet aggregates in INOCA.87
Diagnosis
Invasive Testing
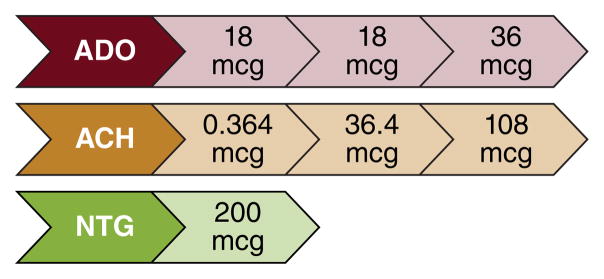
Coronary blood flow (CBF) is driven by the pressure difference between the aorta and the capillary bed and modulated further by physical and neural factors that affect the microcirculation. Different microcirculation compartments are influenced by one main physiological mechanism to control their vascular tone with cardiac metabolism as the final determining factor. Measurement of coronary vascular function includes measurements of CBF and epicardial coronary artery diameter with endothelium-dependent probes: Ach, bradykinin, substence-P, L-NMMA, shear-stress, and predominantly endothelium-independent probes: adenosine or sodium nitroprusside. Doses of test agents and definitions of test findings are summarized in Tables 3 and 4, respectively, in Figure 2, and the protocol in Table 5.
Table 3.
Intra-coronary acetylcholine concentration and infusion
| Prepared Concentration | Infusion Rate | Infusion duration | Infused Dose |
|---|---|---|---|
| 10−6 M (0.182 mcg/mL) | 48 ml/hr | 3 minutes | 0.364 mcg |
| 10−4 M (18.2 mcg/mL) | 48 ml/hr | 3 minutes | 36.4 mcg |
| 10−4 M (18.2 mcg/mL) | 120 ml/hr | 3 minutes | 108 mcg |
Table 4.
Definitions of coronary microvascular and macrovascular dysfunction
| Coronary Microvascular Dysfunction Pathways | Microvascular Dysfunction | Macrovascular Dysfunction |
|---|---|---|
| Non-Endothelial Dependent | CFR in response to adenosine <2.5 | Change in coronary artery diameter in response to nitroglycerin <20% |
| Endothelial Dependent | Change in CBF in response to acetylcholine <50% | Change in coronary artery diameter in response to acetylcholine ≤0% |
| Coronary Spasm | Chest pain + ECG changes Change in coronary artery diameter in response to acetylcholine <90% |
|
Abbreviations: CBF = coronary blood flow, CFR = coronary flow reserve, ECG = electrocardiogram
Figure 2.
Coronary Reactivity Testing Protocol
Table 5.
Protocol for Invasive Coronary Reactivity Testing
| Preparation |
Withhold for 48 hours:
|
| Protocol |
|
ACE = angiotensin-converting enzyme; CAD = coronary artery disease; ECG = electrocardiogram; FFR = fractional flow reserve; LAD = left anterior descending
Exercise, pacing-induced tachycardia, cold pressor test (CPT), and mental stress have also been used to elicit abnormalities in CBF. WISE-CVD project data suggest a strong correlation between Ach and CPT coronary artery diameter changes in women.22 Reports from testing over 1500 patients indicate an excellent safety record with no deaths and <1% procedure-related adverse experiences like those observed with coronary angiography.88–90
Noninvasive Testing
Position Emission Tomography (PET)
PET is a highly accurate, reproducible and modifiable procedure providing comprehensive evaluation of CBF, including perfusion, LV function, and CFR. There is a strong association between impaired CFR and impaired LV myocardial relaxation or elevated filling pressures; strongest among those with cardiac troponin elevations.91 In a study32 of chest pain patients without CAD history, PET-assessed rest and post hyperemic flow (adenosine, dobutamine, dipyridamole) identified significant MACE predictors: Duke clinical risk score (HR, 1.06), LVEF (10% increase, HR, 0.56), CFR (HR, 0.80 for each 10% increase).
Transthoracic Echo-Doppler
In another study of patients with chest pain without obstructive CAD and coronary flow velocity (CFV), by pulsed wave Doppler of the LAD at rest and after dipyridamole, 26% had CFV reserve <2.0.92 Those with low CFV reserve had significantly greater physical limitation and disease perception scores using the Seattle Angina Questionnaire.
Cardiac Magnetic Resonance Imaging (cMRI)
Failure of subendocardial perfusion to increase appropriately in response to stress can be detected by cMRI among INOCA subjects.93, 94 A semi-quantitative approach with measurement of myocardial perfusion reserve index (MPRI) detects CMD in women with INOCA.95 Those with CMD, by abnormal invasive CRT, had reduced MPRI vs normal women. Because the methodology uses standard equipment and protocol available in tertiary hospitals without radiation, MPRI appears useful for diagnosis and management of INOCA and deserves additional evaluation.
Management
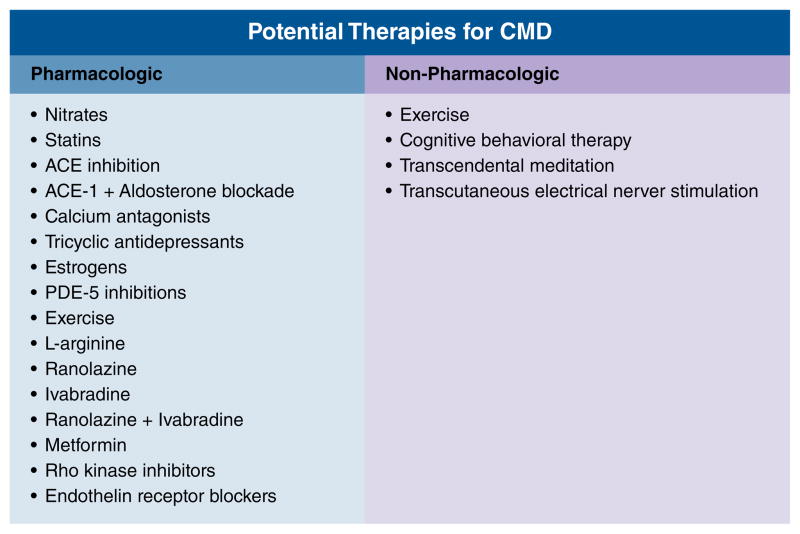
At present, the management strategy for INOCA remains unclear, largely due to absence of an evidence base needed for guidelines. Figure 3 outlines potential therapies for CMD.
Figure 3.
Potential Therapies for CMD
Statins, ACE-Inhibitors (ACE-I), and aspirin (ASA)
Multiple prior statin trials using IVUS have documented prevention of progression, or even regression, of atherosclerosis in coronary arteries, as well as coronary endothelial and/or vascular smooth muscle function in subjects with non-obstructive CAD.96, 97 Statins not only lower cholesterol, but also have anti-atherosclerotic and anti-inflammatory effects.98 Data support the use of statins to improve CFR. Fluvastatin alone showed improvement in CFR and even greater improvement in combination with diltiazem.99 Two small pilot studies have shown administration of atorvastatin improved CFR after 2 months100 and 6 months.101 Angiotensin converting enzyme (ACE) inhibitors have been shown to improve exercise tolerance and angina symptoms.102 In the WISE control trial, women who received quinapril had improved CFR after 16 weeks compared to the placebo group. In addition, the experimental group also had improvement in angina symptoms based on the Seattle Angina Questionnaire.103 Patients with essential hypertension had marked improved coronary blood flow after 12 months of treatment with perindopril, with regression of periarteriolar fibrosis seen on biopsy.104 In patients already on an ACE inhibitor, the addition of an aldosterone blocker did not improve endothelial function.105 In subjects with diabetes, the addition of spironolactone has been shown to improve coronary microvascular function.106 The benefit of spironolactone is explained by the fact that mineralocorticoid receptor activation has been shown to cause vascular damage107 and dysfunction.108 A statin plus ACE-I (atorvastatin and ramipril) was used in a randomized trial of angina patients with “normal coronary angiograms” and ischemia during stress testing; at 6-months, the statin/ACE-I strategy improved Seattle Angina Questionnaire scores and exercise duration vs placebo. Mechanistically, the combination produced greater increases in brachial artery flow-mediated dilation vs placebo and reduced extracellular superoxide dismutase.102
Anti-platelet agents
A majority of patients with CMD have endothelial dysfunction, and while angiography shows no significant plaque burden, IVUS has demonstrated coronary atherosclerosis in most patients22, 64. Therefore, ACC/AHA chronic stable angina guidelines 109 can be extrapolated to use of anti-platelet agents such as aspirin in patients with evidence of ischemia and no obstructive CAD.
Anti-anginal agents
Current approaches to treatment of CMD (Table 6) include the management of risk factors, use of antianginal and anti-atherosclerotic medication and some novel agents. However current literature has little evidence for effective therapy for CMD for the following two reasons: first, studies often comprise patients with cardiac chest pain that may be attributed to clinical entities other than CMD, such as cardiac syndrome X111; secondly, studies have used various CFR cutoff criteria for CMD, as there is no consensus definition thus far.112
Table 6.
Treatment of Subjects with Angina, Evidence of Myocardial Ischemia, and No Obstructive CAD Reproduced from 110
| Microvascular Coronary Dysfunction (CMD) |
| Abnormal Endothelial Function |
| Angiotensin Converting Enzyme Inhibitors (ACE-I) |
| HMG CoA Reductase Inhibitors (Statins) |
| L-arginine supplementation |
| Aerobic Exercise |
| Enhanced External Counterpulsation (EECP) |
| Abnormal Non-Endothelial Function |
| Beta-blockers/alpha-beta blockers |
| Nitrates |
| Anti-Anginal |
| Ranolazine |
| Ivabradine |
| Xanthine derivatives |
| Abnormal Smooth Muscle Function (Prinzmetal’s Angina) |
| Calcium Channel Blockers |
| Nitrates |
| Abnormal Cardiac Nociception |
| Low Dose Tricyclic Medication |
| Spinal Cord Stimulation |
| Cognitive Behavioral Therapy |
Management of risk factors includes control of diabetes and hypertension. Therapeutically lowering blood pressure can improve CFR, but excess lowering of diastolic blood pressure attenuates the benefit.113 The insulin sensitizer metformin has been shown to improve endothelial function.114 Lifestyle modifications include weight loss,115 smoking cessation, high-fiber diet, fruits and vegetables consumption and regular physical activity.116–118
Few studies addressed the use of beta-blockers. The existing studies included patients with signs and symptoms of ischemia, but without definitive diagnosis of CMD. Beta-blockers reduce myocardial oxygen consumption and increase diastolic filling time. Atenolol has been shown to reduce number of angina episodes119 and also improve ischemic threshold.120 Carvedilol has been shown to improve endothelial function.121
Another class of vasodilator is calcium channel blockers, which are a reasonable first line treatment for CMD given the underlying pathophysiology. However, one study of intracoronary diltiazem did not improve CFR in CMD patients, but rather had a predominant vasodilatory effect on the epicardial artery.122 Despite these findings, patients with abnormal vasodilator reserve have improved symptoms, less nitrate usage, and improved exercise tolerance after being treated with verapamil or nifedipine.123
Nitrates achieve anti-anginal effect through venodilation to reduce preload; in addition, they may have some coronary vasodilatory effect. The use of nitrates may improve patient’s symptoms, but there are limited data on their effect on endothelial function.
Ranolazine is an antianginal that inhibits the late sodium current, and overall reduces intracellular calcium levels in cardiomyocytes, thus leading to improved ventricular relaxation.124 Results on CMD have been conflicting. One pilot study showed improved symptoms in women with angina and evidence of ischemia but no obstructive CAD, and patients with low CFR demonstrated improved CFR with treatment.125 A similar sized study showed some improvement with symptoms but no effect on coronary microvascular function.126 A recent large randomized trial of two-week course of ranolazine vs. placebo found no difference in symptoms or myocardial perfusion reserve.127
Ivabradine reduces heart rate through its effect on If of the sinoatrial node. In patients with stable CAD, it is found to improve CFR.128 Another study showed improvement of symptoms, but no effect on coronary microvascular function.126 Ivabradine may have a therapeutic role in CMD patients.
Aminophylline, a nonselective adenosine-receptor antagonist, blocks the mediation of nociception. It is postulated to benefit CMD by attenuating the excess dilation of the microvasculature in a relatively well-perfused area, thus shunting blood to a poorly perfused area. Some improvement in symptoms and exercise capacity were seen with short-term intravenous129 and oral aminophylline130 in patients with signs and symptoms of ischemia but normal coronary angiogram.
Fasudil, a rho kinase inhibitor, reduces smooth muscle cell hypercontraction131 and is being investigated currently and has potential for CMD. It has been shown to be effective for vasospastic angina. Preliminary studies showed patients pretreated with fasudil did not manifest evidence of ischemia with acetylcholine infusion, compared to saline pretreatment.132
There may be a role for L-Arginine supplementation to improve endothelial dysfunction, as L-Arginine is the precursor of nitric oxide.133 Two studies have found improvement of CFR after one time infusion of L-Arginine.134, 135 However Lerman et al. found that after 6-month oral supplementation, there is symptom improvement, decreased endothelin concentration, improvement in CBF, but no improvement in CFR.136
Given that impaired cardiac nociception may be involved in CMD, tricyclic antidepressants can be considered, as they are thought to have modulatory effects on norepinephrine uptake and anticholinergic effect that can cause analgesia. Imipramine has been shown to reduce frequency of pain,137, 138 but in one of the studies did not show any improvement in quality of life,138 likely from its significant side effects.
Non-pharmacologic treatments can be effective in controlling patient symptoms. Spinal cord stimulation has been shown to normalize abnormal pain perception139 and improve angina symptoms and increase exercise tolerance.140 Enhanced external counterpulsation uses pneumatic cuffs applied to patient’s legs. Sequential inflation and deflation synchronized to the cardiac cycle improves hemodynamics.141 It has been shown to improve angina in a small case series.142 Cognitive behavioral therapy can reduce symptom severity and frequency.143 Cardiac rehabilitation involves multiple sessions of cardiovascular exercise, psychological counseling and nutritional planning and can be helpful as it improves blood pressure, BMI and exercise capacity.144
In summary, small studies support the use of statins and ACE-Is to prevent progression of non-obstructive coronary atherosclerosis, improve endothelial and microvascular function, and symptoms. However, treatments for INOCA have not been studied in clinical outcome trials adequately powered to inform evidence-based guidelines.
Knowledge Gaps
Definition
Gaps in current knowledge related to patient phenotype(s), mechanistic understanding, and management of patients with INOCA are numerous. To advance this field, it is essential to develop a uniform definition of the patient with INOCA. Prevailing elements of a definition, summarized from presentations by Think Tank members, include patients with:
stable, chronic (several weeks or longer) symptoms suggesting IHD, such as chest discomfort, both with classic (e.g. angina pectoris) and atypical features in terms of location, quality, and inciting factors;
objective evidence for myocardial ischemia from the ECG and/or a cardiac imaging study (echocardiography, nuclear imaging, MRI or spectroscopy) at rest or during stress (exercise, mental, or pharmacologic);
absence of flow-limiting obstruction by coronary angiography (invasive or CTA) as defined by any epicardial coronary artery diameter reduction ≥50% and/or fractional flow reserve <0.8.
Diagnosis and phenotyping
Given the likelihood that multiple mechanisms may contribute to INOCA, improved understanding by specific phenotyping of these individuals beyond symptoms and ischemia is needed. For example, although epicardial coronary spasm has been recognized for decades, its specific role in patients with INOCA is unclear. While recent data suggest that ~5% of clinically stable women with angina and INOCA have epicardial spasm with intracoronary Ach testing, the role of microvascular coronary spasm requires additional study. The potential for INOCA to evolve into an ACS (e.g. MINOCA) and/or HF with preserved ejection fraction (HFpEF) requires additional study from large, prospective cohorts.
Management
Knowledge gaps exist regarding the pathogenesis and management of INOCA. For example, why are certain CV risk factors associated with CMD in some patients but not in others? What are the clinical, therapeutic, and prognostic implications of using a classification based on measures of non-obstructive CAD, CFR, MPRI, PET in these patients? How often does INOCA/CMD progress to HFpEF, and if so, what is the mechanistic pathway? Are there novel provocative tests for earlier diagnosis and treatment of INOCA? The questions are numerous, but they need to be addressed if we are to make progress in understanding and managing this common syndrome that is increasing in prevalence.
Research Agenda for the Next Decade
The following recommendations address three overarching goals: 1) to formulate phenotypic classification of INOCA patients, based on clinical presentation, pathophysiological mechanisms, and prognosis; 2) to develop diagnostic algorithms based on this classification system and; 3) to develop management approaches to reduce or prevent symptoms and modify risk for adverse outcomes.
Design adequately powered, population-based natural history studies/registries of INOCA patients, using consecutive case cohorts from the large numbers of patients undergoing stress testing and coronary angiography with the definition for INOCA proposed above. Specific attention should be directed to detection and quantitation of non-obstructive atherosclerosis using invasive coronary angiography, CCTA and other modalities. Within these studies, obtain comprehensive clinical (including detailed symptom tools such as the Seattle Angina Questionnaire, the Kansas Heart Failure Questionnaire, and other standardized quality of life measures) and biological information (such as functional capacity, left ventricular function, and filling pressures), including cells, tissue, and body fluids, when feasible. Collect outcomes data and develop rank-ordered adverse outcomes metrics (angina exacerbation and hospitalization, HFpEF-hospitalization, MI, cardiac death) in INOCA patients.
Develop markers as “risk reporters” among “high-risk INOCA patients” that include clinical and advanced technology variables (e.g., proteomic, gene expression, cell-based, exosomes, miRNAs, etc.). Validate them in clinical settings, develop informatics platforms for prediction modeling that may require monitoring specific biological “signatures” periodically to discern which are perturbed before a clinical event (e.g., angina, HF-hospitalization) and will be useful for predicting risk and directing new therapies. This should include, but not be limited to, new methods for predictive modeling using multidimensional data sets. For example, new scores could be developed and validated from coronary or CT angiography and other sources to estimate near-term risk that also include clinical and behavioral variables, existing biomarkers, genetic, omic, and imaging markers. The scoring system should allow addition of new variables when they become available. It would be used as a “targeted screening tool” for patients deemed intermediate or higher CVD risk by traditional risk scores to determine who would benefit from more intensive testing, monitoring, and therapeutic interventions. Use this information to better understand underlying pathophysiologic mechanisms of INOCA, including CMD. Discover “reporters” for these mechanisms to investigate environmental and biological determinants that may account for individual differences in vasomotor function, plaque micro-disruption, enhanced thrombus formation, sympathetic nervous system activation, and other potential triggering mechanisms for ACS.
Conduct adequately powered clinical trials, using standardized INOCA and CMD definitions, on risk outcomes with existing strategies effective in atherosclerotic CVD, such as aspirin, statins, ACE-I and lifestyle modification. Conduct exploratory trials using novel interventions based on new phenotypic and mechanistic understanding on risk outcomes.
Construct evidence-based diagnostic and therapeutic guidelines for INOCA. Develop physician education and fellowship training programs to enhance understanding of this syndrome and encourage use of novel risk assessment and management strategies. Develop programs to understand and overcome barriers to clinical implementation of these guidelines.
Conclusion
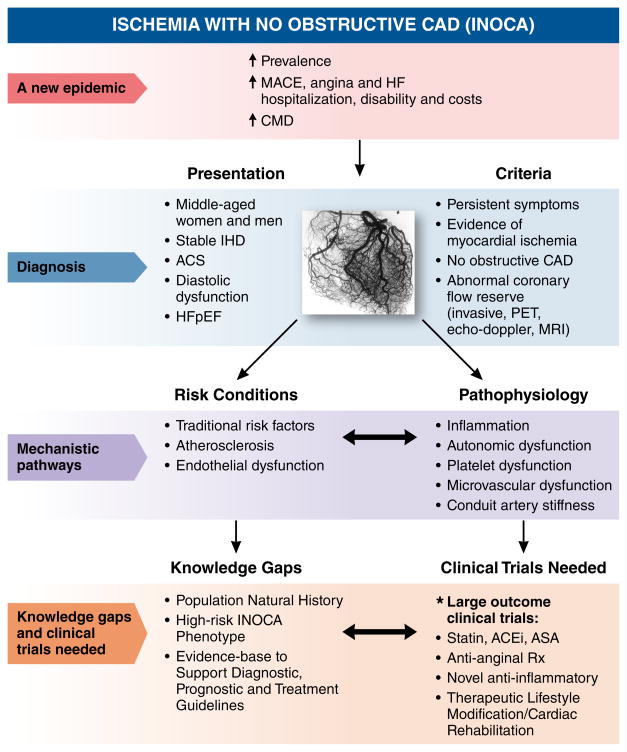
The prevalence of non-obstructive CAD among clinically ordered coronary angiograms conducted for evidence of suspected myocardial ischemia (INOCA) is increasing.1–4 A subgroup of these patients has coronary microvascular dysfunction (CMD), an elevated risk for a cardiovascular event (including acute coronary syndrome and repeated cardiovascular procedures), and higher risk for the development of heart failure hospitalization. At present, there is no uniform, comprehensive diagnostic strategy or algorithm for risk stratification for these patients; however invasive and noninvasive coronary flow reserve testing can be useful. Although small trials have suggested benefit from ACE inhibitors and statins, there is a lack of appropriately designed clinical outcome trials to inform evidence-based therapeutic strategies. Next steps needed to address knowledge gaps include evidence-based approaches to the definition, diagnostic evaluation, risk stratification, and management of INOCA patients, including large outcome clinical trials. This summary of our current understanding of INOCA (Figure 4) support the need for a research agenda for the next decade to facilitate development of evidence-based risk assessment tools and effective therapies for this rapidly growing patient population.
Figure 4.
Ischemia with No Obstructive CAD (INOCA)
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, RO1-HL-073412-01, grants U01-64829, U01-HL649141, U01-HL649241, UL1-TR001427, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, New Jersey, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania, and QMED, Inc., Laurence Harbor, New Jersey, and the Edythe L. Broad Endowment, the Barbra Streisand Women’s Cardiovascular Research and Education Program, the Linda Joy Pollin Women’s Heart Health Program, and the Constance Austin Fellowship Endowment, Cedars-Sinai Medical Center, Los Angeles and the Erika Glazer Women’s Heart Health Project, Cedars-Sinai Medical Center, Los Angeles, California.
Appendix. Working Group Members
Co-Chairs
C. Noel Bairey Merz, MD, Cedars-Sinai Heart Institute
Carl J. Pepine, MD, University of Florida School of Medicine
Mary Norine Walsh, MD, St Vincent Heart
Jerome L. Fleg, MD, National Heart, Lung, and Blood Institute
Members
Paolo G. Camici, MD, Università Vita Salute San Raffaele
William M. Chilian, PhD, Northeast Ohio Medical University
Janine Austin Clayton, MD, National Institutes of Health
Lawton S. Cooper, MD, National Heart, Lung, and Blood Institute
Filippo Crea, MD, Università Cattolica del Sacro Cuore
Marcelo Di Carli, MD, Brigham and Women’s Hospital
Pamela S. Douglas, MD, Duke University School of Medicine
Zorina S. Galis, PhD, National Heart, Lung, and Blood Institute
Paul Gurbel, MD, Inova Heart and Vascular Institute
Eileen M. Handberg, PhD, University of Florida School of Medicine
Ahmed Hasan, MD, National Heart, Lung, and Blood Institute
Joseph A. Hill, MD, PhD, University of Texas Southwestern Medical Center
Judith S. Hochman, MD, New York University School of Medicine
Erin Iturriaga, BS, MSN, National Heart, Lung, and Blood Institute
Ruth Kirby, BS, RN, National Heart, Lung, and Blood Institute
Glenn N. Levine, MD, Baylor College of Medicine
Peter Libby, MD, Brigham and Women’s Hospital
Joao Lima, MD, Johns Hopkins University School of Medicine
Puja Mehta, MD, Emory University School of Medicine
Patrice Desvigne-Nickens, MD, National Heart, Lung, and Blood Institute
Michelle Olive, PhD, National Heart, Lung, and Blood Institute
Gail D. Pearson, MD, National Heart, Lung, and Blood Institute
Arshed A. Quyyumi, MD, Emory University School of Medicine
Harmony Reynolds, MD, New York University Langone Medical Center
British Robinson, MA, Women’s Heart Alliance
George Sopko, MD, National Heart, Lung, and Blood Institute
Viviany Taqueti, MD, Brigham and Women’s Hospital
Janet Wei, MD, Cedars-Sinai Heart Institute
Nanette Wenger, MD, Emory University School of Medicine
Footnotes
Disclaimer
The views expressed in this document are the authors’ and do not necessarily reflect those of the National Institutes of Health or the Department of Health and Human Services or the ACCF, AHA or ESC.
Disclosures
Dr. Bairey Merz reports receiving consulting monies from Gilead, Medscape, and Research Triangle Institute (RTI) International and research grants from the NIH. Dr. Bairey Merz also reports receiving payment for lectures from Beaumont 7th Annual Heart Disease, C2, European Horizon 2020, Florida Hospital, 5th Annual Flagstaff Cardiology Symposium, Gilead, INOVA, Korean Cardiology Society, Medscape, PCP Symposium – Santa Rosa, Practice Point Communications, Pri-Med, Valley Health Grand Rounds, VBWG, University of Colorado, University of Utah, Washington University Grand Rounds, and WomenHeart. Dr. Pepine has received unrestricted educational grants to the University of Florida for the Vascular Biology Working Group—Amgen, AstraZeneca, Bayer Healthcare, Boehringer Ingleheim, Daiichi Sankyo, Gilead Sciences, Pfizer, United Therapeutics; Grant support from Bayer Healthcare (FINESSE HF), Baxter Healthcare (CMI-RENEW), Capricor (ALLSTAR), Cytori Therapeutics (ATHENA and ATHENA Ancillary), Florida Health Equity Research Inst. (HERI), Gilead (RWISE), inVentive Health Clinical LLC (TEVA), Sanofi-Aventis (ODYSSEY); and consulting fees from Amgen, AstraZeneca, Bayer HealthCare, FACT (Foundation for the Accreditation of Cellular Therapy), Gilead, Merck, and SLACK Inc. Dr. Walsh has no disclosures to report. Dr. Fleg has no disclosures to report.
References
- 1.Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–44. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 3.Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, Leon B, Bhatt DL, Fihn SD, Rumsfeld JS. Nonobstructive coronary artery disease and risk of myocardial infarction. Jama. 2014;312:1754–63. doi: 10.1001/jama.2014.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, Duvernoy C, Walsh MN, Bairey Merz CN. Emergence of Nonobstructive Coronary Artery Disease: A Woman’s Problem and Need for Change in Definition on Angiography. J Am Coll Cardiol. 2015;66:1918–33. doi: 10.1016/j.jacc.2015.08.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouldzein H, Elbaz M, Roncalli J, Cagnac R, Carrie D, Puel J, Alibelli-Chemarin MJ. Plaque rupture and morphological characteristics of the culprit lesion in acute coronary syndromes without significant angiographic lesion: analysis by intravascular ultrasound. Ann Cardiol Angeiol (Paris) 2012;61:20–6. doi: 10.1016/j.ancard.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, Pena-Sing I, Axel L, Attubato MJ, Yatskar L, Kalhorn RT, Wood DA, Lobach IV, Hochman JS. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–25. doi: 10.1161/CIRCULATIONAHA.111.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, De Caterina R, Zimarino M, Roffi M, Kjeldsen K, Atar D, Kaski JC, Sechtem U, Tornvall P. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw149. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G Investigators W. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–9. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 9.Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, Block PC, Hewitt K, Weintraub WS, Peterson ED. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117:1787–801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 10.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–50. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jespersen L, Abildstrom SZ, Hvelplund A, Madsen JK, Galatius S, Pedersen F, Hojberg S, Prescott E. Burden of hospital admission and repeat angiography in angina pectoris patients with and without coronary artery disease: a registry-based cohort study. PLoS One. 2014;9:e93170. doi: 10.1371/journal.pone.0093170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 13.Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G Investigators W. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan AK, Holdright DR, Wright CA, Sparrow JL, Cunningham D, Fox KM. Chest pain in women: clinical, investigative, and prognostic features. Bmj. 1994;308:883–6. doi: 10.1136/bmj.308.6933.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eastwood JA, Johnson BD, Rutledge T, Bittner V, Whittaker KS, Krantz DS, Cornell CE, Eteiba W, Handberg E, Vido D, Bairey Merz CN. Anginal symptoms, coronary artery disease, and adverse outcomes in Black and White women: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. J Womens Health (Larchmt) 2013;22:724–32. doi: 10.1089/jwh.2012.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kothawade K, Bairey Merz CN. Microvascular coronary dysfunction in women: pathophysiology, diagnosis, and management. Curr Probl Cardiol. 2011;36:291–318. doi: 10.1016/j.cpcardiol.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharaf B, Wood T, Shaw L, Johnson BD, Kelsey S, Anderson RD, Pepine CJ, Bairey Merz CN. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166:134–41. doi: 10.1016/j.ahj.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sedlak TL, Lee M, Izadnegahdar M, Merz CN, Gao M, Humphries KH. Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. Am Heart J. 2013;166:38–44. doi: 10.1016/j.ahj.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Maffei E, Raff G, Shaw LJ, Villines T, Berman DS. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–60. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 20.Johnson BD, Shaw LJ, Pepine CJ, Reis SE, Kelsey SF, Sopko G, Rogers WJ, Mankad S, Sharaf BL, Bittner V, Bairey Merz CN. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27:1408–15. doi: 10.1093/eurheartj/ehl040. [DOI] [PubMed] [Google Scholar]
- 21.Leipsic J, Taylor CM, Gransar H, Shaw LJ, Ahmadi A, Thompson A, Humphries K, Berman DS, Hausleiter J, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chow BJ, Cury RC, Delago AJ, Dunning AL, Feuchtner GM, Hadamitzky M, Kaufmann PA, Lin FY, Chinnaiyan KM, Maffei E, Raff GL, Villines TC, Gomez MJ, Min JK. Sex-based prognostic implications of nonobstructive coronary artery disease: results from the international multicenter CONFIRM study. Radiology. 2014;273:393–400. doi: 10.1148/radiol.14140269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–60. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepine CJ. Multiple causes for ischemia without obstructive coronary artery disease: not a short list. Circulation. 2015;131:1044–6. doi: 10.1161/CIRCULATIONAHA.115.015553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camici PG, Crea F. Coronary microvascular dysfunction. New Engl J Med. 2007;356:830–40. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 25.Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RS. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58:740–8. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 26.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–24. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukushima K, Javadi MS, Higuchi T, Lautamaki R, Merrill J, Nekolla SG, Bengel FM. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med. 2011;52:726–32. doi: 10.2967/jnumed.110.081828. [DOI] [PubMed] [Google Scholar]
- 28.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. doi: 10.1161/CIRCULATIONAHA.114.011939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–68. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–32. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balazs E, Pinter KS, Egyed A, Csanady M, Forster T, Nemes A. The independent long-term prognostic value of coronary flow velocity reserve in female patients with chest pain and negative coronary angiograms (results from the SZEGED study) Int J Cardiol. 2011;146:259–61. doi: 10.1016/j.ijcard.2010.10.071. [DOI] [PubMed] [Google Scholar]
- 32.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–27. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Britten MB, Zeiher AM, Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis. 2004;15:259–64. doi: 10.1097/01.mca.0000134590.99841.81. [DOI] [PubMed] [Google Scholar]
- 34.Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, Sayre J, Dahlbom M, Licinio J, Schelbert HR. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol. 2006;47:1188–95. doi: 10.1016/j.jacc.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 35.Rigo F, Sicari R, Gherardi S, Djordjevic-Dikic A, Cortigiani L, Picano E. Prognostic value of coronary flow reserve in medically treated patients with left anterior descending coronary disease with stenosis 51% to 75% in diameter. Am J Cardiol. 2007;100:1527–31. doi: 10.1016/j.amjcard.2007.06.060. [DOI] [PubMed] [Google Scholar]
- 36.Nemes A, Forster T, Geleijnse ML, Soliman OI, Cate FJ, Csanady M. Prognostic role of aortic atherosclerosis and coronary flow reserve in patients with suspected coronary artery disease. Int J Cardiol. 2008;131:45–50. doi: 10.1016/j.ijcard.2007.08.137. [DOI] [PubMed] [Google Scholar]
- 37.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–6. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 38.Tio RA, Dabeshlim A, Siebelink HM, de Sutter J, Hillege HL, Zeebregts CJ, Dierckx RA, van Veldhuisen DJ, Zijlstra F, Slart RH. Comparison between the prognostic value of left ventricular function and myocardial perfusion reserve in patients with ischemic heart disease. J Nucl Med. 2009;50:214–9. doi: 10.2967/jnumed.108.054395. [DOI] [PubMed] [Google Scholar]
- 39.Cortigiani L, Rigo F, Gherardi S, Galderisi M, Bovenzi F, Picano E, Sicari R. Prognostic effect of coronary flow reserve in women versus men with chest pain syndrome and normal dipyridamole stress echocardiography. Am J Cardiol. 2010;106:1703–8. doi: 10.1016/j.amjcard.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Ziadi MC, deKemp RA, Williams KA, Guo A, Chow BJW, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RSB. Impaired Myocardial Flow Reserve on Rubidium-82 Positron Emission Tomography Imaging Predicts Adverse Outcomes in Patients Assessed for Myocardial Ischemia. J Am Coll Cardiol. 2011;58:740–748. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 41.Jones E, Eteiba W, Merz NB. Cardiac syndrome X and microvascular coronary dysfunction. Trends Cardiovasc Med. 2012;22:161–8. doi: 10.1016/j.tcm.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography is Associated with Severely Impaired Coronary Flow Reserve, not Obstructive Disease. Circulation. 2016 doi: 10.1161/CIRCULATIONAHA.116.023266. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–11. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreau P, d’Uscio LV, Luscher TF. Structure and reactivity of small arteries in aging. Cardiovasc Res. 1998;37:247–53. doi: 10.1016/s0008-6363(97)00225-3. [DOI] [PubMed] [Google Scholar]
- 45.Rizzoni D, Palombo C, Porteri E, Muiesan ML, Kozakova M, La Canna G, Nardi M, Guelfi D, Salvetti M, Morizzo C, Vittone F, Rosei EA. Relationships between coronary flow vasodilator capacity and small artery remodelling in hypertensive patients. J Hypertens. 2003;21:625–31. doi: 10.1097/00004872-200303000-00030. [DOI] [PubMed] [Google Scholar]
- 46.Smith SM, Huo T, Delia Johnson B, Bittner V, Kelsey SF, Vido Thompson D, Noel Bairey Merz C, Pepine CJ, Cooper-Dehoff RM. Cardiovascular and mortality risk of apparent resistant hypertension in women with suspected myocardial ischemia: a report from the NHLBI-sponsored WISE Study. J Am Heart Assoc. 2014;3:e000660. doi: 10.1161/JAHA.113.000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sucato V, Evola S, Novo G, Novo S. Diagnosis of coronary microvascualar dysfunction in diabetic patients with cardiac syndrome X: comparison by current methods. Recenti Prog Med. 2013;104:63–8. doi: 10.1701/1241.13706. [DOI] [PubMed] [Google Scholar]
- 48.Maseri A, Crea F, Kaski JC, Crake T. Mechanisms of angina pectoris in syndrome X. J Am Coll Cardiol. 1991;17:499–506. doi: 10.1016/s0735-1097(10)80122-6. [DOI] [PubMed] [Google Scholar]
- 49.Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41:1387–93. doi: 10.1016/s0735-1097(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 50.Kaufmann PA, Gnecchi-Ruscone T, Schafers KP, Luscher TF, Camici PG. Low density lipoprotein cholesterol and coronary microvascular dysfunction in hypercholesterolemia. J Am Coll Cardiol. 2000;36:103–9. doi: 10.1016/s0735-1097(00)00697-5. [DOI] [PubMed] [Google Scholar]
- 51.Wessel TR, Arant CB, McGorray SP, Sharaf BL, Reis SE, Kerensky RA, von Mering GO, Smith KM, Pauly DF, Handberg EM, Mankad S, Olson MB, Johnson BD, Merz CN, Sopko G, Pepine CJ. Coronary microvascular reactivity is only partially predicted by atherosclerosis risk factors or coronary artery disease in women evaluated for suspected ischemia: results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE) Clin Cardiol. 2007;30:69–74. doi: 10.1002/clc.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Recio-Mayoral A, Rimoldi OE, Camici PG, Kaski JC. Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. JACC Cardiovasc Imaging. 2013;6:660–7. doi: 10.1016/j.jcmg.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Cosin-Sales J, Pizzi C, Brown S, Kaski JC. C-reactive protein, clinical presentation, and ischemic activity in patients with chest pain and normal coronary angiograms. J Am Coll Cardiol. 2003;41:1468–74. doi: 10.1016/s0735-1097(03)00243-2. [DOI] [PubMed] [Google Scholar]
- 54.Sakr SA, Abbas TM, Amer MZ, Dawood EM, El-Shahat N, Abdel Aal IA, Ramadan MM. Microvascular angina. The possible role of inflammation, uric acid, and endothelial dysfunction. Int Heart J. 2009;50:407–19. doi: 10.1536/ihj.50.407. [DOI] [PubMed] [Google Scholar]
- 55.Ong P, Sivanathan R, Borgulya G, Bizrah M, Iqbal Y, Andoh J, Gaze D, Kaski JC. Obesity, inflammation and brachial artery flow-mediated dilatation: therapeutic targets in patients with microvascular angina (cardiac syndrome X) Cardiovasc Drugs Ther. 2012;26:239–44. doi: 10.1007/s10557-012-6382-4. [DOI] [PubMed] [Google Scholar]
- 56.Teragawa H, Fukuda Y, Matsuda K, Ueda K, Higashi Y, Oshima T, Yoshizumi M, Chayama K. Relation between C reactive protein concentrations and coronary microvascular endothelial function. Heart. 2004;90:750–4. doi: 10.1136/hrt.2003.022269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ishimori ML, Martin R, Berman DS, Goykhman P, Shaw LJ, Shufelt C, Slomka PJ, Thomson LE, Schapira J, Yang Y, Wallace DJ, Weisman MH, Bairey Merz CN. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging. 2011;4:27–33. doi: 10.1016/j.jcmg.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 58.Hahn VS, Lenihan DJ, Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc. 2014;3:e000665. doi: 10.1161/JAHA.113.000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30:1837–43. doi: 10.1093/eurheartj/ehp205. [DOI] [PubMed] [Google Scholar]
- 60.Nichols WW, Denardo SJ, Davidson JB, Huo T, Bairey Merz CN, Pepine CJ. Association of aortic stiffness and wave reflections with coronary flow reserve in women without obstructive coronary artery disease: An ancillary study from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Am Heart J. 2015;170:1243–54. doi: 10.1016/j.ahj.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Redheuil A, Yu WC, Wu CO, Mousseaux E, de Cesare A, Yan R, Kachenoura N, Bluemke D, Lima JA. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010;55:319–26. doi: 10.1161/HYPERTENSIONAHA.109.141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Libby P, Pasterkamp G. Requiem for the ‘vulnerable plaque’. Eur Heart J. 2015;36:2984–7. doi: 10.1093/eurheartj/ehv349. [DOI] [PubMed] [Google Scholar]
- 63.Vitiello L, Spoletini I, Gorini S, Pontecorvo L, Ferrari D, Ferraro E, Stabile E, Caprio M, La Sala A. Microvascular inflammation in atherosclerosis. IJC Metab Endocr. 2014;3:1–7. [Google Scholar]
- 64.Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, Denardo SJ, McGorray SP, Smith KM, Sharaf BL, Nicholls SJ, Nissen SE, Anderson RD. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) J Interv Cardiol. 2010;23:511–9. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davis KB, Chaitman B, Ryan T, Bittner V, Kennedy JW. Comparison of 15-year survival for men and women after initial medical or surgical treatment for coronary artery disease: a CASS registry study. Coronary Artery Surgery Study. J Am Coll Cardiol. 1995;25:1000–9. doi: 10.1016/0735-1097(94)00518-u. [DOI] [PubMed] [Google Scholar]
- 66.Fisher LD, Judkins MP, Lesperance J, Cameron A, Swaye P, Ryan T, Maynard C, Bourassa M, Kennedy JW, Gosselin A, Kemp H, Faxon D, Wexler L, Davis KB. Reproducibility of coronary arteriographic reading in the coronary artery surgery study (CASS) Cathet Cardiovasc Diagn. 1982;8:565–75. doi: 10.1002/ccd.1810080605. [DOI] [PubMed] [Google Scholar]
- 67.Kemp HG, Kronmal RA, Vlietstra RE, Frye RL. Seven year survival of patients with normal or near normal coronary arteriograms: a CASS registry study. J Am Coll Cardiol. 1986;7:479–83. doi: 10.1016/s0735-1097(86)80456-9. [DOI] [PubMed] [Google Scholar]
- 68.Petersen JW, Johnson BD, Kip KE, Anderson RD, Handberg EM, Sharaf B, Mehta PK, Kelsey SF, Merz CN, Pepine CJ. TIMI frame count and adverse events in women with no obstructive coronary disease: a pilot study from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) PLoS One. 2014;9:e96630. doi: 10.1371/journal.pone.0096630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baller D, Notohamiprodjo G, Gleichmann U, Holzinger J, Weise R, Lehmann J. Improvement in coronary flow reserve determined by positron emission tomography after 6 months of cholesterol-lowering therapy in patients with early stages of coronary atherosclerosis. Circulation. 1999;99:2871–5. doi: 10.1161/01.cir.99.22.2871. [DOI] [PubMed] [Google Scholar]
- 70.Wei J, Nelson MD, Szczepaniak EW, Smith L, Mehta PK, Thomson LE, Berman DS, Li D, Bairey Merz CN, Szczepaniak LS. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am J Physiol Heart Circ Physiol. 2016;310:H14–9. doi: 10.1152/ajpheart.00612.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colom B, Oliver J, Garcia-Palmer FJ. Sexual Dimorphism in the Alterations of Cardiac Muscle Mitochondrial Bioenergetics Associated to the Ageing Process. J Gerontol A Biol Sci Med Sci. 2015;70:1360–9. doi: 10.1093/gerona/glu014. [DOI] [PubMed] [Google Scholar]
- 72.Eroglu S, Sade LE, Bozbas H, Haberal A, Ozbicer S, Demir O, Muderrisoglu H. Association of serum adiponectin levels and coronary flow reserve in women with normal coronary angiography. Eur J Cardiovasc Prev Rehabil. 2009;16:290–6. doi: 10.1097/HJR.0b013e32831f1b8a. [DOI] [PubMed] [Google Scholar]
- 73.Regensteiner JG, Golden S, Huebschmann AG, Barrett-Connor E, Chang AY, Chyun D, Fox CS, Kim C, Mehta N, Reckelhoff JF, Reusch JE, Rexrode KM, Sumner AE, Welty FK, Wenger NK, Anton B. Sex Differences in the Cardiovascular Consequences of Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation. 2015;132:2424–47. doi: 10.1161/CIR.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 74.Di Carli MF, Charytan D, McMahon GT, Ganz P, Dorbala S, Schelbert HR. Coronary circulatory function in patients with the metabolic syndrome. J Nucl Med. 2011;52:1369–77. doi: 10.2967/jnumed.110.082883. [DOI] [PubMed] [Google Scholar]
- 75.Nahser PJ, Jr, Brown RE, Oskarsson H, Winniford MD, Rossen JD. Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation. 1995;91:635–40. doi: 10.1161/01.cir.91.3.635. [DOI] [PubMed] [Google Scholar]
- 76.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H, Narula J. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212–21. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 77.Lanza GA, Giordano A, Pristipino C, Calcagni ML, Meduri G, Trani C, Franceschini R, Crea F, Troncone L, Maseri A. Abnormal cardiac adrenergic nerve function in patients with syndrome X detected by [123I]metaiodobenzylguanidine myocardial scintigraphy. Circulation. 1997;96:821–6. doi: 10.1161/01.cir.96.3.821. [DOI] [PubMed] [Google Scholar]
- 78.Mehta PK, Nelson M, Thomson L, Friedman J, Hayes S, Hermel D, Slomka P, Swift A, Wei J, Cook-Wiens G, Sayari S, Irwin MR, Krantz D, Travin M, Berman D, Bairey Merz CN. Abnormal cardiac sympathetic activity detected by 123-I-meta-iodobenzylguanidine imaging in women with signs and symptoms of ischemia and no obstructive coronary artery disease. J Am Coll Cardiol. 2016;67(13_S):1619. [Google Scholar]
- 79.Klabunde RE. Cardiovascular Physiology Concepts. 1. Philadelphia: Lippincott; 2005. [Google Scholar]
- 80.Muller MD, Gao Z, McQuillan PM, Leuenberger UA, Sinoway LI. Coronary responses to cold air inhalation following afferent and efferent blockade. Am J Physiol Heart Circ Physiol. 2014;307:H228–35. doi: 10.1152/ajpheart.00174.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Carli MF, Tobes MC, Mangner T, Levine AB, Muzik O, Chakroborty P, Levine TB. Effects of cardiac sympathetic innervation on coronary blood flow. N Engl J Med. 1997;336:1208–15. doi: 10.1056/NEJM199704243361703. [DOI] [PubMed] [Google Scholar]
- 82.Harris KF, Matthews KA. Interactions between autonomic nervous system activity and endothelial function: a model for the development of cardiovascular disease. Psychosom Med. 2004;66:153–64. doi: 10.1097/01.psy.0000116719.95524.e2. [DOI] [PubMed] [Google Scholar]
- 83.Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO., 3rd Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am J Cardiol. 1995;76:125–30. doi: 10.1016/s0002-9149(99)80043-5. [DOI] [PubMed] [Google Scholar]
- 84.Rimoldi O, Spyrou N, Foale R, Hackett DR, Gregorini L, Camici PG. Limitation of coronary reserve after successful angioplasty is prevented by oral pretreatment with an alpha1-adrenergic antagonist. J Cardiovasc Pharmacol. 2000;36:310–5. doi: 10.1097/00005344-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 85.Lanza GA, Andreotti F, Sestito A, Sciahbasi A, Crea F, Maseri A. Platelet aggregability in cardiac syndrome X. Eur Heart J. 2001;22:1924–30. doi: 10.1053/euhj.2001.2624. [DOI] [PubMed] [Google Scholar]
- 86.Lanza GA, Aurigemma C, Fattorossi A, Scambia G, Crea F. Changes in platelet receptor expression and leukocyte-platelet aggregate formation following exercise in Cardiac Syndrome X. J Thromb Haemost. 2006;4:1623–5. doi: 10.1111/j.1538-7836.2006.02003.x. [DOI] [PubMed] [Google Scholar]
- 87.Aurigemma C, Scalone G, Fattorossi A, Sestito A, Lanza GA, Crea F. Adenosine inhibition of adenosine diphosphate and thrombin-induced monocyte-platelet aggregates in cardiac syndrome X. Thromb Res. 2009;124:116–20. doi: 10.1016/j.thromres.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 88.Ong P, Athanasiadis A, Borgulya G, Vokshi I, Bastiaenen R, Kubik S, Hill S, Schaufele T, Mahrholdt H, Kaski JC, Sechtem U. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129:1723–30. doi: 10.1161/CIRCULATIONAHA.113.004096. [DOI] [PubMed] [Google Scholar]
- 89.Reriani M, Sara JD, Flammer AJ, Gulati R, Li J, Rihal C, Lennon R, Lerman LO, Lerman A. Coronary endothelial function testing provides superior discrimination compared with standard clinical risk scoring in prediction of cardiovascular events. Coron Artery Dis. 2016;27:213–20. doi: 10.1097/MCA.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, Shufelt C, Kothawade K, Sopko G, Lerman A, Shaw L, Kelsey SF, Pepine CJ, Merz CN. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;5:646–53. doi: 10.1016/j.jcin.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation. 2015;131:528–35. doi: 10.1161/CIRCULATIONAHA.114.009716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mygind ND, Michelsen MM, Pena A, Frestad D, Dose N, Aziz A, Favber R, Høst N, Gustafsson I, Hansen PR, Hansen HS, Bairey Merz CN, Kastrup J, Prescott E. Coronary microvascular function and cardiovascular risk factors in women with angina pectoris and no obstructive coronary artery disease: The iPOWER Study. J Am Heart Assoc. 2016;5:e003064. doi: 10.1161/JAHA.115.003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lanza GA, Buffon A, Sestito A, Natale L, Sgueglia GA, Galiuto L, Infusino F, Mariani L, Centola A, Crea F. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51:466–72. doi: 10.1016/j.jacc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 94.Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, Pennell DJ. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–53. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 95.Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK, Gill EB, Johnson BD, Kenkre T, Handberg EM, Li D, Sharif B, Berman DS, Petersen JW, Pepine CJ, Bairey Merz CN. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8:e002481. doi: 10.1161/CIRCIMAGING.114.002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ballantyne CM, Raichlen JS, Nicholls SJ, Erbel R, Tardif JC, Brener SJ, Cain VA, Nissen SE. Effect of rosuvastatin therapy on coronary artery stenoses assessed by quantitative coronary angiography: a study to evaluate the effect of rosuvastatin on intravascular ultrasound-derived coronary atheroma burden. Circulation. 2008;117:2458–66. doi: 10.1161/CIRCULATIONAHA.108.773747. [DOI] [PubMed] [Google Scholar]
- 97.Dupuis J, Tardif JC, Cernacek P, Theroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. The RECIFE (reduction of cholesterol in ischemia and function of the endothelium) trial. Circulation. 1999;99:3227–33. doi: 10.1161/01.cir.99.25.3227. [DOI] [PubMed] [Google Scholar]
- 98.Ridker PM, MacFadyen J, Libby P, Glynn RJ. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) Am J Cardiol. 2010;106:204–9. doi: 10.1016/j.amjcard.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X, Li Q, Zhao J, Li X, Sun X, Yang H, Wu Z, Yang J. Effects of combination of statin and calcium channel blocker in patients with cardiac syndrome X. Coron Artery Dis. 2014;25:40–4. doi: 10.1097/MCA.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 100.Caliskan M, Erdogan D, Gullu H, Topcu S, Ciftci O, Yildirir A, Muderrisoglu H. Effects of atorvastatin on coronary flow reserve in patients with slow coronary flow. Clin Cardiol. 2007;30:475–9. doi: 10.1002/clc.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eshtehardi P, McDaniel MC, Dhawan SS, Binongo JN, Krishnan SK, Golub L, Corban MT, Raggi P, Quyyumi AA, Samady H. Effect of intensive atorvastatin therapy on coronary atherosclerosis progression, composition, arterial remodeling, and microvascular function. The Journal of invasive cardiology. 2012;24:522–9. [PubMed] [Google Scholar]
- 102.Pizzi C, Manfrini O, Fontana F, Bugiardini R. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac Syndrome X: role of superoxide dismutase activity. Circulation. 2004;109:53–8. doi: 10.1161/01.CIR.0000100722.34034.E4. [DOI] [PubMed] [Google Scholar]
- 103.Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, Sopko G, Sharaf BM, Kelsey SF, Merz CN, Pepine CJ. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE) Am Heart J. 2011;162:678–84. doi: 10.1016/j.ahj.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schwartzkopff B, Brehm M, Mundhenke M, Strauer BE. Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension. 2000;36:220–5. doi: 10.1161/01.hyp.36.2.220. [DOI] [PubMed] [Google Scholar]
- 105.Bavry AA, Handberg EM, Huo T, Lerman A, Quyyumi AA, Shufelt C, Sharaf B, Merz CN, Cooper-DeHoff RM, Sopko G, Pepine CJ. Aldosterone inhibition and coronary endothelial function in women without obstructive coronary artery disease: an ancillary study of the national heart, lung, and blood institute-sponsored women’s ischemia syndrome evaluation. Am Heart J. 2014;167:826–32. doi: 10.1016/j.ahj.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garg R, Rao AD, Baimas-George M, Hurwitz S, Foster C, Shah RV, Jerosch-Herold M, Kwong RY, Di Carli MF, Adler GK. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes. 2015;64:236–42. doi: 10.2337/db14-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oestreicher EM, Martinez-Vasquez D, Stone JR, Jonasson L, Roubsanthisuk W, Mukasa K, Adler GK. Aldosterone and not plasminogen activator inhibitor-1 is a critical mediator of early angiotensin II/NG-nitro-L-arginine methyl ester-induced myocardial injury. Circulation. 2003;108:2517–23. doi: 10.1161/01.CIR.0000097000.51723.6F. [DOI] [PubMed] [Google Scholar]
- 108.Farquharson CA, Struthers AD. Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone-induced vasculopathy. Clinical science. 2002;103:425–31. doi: 10.1042/cs1030425. [DOI] [PubMed] [Google Scholar]
- 109.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014;130:1749–67. doi: 10.1161/CIR.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 110.Mehta PK, Bairey Merz CN. Braunwald’s Heart Disease. 9. Philadelphia, PA: Elsevier; 2011. Treatment of angina in subjects with evidence of myocardial ischemia and no obstructive coronary artery disease. [Google Scholar]
- 111.Kemp HG, Jr, Vokonas PS, Cohn PF, Gorlin R. The anginal syndrome associated with normal coronary arteriograms. Report of a six year experience. The American journal of medicine. 1973;54:735–42. doi: 10.1016/0002-9343(73)90060-0. [DOI] [PubMed] [Google Scholar]
- 112.Marinescu MA, Loffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging. 2015;8:210–20. doi: 10.1016/j.jcmg.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mizuno R, Fujimoto S, Saito Y, Okamoto Y. Optimal antihypertensive level for improvement of coronary microvascular dysfunction: the lower, the better? Hypertension. 2012;60:326–32. doi: 10.1161/HYPERTENSIONAHA.111.189209. [DOI] [PubMed] [Google Scholar]
- 114.Jadhav S, Ferrell W, Greer IA, Petrie JR, Cobbe SM, Sattar N. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2006;48:956–63. doi: 10.1016/j.jacc.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 115.Lim TK, Choy AJ, Khan F, Belch JJ, Struthers AD, Lang CC. Therapeutic development in cardiac syndrome X: a need to target the underlying pathophysiology. Cardiovascular therapeutics. 2009;27:49–58. doi: 10.1111/j.1755-5922.2008.00070.x. [DOI] [PubMed] [Google Scholar]
- 116.Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S Investigators I. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29:932–40. doi: 10.1093/eurheartj/ehn018. [DOI] [PubMed] [Google Scholar]
- 117.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 118.Parikh P, McDaniel MC, Ashen MD, Miller JI, Sorrentino M, Chan V, Blumenthal RS, Sperling LS. Diets and cardiovascular disease: an evidence-based assessment. J Am Coll Cardiol. 2005;45:1379–87. doi: 10.1016/j.jacc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- 119.Lanza GA, Colonna G, Pasceri V, Maseri A. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol. 1999;84:854–6. A8. doi: 10.1016/s0002-9149(99)00450-6. [DOI] [PubMed] [Google Scholar]
- 120.Leonardo F, Fragasso G, Rossetti E, Dabrowski P, Pagnotta P, Rosano GM, Chierchia SL. Comparison of trimetazidine with atenolol in patients with syndrome X: effects on diastolic function and exercise tolerance. Cardiologia. 1999;44:1065–9. [PubMed] [Google Scholar]
- 121.Matsuda Y, Akita H, Terashima M, Shiga N, Kanazawa K, Yokoyama M. Carvedilol improves endothelium-dependent dilatation in patients with coronary artery disease. Am Heart J. 2000;140:753–9. doi: 10.1067/mhj.2000.110093. [DOI] [PubMed] [Google Scholar]
- 122.Sutsch G, Oechslin E, Mayer I, Hess OM. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. Int J Cardiol. 1995;52:135–43. doi: 10.1016/0167-5273(95)02458-9. [DOI] [PubMed] [Google Scholar]
- 123.Cannon RO, 3rd, Watson RM, Rosing DR, Epstein SE. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol. 1985;56:242–6. doi: 10.1016/0002-9149(85)90842-2. [DOI] [PubMed] [Google Scholar]
- 124.Hasenfuss G, Maier LS. Mechanism of action of the new anti-ischemia drug ranolazine. Clinical research in cardiology : official journal of the German Cardiac Society. 2008;97:222–6. doi: 10.1007/s00392-007-0612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mehta PK, Goykhman P, Thomson LE, Shufelt C, Wei J, Yang Y, Gill E, Minissian M, Shaw LJ, Slomka PJ, Slivka M, Berman DS, Bairey Merz CN. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging. 2011;4:514–22. doi: 10.1016/j.jcmg.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Villano A, Di Franco A, Nerla R, Sestito A, Tarzia P, Lamendola P, Di Monaco A, Sarullo FM, Lanza GA, Crea F. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. 2013;112:8–13. doi: 10.1016/j.amjcard.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 127.Bairey Merz CN, Handberg EM, Shufelt CL, Mehta PK, Minissian MB, Wei J, Thomson LE, Berman DS, Shaw LJ, Petersen JW, Brown GH, Anderson RD, Shuster JJ, Cook-Wiens G, Rogatko A, Pepine CJ. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J. 2016;37:1504–13. doi: 10.1093/eurheartj/ehv647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Skalidis EI, Hamilos MI, Chlouverakis G, Zacharis EA, Vardas PE. Ivabradine improves coronary flow reserve in patients with stable coronary artery disease. Atherosclerosis. 2011;215:160–5. doi: 10.1016/j.atherosclerosis.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 129.Yesildag O, Yazici M, Yilmaz O, Ucar R, Sagkan O. The effect of aminophylline infusion on the exercise capacity in patients with syndrome X. Acta cardiologica. 1999;54:335–7. [PubMed] [Google Scholar]
- 130.Elliott PM, Krzyzowska-Dickinson K, Calvino R, Hann C, Kaski JC. Effect of oral aminophylline in patients with angina and normal coronary arteriograms (cardiac syndrome X) Heart. 1997;77:523–6. doi: 10.1136/hrt.77.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi J, Wei L. Rho kinases in cardiovascular physiology and pathophysiology: the effect of fasudil. J Cardiovasc Pharmacol. 2013;62:341–54. doi: 10.1097/FJC.0b013e3182a3718f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003;41:15–9. doi: 10.1016/s0735-1097(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 133.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–6. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 134.Egashira K, Hirooka Y, Kuga T, Mohri M, Takeshita A. Effects of L-arginine supplementation on endothelium-dependent coronary vasodilation in patients with angina pectoris and normal coronary arteriograms. Circulation. 1996;94:130–4. doi: 10.1161/01.cir.94.2.130. [DOI] [PubMed] [Google Scholar]
- 135.Gellman J, Hare JM, Lowenstein CJ, Gerstenblith G, Coombs V, Langenberg P, Brinker JA, Resar JR. L-arginine ameliorates the abnormal sympathetic response of the dysfunctional human coronary microvasculature. Angiology. 2004;55:1–8. doi: 10.1177/000331970405500101. [DOI] [PubMed] [Google Scholar]
- 136.Lerman A, Burnett JC, Jr, Higano ST, McKinley LJ, Holmes DR., Jr Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123–8. doi: 10.1161/01.cir.97.21.2123. [DOI] [PubMed] [Google Scholar]
- 137.Cannon RO, 3rd, Quyyumi AA, Mincemoyer R, Stine AM, Gracely RH, Smith WB, Geraci MF, Black BC, Uhde TW, Waclawiw MA, Maher K, Benjamin SB. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. 1994;330:1411–7. doi: 10.1056/NEJM199405193302003. [DOI] [PubMed] [Google Scholar]
- 138.Cox ID, Hann CM, Kaski JC. Low dose imipramine improves chest pain but not quality of life in patients with angina and normal coronary angiograms. Eur Heart J. 1998;19:250–4. doi: 10.1053/euhj.1997.0615. [DOI] [PubMed] [Google Scholar]
- 139.Sestito A, Lanza GA, Le Pera D, De Armas L, Sgueglia GA, Infusino F, Miliucci R, Tonali PA, Crea F, Valeriani M. Spinal cord stimulation normalizes abnormal cortical pain processing in patients with cardiac syndrome X. Pain. 2008;139:82–9. doi: 10.1016/j.pain.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 140.Lanza GA, Sestito A, Sandric S, Cioni B, Tamburrini G, Barollo A, Crea F, De Seta F, Meglio M, Bellocci F, Maseri A. Spinal cord stimulation in patients with refractory anginal pain and normal coronary arteries. Italian heart journal : official journal of the Italian Federation of Cardiology. 2001;2:25–30. [PubMed] [Google Scholar]
- 141.Kitsou V, Xanthos T, Roberts R, Karlis GM, Padadimitriou L. Enhanced external counterpulsation: mechanisms of action and clinical applications. Acta cardiologica. 2010;65:239–47. doi: 10.2143/AC.65.2.2047060. [DOI] [PubMed] [Google Scholar]
- 142.Kronhaus KD, Lawson WE. Enhanced external counterpulsation is an effective treatment for Syndrome X. Int J Cardiol. 2009;135:256–7. doi: 10.1016/j.ijcard.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 143.Asbury EA, Kanji N, Ernst E, Barbir M, Collins P. Autogenic training to manage symptomology in women with chest pain and normal coronary arteries. Menopause. 2009;16:60–5. doi: 10.1097/GME.0b013e318184762e. [DOI] [PubMed] [Google Scholar]
- 144.Samim A, Nugent L, Mehta PK, Shufelt C, Bairey Merz CN. Treatment of angina and microvascular coronary dysfunction. Current treatment options in cardiovascular medicine. 2010;12:355–64. doi: 10.1007/s11936-010-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.