TGFβ signaling is linked to posterior capsule opacification (PCO), the most common complication of cataract surgery. TGFβ can induce primary lens epithelial cells to differentiate into two disparate, PCO-causing abnormal cell phenotypes, a variation of the TGFβ paradox. Analysis of the signaling pathways downstream of TGFβ reveals novel therapeutic targets for PCO.
Abstract
The most common vision-disrupting complication of cataract surgery is posterior capsule opacification (PCO; secondary cataract). PCO is caused by residual lens cells undergoing one of two very different cell fates: either transdifferentiating into myofibroblasts or maturing into lens fiber cells. Although TGFβ has been strongly implicated in lens cell fibrosis, the factors responsible for the latter process have not been identified. We show here for the first time that TGFβ can induce purified primary lens epithelial cells within the same culture to undergo differentiation into either lens fiber cells or myofibroblasts. Marker analysis confirmed that the two cell phenotypes were mutually exclusive. Blocking the p38 kinase pathway, either with direct inhibitors of the p38 MAP kinase or a small-molecule therapeutic that also inhibits the activation of p38, prevented TGFβ from inducing epithelial–myofibroblast transition and cell migration but did not prevent fiber cell differentiation. Rapamycin had the converse effect, linking MTOR signaling to induction of fiber cell differentiation by TGFβ. In addition to providing novel potential therapeutic strategies for PCO, our findings extend the so-called TGFβ paradox, in which TGFβ can induce two disparate cell fates, to a new epithelial disease state.
INTRODUCTION
The lens consists of a monolayer of epithelial cells at the anterior face of the organ and highly elongated, crystallin-rich fiber cells that differentiate from these epithelial cells at a region of the lens termed the lens equator (Cvekl and Ashery-Padan, 2014). The lens is encased by the acellular lens capsule, which is the thickest basement membrane in the body. A loss of transparency of the lens that disrupts its ability to focus light on the retina is referred to as a cataract. Cataracts are a leading cause of visual impairment worldwide, estimated to be responsible for 10.8 million cases of blindness in 2010 (Khairallah et al., 2015). Rates of cataract are increasing due in part to an aging population in many countries.
The only treatment for cataract is cataract surgery, in which a hole is made in the anterior of the lens capsule through which a probe is inserted that ultrasonically disrupts the lens fiber cell mass. The lens fragments are removed by aspiration, after which an artificial lens is inserted into the natural lens capsule. Despite nearly 50 yr of optimization, it remains exceedingly difficult to remove all of the lens epithelial cells from the anterior capsule during cataract surgery (Apple et al., 2000, 2011; Awasthi et al., 2009; Wormstone et al., 2009; Vasavada and Praveen, 2014). Some residual lens cells become myofibroblasts. When these abnormal cells accumulate at the rear of the lens capsule, they obscure the light path by wrinkling the capsule and depositing large amounts of opaque, fibronectin- and collagen-enriched extracellular matrix. Other residual lens epithelial cells take on a very different fate, differentiating into immature lens fiber cells that undergo a large increase in cell volume and that express lens fiber cell–specific proteins (e.g., aquaporin 0; beaded filament proteins). In the absence of normal lens architecture, these enlarged cells can take on a globular shape. The presence of such crystallin-rich, so-called Elschnig pearls at the rear of the lens capsule interferes with vision due to light scattering.
The disorder resulting from the pathological conversion of lens cells to myofibroblasts or to lens fiber cells after cataract surgery is referred to as posterior capsule opacification (PCO), commonly referred to as secondary cataract. The fibrotic/myofibroblast and lens fiber cell types of PCO have been reported to coexist within the same eye, in which the two populations of cells can be adjacent to each other (Raj et al., 2007). Cells in the anterior epithelium of the intact lens can also take on both of these abnormal cell fates in response to injury or disease, forming anterior subcapsular cataracts (ASCs; Lovicu et al., 2004a, b).
PCO is the most common vision-disrupting complication of cataract surgery, with a reported incidence of ∼4–12% after uncomplicated standard cataract surgery in healthy adults (Findl et al., 2010) and a much higher prevalence in certain other populations (e.g., very young children; Medsinge and Nischal, 2015). The only standard treatment for PCO is YAG capsulotomy, in which an externally applied laser is used to ablate the posterior lens capsule and any associated cells. Drawbacks of this procedure include its high cost ($158 million billed to Medicare in 2003; Cleary et al., 2007), limited availability in underdeveloped/economically disadvantaged areas, the potential for reoccurrence of PCO (Menapace, 2008), and the risk of serious ocular complications, including cystoid macular edema, secondary glaucoma, and retinal detachment (Javitt et al., 1992; Pandey et al., 2004). These concerns have led to an intense interest in developing strategies to prevent the formation of PCO, thereby precluding the need for laser capsulotomy.
Essential to the rational design of novel anti-PCO therapies is an understanding of the molecular mechanisms responsible for the vision-disrupting features of this disease. It is known that the level of transforming growth factor β (TGFβ) signaling is increased in lens epithelial cells remaining after cataract surgery, presumably as part of a wound-healing response (Saika et al., 2002). A large body of evidence implicates TGFβ in the fibrotic form of PCO (Meacock et al., 2000; Wormstone et al., 2002, 2006; de Iongh et al., 2005; Walker and Menko, 2009). TGFβ is the only factor known to be able to induce lens cells to undergo epithelial–mesenchymal transition (EMT) into α−smooth muscle actin (αSMA)–expressing myofibroblasts in the three major in vitro systems heretofore used to study PCO, namely weanling rat lens central epithelial explants, lens epithelial cell–derived cell lines, and capsular bags prepared from human cadaver lenses (Wormstone and Eldred, 2016). Overexpression of a self-activating form of TGFβ in the intact rodent lens in vivo results in the formation of ASC containing fibrotic, myofibroblastic cells (Srinivasan et al., 1998; Lovicu et al., 2004a, b; Robertson et al., 2007).
In contrast, little is known about the factors involved in lens fiber cell–type PCO, considered to be the major cause of vision loss after cataract surgery (Apple et al., 2000; Findl et al., 2010). The primary inducer of epithelial-to-fiber cell differentiation in the intact lens during normal lens development is fibroblast growth factor (FGF), which is found in levels sufficient to promote fiber cell differentiation in the vitreous humor but not in the aqueous humor (reviewed in Lovicu and McAvoy, 2005; Robinson, 2006), It has generally been assumed, but never experimentally proven, that the high levels of FGF in the vitreous humor are also responsible for the differentiation of residual lens epithelial cells into fiber cells after cataract surgery (Wormstone et al., 2009; Wormstone and Eldred, 2016; Shirai et al., 2014). This cannot, however, explain how lens epithelial cells that are not exposed to vitreous humor can nonetheless undergo lens fiber cell differentiation. For example, cells expressing lens fiber cell–specific proteins have been reported on the anterior (e.g., aqueous humor-facing) surface of the lens in TGFβ-induced ASCs in rodents, as well as in naturally occurring ASC in humans (Lovicu et al., 2004b; Banh et al., 2006; Johar and Vasavsda, 2008). Formation of fiber-like Elschnig pearls has been described after posterior capsule buttonholing, a modified cataract surgery procedure in which residual lens epithelial cells on both the posterior and anterior lens capsules are exposed to aqueous, but not to vitreous, humor (Menapace et al., 2008). Although it has been reported that the amount of FGF2 in aqueous humor increases after cataract surgery, the increase measured in rabbits (Wallentin et al., 1998) and human patients (Chen et al., 2015) is well below the level (∼10–15 ng/ml) required to induce fiber differentiation in isolated lens epithelial cells. Finally, it is noteworthy that the central posterior pole of the lens is not in direct contact with the vitreous body but is instead separated from it by a region (Berger’s space; capsulohyaloidal interspace) believed to be filled with aqueous humor (Millodot, 2008). Residual lens cells migrating into this region after cataract surgery to cause PCO may therefore be in an environment in which the level of FGF is too low to sustain fiber cell differentiation.
We show here for the first time that TGFβ, the main factor implicated in fibrotic PCO, can also induce lens epithelial cells to undergo differentiation into lens fiber cells in a purified primary lens cell system. As observed in human PCO (Raj et al., 2007) and ASC (Lovicu et al., 2004b), TGFβ-treated lens cultures have populations of lens fiber–like cells adjacent to cells stimulated by TGFβ to become myofibroblasts. Remarkably, however, the signal transduction cascades downstream of TGFβ receptors (TGFβRs) responsible for these two cell fates are very different. In cancer, TGFβ is well known to act first as a tumor suppressor and then switch to being a tumor promoter in later stages of the disease (the “TGFβ paradox”; Roberts and Wakefield, 2003; Principe et al., 2014). Our studies show that TGFβ is capable of concurrently directing two disparate cell fates in nontransformed lens epithelial cells, revealing a novel dual role for TGFβ in a very different type of disorder.
RESULTS
TGFβ induces both myofibroblast and lens fiber cell differentiation of lens epithelial cells in dissociated cell-derived monolayers
Compared to mammalian species, the embryonic chick is a practical source of a large number of lens epithelial cells that can be cultured under a variety of conditions and are amenable to many types of experimental manipulations (Musil, 2012; Wormstone and Eldred, 2016). Because of the structural and developmental similarities between avian and mammalian lenses, primary cultures of embryonic chick lens cells have been used as a model system to study vertebrate lens cell development and function for >40 yr (e.g., Piatigorsky et al., 1973). In our culture system (referred to as dissociated cell-derived monolayers [DCDMLs]), cells are grown on laminin, the major extracellular matrix component of the natural lens capsule, in a defined medium (M199 + BOTS) in the absence of serum to replicate the avascular environment of the lens in vivo. To further preserve their native state, the cells are never passaged.
As previously reported in mammalian lens cell systems (Lovicu et al., 2004a, b; Stump et al., 2006), addition of TGFβ to primary lens cell DCDML cultures stimulated the expression of the myofibroblast marker αSMA in stress fibers and down-regulated the lens epithelial cell markers Pax6, ZO-1, and Cx43 (Figure 1). This was accompanied by other features characteristic of classic EMT, including up-regulation of the fibrotic markers fibronectin, procollagen I, and α5 integrin, and redistribution of vinculin to focal adhesions. Induction of αSMA stress fiber-positive myofibroblasts required >3 d of treatment with ≥0.4 ng/ml TGFβ1 or β2. Of note, TGFβ did not adversely affect cell viability during the 6-d culture period (Supplemental Figure S1). No other growth factor tested (insulin, insulin-like growth factor-1 [IGF1], bone morphogenetic protein [BMP] 2, 4, 6, and 7; FGF 1, 2, and 9; heparin-binding epidermal growth factor–like growth factor [HB-EGF]; TGFα; or platelet-derived growth factor [PDGF]) promoted a fibrotic phenotype in DCDMLs. Using the nomenclature of Masszi et al. (2010), we refer to this process as the epithelial–myofibroblast transition (EMyT).
FIGURE 1:

TGFβ induces a loss of lens epithelial markers and a gain of EMT/EMyT markers. DCDMLs were cultured for 6 d with or without 4 ng/ml TGFβ1 before fixation and immunostaining for vinculin, the lens epithelial cell markers ZO-1, connexin43, and Pax6, the mesenchymal proteins fibronectin, procollagen 1, and α5 integrin, or the myofibroblast marker αSMA. Note that TGFβ induced a redistribution of vinculin from cell–cell interfaces to focal adhesions, indicative of EMT. Intracellular accumulation of procollagen I is due to low levels of ascorbic acid in the culture medium; supplementation with ascorbic acid stimulated secretion of procollagen I but did not otherwise detectably change the phenotype of myofibroblastic cells in TGFβ-treated DCDMLs (not shown). All markers assessed in a minimum of three independent experiments with similar results.
In addition to the very flat myofibroblastic cells, TGFβ-treated DCDMLs also contained phase-refractile clusters of enlarged cells with the morphological appearance of large lentoids, the structures formed by differentiating primary lens fiber cells in culture (Menko et al., 1984; Tenbroek et al., 1997; Figure 2A, left). Their identity was confirmed by the presence of aquaporin 0 (AQP0; MP28) and the beaded filament proteins CP49 and CP115, all of which are unique to differentiating and fully mature lens fiber cells (Cvelk and Ashery, 2014), as well as high levels of δ-crystallin (Figure 2A, right). Double-labeling immunofluorescence microscopy demonstrated that lentoids never expressed EMT/EMyT markers such as αSMA and procollagen I, whereas the αSMA-positive, flat myofibroblastic cells were always negative for the aforementioned lens fiber cell markers (Figure 2B). The fiber-like cells retained their nuclei and therefore more closely resembled cortical lens fiber cells than the fully mature, organelle-free fibers that form the lens core. Further demonstration that the lens fiber and myofibroblast cell fates are mutually exclusive is provided in Supplemental Figure S2. Of importance, TGFβ failed to up-regulate expression of both myofibroblast and lens fiber cell markers when cells were cultured in the presence of the highly selective TGFβ type 1 receptor (ALK5) kinase inhibitor SB-431542, indicating that both fates are downstream of TGFβR activation.
FIGURE 2:

TGFβ also induces lens fiber cell differentiation. (A, B) DCDMLs were cultured for 6 d with no additions (control), 4 ng/ml TGFβ1, or TGFβ1 plus the TGFβR inhibitor SB-431542 before phase-contrast microscopy (phase) or fixation and immunostaining for proteins exclusive to (AQP0 and the beaded filament proteins CP49/filensin and CP115/phakinin), or highly enriched in (δ−crystallin), differentiating lens fiber cells. In B, some cultures were double stained with the EMT/EMyT marker αSMA or procollagen 1 (procol). Hoechst 33342 was used to detect nuclei. Representative of at least four experiments, except for the SB-431542 data, which are representative of three experiments.
To quantitate expression of lens fiber cell markers, we used Western blotting (CP49 and CP115) and [35S]methionine labeling (δ-crystallin; Le and Musil, 2001; Musil, 2012). Normalized to β-actin, treatment of DCDMLs with 4 ng/ml TGFβ1 stimulated the expression of all three proteins to levels comparable to those obtained with FGF, the major inducer of lens fiber cell differentiation in normal lens development (Boswell et al., 2008; Boswell and Musil 2015; Figure 3). No increase in fiber cell marker expression was obtained if cells were cultured with PDGF or HB-EGF despite the presence of functional receptors for both of these growth factors in DCDMLs (unpublished data). As expected (Figure 2B), the TGFβR blocker SB-431542 reduced expression of all three lens fiber cell proteins by >85% when cells were cultured with TGFβ but had no significant effect on FGF-induced expression (Figure 3C).
FIGURE 3:
Biochemical assessment of lens fiber and EMT/EMyT marker expression in DCDMLs. DCDMLs were incubated without growth factor (control) or with 4 ng/ml TGFβ1 or 10 ng/ml FGF2 in either the absence or presence of the TGFβR inhibitor SB-431542. After 6 d of culture, cells were assayed for synthesis of the fiber cell differentiation markers δ-crystallin (by [35S]methionine labeling; entire lane shown in Supplemental Figure S1), CP115, and CP49 (by quantitative Western blotting). The cultures were also assayed for expression of the fibrotic markers fibronectin and αSMA by Western blot. (A) Data from a representative experiment. (B) Quantitation of expression of fiber cell differentiation markers, expressed as fold of control DCDMLs cultured with no additions. (C) Quantitation of the effect of SB-431542 on expression of fiber cell markers, expressed as fold of DCDMLs cultured with growth factor alone. Hashtags denote data sets in which SB-431542 completely blocked the ability of TGFβ to up-regulate fiber marker expression in at least three independent experiments. *p ≤ 0.01.
In keeping with recent studies in mammalian cells (Carthy et al., 2016), we found that the most robust and reproducible marker of fibrosis on Western blots was fibronectin, the amount of which was below the level of detection in the absence of TGFβ. As observed in other primary lens epithelial cells (Saika et al., 2004; Rungger-Brändle et al., 2005; Garcia et al., 2006), DCDMLs express a variable amount of αSMA in the absence of exogenously added TGFβ, albeit generally not organized in stress fibers as in myofibroblasts. Neither protein was detectable in FGF-treated cultures (Figure 3A).
The finding that myofibroblast and lens fiber cell differentiation are both inhibited by SB-431542 raised the question of whether blocking one cell fate downstream of TGFβ receptor activation would also inhibit the other. To address this issue, we examined the effect of two previously characterized, mechanistically distinct inhibitors of EMT on the response of DCDMLs to TGFβ. In several cell types, RGD-binding integrins and their ligand fibronectin are required for EMT (Serini et al., 1998; Jester et al., 1999). Given that TGFβ induces expression of fibronectin and of at least one RGD-binding integrin subunit (α5; Figure 1), we tested whether this is also the case in lens cells by using an RGDS peptide to disrupt integrin–fibronectin interactions. Culture of DCDMLs with a RGDS peptide blocked the ability of TGFβ to induce myofibroblast differentiation without killing the cells or causing them to disadhere (Figure 4A). It has been reported in several systems, including the mammalian lens (Dwivedi et al., 2006), that the activity of matrix metalloproteases (MMPs) is essential for TGFβ to stimulate myofibroblast differentiation. The general MMP inhibitor GM6001 also effectively prevented TGFβ from inducing EMT in DCDMLs (Figure 4A). Relative to TGFβ-only controls, expression of both myofibroblast markers was decreased by >80% when cells were cultured with TGFβ in the presence of either RGDS peptide or GM6001 (Figure 4B). In contrast, neither treatment reduced the expression of any of the three lens fiber markers measured, demonstrating that the two cell fates are independently regulated.
FIGURE 4:

Two different inhibitors of myofibroblast differentiation do not abrogate the ability of TGFβ to induce expression of lens fiber cell markers. DCDMLs were incubated without (control) or with 4 ng/ml TGFβ1 for 6 d in either the absence or presence of vehicle (0.1% DMSO), 200 μM RGDS peptide, or 20 μM GM6001. (A) Cells were fixed and stained with antibodies against procollagen 1 (green) or αSMA (red). Typical of five (GM6001) or three (RGDS) experiments. (B) Cells were lysed and assayed for the EMT/EMyT markers αSMA and fibronectin and the fiber cell differentiation markers δ-crystallin, CP115, and CP49 as in Figure 3. The extent to which each treatment reduced the ability of TGFβ to up-regulate the expression of the indicated protein is graphed relative to TGFβ plus DMSO-only cultures. *p ≤ 0.000.
Promotion of myofibroblast differentiation by TGFβ requires p38 and extracellular signal–regulated kinase activity
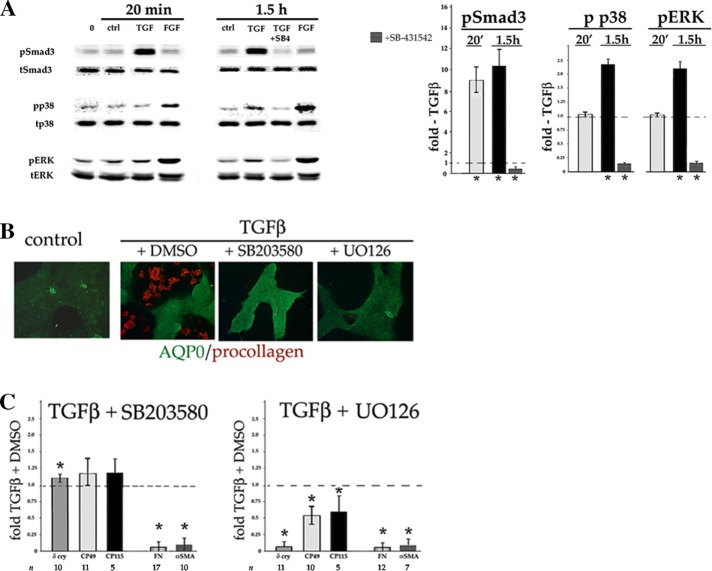
Next we addressed the signaling pathways underlying TGFβ’s dual effect on lens epithelial cell differentiation. In the canonical TGFβ signaling pathway, ligand-activated receptors phosphorylate the C-terminal SSXS motif of Smad2 and 3 proteins, which enhance or repress specific gene expression. TGFβ has also been reported to activate certain non-Smad pathways, notably mitogen-activated protein kinases (MAPKs), in a cell type– and context-dependent manner (Zhang, 2009; Derynck et al., 2014), including in primary lens cells (Lovicu et al., 2012; Tiwari et al, 2016) and lens cell lines (Dawes et al., 2009). As previously reported (Boswell et al., 2010), TGFβ stimulates C-terminal phosphorylation (activation) of Smad3 within 20 min of addition to DCDMLs (Figure 5A). Time-course studies showed that TGFβ also induced a 2.3 ± 0.2–fold (n = 59) activation (phosphorylation on Thr-180/Tyr-182) of p38 MAPK without affecting total p38 levels, but only after 1.5 h of treatment (Figure 5A). Assessing the rate at which TGFβ stimulates extracellular signal–regulated kinase (ERK) was confounded by fact that removing and replacing the same medium with no additions induced a variably large (up to threefold) transient activation (phosphorylation on Thr-202/Tyr-204) of ERK detectable within 5 min (compare pERK in lanes 0 and ctrl; Figure 5A). This phenomenon has been described in other mechanosensitive cell types and been considered as a response to shear stress (Li et al., 2003). At 1.5 h after medium removal and replacement, pERK levels in otherwise-untreated cells returned to near baseline and were 2.2 ± 0.3–fold (n = 21) higher in cells exposed to TGFβ (Figure 5A). Compared to fiber-differentiating levels of FGF (10 ng/ml), TGFβ induced a much weaker stimulation of ERK. Activation of Smad3, p38, and ERK by TGFβ was blocked by the TGFβR-specific inhibitor SB-431542 (Figure 5A).
FIGURE 5:
Inhibitors of p38 and ERK prevent TGFβ from inducing myofibroblast, but not lens fiber cell, differentiation. (A) A 10× stock of either TGFβ or FGF2 in culture medium was added to the growth medium of DCDMLs to reach a final concentration of 4 or 10 ng/ml, respectively. Control cultures received an equal volume of culture medium without growth factor (ctrl), or were left undisturbed (0). Where indicated, cells were pretreated with SB-431542 (SB4) before addition of TGFβ. After a 20-min or 1.5-h incubation, whole-cell lysates were prepared and probed with antibodies specific for the total or phosphorylated (activated) forms of Smad3, p38, or ERK. Fold activation induced by TGFβ over medium-only control (ctrl) is graphed, as is the value obtained in the presence of SB-431542. *p ≤ 0.004. For the remaining data sets, p ≥ 0.2. Representative of three or more independent experiments. Avian species only express ERK2. (B, C) DCDMLs were preincubated with 20 μM SB203580, 15 μM UO126, or vehicle (0.1% DMSO) for 1 h before addition of 4 ng/ml TGFβ, after which cells were cultured in the continuous presence of both drug and TGFβ for 6 d. Cells were processed for either (B) immunofluorescence microscopy (n = 6) or (C) Western blot/metabolic labeling analysis of EMT/EMyT and lens fiber cell markers as in Figure 4. *p ≤ 0.003. For the remaining data sets, p ≤ 0.05.
In some cell types, induction of EMT by TGFβ requires p38 and/or ERK activity (Derynck et al., 2014; Gonzalez and Medici, 2014). Blocking p38 activity with SB203580 (Davies et al., 2000) resulted in near-complete inhibition of expression of EMT/EMyT markers, including αSMA, fibronectin, and (as assessed by immunofluorescence microscopy) procollagen I (Figure 5, B and C), without a detectable effect on cell viability (Supplemental Figure S1). SB203580 did not, however, abrogate the ability of TGFβ to stimulate lens fiber cell differentiation. Similar results were obtained with other p38 inhibitors, including SB202190 and the mechanistically distinct p38 blocker BIRB 796 (unpublished data).
To test the role of ERK, we preincubated DCDMLs with UO126, a potent and highly specific inhibitor of MEK1/2, the kinases immediately upstream of ERK1/2 in the ERK signaling cascade (Davies et al., 2000). Western blotting demonstrated that the ratio of active to total ERK was rapidly reduced by ∼99% in either the absence or presence of TGFβ (Supplemental Figure S3). Cells cultured with UO126 and TGFβ failed to up-regulate EMT/EMyT markers over a 6-d period (Figure 5, C and D). Addition of UO126 also reduced expression of the fiber cell markers CP49 and CP115 by ∼50% and δ-crystallin by ∼100%. We conclude that p38 and ERK have distinct functions in DCDML cell fate, with the p38 pathway playing a specific, essential role in TGFβ-induced EMyT and ERK activity supporting both myofibroblast and lens fiber cell differentiation.
In mice, genetic ablation of Smad3 has been reported to greatly decrease (Banh et al., 2006) or completely block (Saika et al., 2004) the ability of TGFβ to induce myofibroblast differentiation of lens cells both in vivo and in ex vivo culture systems. One mechanism by which treatment of DCDMLs with p38 and/or ERK inhibitors could block TGFβ-induced myofibroblast differentiation would be if these compounds abrogated canonical Smad3 signaling. To test this possibility, we transiently transfected DCDMLs with a well-established reporter of Smad3-dependent gene expression (Jonk et al., 1998; Piek et al., 2001) and measured luciferase levels 48 h later. Unlike the TGFβR inhibitor SB431542, the p38 inhibitors SB203580 and BIRB 796, and the ERK pathway inhibitor UO126 did not prevent TGFβ from up-regulating SBE4-Luc expression (Figure 6). The small reduction in SBE-Luc levels in SB203580-treated cells could be attributed to the previously reported partial inhibition of the ALK5 TGFβR by micromolar levels of this drug (Laping et al., 2002).
FIGURE 6:
p38 and ERK activity are not required for TGFβ to induce Smad3-dependent gene expression. DCDMLs were transfected with the SBE4-Luc reporter construct. Where indicated, the cells were preincubated for 1 h with SB-431542, UO126, SB203580, or BIRB 0796 before culture for 2 d with no additions (ctrl) or 4 ng/ml TGFβ1. Expression of luciferase was assessed by Western blot analysis and normalized to β-actin in the same sample. Fold increase expression of reporter by TGFβ over control was 12.7 ± 5.2 (n = 7). The extent to which each treatment inhibited the ability of TGFβ to up-regulate the expression of the reporter is graphed for each condition. *p ≤ 0.000.
Promotion of lens fiber cell differentiation by TGFβ requires mammalian target of rapamycin activity
In vivo and in vitro studies demonstrated an essential role for FGF signaling in epithelial-to–fiber cell differentiation during normal lens development (reviewed in Lovicu and McAvoy, 2005; Robinson, 2006). The simplest mechanism by which TGFβ could promote lens fiber cell formation would be by increasing the expression and/or secretion of FGF to the level required for fiber differentiation. In DCDMLs, we showed that the ability of FGF to induce fiber cells depends on signaling via endogenously expressed BMP4 and/or 7 such that inhibiting BMP binding with noggin abolishes fiber cell differentiation in response to FGF (Boswell et al., 2008; Boswell and Musil, 2015). We found that noggin has little effect on induction of fiber cell marker expression by TGFβ (Figure 7A). Moreover, the FGF receptor inhibitor PD173074, although capable of abolishing fiber cell differentiation after addition of FGF (Boswell et al., 2008), only partially reduced induction of CP49 and CP115 by TGFβ. Of note, PD173074 also attenuated expression of the EMT/EMyT marker fibronectin (Figure 7, A and B). The inhibitor did not reduce activation of Smad3 by TGFβ, ruling out an off-target effect on TGFβR activation (Supplemental Figure S4A). Incubation of DCDMLs with PD173074, did, however, decrease the basal (e.g., no added growth factor) level of active ERK by ∼45% (Supplemental Figure S4B), in keeping with the fact that lens cells endogenously produce FGF and that FGF is a major inducer of pERK in the lens (Govindarajan and Overbeek, 2001; Zhao et al., 2008). Given that ERK signaling contributes to CP49 and CP115 expression downstream of TGFβ and is essential for up-regulation of δ-crystallin (Figure 5), it is possible that blocking endogenous FGF signaling with PD173074 blunts the ability of TGFβ to stimulate fiber formation (Figure 7) by reducing the level of active ERK, explaining why PD173074 phenocopies the effects of UO126 on fiber differentiation. The lesser inhibition of ERK by PD173074 (45%; Supplemental Figure S4) relative to that obtained with UO126 (99%; Supplemental Figure S3) could account for the ability of PD173074 to reduce but not abolish induction of fibronectin by TGFβ. Taken together, these findings suggest that the activity of FGF receptors, like that of their main downstream effector ERK, plays a general, permissive role in TGFβ-induced responses in lens cells instead of specifically directing fiber cell differentiation.
FIGURE 7:

FGFR signaling is not essential for induction of fiber cell differentiation by TGFβ. (A) DCDMLs were preincubated with noggin, PD173074, or vehicle (0.1% DMSO) for 1 h before addition of 4 ng/ml TGFβ1. Six days later, cells were processed for Western blot/metabolic labeling analysis of lens fiber cell markers and fibronectin as in Figure 4. *p ≤ 0.000. For the remaining data sets, p ≥ 0.4. (B) Representative Western blot data showing that PD173074 abolishes fiber cell marker expression by FGF but only partially reduces induction by TGFβ. PD173074 also reduces expression of fibronectin by TGFβ.
On the basis of these findings, we considered the possibility that TGFβ induction of fiber cell differentiation involves a signaling pathway not previously linked to fiber cell formation. Mammalian target of rapamycin (MTOR), a kinase central to developmental processes in a variety of cell types, is assembled into two complexes with distinct roles, MTORC1 and MTORC2. Rapamycin specifically blocks the activity of MTORC1 by binding to the obligatory MTORC1 subunit FKBP12, which is absent in MTORC2 (Laplante and Sabatini, 2012). In mammary epithelial NMuMG cells, rapamycin inhibits the ability of TGFβ to increase cell volume without blocking EMT (Lamouille and Derynck, 2007). Because fiber cell formation involves a large increase in cell size, we used rapamycin to investigate the role of MTOR in TGFβ-induced fiber cell differentiation in DCDMLs. We found that addition of 100 nM rapamycin greatly decreased the size of AQPO-positive lentoids (Figure 8A) and profoundly (≥90%) reduced the expression of the fiber cell markers δ-crystallin, CP115, and CP49 in the presence of TGFβ (Figure 8B). In contrast, rapamycin had only a minimal effect on induction of the same marker proteins by FGF (Figure 8B). Strikingly, myofibroblast differentiation was not inhibited by rapamycin, as assessed by Western blotting and immunofluorescence microscopy. Suppression of lens fiber cell, but not myofibroblast, differentiation by TGFβ was also observed with Ku-0063794, an inhibitor of MTORC1 and C2 that directly blocks the MTOR kinase active site (Garcia-Martinez et al., 2009; Figure 8B).
FIGURE 8:
Inhibitors of MTOR prevent TGFβ from inducing lens fiber cell, but not myofibroblast, differentiation. (A, B) DCDMLs were preincubated with 100 nM rapamycin, 20 μM Ku-0063794, rapamycin + 20 μM SB203580, or vehicle (0.1% DMSO) for 1 h before addition of 4 ng/ml TGFβ or 10 ng/ml FGF2. (A) After 6 d, cells were processed for immunofluorescence staining of AQP0 and αSMA (i–iv) or either AQP0 or CP49 and counterstained with Hoechst 33342 (v–viii). n = 3. (B) After 6 d, cells were processed for Western blot/metabolic labeling analysis of lens fiber cell and EMT/EMyT markers as in Figure 4. (C) A 1-h preincubation with 100 nM rapamycin does not block the ability of a 1.5-h treatment with TGFβ to activate Smad3 or ERK as assessed by Western blotting (p ≥ 0.46). (D, E) DCDMLs were incubated with KU-0063794, rapamycin, TGFβ1, IGF1, or FGF2 for the indicated period before Western blot assessment of phosphorylation (p) of AKT on serine 473 and total (t) AKT levels. Fold p/t AKT ratio relative to that obtained in DMSO-only controls in the same experiment. *p ≤ 0.02.
Rapamycin did not affect the ability of TGFβ to activate Smad3 or ERK (Figure 8C). Although exquisitely specific for MTORC1 after a short-term exposure, rapamycin is known to block the assembly, and therefore the function, of MTORC2 in some cell types after ≥12-h treatment (Sarbassov et al., 2006; Schreiber et al., 2015). AKT is a direct substrate of MTORC2, and its phosphorylation on Ser-473 is routinely used as readout of MTORC2 activity (Sarbassov et al., 2005). As expected, a 2-h exposure of DCDMLs to the direct MTORC1 and MTORC2 inhibitor Ku-0063794 reduced the levels of basal (e.g., no added growth factor) AKT pSer-473 by >90%, whereas rapamycin had no inhibitory effect (Figure 8D). Rapamycin did, however, reduce phosphorylation of AKT on Ser-473 below basal levels when the treatment was extended to ≥48 h in either the absence or presence of TGFβ. Thus, under the conditions in which it inhibits TGFβ-induced fiber cell differentiation, rapamycin blocks MTORC2 as well as MTORC1.
As expected, addition of the MTOR C1/2 agonist IGF1 to DCDMLs stimulated phosphorylation of Ser-473 AKT within 20 min. In contrast, we did not detect increased levels of pSer-473 AKT in response to TGFβ until 24 h of treatment (Figure 8E). Attempts to detect the phosphorylation of MTOR or of the canonical MTORC1 substrate p70S6K in response to TGFβ failed, most likely due to the limited sensitivity of the available antibodies. Taken together, the data shown in Figure 8 are in keeping with a requirement for MTORC1 and/or MTORC2 in lens fiber differentiation induced by TGFβ, although whether MTORC1/2 play a direct or a permissive role in this process remains to be determined (see the Discussion). AKT does not appear to be the sole effector of MTOR, given that two mechanistically distinct direct inhibitors of AKT did not phenocopy the effects of rapamycin on TGFβ-induced fiber cell differentiation (Supplemental Figure S5).
An important question is whether the previously unrecognized ability of TGFβ to up-regulate lens fiber cell marker expression in an MTOR-dependent manner is confined to chick genes. This was ruled out in a series of experiments in which DCDMLs were transfected with a reporter construct (DCR1-αA-promoter–enhanced green fluorescent protein [EGFP]) driven by upstream elements from mouse αA crystallin, a marker of fiber cell differentiation in the mammalian lens. The DCR1 lens-specific enhancer confers high-level expression on the reporter in fiber cells of transgenic mice (Yang et al., 2006), as well as the ability to be up-regulated by fiber-inducing levels of FGF2 in rat lens central epithelial explants (Yang et al., 2006) and in DCDMLs (Boswell and Musil, 2015). Expression of DCR1-αA-promoter–EGFP in response to TGFβ on day 9 was comparable to that induced by FGF2 (Figure 9). Similar to endogenously expressed fiber cell markers (Figures 2, 3, and 8), up-regulation of DCR1-αA-promoter–EGFP by TGFβ was blocked by the TGFβR inhibitor SB-431542, as well as by the MTOR inhibitor rapamycin. DCR1-αA-promoter–EGFP levels were not decreased, however, by the p38 inhibitor SB203580 at concentrations at which it abolished TGFβ-induced EMyT.
FIGURE 9:

TGFβ induces rapamycin-sensitive expression of a reporter of mammalian fiber differentiation in DCDMLs. DCDMLs were transfected with the DCR1-αA promoter-EGFP reporter construct and cultured for 8 d with no additions (control), 10 ng/ml FGF2, or 4 ng/ml FGF2. Where indicated, the cells were incubated with TGFβ in the presence of DMSO (0.1%), SB-431542 (SB4), rapamycin (rap), or SB203580 (SB2). Expression of EGFP was assessed by live-cell imaging of confluent regions of the cultures or by Western blot analysis. Fold increase over untreated control as measured by Western blot is graphed for each condition.
Typically, expression of myofibroblast markers is considerably greater in the presence of rapamycin and TGFβ than with TGFβ only (Figure 8B). It could therefore be argued that rapamycin acts by promoting the ability of TGFβ to induce EMyT and that the concomitant decrease in lens fiber differentiation is merely a downstream consequence of the fact that an individual cell can only undergo one cell fate or the other. If so, then inhibiting the ability of TGFβ to induce myofibroblast differentiation should allow cells treated with TGFβ and rapamycin to undergo fiber differentiation. To test this possibility, we used SB203580, which prevents TGFβ from inducing fibrosis without inhibiting fiber cell marker expression (Figure 5). As would be expected, cells treated with TGFβ + SB203580 + rapamycin express much lower levels of myofibroblast markers than cells treated with only TGFβ and rapamycin (Figure 8, A and B). If the SB203580-induced reduction in EMyT were sufficient to allow TGFβ/rapamycin to induce fiber differentiation, then such triply treated cells should have high levels of fiber cell marker expression. Instead, DCDMLs simultaneously treated with TGFβ, rapamycin, and SB203580 remained mainly as undifferentiated lens epithelial cells (Figure 8, A and B). We conclude that rapamycin exerts its effect on lens cell fate by inhibiting TGFβ from inducing fiber differentiation instead of by promoting myofibroblast formation. It also follows from this experiment that SB203580 blocks TGFβ-induced EMyT directly instead of indirectly by promoting fiber cell differentiation.
Promotion of cell migration by TGFβ is associated with myofibroblast differentiation
Migration of lens cells to the posterior pole of the lens capsule is essential to vision-disrupting PCO, making it a potential therapeutic target. TGFβ has been shown to be a promigratory factor for many cell types (Giehl and Menke, 2006), including human lens epithelial cell lines (Chang and Petrash, 2015). To study migration in DCDMLs, we adapted a previously developed semiquantitative assay (Afshari and Fawcett, 2012) in which coverslips containing confluent cultures of lens cells were placed cell side down into larger, laminin-coated wells. The cells were then cultured in the continuous presence of the mitotic inhibitor aphidicolin. As detected by phase-contrast microscopy, 4 ng/ml TGFβ induced robust migration of cells from the edge of the coverslip onto the previously cell-free surface. The ability of TGFβ to enhance migration was blocked by the TGFβR inhibitor SB-431542 and was not shared by the TGFβ superfamily member BMP4, demonstrating the specificity of the effect (Figure 10A).
FIGURE 10:

Up-regulation of cell migration by TGFβ in DCDMLs is associated with EMT/EMyT. Confluent DCDMLs grown on a laminin-coated 96-well coverslip were transferred cell side down onto a laminin-coated 48-well plate. The cells were then cultured for 4–6 d with vehicle only (0.1% DMSO; control) or in the continuous presence of either 4 ng/ml TGFβ1 or 5 ng/ml BMP4, with or without inhibitor (SB-431542, SB203580, UO126, or rapamycin) as indicated. Cells migrating from the edge of the coverslip were visualized by phase contrast microscopy (A, C), or after fixation and double staining for α−tubulin (to label all cells) and αSMA (to label lens cells that had undergone EMyT); edge of coverslip indicated by white line (B). Typical of at least three independent experiments.
Immunofluorescence microscopy revealed that most of the migrating cells in TGFβ-treated cultures expressed αSMA in stress fibers (Figure 10B). We found that the ability of TGFβ to enhance cell migration was blocked by inhibitors of myofibroblast differentiation (SB203580 and UO126) but not by the fiber cell differentiation inhibitor rapamycin (Figure 10C). We conclude that under the conditions tested, up-regulation of lens cell migration by TGFβ is mechanistically linked to myofibroblast differentiation instead of to fiber cell formation, in keeping with the acquisition of a migratory phenotype during EMT (Lamouille et al., 2014).
Multikinase inhibitors as potential anti-PCO therapeutics
Recently small-molecule multikinase inhibitors have emerged as therapeutics for several cancers. It is believed that by simultaneously affecting multiple signaling kinases, such compounds can produce a stronger and more durable clinical response than a monotherapy (Wilhelm et al., 2006; Leonard et al., 2016; Mirantes et al., 2016). To investigate whether this concept might be applicable to PCO, we screened commercially available small-molecule inhibitors with activity against multiple kinases for their effect on TGFβ-induced differentiation in DCDMLs. We found that one such compound, rebastinib (DCC-2036; Chan et al., 2011; Eide et al., 2011), inhibited TGFβ from inducing myofibroblast formation at concentrations ≥100 nM (data for 1 μM rebastinib are shown in Figure 11). Rebastinib did not, however, block TGFβ from up-regulating lens fiber cell differentiation (Figure 11B). To test whether rebastinib also inhibited EMyT in a mammalian system, we used rat lens central epithelial explants. In this well-accepted model for fibrotic PCO (West-Mays et al., 2010; Wormstone and Eldred, 2016), TGFβ causes lens epithelial cells to become spindle shaped and express αSMA in stress fibers within 48 h. Acquisition of this canonical marker of EMyT was blocked when explants were pretreated with 2.5 μM rebastinib before addition of TGFβ (Figure 11C). Longer treatment of explants with TGFβ was not possible because of the onset of cell death after 3–4 d (see the Discussion).
FIGURE 11:
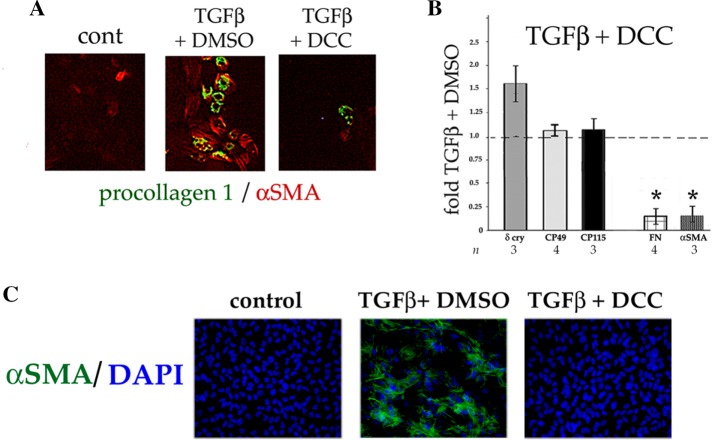
The multikinase inhibitor rebastinib (DCC-2036) prevents TGFβ from inducing myofibroblast, but not lens fiber cell, differentiation. (A, B) DCDMLs were preincubated with 1 μM rebastinib (DCC) or vehicle (0.1% DMSO) for 1 h before addition of 4 ng/ml TGFβ1, after which cells were cultured in the continuous presence of both drug and TGFβ for 6 d. Cells were processed for (A) immunofluorescence microscopy (n = 4) or (B) Western blot/metabolic labeling analysis of EMT/EMyT and lens fiber cell markers as in Figure 4. The extent to which each treatment affected the ability of TGFβ to up-regulate the expression of the indicated protein is graphed relative to TGFβ plus DMSO-only positive controls. *p < 0.01. (C) Rat lens explants were incubated with 2.5 μM rebastinib for 1 h before a 48-h treatment with or without 4 ng/ml TGFβ2. Fixed explants were stained for α SMA and mounted in medium with DAPI to localize nuclei. For all conditions, n ≥ 8.
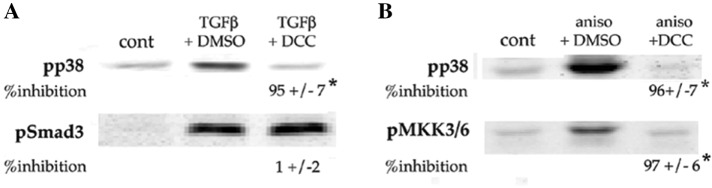
Rebastinib was developed as a therapeutic against chronic myelogenous leukemia driven by bcr-Abl, an oncogenic fusion protein not found in lens cells. In in vitro kinase assays, rebastinib blocked 39 additional kinases with IC50 ≤ 100 nM (Chan et al., 2011). These include the α and β isoforms of p38, which play an essential role in induction of EMyT by TGFβ in DCDMLs (Figure 5). Canonical signaling by p38 α/β requires activation (i.e., phosphorylation of Thr-180 and Tyr-182) of p38 by the p38 MAPK kinases MKK3 and 6. MKK3 and 6 are in turn activated by a variety of enzymes collectively referred to as p38 MAPK kinase kinases (MKKKs; Cuadrado and Nebreda, 2010). We found that ≥100 nM rebastinib decreases the level of pT180/Y182 p38 induced by TGFβ; we obtained similar results with the TGFβ-unrelated p38 signaling agonist anisomycin (Figure 12, A and B). Rebastinib did not, however, inhibit canonical Smad signaling as assessed by anti–phospho Smad3 levels (Figure 12A). The simplest mechanism by which rebastinib could specifically reduce the level of active p38 is by inhibiting one or more upstream kinases in the p38 pathway. Consistent with this possibility, rebastinib inhibited the ability of anisomycin to induce the activation of MKK3 and/or 6 as assessed with an antibody specific for the phosphorylated (activated) forms of the two p38 MKKs (Figure 12B). Rebastinib therefore blocks p38 signaling in DCDMLs at least two steps: at the level of p38 kinase and at the level of one or more p38 MKKKs, several of which are potently inhibited by rebastinib in in vitro kinase assays and have been reported to be downstream of TGFβ in various cell types (e.g., MLK1, 3, and 7 and TAK1; Chan et al., 2011).
FIGURE 12:
Rebastinib prevents TGFβ from activating the p38 pathway but not Smad3. (A) DCDMLs were treated for 3 h with rebastinib (DCC) or vehicle before a 1.5-h incubation with or without 4 ng/ml TGFβ. Whole-cell lysates were prepared and Western blots probed with antibodies specific for the phosphorylated (activated) forms of p38 or Smad3. (B) DCDMLs were treated for 3 h with rebastinib or vehicle before a 30-min incubation with or without the p38 agonist anisomycin (3 μg/ml). Whole-cell lysates were analyzed by Western blotting with antibodies against activated forms of p38 or MKK3/6. (A, B) The percentage inhibition by rebastinib compared with cultures treated with TGFβ + DMSO; for all conditions, n = 4; *p ≤ 0.000. Experiments using TGFβ instead of anisomycin as a p38 agonist were uninformative due to the limited sensitivity of the phospho-MKK3/6 antibody.
To be clinically practical, an anti-PCO therapeutic would ideally be administered once at the time of cataract surgery. Our finding that rebastinib targets multiple kinases in the p38 signaling pathway led us to consider whether the drug could prevent TGFβ from inducing EMyT after a single application. We found that treating DCDMLs with 10 μM rebastinib for 1 h on day 1 of culture followed by removal of the drug and replacement with fresh, unsupplemented medium completely prevented activation of p38 by TGFβ on day 7 (Figure 13A) without an appreciable effect on activation of Smad3 (unpublished data). Strong, albeit incomplete, inhibition of p38 activation was also observed when the more potent p38 agonist anisomycin was used instead of TGFβ (Figure 13A). Strikingly, such a short-term exposure to rebastinib also markedly reduced myofibroblast differentiation (Figure 13, B and C), as well as cell migration (Figure 13D) in response to TGFβ assessed 4–6 d later. Similar to what occurred after continuous exposure to 1 μM rebastinib (Figure 11), fiber cell differentiation was not abrogated (Figure 13C), nor was cell viability detectably affected (Supplemental Figure S1).
FIGURE 13:
A single 1-h treatment with rebastinib has long-term inhibitory effects on p38 activation and induction of myofibroblast differentiation and cell migration by TGFβ. (A–D) On day 1 of culture, DCDMLS were treated with 10 μM rebastinib (DCC) or vehicle (0.1% DMSO) for 1 h, after which the medium was removed and replaced with fresh, drug-free medium. Medium was replaced on days 3 and 5. (A) After 6 d of culture in TGFβ-free medium, cells were incubated with TGFβ1 (4 ng/ml for 1.5 h; n = 3) or anisomycin (3 μg/ml for 30 min; n = 5). Whole-cell lysates were probed with antibodies specific for the phosphorylated (activated) forms of p38 or Smad3. Percentage inhibition of activation of p38 by rebastinib compared with cultures treated with TGFβ + DMSO. *p ≤ 0.000. (B, C) After 6 d of culture in TGFβ-containing medium, cells were processed for immunofluorescence microscopy (B; typical of five experiments) or Western blot/metabolic labeling analysis (C) of EMT/EMyT and lens fiber cell markers. The extent to which rebastinib pretreatment reduced the ability of TGFβ to up-regulate the expression of the indicated protein is graphed relative to cells pretreated with DMSO before addition of TGFβ. *p ≤ 0.001. (D) DCDMLs grown on coverslips were treated with rebastinib or DMSO for 1 h and cultured cell side down for 4 d in the presence of 4 ng/ml TGFβ1 to assess cell migration as in Figure 10. (E) On day 1 of culture, DCDMLs were treated with 25 μM SB203580 (SB2) or 0.1% DMSO for 1 h before drug removal and culture for 6 d with 4 ng/ml TGFβ. Unlike rebastinib (B), short-term treatment with a p38 kinase inhibitor did not block expression of the EMT/EMyT markers αSMA and procollagen 1. The experiment was repeated three times.
Unlike rebastinib, SB203580 is considered to be relatively specific for p38 α/β kinases (Davies et al., 2000). We found that a 1-h exposure to 25 μM SB203580 followed by drug washout did not block induction of EMyT by TGFβ assessed 6 d later (Figure 13E). We conclude that short-term inhibition of only p38 kinase is insufficient to effect a long-term block in myofibroblast differentiation, consistent with our hypothesis that rebastinib has multiple inhibitory effects on this process.
DISCUSSION
In cancer, TGFβ can act as a tumor supressor, as well as a promoter of tumor progression (the so-called TGFβ paradox; Tian and Schiemann, 2009; Roberts and Wakefield, 2003; Principe et al., 2014). Here we report that TGFβ can also direct two mutually exclusive cell fates in a very different, cancer-unrelated disease, namely posterior capsule opacification.
Up-regulation of myofibroblast differentiation by TGFβ in lens cells
In mammalian systems, considerable evidence has implicated TGFβ in lens cell fibrosis in PCO and ASC (Meacock et al., 2000; Wormstone et al., 2002, 2006; de Iongh et al., 2005). Inhibitors p38, ERK, and MMPs have been reported to inhibit TGFβ-induced EMyT in rodent central epithelial explants and/or human lens cells, in keeping with our findings in DCDMLs (Tiwari et al., 2016; Lovicu et al., 2012; Dwivedi et al., 2006). A role for RGD-binding integrins in PCO has been suggested based on studies in human lens cells showing up-regulation of expression of α5 integrin and its ligand, fibronectin, by TGFβ (Marcantonio and Reddan, 2004) and inhibition of cell attachment and/or migration by RGD peptides (Oharazawa et al., 2005; Tiwari et al., 2016). To our knowledge, however, this is the first demonstration that an RGD peptide can block TGFβ from inducing myofibroblast differentiation in primary lens cells. It is well known that α5 integrin, as well as certain other RGD-binding integrins implicated in PCO (e.g., αV; Sponer et al., 2005; Mamuya et al., 2014), are able to activate latent TGFβ (Fontana et al., 2005). Our finding that an RGD peptide reduces EMyT in DCDMLs even in the presence of an excess of exogenously added active TGFβ (Figure 4) implies that these integrins must play one or more other essential roles in myofibroblast differentiation. Possible additional functions for RGD-binding integrins based on findings in other systems include up-regulation of expression of TGFβ receptors or of their downstream signaling cascades (Galliher and Schiermann, 2006).
Up-regulation of lens fiber cell differentiation by TGFβ in lens cells
In the same cultures in which it induced myofibroblast formation, TGFβ stimulated other epithelial cells in DCDMLs to differentiate into lens fiber cells. Despite leading to comparable levels of fiber cell marker expression, up-regulation of fiber formation by TGFβ is pharmacologically distinct from that induced by FGF or BMP in its response to noggin, PD173074, and UO126 (Figures 5 and 7; Le and Musil, 2001; Boswell et al., 2008; Boswell and Musil, 2015). As first shown by Menko et al. (1984), DCDML cells are capable of degrading their organelles to become terminally differentiated, mature lens fiber cells when grown in the presence of fetal calf serum (FCS). In contrast, we found that the fiber-like cells induced by TGFβ retain their nuclei (Figure 2) and capacity for protein synthesis (Supplemental Figure S2). After cataract surgery, the lens fiber-like cells that form Sommering’s ring and Elschnig pearls contain nuclei and appear to be metabolically active (Kappelhof et al., 1986). TGFβ therefore induces in DCDMLs a form of epithelial-to-fiber differentiation that more closely resembles that obtained after cataract surgery than after completion of normal lens development, supporting the appropriateness of the TGFβ/DCDML system as a model for PCO. Active TGFβRs appear to be dispensable for initiation of lens epithelial-to–fiber cell differentiation in embryonic mice (Beebe et al., 2004; de Iongh et al., 2001), further suggesting that stimulation of lens fiber formation by TGFβ is a pathological instead of a normal developmental process.
MTOR inhibitors such as rapamycin and KU-0063794 are the only compounds we found that can prevent TGFβ from up-regulating lens fiber differentiation without also reducing myofibroblast formation. Rapamycin has been reported to hasten the loss of organelles during terminal differentiation of embryonic chick lens fiber cells cultured in 10% FCS (Basu et al., 2014). The small population of cells in our (serum-free) DCDML system that express fiber cell markers in the presence of TGFβ and rapamycin retain their nuclei (Figure 8A), indicating that they never attain the level of fiber differentiation that leads to organelle loss. This difference in maturation state can explain the apparently contradictory effects of rapamycin in the presence of serum (in which it promotes terminal lens fiber maturation) and in the presence of TGFβ (in which it inhibits epithelial-to-fiber differentiation).
Lamouille et al. (2012) reported that in mammary epithelial NMuMG cells, TGFβ induces activation of MTORC2 within 30 min, as monitored by phosphorylation of AKT on Ser-473, an event causally linked to induction of EMT (MTORC1 is not required for the EMT phenotype in these cells; Lamouille and Derynck, 2007). In contrast, we did not detect Ser-473 phosphorylation in DCDMLs until a minimum of 24 h after addition of TGFβ (Figure 8). We suspect that this difference in time course reflects the different roles of MTOR downstream of TGFβ in the two cell types: to rapidly induce EMT in NMuMG cells (via AKT and MTORC2), and to support lens fiber cell differentiation in DCDMLs (via MTORC1 and/or 2 in a process in which AKT activity is not essential; Supplemental Figure S5). Given that rapamycin and KU-0063794 both decrease the relatively high levels of pAKT Ser-473 in DCDMLs in the absence of TGFβ (Figure 8), we cannot determine whether activation of MTORC2 by TGFβ is required for lens fiber differentiation or whether basal levels of MTOR C1 and/or C2 activity are sufficient. If the latter, then another, as-yet-unknown, signal must be triggered by TGFβ to initiate fiber differentiation.
As previously mentioned, addition of TGFβ induces EMT in the three main in vitro systems used to study PCO, namely weanling rat lens central epithelial explants, lens epithelial cell–derived cell lines, and capsular bags prepared by subjecting human cadaver lenses to mock cataract surgery. Why is there no induction of fiber cell differentiation, as we observe in DCDMLs? Lens cell lines and capsular bags are unable to undergo appreciable fiber cell differentiation when cultured with either FCS or FGF, considered to be the strongest stimulators of fiber cell formation in various primary lens cell systems (Wormstone and Eldred, 2016). It is therefore not surprising that they also fail to form fibers in response to TGFβ. In contrast, rodent lens central epithelial explants undergo bona fide fiber cell differentiation when treated with FGF or serum for 5 d (Lovicu and McAvoy, 2001). Exposure of explants from weanling rats to TGFβ induces a subset of cells to express αSMA within 2 d and causes all cells to die by apoptosis within 5 d (Schulz et al., 1996). The fact that treatment of intact isolated lens from comparably aged rats with TGFβ (Maruno et al., 2002) or exogenous expression of TGFβ in mice in vivo (Banh et al., 2006) results in the formation of anterior subcapsular cataracts without massive cell death suggests that apoptosis is exacerbated by lens central epithelial explant preparation and/or culture (of note, TGFβ does not induce cell death in DCDMLs; Supplemental Figure S1). Mansfield et al. (2004) reported that a 2-d treatment of rat lens central epithelial explants with TGFβ causes virtually all cells to lose expression of the transcription factor Pax6, indicative of loss of the lens epithelial phenotype. Prolonging the viability of TGFβ-treated cultures to 5 d with low levels of FGF did not markedly increase the proportion of αSMA-positive cells. One untested possibility is that the Pax6- and αSMA-negative cells in these lens explants represent lens cells committed to the only reported nonepithelial, nonmyofibroblast fate of epithelial cells in the lens in vivo, namely lens fiber cells. If so, then epithelial explants may share with DCDMLs and anterior lens epithelial in vivo (Lovicu et al., 2004b; Banh et al., 2006) the capacity to respond to TGFβ by initiating lens fiber cell as well as myofibroblast differentiation.
Dual response of DCDMLs to TGFβ
How can TGFβ up-regulate both myofibroblast and lens fiber cell fates? A potential clue comes from the stereotypic distribution of these two cell populations in DCDML cultures, with multilayered islands of fiber marker–expressing lentoids surrounded by a continuous fringe of monolayered, flattened myofibroblasts (e.g., Figure 2B). It is possible that high local cell density favors differentiation of lens epithelial cells to the fiber cell lineage by increasing cell–cell contact and/or by enhancing the accumulation of fiber cell–promoting autocrine or paracrine factors. Alternately or in addition, low cell density could stimulate myofibroblast differentiation by promoting cell spreading, increasing cell contractility, or minimizing the concentration of soluble myofibroblast-inhibitory factors. Studies are underway to address these possibilities. Of interest, the density of viable lens epithelial cells remaining after cataract surgery appears to be higher in the equatorial region of the lens capsule (where lens fiber cell–type PCO predominates) than in the anterior capsule (where fibrotic PCO is much more prevalent; Quinlan et al., 1997).
Fibrosis of lens cells can be blocked by a multikinase inhibitor
A recent concept in the study of cancer and other complex diseases is that multikinase inhibitors can lead to stronger and more durable clinical response than a monotherapy, in part because multiple blocks in one or more signaling pathways must be overcome or circumvented to render the former ineffective (Frantz, 2005; Lu et al., 2012; Ramsay et al., 2016). We found that one human therapeutic multikinase inhibitor, rebastinib, strongly and specifically inhibited the ability of TGFβ to up-regulate the p38 pathway and myofibroblast differentiation in DCDMLs. Rebastinib also blocked TGFβ-induced EMyT in rat lens epithelial explants, extending our findings to a mammalian ex vivo model of PCO (Figure 11C). Most strikingly, we found that a single 1-h treatment with 10 μM rebastinib inhibited the ability of TGFβ to induce myofibroblast differentiation of DCDMLs for at least 6 d, whereas a comparable acute treatment with the p38 α/β kinase inhibitor SB203580 at 25 μM had no such long-term effect. What could account for this difference in persistence? Studies by O’Hare et al. (2013) rule out the possibility that rebastinib is exceptionally well retained within cells. It has been proposed that partial inhibition of multiple signaling components can be more effective in combating a disorder than the complete arrest of a single essential component (Csermely et al., 2005). We suspect that the substantial (albeit incomplete; Figure 12A) inhibition of activation of p38 detectable 1 wk after short-term rebastinib exposure is due to the partial block of more than one p38 MKKK. Rebastinib may also continue to partly inhibit p38 kinase itself. A partial blockade by rebastinib of multiple processes that contribute to myofibroblast differentiation including, but perhaps not limited to, p38 activity could lead to a long-term inhibition of fibrosis not achievable with a single-target inhibitor of p38.
What is the potential therapeutic significance of these results? It has been reported that within 1 mo after cataract surgery, the square posterior edge of the IOL “shrink wraps” to the lens capsule, forming a capsular bend that physically blocks the further movement of lens cells to the posterior capsule, thereby stopping the progression of PCO (Nishi et al., 2002; Buehl et al., 2004; Nixon, 2004). Our finding that a single 1-h exposure to rebastinib inhibits EMyT and cell migration in lens cells for at least 1 wk raises the possibility that such a treatment at the time of cataract surgery could block fibrosis during the critical first few postoperative weeks, leading to a long-term reduction in PCO. Possible modes of delivery of the drug include sealed capsule irrigation (Rabsilber et al., 2007), loading of rebastinib into an intraocular lens (Davis et al., 2012), or its incorporation into the viscoelastic solution used during cataract surgery.
MATERIALS AND METHODS
Materials
Recombinant human TGFβ1, TGFβ2, bovine FGF2, mouse noggin/Fc chimera, and human BMP4 were from R & D Systems (Minneapolis, MN). R3IGF-1, an analogue of human IGF1, was from GroPep (Adelaide, Australia). The following antibodies were all purchased from Cell Signaling Technology (Danvers, MA): anti–phospho-p44/42 MAPK (9106), anti–total p44/42 MAPK (9106), anti–phospho-p38 (9211), anti-phospho (Ser-473) AKT (9275), anti–phospho MKK3/6 (9231), cleaved caspase 3 (9661), and total AKT (9272). Other commercial antibodies used in this study were, for luciferase, G745A from Promega (Madison, WI); for phospho-Smad3, ab51451 from Abcam (Cambridge, MA); for total-Smad3, ab84177 from Abcam; for total p38, sc-535 from Santa Cruz Biotechnology (Santa Cruz, CA); for connexin43, C8093 from Sigma-Aldrich (St. Louis, MO); for vinculin, V9131 from Sigma-Aldrich; for α−tubulin, T5168 from Sigma-Aldrich; for αSMA (DCDML studies), clone 1A4 from Dako (Carpinteria, CA); and for β-actin, clone C4 (MilliporeSigma, Billerica, MA). The following antibodies were from the Developmental Studies Hybridoma Bank, University of Iowa: anti–chick Pax6 (contributed by A. Kamakawi, Tokyo Institute of Technology), anti–chick fibronectin B3/D6 (from D. Fambrough, Johns Hopkins University), anti–chick α5 integrin clone D71E2 (from A. Horowitz, University of Virginia), and procollagen 1 SP1.D8 (from H. Furthmayr, Stanford University). Rabbit anti-mouse CP49 polyclonal serum (899 or 900) for Western blots and affinity-purified C1 for immunocytochemistry were generous gifts of Paul FitzGerald (University of California, Davis), as was the rabbit anti-CP115 antiserum (76). Rabbit anti–chicken MP28 antibodies were from Ross Johnson (University of Minnesota), and rat anti–dog ZO-1 monoclonal antibody R40.76 was provided by Daniel Goodenough (Harvard Medical School). Sheep anti–δ-crystallin antibody was produced in the laboratory of Joram Piatigorsky (National Institutes of Health) and was a gracious gift of Steve Bassnett (Washington University School of Medicine). The LIVE/DEAD kit (L3224) was from Molecular Probes (Eugene, OR). UO126, PD173074, SB-431542, GM6001, rapamycin, and SB203580 were from Calbiochem (La Jolla, CA). BIRB 0796, MK-2206, and GSK690693 were from Axon Medchem (Reston, VA); rebastinib (DCC-2036) was from Selleckchem (Houston, TX), and Ku-0063794 was purchased from Chemdea (Ridgewood, NJ). RGDS peptide (A9041) was from Sigma-Aldrich, as were all other reagents.
DCDML cell culture and treatments
DCDML cultures were prepared from E10 chick lenses as previously described in Le and Musil (1998). During this process, cells exterior to the lens capsule are removed and mature lens fiber cells die, leaving a preparation of purified lens epithelial cells. Cells were plated at (1.0–1.2) × 105 cells/well onto laminin-coated 96-well tissue culture plates and cultured in the absence of serum in M199 medium plus BOTS (2.5 mg/ml bovine serum albumin, 25 mg/ml ovotransferrin, 30 nM selenium), penicillin G, and streptomycin (M199/BOTS), with or without additives at 37°C in a 5% CO2 incubator. Cells were fed every 2 d with fresh medium. We refer to these cultures as DCDMLs to distinguish them from related but functionally distinct systems such as central epithelial explants and immortalized lens-derived cell lines (Musil, 2012). Where indicated, DCDMLs were incubated starting day 2 of culture with (final concentration) 15 µM UO126, 100 nM PD173074, 3 μM SB-431542, 20 μM SB203580, 2 μM Ku-0063794, 100 nM rapamycin, or 20 μM GM6001 for 1 h at 37°C before addition of growth factors. Noggin was used at 0.5 µg/ml and the RGDS peptide at 200 μM. The concentration of other inhibitors is specified in the text. For experiments with BIRB 0796 (1 μM), cultures were incubated for 2 h before addition of growth factor (Pargellis et al., 2002).
Plasmids and transient transfection of lens cells
One day after plating, DCDML cultures were transfected in M199 medium without BOTS or antibiotics using Lipofectamine 2000 (GibcoBRL) following the manufacture’s suggested protocol. Control experiments confirmed that the efficiency of transient transfection of DCDMLs is consistently ∼70% (Boswell et al., 2009). The DCR1-αA-promoter–EGFP reporter (Yang et al., 2006) was a kind gift of Ales Cvekl (Albert Einstein College of Medicine). The SBE4-Luc reporter construct (Zawel et al., 1998) was provided by Bert Vogelstein (Johns Hopkins University; Addgene plasmid 16495).
Immunofluorescence microscopy
DCDMLs grown on glass coverslips were fixed in 2% paraformaldehyde in phosphate-buffered saline and processed as previously described (Le and Musil, 1998, 2001). Images were taken of confluent regions of the culture and captured using a Leica DM LD photomicrography system and Scion Image 1.60 software.
Assessment of cell migration
Confluent DCDMLs grow on a laminin-coated 96-well coverslip were transferred, cell side down, onto a laminin-coated 48-well plate. The medium was completely removed to facilitate the formation of a tight seal between the cells and the bottom of the tissue culture well, after which fresh medium was immediately added to the well. The cells were then cultured for 4 d with or without 4 ng/ml TGFβ in the continuous presence of the mitotic inhibitor aphidocolin (10 μg/ml) to ensure that the spreading of cells from the coverslip edge was due solely to cell migration instead of proliferation. To assess the effect of SB203580, UO126, rapamycin, or rebastinib on cell migration, DCDMLs were preincubated with the drug for 1 h before coverslip inversion. Unless otherwise indicated, the drug was present throughout the culture period. Qualitatively similar results were obtained in the absence of aphidocolin.
[35S]methionine metabolic labeling
DCDML cultures were labeled at 37°C with [35S]methionine for 4 h in methionine- and serum-free DMEM (GibcoBRL) and solubilized as previously described (Le and Musil, 1998, 2001). [35S]methionine incorporation into total cellular protein and into δ-crystallin was quantitated after SDS–PAGE using a PhosphorImager (Molecular Dynamics) and IPLab Gel software (Signal Analytics).
Immunoblot analysis
Cultures were solubilized directly into SDS–PAGE sample buffer and boiled. Equal volumes of total cell lysate were transferred to polyvinylidene fluoride membranes, and the blots were probed with primary antibodies. Immunoreactive proteins were detected using secondary antibodies conjugated to either IRDye800 (Rockland Immunochemicals) or Alexa Fluor 680 (Molecular Probes, Eugene, OR) and directly quantified using the LI-COR Biosciences Odyssey infrared imaging system (Lincoln, NE) and associated software. The level of each protein was normalized to the level of β-actin in the same sample.
Quantitation
For fiber cell markers (δ-crystallin; CP49, and CP115), the fold to which TGFβ increased expression was calculated as expression with TGFβ/expression without TGFβ in the same experiment. The effect of a treatment on the ability of TGFβ to up-regulate expression of that marker was graphed normalized to this fold increase. Because the amount of fibronectin in untreated control cultures was undetectable and that of αSMA was inconsistent (Figure 3), it was not possible to calculate meaningfully the fold increase in fibronectin or αSMA expression induced by TGFβ. The effect of a treatment on the ability of TGFβ to up-regulate these proteins was therefore graphed normalized to the value obtained in cultures treated with TGFβ and vehicle (usually 0.1% dimethyl sulfoxide [DMSO]) in the same experiment. Data are graphed as means ± SD obtained in the number of experiments indicated in the figures. Asterisks denote values significantly different from TGFβ without inhibitor as assessed by the two-tailed paired Student’s t test. Unless otherwise indicated, all experiments were performed a minimum of three times and data from typical experiments presented.
Ex vivo rat lens epithelial explant preparation and treatments
Lens epithelial explants were prepared from 17- to 19-d-old Wistar rats as described in Korol et al. (2016). At 24 h after preparation, confluent epithelial explants were incubated with 2.5 μM rebastinib for 1 h in serum-free M199 medium. TGFβ2 (4 ng/ml) was added directly to the drug-containing tissue culture medium. Explants were cultured for an additional 48 h, a period known to be sufficient for TGFβ to induce EMyT without causing significant cell death. Explants were fixed and stained for αSMA and 4′,6-diamidino-2-phenylindole (DAPI) before immunofluorescence microscopy as described in Korol et al. (2016) using fluorescein isothiocyanate conjugated anti-αSMA antibody (Sigma-Aldrich) and Prolong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA).
Supplementary Material
Acknowledgments
L.M. and B.B. were supported by National Institutes of Health Grants R01EY022113 (National Eye Institute) and TR000128 (Oregon Clinical and Translational Research Institute, from the National Center for Advancing Translational Sciences and National Institutes of Health Roadmap for Medical Research). A.K. and J.W.-M. were supported by National Institutes of Health Grant EY017146.
Abbreviations used:
- αSMA
α−smooth muscle actin
- AQP0
aquaporin 0
- ASC
anterior subcapsular cataract
- DCDML
dissociated cell-derived monolayer
- EMT
epithelial–mesenchymal transition
- EMyT
epithelial–myofibroblast transition
- ERK
extracellular signal–regulated kinase
- FGF
fibroblast growth factor
- MTOR
mammalian target of rapamycin
- PCO
posterior capsule opacification
- TGFβ
transforming growth factor β
- TGFβR
transforming growth factor β receptor
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E16-12-0865) on February 16, 2017.
REFERENCES
- Afshari F, Fawcett J. An in vitro assay to examine oligodendrocyte precursor cell migration on astrocyes. Methods Mol Biol. 2012;814:393–399. doi: 10.1007/978-1-61779-452-0_26. [DOI] [PubMed] [Google Scholar]
- Apple DJ, Escobar-Gomez M, Zaugg B, Kleinmann G, Borkenstein AF. Modern cataract surgery: unfinished business and unanswered questions. Surv Ophthalmol. 2011;56:S3–S53. doi: 10.1016/j.survophthal.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Apple DJ, Ram J, Foster A, Peng Q. Elimination of cataract blindness: a global perspective entering the new millenium. Surv Ophthalmol. 2000;45:S1–S196. [PubMed] [Google Scholar]
- Awasthi N, Guo S, Wagner BJ. Posterior capsular opacification: a problem reduced but not yet eradicated. Arch Ophthalmol. 2009;127:555–562. doi: 10.1001/archophthalmol.2009.3. [DOI] [PubMed] [Google Scholar]
- Banh A, Deschamps PA, Gauldie J, Overbeek PA, Sivak JG, West-Mays JA. Lens-specific expression of TGF-beta induces anterior subcapsular cataract formation in the absence of Smad3. Invest Ophthalmol Vis Sci. 2006;47:3450–3460. doi: 10.1167/iovs.05-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Rajakaruna S, Reyes B, Van Bockstaele E, Menko AS. Suppression of MAPK/JNK-MTORC1 signaling leads to premature loss of organelles and nuclei by autophagy during terminal differentiation of lens fiber cells. Autophagy. 2014;10:1193–1211. doi: 10.4161/auto.28768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D, Garcia C, Wang X, Rajagopal R, Feldmeier M, Kim JY, Chytil A, Moses H, Ashery-Padan R, Rauchman M. Contributions by members of the TGFbeta superfamily to lens development. Int J Dev Biol. 2004;48:845–856. doi: 10.1387/ijdb.041869db. [DOI] [PubMed] [Google Scholar]
- Boswell BA, Le AC, Musil LS. Upregulation and maintenance of gap junctional communication in lens cells. Exp Eye Res. 2009;88:919–927. doi: 10.1016/j.exer.2008.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell BA, Musil LS. Synergistic interaction between the fibroblast growth factor and bone morphogenetic protein signaling pathways in lens cells. Mol Biol Cell. 2015;26:2561–2572. doi: 10.1091/mbc.E15-02-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell BA, Overbeek PA, Musil LS. Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev Biol. 2008;324:202–212. doi: 10.1016/j.ydbio.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell BA, VanSlyke JK, Musil LS. Regulation of lens gap junctions by transforming growth factor beta. Mol Biol Cell. 2010;21:1686–1697. doi: 10.1091/mbc.E10-01-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehl W, Menapace R, Sacu S, Kriechbaum K, Koeppl C, Wirtitsch M, Georgopoulos M, Findl O. Effect of a silicone intraocular lens with a sharp posterior optic edge on posterior capsule opacification. J Cataract Refract Surg. 2004;30:1661–1667. doi: 10.1016/j.jcrs.2004.02.051. [DOI] [PubMed] [Google Scholar]
- Carthy JM, Stöter M, Bellomo C, Vanlandewijck M, Heldin A, Morén A, Kardassis D, Gahman TC, Shiau AK, Bickle M, et al. Chemical regulators of epithelial plasticity reveal a nuclear receptor pathway controlling myofibroblast differentiation. Sci Rep. 2016;6:29868. doi: 10.1038/srep29868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WW, Wise SC, Kaufman MD, Ahn YM, Ensinger CL, Haack T, Hood MM, Jones J, Lord JW, Lu WP, et al. Conformational control inhibition of the BCR-ABL1 tyrosine kinase, including the gatekeeper T315I mutant, by the switch-control inhibitor DCC-2036. Cancer Cell. 2011;19:556–68. doi: 10.1016/j.ccr.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KC, Petrash JM. Aldose reductase mediates transforming growth factor β2 (TGF-β2)-induced migration and epithelial-to-mesenchymal transition of lens-derived epithelial cells. Invest Ophthalmol Vis Sci. 2015;56:4198–4210. doi: 10.1167/iovs.15-16557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lin H, Zheng D, Liu Y, Chen W, Liu Y. Expression of cytokines, chemokines and growth factors in patients undergoing cataract surgery with femtosecond laser pretreatment. PLoS One. 2015;10:e0137227. doi: 10.1371/journal.pone.0137227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary G, Spalton DJ, Koch DD. Effect of square-edged intraocular lenses on neodymium: YAG laser capsulotomy rates in the United States. J Cataract Refract Surg. 2007;33:1899–1906. doi: 10.1016/j.jcrs.2007.06.056. [DOI] [PubMed] [Google Scholar]
- Csermely P, Agoston V, Pongor S. The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharmacol Sci. 2005;26:178–182. doi: 10.1016/j.tips.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Ashery-Padan R. The cellular and molecular mechanisms of vertebrate lens development. Development. 2014;141:4432–4447. doi: 10.1242/dev.107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JL, Yi NY, Salmon JH, Charlton AN, Colitz CM, Gilger BC. Sustained-release celecoxib from incubated acrylic intraocular lenses suppresses lens epithelial cell growth in an ex vivo model of posterior capsule opacity. J Ocul Pharmacol Ther. 2012;28:359–368. doi: 10.1089/jop.2011.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes LJ, Sleeman MA, Anderson IK, Reddan JR, Wormstone IM. TGFbeta/Smad4-dependent and -independent regulation of human lens epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:5318–5327. doi: 10.1167/iovs.08-3223. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Lovicu FJ, Overbeek PA, Schneider MD, Joya J, Hardeman ED, McAvoy JW. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development. 2001;128:3995–4010. doi: 10.1242/dev.128.20.3995. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- Derynck R, Muthusamy BP, Saeteurn KY. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr Opin Cell Biol. 2014;31:56–66. doi: 10.1016/j.ceb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi DJ, Pino G, Banh A, et al. Matrix metalloproteinase inhibitors suppress transforming growth factor-beta-induced subcapsular cataract formation. Am J Pathol. 2006;168:69–79. doi: 10.2353/ajpath.2006.041089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide CA, Adrian LT, Tyner JW, Mac Partlin M, Anderson DJ, Wise SC, Smith BD, Petillo PA, Flynn DL, Deininger MW, et al. The ABL switch control inhibitor DCC-2036 is active against the chronic myeloid leukemia mutant BCR-ABLT315I and exhibits a narrow resistance profile. Cancer Res. 2011;71:3189–3195. doi: 10.1158/0008-5472.CAN-10-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findl O, Buehl W, Bauer P, Sycha T. Interventions for preventing posterior capsule opacification. Cochrane Database Syst Rev. 2010;2:CD003738. doi: 10.1002/14651858.CD003738.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Chen Y, Prijatelj P, Sakai T, Fässler R, Sakai LY, Rifkin DB. Fibronectin is required for integrin alphavbeta6-mediated activation of latent TGF-beta complexes containing LTBP-1. FASEB J. 2005;19:1798–1808. doi: 10.1096/fj.05-4134com. [DOI] [PubMed] [Google Scholar]
- Frantz S. Drug discovery: playing dirty. Nature. 2005;437:942–943. doi: 10.1038/437942a. [DOI] [PubMed] [Google Scholar]
- Galliher AJ, Schiemann WP. Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CM, Kwon GP, Beebe DC. Alpha-smooth muscle actin is constitutively expressed in the lens epithelial cells of several species. Exp Eye Res. 2006;83:999–1001. doi: 10.1016/j.exer.2006.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Martínez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, Alessi DR. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR) Biochem J. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giehl K, Menke A. Moving on: molecular mechanisms in TGFβ-induced epithelial cell migration. Signal Transduction. 2006;6:355–364. [Google Scholar]
- Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;76:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan V, Overbeek PA. Secreted FGFR3, but not FGFR1, inhibits lens fiber differentiation. Development. 2001;1286:1617–1627. doi: 10.1242/dev.128.9.1617. [DOI] [PubMed] [Google Scholar]
- Javitt JC, Tielsch JM, Canner JK, Kolb MM, Sommer A, Steinberg EP. National outcomes of cataract extraction: increased risk of retinal complications associated with Nd:YAG laser capsulotomy. Ophthalmology. 1992;996:1487–1498. doi: 10.1016/s0161-6420(92)31775-0. [DOI] [PubMed] [Google Scholar]
- Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999;406:1959–1967. [PubMed] [Google Scholar]
- Johar KS, Vasavada AR. Cells of human anterior capsular plaques. Invest Ophthalmol Vis Sci. 2008;49 doi: 10.1167/iovs.07-0312. [DOI] [PubMed] [Google Scholar]
- Jonk LJ, Itoh S, Heldin CH, ten Dijke P, Kruijer W. Identification and functional characterization of a Smad binding element (SBE) in the JunB promoter that acts as a transforming growth factor-beta, activin, and bone morphogenetic protein-inducible enhancer. J Biol Chem. 1998;2736:21145–21152. doi: 10.1074/jbc.273.33.21145. [DOI] [PubMed] [Google Scholar]
- Kappelhof JP, Vrensen GF, de Jong PT, Pameyer J, Willekens B. An ultrastructural study of Elschnig’s pearls in the pseudophakic eye. Am J Ophthalmol. 1986;1016:58–69. doi: 10.1016/0002-9394(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Khairallah M, Kahloun R, Bourne R, Limburg H, Flaxman SR, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K, et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis Sci. 2015;566:6762–6769. doi: 10.1167/iovs.15-17201. [DOI] [PubMed] [Google Scholar]
- Korol A, Taiyab A, West-Mays J. RhoA/ROCK signaling regulates TGFB-induced epithelial-mesenchymal transition of lens epithelial cells through MRTF-A. Mol Med. 2016;22:713–723. doi: 10.2119/molmed.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. TGF-β-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J Cell Sci. 2012;1256:1259–7123. doi: 10.1242/jcs.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;1786:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;156:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, Martin W, Fornwald J, Lehr R, Harling J, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AC, Musil LS. Normal differentiation of cultured lens cells after inhibition of gap junction-mediated intercellular communication. Dev Biol. 1998;204:80–96. doi: 10.1006/dbio.1998.9030. [DOI] [PubMed] [Google Scholar]
- Le AC, Musil LS. FGF signaling in chick lens development. Dev Biol. 2001;233:394–411. doi: 10.1006/dbio.2001.0194. [DOI] [PubMed] [Google Scholar]
- Leonard JT, Rowley JS, Eide CA, Traer E, Hayes-Lattin B, Loriaux M, Spurgeon SE, Druker BJ, Tyner JW, Chang BH. Targeting BCL-2 and ABL/LYN in Philadelphia chromosome-positive acute lymphoblastic leukemia. Sci Transl Med. 2016;8:354ra114. doi: 10.1126/scitranslmed.aaf5309. [DOI] [PubMed] [Google Scholar]
- Li KW, Wang AS, Sah RL. Microenvironment regulation of extracellular signal-regulated kinase activity in chondrocytes: effects of culture configuration, interleukin-1, and compressive stress. Arthritis Rheum. 2003;48:689–699. doi: 10.1002/art.10849. [DOI] [PubMed] [Google Scholar]
- Lovicu F, Mahmutovic L, Wojciechowski M, Dawes L, McAvoy J. MAPK/ERK1/2 signaling In Tgfß-induced lens epithelial to mesenchymal transition (EMT) Invest Ophthalmol Vis Sci. 2012;53:1061. [Google Scholar]
- Lovicu FJ, Ang S, Chorazyczewska M, McAvoy JW. Deregulation of lens epithelial cell proliferation and differentiation during the development of TGFbeta-induced anterior subcapsular cataract. Dev Neurosci. 2004a;26:446–455. doi: 10.1159/000082286. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development. 2001;128:5075–5084. doi: 10.1242/dev.128.24.5075. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Steven P, Saika S, McAvoy JW. Aberrant lens fiber differentiation in anterior subcapsular cataract formation: a process dependent on reduced levels of Pax6. Invest Ophthalmol Vis Sci. 2004b;45:1946–1953. doi: 10.1167/iovs.03-1206. [DOI] [PubMed] [Google Scholar]
- Lu JJ, Pan W, Hu YJ, Wang YT. Multi-target drugs: the trend of drug research and development. PLoS One. 2012;7:e40262. doi: 10.1371/journal.pone.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamuya FA, Wang Y, Roop VH, Scheiblin DA, Zajac JC, Duncan MK. The roles of αV integrins in lens EMT and posterior capsular opacification. J Cell Mol Med. 2014;18:656–670. doi: 10.1111/jcmm.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield KJ, Cerra A, Chamberlain CG. FGF-2 counteracts loss of TGFbeta affected cells from rat lens explants: implications for PCO (after cataract) Mol Vis. 2004;10:521–532. [PubMed] [Google Scholar]
- Marcantonio JM, Reddan JR. TGFbeta2 influences alpha5-beta1 integrin distribution in human lens cells. Exp Eye Res. 2004;79:437–442. doi: 10.1016/j.exer.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Maruno KA, Lovicu FJ, Chamberlain CG, McAvoy JW. Apoptosis is a feature of TGF beta-induced cataract. Clin Exp Optom. 2002;85:76–82. doi: 10.1111/j.1444-0938.2002.tb03012.x. [DOI] [PubMed] [Google Scholar]
- Masszi A, Speight P, Charbonney E, Lodyga M, Nakano H, Szászi K, Kapus A. Fate-determining mechanisms in epithelial-myofibroblast transition: major inhibitory role for Smad3. J Cell Biol. 2010;188:383–399. doi: 10.1083/jcb.200906155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacock WR, Spalton DJ, Stanford MR. Role of cytokines in the pathogenesis of posterior capsule opacification. Br J Ophthalmol. 2000;84:332–336. doi: 10.1136/bjo.84.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medsinge A, Nischal KK. Pediatric cataract: challenges and future directions. Clin Ophthalmol. 2015;9:77–90. doi: 10.2147/OPTH.S59009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menapace R. Posterior capsulorhexis combined with optic buttonholing: an alternative to standard in-the-bag implantation of sharp-edged intraocular lenses? A critical analysis of 1000 consecutive cases. Graefes Arch Clin Exp Ophthalmol. 2008;246:787–801. doi: 10.1007/s00417-008-0779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko AS, Klukas KA, Johnson RG. Chicken embryo lens cultures mimic differentiation in the lens. Dev Biol. 1984;103:129–141. doi: 10.1016/0012-1606(84)90014-9. [DOI] [PubMed] [Google Scholar]
- Millodot M. Dictionary of Optometry and Visual Science. 7th ed. Boston: Butterworth-Heinemann; 2008. [Google Scholar]
- Mirantes C, Dosil MA, Eritja N, Felip I, Gatius S, Santacana M, Matias-Guiu X, Dolcet X. Effects of the multikinase inhibitors Sorafenib and Regorafenib in PTEN deficient neoplasias. Eur J Cancer. 2016;63:74–87. doi: 10.1016/j.ejca.2016.04.019. [DOI] [PubMed] [Google Scholar]
- Musil LS. Primary cultures of embryonic chick lens cells as a model system to study lens gap junctions and fiber cell differentiation. J Membr Biol. 2012;245:357–368. doi: 10.1007/s00232-012-9458-y. [DOI] [PubMed] [Google Scholar]
- Nishi O, Nishi K, Akura J. Speed of capsular bend formation at the optic edge of acrylic, silicone, and poly(methyl methacrylate) lenses. J Cataract Refract Surg. 2002;28:431–437. doi: 10.1016/s0886-3350(01)01094-x. [DOI] [PubMed] [Google Scholar]
- Nixon DR. In vivo digital imaging of the square-edged barrier effect of a silicone intraocular lens. J Cataract Refract Surg. 2004;30:2574–2584. doi: 10.1016/j.jcrs.2004.06.049. [DOI] [PubMed] [Google Scholar]
- Oharazawa H, Ibaraki N, Ohara K, Reddy VN. Inhibitory effects of Arg-Gly-Asp (RGD) peptide on cell attachment and migration in a human lens epithelial cell line. Ophthalmic Res. 2005;37:191–196. doi: 10.1159/000086595. [DOI] [PubMed] [Google Scholar]
- O’Hare T, Eide CA, Agarwal A, Adrian LT, Zabriskie MS, Mackenzie RJ, Latocha DH, Johnson KJ, You H, Luo J, et al. Threshold levels of ABL tyrosine kinase inhibitors retained in chronic myeloid leukemia cells determine their commitment to apoptosis. Cancer Res. 2013;73:3356–3370. doi: 10.1158/0008-5472.CAN-12-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SK, Apple DJ, Werner L, Maloof AJ, Milverton EJ. Posterior capsule opacification: a review of the aetiopathogenesis, experimental and clinical studies and factors for prevention. Indian J Ophthalmol. 2004;52:99–112. [PubMed] [Google Scholar]
- Pargellis C, Tong L, Churchill L, Cirillo PF, Gilmore T, Graham AG, Grob PM, Hickey ER, Moss N, Pav S, Regan J. Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nat Struct Biol. 2002;9:268–272. doi: 10.1038/nsb770. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J, Rothschild SS, Wollberg M. Stimulation by insulin of cell elongation and microtubule assembly in embryonic chick-lens epithelia. Proc Natl Acad Sci USA. 1973;70:1195–1198. doi: 10.1073/pnas.70.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek E, Ju WJ, Heyer J, Escalante-Alcalde D, Stewart CL, Weinstein M, Deng C, Kucherlapati R, Bottinger EP, Roberts AB. Functional characterization of transforming growth factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J Biol Chem. 2001;276:19945–19953. doi: 10.1074/jbc.M102382200. [DOI] [PubMed] [Google Scholar]
- Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L, Pasche B, Lee C, Grippo PJ. TGF-β: duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst. 2014;106:djt369. doi: 10.1093/jnci/djt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M, Wormstone IM, Duncan G, Davies PD. Phacoemulsification versus extracapsular cataract extraction: a comparative study of cell survival and growth on the human capsular bag in vitro. Br J Ophthalmol. 1997;81:907–910. doi: 10.1136/bjo.81.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabsilber TM, Limberger IJ, Reuland AJ, Holzer MP, Auffarth GU. Long-term results of sealed capsule irrigation using distilled water to prevent posterior capsule opacification: a prospective clinical randomised trial. Br J Ophthalmol. 2007;91:912–915. doi: 10.1136/bjo.2006.106468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj SM, Vasavada AR, Johar SR, Vasavada VA, Vasavada VA. Post-operative capsular opacification: a review. Int J Biomed Sci. 2007;3:237–250. [PMC free article] [PubMed] [Google Scholar]
- Ramsay RR, Majekova M, Medina M, Valoti M. Key targets for multi-target ligands designed to combat neurodegeneration. Front Neurosci. 2016;10:375. doi: 10.3389/fnins.2016.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci USA. 2003;100:8621–8623. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JV, Nathu Z, Najjar A, Dwivedi D, Gauldie J, West-Mays JA. Adenoviral gene transfer of bioactive TGFbeta1 to the rodent eye as a novel model for anterior subcapsular cataract. Mol Vis. 2007;13:457–469. [PMC free article] [PubMed] [Google Scholar]
- Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17:726–740. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungger-Brändle E, Conti A, Leuenberger PM, Rungger D. Expression of alphasmooth muscle actin in lens epithelia from human donors and cataract patients. Exp Eye Res. 2005;81:539–550. doi: 10.1016/j.exer.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Flanders KC, Yoo J, Anzano M, Liu CY, et al. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol. 2004;164:651–663. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Miyamoto T, Ishida I, Shirai K, Ohnishi Y, Ooshima A, McAvoy JW. TGFbeta-Smad signalling in postoperative human lens epithelial cells. Br J Ophthalmol. 2002;86:1428–1433. doi: 10.1136/bjo.86.12.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schreiber KH, Ortiz D, Academia EC, Anies AC, Liao CY, Kennedy BK. Rapamycin-mediated mTORC2 inhibition is determined by the relative expression of FK506-binding proteins. Aging Cell. 2015;14:265–273. doi: 10.1111/acel.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz MW, Chamberlain CG, McAvoy JW. Inhibition of transforming growth factor-beta-induced cataractous changes in lens explants by ocular media and alpha 2-macroglobulin. Invest Ophthalmol Vis Sci. 1996;37:1509–1519. [PubMed] [Google Scholar]
- Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai K, Kitano-Izutani A, Miyamoto T, Tanaka S, Saika S. Wound healing and epithelial–mesenchymal transition in the lens epithelium: roles of growth factors and extracellular matrix. In: Saika S, Werner L, Lovicu FJ, editors. Lens Epithelium and Posterior Capsular Opacification. New York: Springer; 2014. pp. 159–174. [Google Scholar]
- Sponer U, Pieh S, Soleiman A, Skorpik C. Upregulation of alphavbeta6 integrin, a potent TGF-beta1 activator, and posterior capsule opacification. J Cataract Refract Surg. 2005;31:595–606. doi: 10.1016/j.jcrs.2004.05.058. [DOI] [PubMed] [Google Scholar]
- Srinivasan Y, Lovicu FJ, Overbeek PA. Lens-specific expression of transforming growth factor beta1 in transgenic mice causes anterior subcapsular cataracts. J Clin Invest. 1998;101:625–634. doi: 10.1172/JCI1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump RJ, Lovicu FJ, Ang SL, Pandey SK, McAvoy JW. Lithium stabilizes the polarized lens epithelial phenotype and inhibits proliferation, migration, and epithelial mesenchymal transition. J Pathol. 2006;210:249–257. doi: 10.1002/path.2049. [DOI] [PubMed] [Google Scholar]
- Tenbroek EM, Louis CF, Johnson R. The differential effects of 12-O-tetradecanoylphorbol-13-acetate on the gap junctions and connexins of the developing mammalian lens. Dev Biol. 1997;191:88–102. doi: 10.1006/dbio.1997.8703. [DOI] [PubMed] [Google Scholar]
- Tian M, Schiemann WP. The TGF-beta paradox in human cancer: an update. Future Oncol. 2009;5:259–271. doi: 10.2217/14796694.5.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A, Kumar R, Ram J, Sharma M, Luthra-Guptasarma M. Control of fibrotic changes through the synergistic effects of anti-fibronectin antibody and an RGDS-tagged form of the same antibody. Sci Rep. 2016;6:30872. doi: 10.1038/srep30872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasavada AR, Praveen MR. Posterior capsule opacification after phacoemulsification: annual review. Asia Pac J Ophthalmol (Phila) 2014;3:235–240. doi: 10.1097/APO.0000000000000080. [DOI] [PubMed] [Google Scholar]
- Walker J, Menko AS. Integrins in lens development and disease. Exp Eye Res. 2009;88:216–225. doi: 10.1016/j.exer.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallentin N, Wickstrom K, Lundberg C. Effect of cataract surgery on aqueous TGF-beta and lens epithelial cell proliferation. Invest Ophthalmol Vis Sci. 1998;39:1410–1418. [PubMed] [Google Scholar]
- West-Mays JA, Pino G, Lovicu FJ. Development and use of the lens epithelial explant system to study lens differentiation and cataractogenesis. Prog Retin Eye Res. 2010;29:135–143. doi: 10.1016/j.preteyeres.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- Wormstone IM, Anderson IK, Eldred JA, Dawes LJ, Duncan G. Short-term exposure to transforming growth factor beta induces long-term fibrotic responses. Exp Eye Res. 2006;83:1238–1245. doi: 10.1016/j.exer.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Wormstone IM, Eldred JA. Experimental models for posterior capsule opacification research. Exp Eye Res. 2016;142:2–12. doi: 10.1016/j.exer.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Wormstone IM, Tamiya S, Anderson I, Duncan G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest Ophthalmol Vis Sci. 2002;43:2301–2308. [PubMed] [Google Scholar]
- Wormstone IM, Wang L, Liu CS. Posterior capsule opacification. Exp Eye Res. 2009;88:257–269. doi: 10.1016/j.exer.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Yang Y, Stopka T, Golestaneh N, Wang Y, Wu K, Li A, Chauhan BK, Gao CY, Cveklová K, Duncan MK, et al. Regulation of alphaA-crystallin via Pax6, c-Maf, CREB and a broad domain of lens-specific chromatin. EMBO J. 2006;25:2107–2118. doi: 10.1038/sj.emboj.7601114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell. 1998;1:611–617. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–288. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.