Cytokinins stimulate stomatal immunity and plant resistance to bacteria by the ARR2-mediated transcriptional regulation of apoplastic peroxidase genes, which control ROS homeostasis in guard cells.
Abstract
Stomata play an important role in preinvasive defense responses by limiting pathogen entry into leaves. Although the stress hormones salicylic acid (SA) and abscisic acid (ABA) are known to regulate stomatal immunity, the role of growth promoting hormones is far from understood. Here, we show that in Arabidopsis thaliana, cytokinins (CKs) function in stomatal defense responses. The cytokinin receptor HISTIDINE KINASE3 (AHK3) and RESPONSE REGULATOR2 (ARR2) promote stomatal closure triggered by pathogen-associated molecular pattern (PAMP) and resistance to Pseudomonas syringae pv tomato bacteria. Importantly, the cytokinin trans-zeatin induces stomatal closure and accumulation of reactive oxygen species (ROS) in guard cells through AHK3 and ARR2 in an SA-dependent and ABA-independent manner. Using pharmacological and reverse genetics approaches, we found that CK-mediated stomatal responses involve the apoplastic peroxidases PRX4, PRX33, PRX34, and PRX71, but not the NADPH oxidases RBOHD and RBOHF. Moreover, ARR2 directly activates the expression of PRX33 and PRX34, which are required for SA- and PAMP-triggered ROS production. Thus, the CK signaling pathway regulates ROS homeostasis in guard cells, which leads to enhanced stomatal immunity and plant resistance to bacteria.
INTRODUCTION
Guard cells are specialized cells that form stomatal pores at the leaf epidermis and mediate gas exchange between the plant and the environment. Environmental and internal signals such as light, plant hormones, biotic, and abiotic stresses regulate stomatal opening and closing (Melotto et al., 2008; Acharya and Assmann, 2009; Sirichandra et al., 2009). As a part of the innate immune response, stomata play a critical role in plant defense (Melotto et al., 2008). Upon contact with pathogens or pathogen-associated molecular patterns (PAMPs), plants actively close their stomata to prevent pathogen invasion in the apoplast and subsequent colonization of host tissues (Lee et al., 1999; Melotto et al., 2006). In turn, pathogens have evolved virulence factors, such as the phytotoxin coronatine (COR) secreted by the bacterial pathogen Pseudomonas syringae pv tomato (Pst) strain DC3000, to counteract host stomatal defenses by promoting stomatal reopening (Melotto et al., 2006).
The earliest event in stomatal immunity is the recognition of PAMPs by plasma membrane pattern recognition receptors (Arnaud and Hwang, 2015). In Arabidopsis thaliana, the receptor FLAGELLIN-SENSITIVE2 (FLS2) that recognizes the peptide flg22, the biologically active epitope of the bacterial PAMP flagellin, plays a prominent role in stomatal immunity (Melotto et al., 2006; Zeng and He, 2010). Downstream of PAMP perception, reactive oxygen species (ROS) function as secondary messengers to promote stomatal closure. Both the NADPH oxidase RESPIRATORY BURST OXIDASE HOMOLOGD (RBOHD) and apoplastic peroxidases (PRXs) have been proposed to mediate PAMP-triggered ROS production in guard cells (Mersmann et al., 2010; Khokon et al., 2010a, 2010b; Kadota et al., 2014; Li et al., 2014). Still, the identity of these PRXs and the mechanistic details of their transcriptional regulation remain largely unknown.
Stomatal immunity was also shown to be regulated by hormonal pathways, mainly the abscisic acid (ABA) and salicylic acid (SA) signaling pathways (Melotto et al., 2008; Arnaud and Hwang, 2015). Particularly, the SA biosynthesis enzyme SALICYLIC ACID INDUCTION DEFICIENT2 (SID2) and the SA signaling regulator NONEXPRESSER OF PR GENES1 (NPR1) are required for bacterium-induced stomatal closure (Melotto et al., 2006; Zeng and He, 2010). Moreover, COR induces the expression of NAC transcription factors that contribute to COR-mediated stomatal reopening and activate the expression of SA metabolism genes (Zheng et al., 2012; Du et al., 2014). Several components of the core ABA pathway including OPEN STOMATA1 activate stomatal defense responses to bacteria (Melotto et al., 2006; Zeng and He, 2010; Du et al., 2014; Guzel Deger et al., 2015). However, the contribution of the ABA pathway to stomatal immunity was recently questioned (Montillet et al., 2013). Indeed, it was shown that ost1 and aba2 mutants were only partially defective in PAMP-triggered stomatal closure (Montillet et al., 2013; Issak et al., 2013). Moreover, to our knowledge, the role of cytokinin (CK) phytohormones in the regulation of stomatal immunity has not been investigated. Although CKs are considered to promote stomatal opening, the stomatal response to exogenously applied CKs depends on the concentration and CK species (Acharya and Assmann, 2009).
Growing evidence support a role of the plant growth promoting hormones CKs in regulating plant-microbe interactions (Choi et al., 2011; Argueso et al., 2012; Hann et al., 2014). It has been reported that CK stimulates the plant defense response to Pst DC3000 bacteria through the Arabidopsis type-B RESPONSE REGULATOR2 (ARR2), which acts downstream of the CK receptor HISTIDINE KINASE3 (AHK3) in phosphorelay signaling (Kim et al., 2006; Choi et al., 2010). In particular, CK activates the SA signaling pathway in a NPR1-dependent manner and ARR2 interacts with the transcription factor TGA1A-RELATED GENE3 (TGA3) to directly activate PR1 expression (Choi et al., 2010). However, until now, most of these studies have focused on postinvasive defense responses in the apoplast, such as callose deposition, PTI/defense marker gene expression, or phytoalexin production (Choi et al., 2010; Grosskinsky et al., 2011; Hann et al., 2014).
In this article, we have uncovered a new function of CK in promoting stomatal immunity through a signaling cascade involving the CK components AHK3 and ARR2. Our data show that CK induces ROS production and stomatal closure in an SA-dependent and ABA-independent manner. Importantly, we found that ARR2 directly activates the expression of the apoplastic peroxidases PRX33 and PRX34, which play a crucial role in stomatal defense via the SA signaling pathway.
RESULTS
Cytokinin Induces Stomatal Closure through AHK3 and ARR2
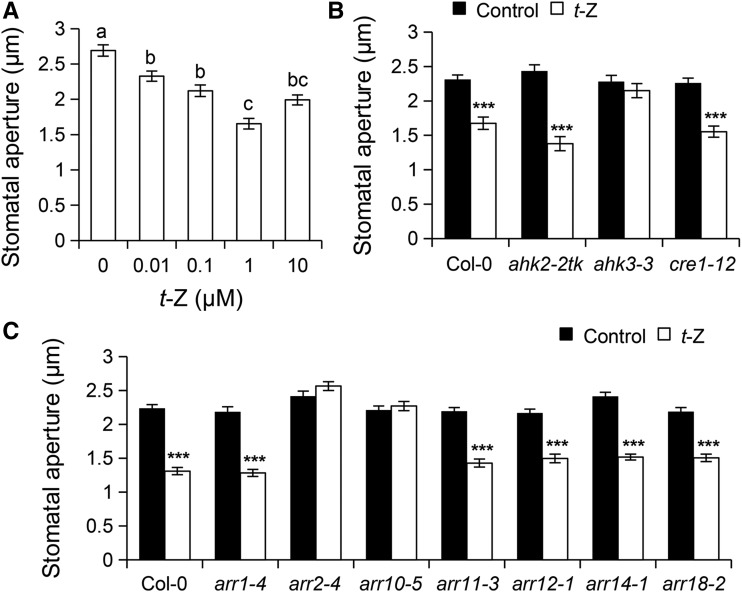
To investigate the role of the CK signaling pathway in stomatal immunity, we first examined the effect of the biologically active CK t-zeatin (t-Z) on stomatal aperture in Arabidopsis Col-0 wild-type epidermal peels. Application of t-Z from 10 nM to 1 µM gradually induced stomatal closure (Figure 1A), indicating that physiological concentrations of exogenous CK promote stomatal closure. We then chose the t-Z concentration of 1 µM for further stomatal assay. In a time-course experiment, significant stomatal closure was observed as early as 30 min after t-Z treatment and stomatal aperture continued to decrease up to 2 h after t-Z treatment (Supplemental Figure 1A). We then examined the relative contribution of CK signaling components in t-Z-induced stomatal closure. Interestingly, stomata of ahk3-3, arr2-4, and arr10-5 mutants did not close in response to 1 µM t-Z, while those of the ahk2-2tk, cre1-12, arr1-4, arr11-3, arr12-1, arr14-1, and arr18-2 mutants exhibited wild-type levels of stomatal closure in response to CK (Figures 1B and 1C). Similar results were obtained with the arr2-5 and arr2-6 mutant alleles (Supplemental Figures 1B), which likely represent null mutants as no full-length transcript can be detected by RT-qPCR (Supplemental Figure 1C). Thus, the histidine kinase AHK3 and the type-B response regulators ARR2 and ARR10 are required for CK-mediated stomatal closure. Consistent with their function in stomatal movements, AHK3, ARR2, and ARR10 are expressed in guard cells (Supplemental Figure 1D).
Figure 1.
Cytokinin Induces Stomatal Closure through AHK3, ARR2, and ARR10.
(A) Stomatal response of Arabidopsis Col-0 wild-type epidermal peels to different concentrations of t-Z. Results are shown as the mean of ≥100 stomata measurements ±se. Different letters indicate significant differences at P < 0.01 based on a Tukey’s HSD test (see ANOVA table; Supplemental Data Set 1).
(B) and (C) Stomatal apertures in epidermal peels of Col-0 wild type, cytokinin receptor mutants (B), and type-B response regulator mutants (C) after 2 h of incubation with control solution (10 µM NaOH) or 1 µM t-Z. Results are shown as the mean of ≥100 stomata measurements ±se. Asterisks indicate significant differences between control and t-Z treatment based on a two-tailed Student's t test (**P < 0.01; ***P < 0.001).
AHK3 and ARR2 Activate Stomatal Immunity
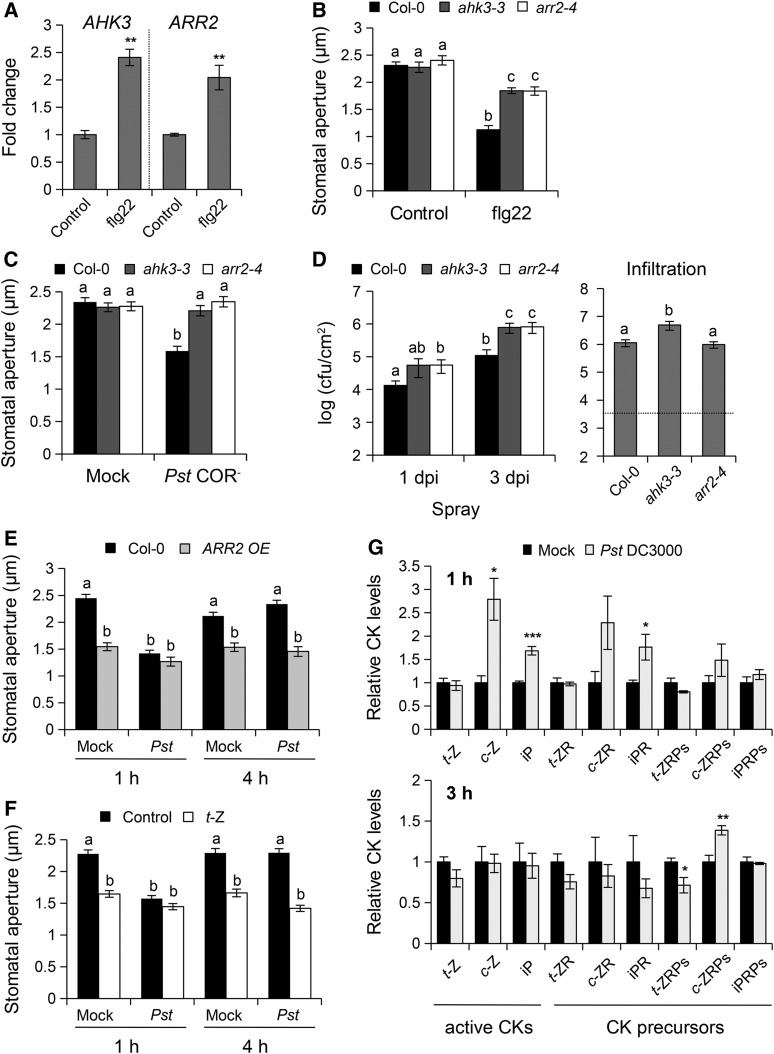
As AHK3 and ARR2 are known to participate in apoplastic defense (Choi et al., 2010) and their expression is induced in guard cells after flg22 treatment (Figure 2A), we evaluated their role in PAMP-triggered stomatal closure and stomatal defense against bacteria. Epidermal peels were incubated with the PAMP flg22, which promotes stomatal closure in wild-type Col-0 plants (Melotto et al., 2006). Interestingly, ahk3-3 and arr2 mutants were defective in flg22-mediated stomatal closure (Figure 2B; Supplemental Figure 2A). We then analyzed stomatal movements in ahk3-3 and arr2-4 mutants after inoculation with the coronatine-deficient Pst DC3000 (Pst COR−) strain, which lacks the ability to reopen stomata during infection (Melotto et al., 2006). At 2 h postinoculation with Pst COR− bacteria, we observed bacteria-induced stomatal closure in wild-type plants, but not in ahk3-3 and arr2-4 mutants (Figure 2C). Because a defect in stomatal defense often results in enhanced susceptibility to bacteria, plants were spray-inoculated with Pst COR− bacteria and bacterial growth was evaluated 1 and 3 d later. As expected, ahk3-3 and arr2-4 mutants were more susceptible than the Col-0 control to surface-inoculated Pst COR− bacteria, with a 4- and 7-fold increase in bacterial population at 1 and 3 d postinoculation, respectively (Figure 2D, left panel). Since the enhanced susceptibility of ahk3-3 and arr2-4 mutants to Pst COR− after spray inoculation could also be caused by a deficiency in postinvasive defenses, plants were also inoculated with Pst COR− by syringe infiltration. Only the ahk3-3 mutant showed higher susceptibility to Pst COR− after infiltration-inoculation (Figure 2D, right panel), with a modest increase in bacterial growth (4-fold) at 3 d postinoculation. Taken together, these results establish a mechanistic connection between stomatal defense and the CK signaling pathway in guard cells.
Figure 2.
The CK Signaling Components AHK3 and ARR2 Participate in Stomatal Immunity.
(A) RT-qPCR analysis of AHK3 and ARR2 transcript levels in guard cell protoplasts after treatment with control solution (MES buffer) or 1 µM flg22 for 2 h. Transcript levels were normalized to UBQ1. Error bars indicate se of three independent experiments (n = 3). Asterisks indicate statistically significant differences between control and flg22 treatments based on a two-tailed Student's t test (**P < 0.01).
(B) and (C) Stomatal apertures in wild-type Col-0, ahk3-3, and arr2-4 mutants exposed to Control solution and 5 µM flg22 (B) or mock control (10 mM MgCl2) and 108 cfu/mL COR-deficient Pst DC3000 (Pst COR−) bacteria (C) for 2 h. Data are means ± se (n ≥ 100).
(D) Bacterial growth in wild-type Col-0, ahk3-3, and arr2-4 mutants assessed at 1 d (1 dpi) and 3 d (3 dpi) after spray inoculation with Pst COR− at 108 cfu/mL (left panel) or at 0 and 3 d after syringe infiltration with Pst COR− at 106 cfu/mL (right panel). The dashed line indicates the apoplastic bacterial population (cfu/cm2) at 0 d postinoculation. Values are the means ± se (n = 4).
(E) and (F) t-Z or ARR2 overexpression inhibits Pst DC3000-mediated stomatal reopening. Stomatal apertures in Col-0 and the ARR2 overexpressing (ARR2 OE) line (E) or Col-0 wild type treated with 1 µM t-Z or control solution (F) exposed to mock control or 108 cfu/mL Pst DC3000 (Pst) for 1 and 4 h. Data are means ± se (n ≥ 100). Different letters indicate significant differences at P < 0.05 (D) and P < 0.001 ([B], [C], [E], and [F]) based on a Tukey’s HSD test (see ANOVA tables; Supplemental Data Set 1).
(G) Relative CK levels in Col-0 wild-type leaves after infiltration with mock control (10 mM MgCl2) and 108 cfu/mL Pst DC3000 bacteria for 1 h (upper panel) and 3 h (lower panel). CK concentration was calculated as pmol/g dry weight, and the relative levels of CKs were normalized to a value of 1 in mock-treated control. Data represent the means and se values of three independent biological replicates. Asterisks indicate significant difference between mock and Pst DC3000 treatments based on a two-tailed Student's t test (*P < 0.05; **P < 0.01; ***P < 0.001).
Cytokinins Compromise COR-Mediated Stomatal Reopening
We observed that activation of the CK signaling pathway in plants overexpressing ARR2 (ARR2 OE) induces constitutive stomatal closure (Figure 2E). Likewise, without any treatments, IPT1 and IPT3 overexpression lines, which have elevated CK concentrations (Choi et al., 2010), exhibited partially closed stomata, indicating that endogenous accumulation of CKs enhances stomatal closure or inhibits stomatal opening (Supplemental Figure 2B). Therefore, we hypothesized that CK- or ARR2-mediated stomatal closure may enhance stomatal immunity against the Pst DC3000 bacterium, which produces COR to counteract PAMP-triggered stomatal closure and reopen stomata (Melotto et al., 2006). Treatments of Col-0 wild-type epidermal peels with 1 µM t-Z impaired Pst DC3000-mediated stomatal reopening observed at 4 h in control treated plants (Figure 2F). Consistently, Pst DC3000-induced stomatal reopening at 4 h was also largely compromised in IPT1 and IPT3 OE lines (Supplemental Figure 2B), as well as in ARR2 OE plants (Figure 2E). Since CK-treated plants or plants overexpressing IPT1, IPT3, and ARR2 were defective in Pst DC3000-triggered stomatal reopening, we expected that these plants are more resistant to Pst DC3000 bacteria. Indeed, in our surface inoculation assays, the multiplication of Pst DC3000 bacteria at 3 d postinoculation in CK-treated plants or plants overexpressing ARR2, IPT1, and IPT3 was greatly reduced as compared with wild-type plants (Supplemental Figure 2). Together, these data suggest that CKs promote resistance to bacteria by inhibiting Pst DC3000-mediated stomatal reopening.
These results imply that the perception of PAMPs carried by bacteria combined with the activation of the CK pathway impedes COR-mediated inhibition of PAMP-induced stomatal closure. To test this hypothesis, epidermal peels of wild-type Col-0 and ARR2 OE plants were treated with COR alone, flg22 alone, or flg22 together with COR, in combination with t-Z or control solution (Supplemental Figures 2F and 2G). As observed previously (Melotto et al., 2006), COR reopened stomata of flg22-treated wild-type controls. Similarly, treatment with COR alone was able to revert t-Z- and ARR2-mediated stomatal closure, suggesting that COR interferes with CK signaling in guard cells. Importantly, when stomatal closure was induced by both flg22 and t-Z or ARR2 overexpression, COR at the concentration used was not able to reopen stomata. Together, these data suggest that the activation of the CK signaling pathway via ARR2 potentiates PAMP signaling and interferes with COR-mediated suppression of PAMP-induced stomatal closure.
As a next step to study the relationship between CK metabolism and plant response to bacterial infection, we examined the CK content in wild-type Col-0 leaves at 1 and 3 h postinoculation with Pst DC3000 bacteria (Figure 2G; Supplemental Table 1). At 1 h postinoculation, the levels of c-Z- and iP-type CKs, particularly the bioactive CK bases and the CK riboside precursors, were higher in Pst-inoculated leaves compared with the mock control. By contrast, no meaningful changes in CK levels were observed between mock- and Pst-inoculated leaves at a later time point (3 h postinoculation). These data further support a function of CKs in activating the early events of pathogen recognition.
Cytokinin Induces ROS Production in Guard Cells
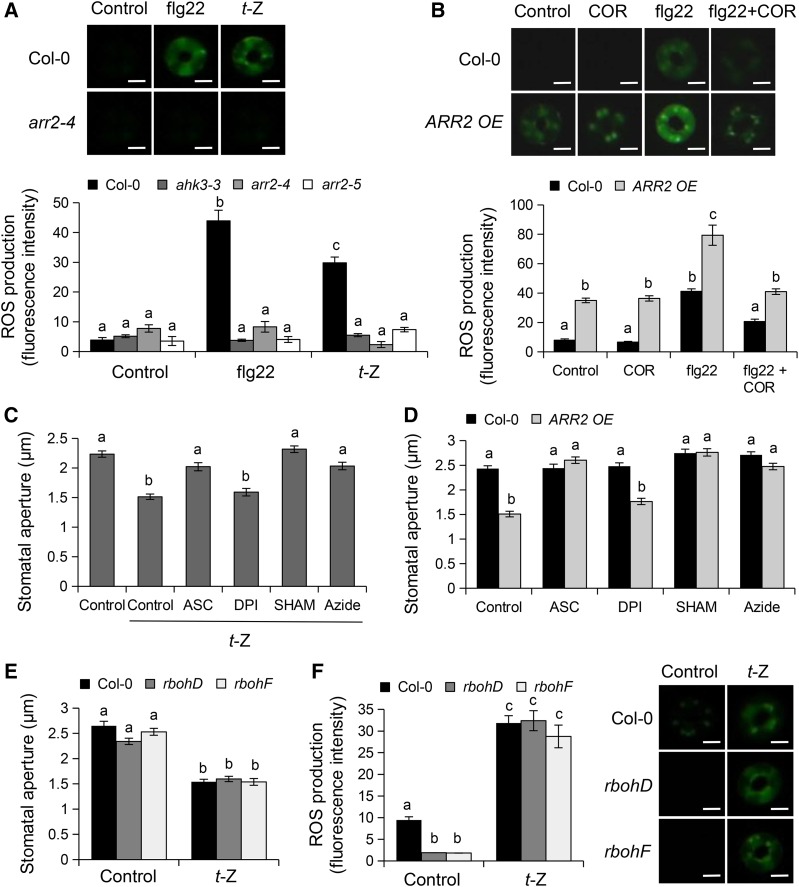
ROS, including superoxide (O2−) and hydrogen peroxide (H2O2), are produced in guard cells after PAMP perception and have been proposed to function as early secondary messengers in the promotion of stomatal closure by PAMPs, as well as by ABA and SA hormones (Lee et al., 1999; Pei et al., 2000; Khokon et al., 2011). Therefore, we tested whether H2O2 is also an important secondary messenger in CK-mediated stomatal immunity. We monitored H2O2 production in epidermal strips treated with 1 µM t-Z or 5 µM flg22 using the fluorescent dye 2',7'-dichlorofluorescein diacetate (H2DCF-DA). An increase in fluorescence was observed in guard cells after t-Z treatment (Figure 3A), indicating that CK is able to induce ROS production. Notably, t-Z-mediated ROS production was compromised in the ahk3-3 and arr2 mutants and these mutants were also defective in flg22-mediated ROS production (Figure 3A). These results indicate that AHK3 and ARR2 mediate t-Z- and flg22-induced ROS accumulation in guard cells. We also observed higher levels of ROS in guard cells of IPT3 and ARR2 overexpression lines compared with Col-0 wild type (Figure 3B; Supplemental Figure 3A), which could explain their constitutive closed stomata phenotype. Importantly, IPT3 and ARR2 overexpression lines showed enhanced ROS production after flg22 treatment, suggesting an overactivation of stomatal immunity. Interestingly, ROS accumulation in response to flg22 was reduced after pretreatment with COR (Figure 3B). This result indicates that COR may act upstream of ROS production in PAMP-mediated stomatal closure. By contrast, COR application did not reduce ARR2-mediated ROS accumulation. As the H2DCF-DA probe becomes irreversibly fluorescent upon reaction with ROS (Swanson et al., 2011), the enhanced ROS accumulation in the ARR2 OE line could have occurred before COR treatment.
Figure 3.
Cytokinin Induces ROS Production and Stomatal Closure through Apoplastic Peroxidases.
(A) ROS production detected by H2DCF-DA fluorescence in guard cells of Col-0 wild type, ahk3-3, arr2-4, and arr2-5 mutants 30 min after treatment with control solution, 5 µM flg22, or 1 µM t-Z.
(B) Coronatine reduces flg22-mediated ROS accumulation. ROS production detected by H2DCF-DA fluorescence in guard cells of Col-0 wild type and ARR2 overexpression (OE) lines 30 min after treatment with control solution or 5 µM flg22. Peels were incubated with 1.6 µM COR for 30 min before the addition of flg22 (flg22+COR). In (A) and (B), a representative stomate is shown (upper panel). Bars = 5 µm.
(C) Stomatal aperture in Col-0 wild type exposed to control solutions, 1 µM t-Z, and 1 µM t-Z together with 1 mM ASC, 20 µM DPI, 2 mM SHAM, or 1 µM sodium azide for 3 h.
(D) Stomatal aperture in Col-0 wild-type and ARR2 OE lines exposed to control solution, 1 mM ASC, 20 µM DPI, 2 mM SHAM, or 1 µM sodium azide for 3 h.
(E) Stomatal apertures in Col-0 wild type, rbohD, and rbohF mutants after 2 h of incubation with control solution or 1 µM t-Z. In (C) to (E), values are means ± se (n ≥ 100).
(F) ROS detected by H2DCF-DA fluorescence in guard cells of wild-type Col-0, rbohD, and rbohF mutants 30 min after treatments with control solution and 1 µM t-Z.
In (A), (B), and (F), values are means ± se (n ≥ 60 stomata). Different letters indicate significant differences at P < 0.001 ([A] to [E]) and P < 0.05 (F) based on a Tukey’s HSD test (see ANOVA tables; Supplemental Data Set 1). A representative stomate is shown (right panel). Bars = 5 µm.
Apoplastic Peroxidases, but Not NADPH Oxidases, Are Involved in CK-Mediated Stomatal Closure
Two types of enzymes, the apoplastic peroxidases (PRXs) and the plasma membrane NADPH oxidases, have been proposed to generate a burst of ROS upon pathogen attack or elicitation with PAMPs (Torres, 2010; O’Brien et al., 2012a). ROS produced by the NADPH oxidases RBOHD and RBOHF are known to be respectively implicated in PAMP- and ABA-mediated stomatal closure, whereas SA- and chitosan-mediated stomatal closure involves ROS generated by yet unknown PRXs (Kwak et al., 2003; Khokon et al., 2010b, 2011; Mersmann et al., 2010). To investigate the role of ROS and the relative contribution of NADPH oxidases and PRXs in CK-mediated stomatal closure, we treated Col-0 epidermal peels with t-Z alone or with t-Z together with ascorbate (ASC), which is known to reduce ROS levels in plants (Lee et al., 1999); diphenylene iodium chloride (DPI), an inhibitor of NADPH oxidases (Pei et al., 2000); or sodium azide and salicylhydroxamic acid (SHAM), two inhibitors of PRXs (O’Brien et al., 2012b; Khokon et al., 2010a). We found that t-Z-induced stomatal closure was inhibited by ASC, SHAM, and sodium azide, but not by DPI (Figure 3C). Likewise, ASC, SHAM, and sodium azide, but not DPI, induced stomatal opening in the lines overexpressing ARR2 or IPT3 (Figure 3D; Supplemental Figure 3B), suggesting that PRXs, but not NADPH oxidases, are responsible for CK-mediated stomatal closure.
To further analyze the role of NADPH oxidases in CK-mediated ROS production and stomatal aperture, we used the rbohD and rbohF mutants. Interestingly, these mutants were not impaired in t-Z-induced stomatal closure (Figure 3E). Even if we noticed a lower basal level of ROS in rbohD and rbohF guard cells relative to the Col-0 wild type, the rbohD and rbohF mutants consistently exhibited a wild-type increase in ROS production after t-Z treatment (Figure 3F). Moreover, the basal expression level of RBOHF was not affected and RBOHD expression was downregulated in ARR2 OE guard cells (Supplemental Figure 3C). Collectively, these results suggest that CK-mediated stomatal closure is independent of the NADPH oxidases RBOHD and F and rather involves apoplastic peroxidases.
A Subset of PRX Genes Is Commonly Upregulated by PAMPs and in the ARR2 Overexpression Line
Apoplastic peroxidases belong to a large multigenic family of 73 members (Valério et al., 2004). To identify the PRX(s) implicated in CK-mediated stomatal immunity, we first analyzed public microarray databases (AtGenExpress and ArrayExpress) to select PRX genes that are both expressed in guard cells and induced by PAMPs or bacterial infection. We found that PRX4, PRX5, PRX32/33/34, PRX37, PRX50/51, PRX62, and PRX71 fulfill these selection criteria, while the PAMPs/bacteria-induced PRX38, PRX69, and PRX70 genes are weakly expressed in guard cells (Supplemental Figure 4).
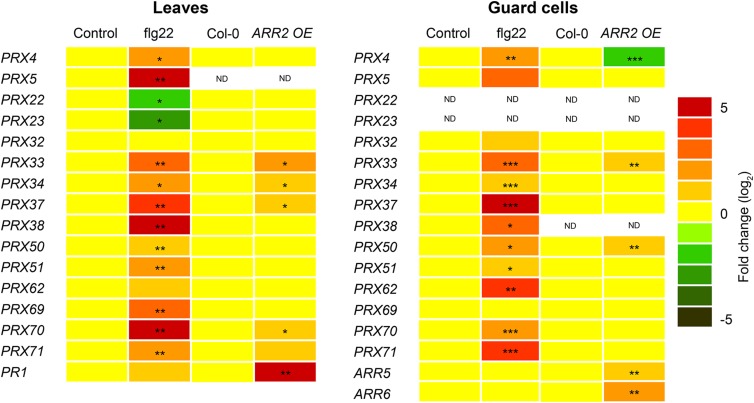
The expression pattern of PRX genes in whole leaves and in guard cell protoplasts after flg22 treatment as well as in ARR2 and IPT3 overexpression lines was further analyzed by RT-qPCR (Figure 4; Supplemental Tables 2 and 3). Except for PRX22, PRX23, and PRX32, which exhibited reduced expression or did not have altered expression after 2 h of flg22 treatment, the expression of all genes selected was induced by flg22 compared with control treatments in both leaves and guard cells. Even if PRX5 and PRX38 expression was highly induced by flg22, their basal expression levels were either very low or undetectable. Interestingly, the basal expression level of PRX33, 34, 37, 70, and 71 was moderately upregulated in leaves of the ARR2 overexpression line relative to the Col-0 wild type. Particularly, PRX33, which showed the highest expression level in the ARR2 OE line, with a 6- and 2-fold induction in leaves and guard cells, respectively (Figure 4), was also 2-fold upregulated in the IPT3 OE line (Supplemental Table 3). Interestingly, PRX4 expression was lower in ARR2 OE guard cells than in the Col-0 wild type. These results suggest that the up- or downregulation of some PRX genes in the ARR2 or IPT3 OE lines might be responsible for their constitutive stomatal closure.
Figure 4.
Expression of PRX Genes in Leaves and Guard Cells after PAMP Treatment or in the ARR2 Overexpression Line.
Expression analysis of PAMP-inducible PRX genes in leaves (left panel) and guard cell protoplasts (right panel) after flg22 or control treatments for 2 h, and in Col-0 wild-type and ARR2-overexpressing lines (ARR2 OE). Transcript levels were determined by RT-qPCR and normalized to both UBQ1 and EF1. PR1, ARR5, and ARR6 were used as positive controls. The changes in transcript levels relative to Col-0 are color-coded. ND, not detected. Asterisks indicate statistically significant differences between wild-type and ARR2 OE lines or between control and flg22 treatments based on a two-tailed Student’s t test (*P < 0.05; **P < 0.01; ***P < 0.01). Numerical expression values are shown in Supplemental Tables 2 and 3.
PRX33 and PRX34 Function in CK-Mediated Stomatal Immunity
To analyze the function of flg22-induced PRX genes in stomatal immunity, we isolated available Arabidopsis lines homozygous for T-DNA insertions (see Methods and Supplemental Figure 5A). Apart from prx4-1, prx4-2, prx33-2, prx34-1, and prx37-1, which are hypo- or hypermorphic mutants depending on the T-DNA insertions, we could not detect any full-length transcript by RT-PCR for the remaining mutants, suggesting loss-of-function mutations (Supplemental Figure 5B). PRX4 expression was 2-fold downregulated in the prx4-1 mutant and 7-fold upregulated in the prx4-2 mutant (Supplemental Figure 5C). In contrast to previous observations (Daudi et al., 2012; Lyons et al., 2015), the expression of PRX33 and PRX34 was not affected in the prx33-2 and prx34-1 mutants, respectively (Supplemental Figure 5B). Surprisingly, in the prx34-1 mutant, the downstream tandem-duplicated PRX33 gene was 20-fold overexpressed. The prx37-1 mutant exhibited a 9-fold reduction of PRX37 expression.
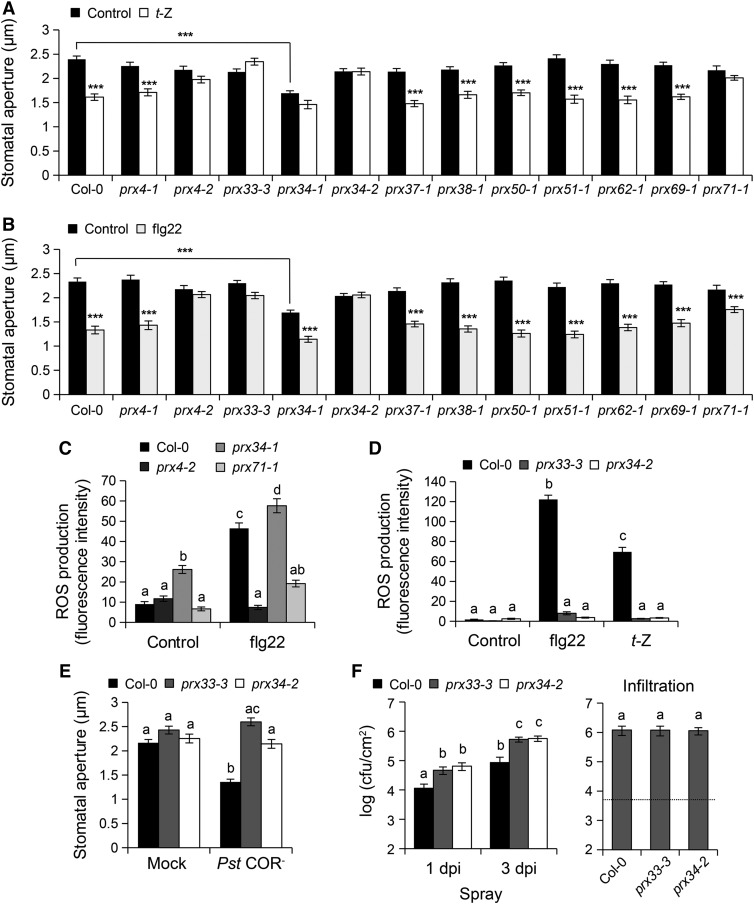
We then screened these mutants for their responsiveness to PAMP- and CK-triggered stomatal closure. We found that the prx4-2 (but not prx4-1), prx33-3, prx34-2, and prx71-1 mutants were not responsive to CK-mediated stomatal closure (Figure 5A). Similarly, these mutants were fully defective in flg22-mediated stomatal closure, except for prx71-1, which was partially impaired in this response (Figure 5B). As PRX4 was overexpressed in the hypermorphic prx4-2 mutant, PRX4 could play a negative role in PAMP- and CK-induced stomatal closure. By contrast, PRX33, PRX34, and PRX71 may activate PAMP- and CK-mediated stomatal closure. The prx4-2 mutant and to a lesser extend the prx71-1 mutant were also defective in flg22-triggered ROS production (Figure 5C). By contrast, the prx34-1 mutant, which exhibits high PRX33 expression, showed constitutive stomatal closure and enhanced ROS accumulation in guard cells (Figures 5A and 5C). Importantly, the prx33-3 and prx34-2 mutants were impaired in flg22- and t-Z-induced ROS production, as well as in bacteria-mediated stomatal closure (Figures 5D and 5E). As expected, the prx33-3 and prx34-2 mutants were highly susceptible to spray-inoculated Pst COR− bacteria with 5- and 7-fold more bacteria compared with the wild type at 1 and 3 days postinoculation, respectively (Figure 5F, left panel). By contrast, both mutants demonstrated wild-type susceptibility levels after infiltration-inoculation (Figure 5F, right panel), indicating that PRX33 and PRX34 play an important role in CK-mediated promotion of stomatal defense responses.
Figure 5.
PRX4, PRX33, PRX34, and PRX71 Are Involved in flg22- and t-Z-Mediated Stomatal Closure.
(A) and (B) Stomatal apertures in Col-0 wild-type and prx mutants exposed to control solution, 5 µM flg22 (A), or 1 µM t-Z (B) for 2 h. Values are means ± se (n ≥ 100). Asterisks indicate significant differences between control and flg22 or t-Z treatments based on a t test (***P < 0.001).
(C) and (D) ROS production detected by H2DCF-DA fluorescence in guard cells of Col-0 wild-type, prx4-2, prx34-1, and prx71-1 mutants (C) and prx33-3 and prx34-2 mutants (D) 30 min after treatments with control solution, 5 µM flg22, or 1 µM t-Z. Values are means ± se (n ≥ 60).
(E) Stomatal apertures in Col-0 wild-type, prx33-3, and prx34-2 mutants exposed to mock control or 108 cfu/mL COR-deficient Pst DC3000 (Pst COR−) bacteria for 2 h. Values are means ± se (n ≥ 100). In (C) to (E), different letters denote significant differences at P < 0.001 (Tukey’s HSD test).
(F) Bacterial growth in Col-0 wild-type, prx33-3, and prx34-2 mutants assessed at 1 d (1 dpi) and 3 d (3 dpi) after spray inoculation with Pst COR− at 108 cfu/mL (left panel) or at 0 and 3 d after syringe infiltration with Pst COR− at 106 cfu/mL (right panel). The dashed line indicates the apoplastic bacterial population (cfu/cm2) at 0 d postinoculation. Values are the means ± se (n = 4). Different letters indicate significant differences at P < 0.05 based on a Tukey’s HSD test (see ANOVA tables; Supplemental Data Set 1).
ARR2 Directly Activates the Expression of PRX33 and PRX34
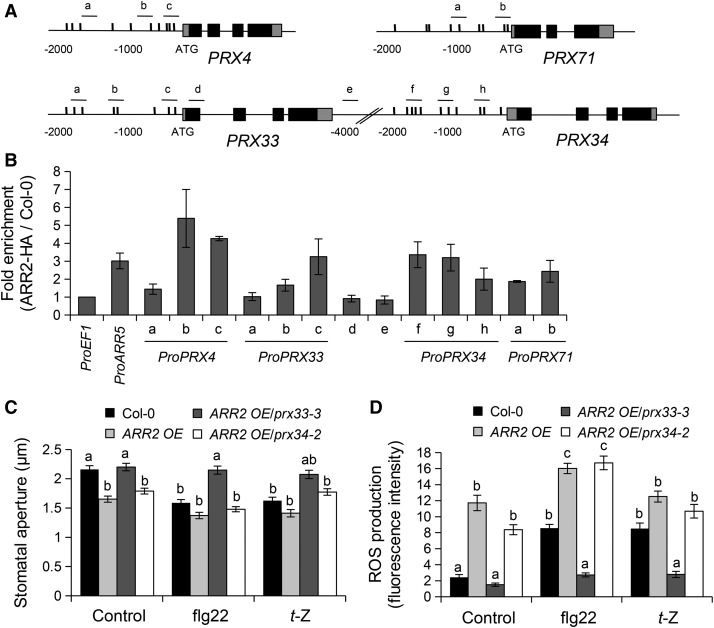
Since PRX4, PRX33, PRX34, and PRX71 contribute to flg22- and t-Z-mediated stomatal responses, we next tested whether ARR2 interacts with the promoters of these PRX genes using the HA-tagged ARR2-overexpressing line for chromatin immunoprecipitation (ChIP) experiments (Kim et al., 2006; Choi et al., 2010). The ARR2 DNA binding domain specifically interacts in vitro with the AGATT sequence (Sakai et al., 2000). Several DNA regions were analyzed to cover all potential ARR2 binding sites in the PRX4, PRX33, PRX34, and PRX71 promoters (Figure 6A; Supplemental Table 4). In addition, the intergenic region, PRX33 coding region, and the EF1 promoter were used as negative controls. The promoter of the CK-inducible ARR5 gene was used as a positive control. As shown in Figure 6B, DNA segments containing potential ARR binding motifs were enriched in the PRX34 promoter. For the PRX4, PRX33, and PRX71 promoters, enrichment was preferentially found in potential binding sites close to the transcriptional start site. These results indicate that ARR2 binds to the PRX4, PRX33, PRX34, and PRX71 promoters.
Figure 6.
ARR2 Directly Regulates PRX4, PRX33, PRX34, and PRX71 Expression.
(A) Schematic representation of PRX4, PRX33, PRX34, and PRX71 genes, with dots indicating putative ARR binding motifs (AGATT), and black and gray boxes the exons and untranslated region, respectively. Positions of PCR primers for ChIP are indicated by letters (a to h).
(B) ChIP in Col-0 and ARR2-HA OE plants followed by qPCR of the PRX4, PRX33, PRX34, and PRX71 promoters. ARR5 and EF1 promoters were used as positive and negative controls, respectively. qPCR results were normalized against the input samples and fold enrichment was calculated by calculating the ratios between normalized results from ARR2-HA OE and Col-0 (wild type) plants. Error bars represent se of three independent experiments.
(C) and (D) Stomatal apertures (C) and ROS production detected by H2DCF-DA fluorescence in guard cells (D) of the wild-type Col-0 and ARR2 OE, ARR2 OE/prx33-3, and ARR2 OE/prx34-2 lines exposed to control solution, 5 µM flg22, or 1 µM t-Z. Values are means ± se, n ≥ 100 in (C) and n ≥ 60 in (D). Different letters indicate significant differences at P < 0.001 based on a Tukey’s HSD test (see ANOVA tables; Supplemental Data Set 1).
To determine whether PRX33 or PRX34 acts downstream of ARR2 in PAMP- and CK-mediated stomatal closure, we generated the ARR2 OE/prx33-3 and ARR2 OE/prx34-2 lines by crossing. Introduction of the prx33-3 mutation in the ARR2 OE line restored stomatal aperture and ROS accumulation to wild-type levels in control conditions (Figures 6C and 6D). By contrast, the ARR2 OE/prx34-2 line still exhibited partially closed stomata and high ROS levels, similar to the ARR2 OE plants. Unlike the prx34-2 mutant, ARR2 OE/prx34-2 plants exhibited stomatal closure in response to flg22 and t-Z (Figures 6C and 6D), albeit to a lesser extend for t-Z treatment compared with the ARR2 OE line. Compared with wild-type Col-0, similar overaccumulation of ROS was observed in ARR2 OE and ARR2 OE/prx34-2 lines after flg22 treatment. In t-Z-treated conditions, ARR2 OE plants showed a modest increase in ROS level and the ARR2 OE/prx34-2 line accumulated wild-type ROS levels. Importantly, ARR2 OE/prx33-3 plants were fully defective in flg22- and t-Z-induced stomatal closure and ROS production. It should be noted that the PRX33 expression level remains high in the ARR2 OE/prx34-2 line, similar to the ARR2 OE line (Supplemental Figure 6). By contrast, the upregulation of PRX34 observed in the ARR2 OE line was reverted to the wild-type level in the ARR2 OE/prx33-3 line. Thus, even though we cannot exclude a role for PRX34 downstream of ARR2, these results suggest that PRX33 plays a prominent role in ARR2-, PAMP-, and CK-mediated ROS production and stomatal closure.
CK-Mediated Stomatal Closure Is Mechanistically Linked to the SA Pathway, but Not the ABA Pathway
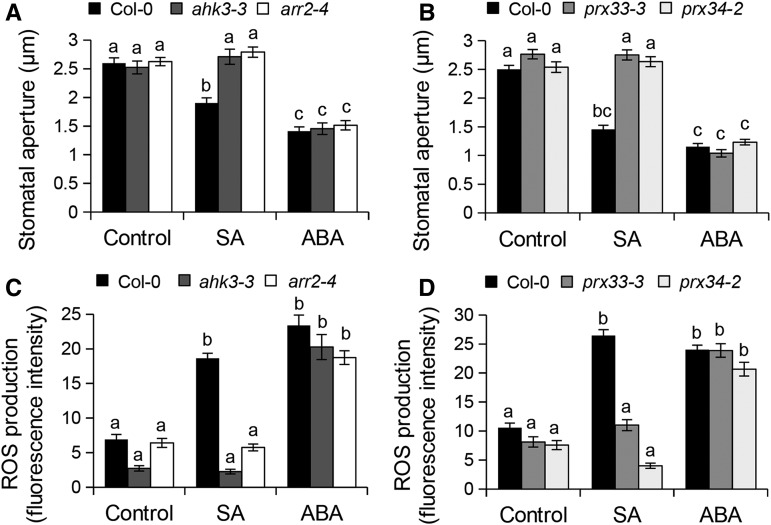
Previous studies have shown that both ABA and SA signaling pathways positively regulate stomatal immunity (Melotto et al., 2006; Zeng and He 2010; Montillet et al., 2013). Moreover, stomatal closure induced by SA, but not by ABA, was impaired by PRX inhibitors (Khokon et al., 2010a, 2011). Interestingly, ahk3-3, arr2-4, prx33-3, and prx34-2 mutants were impaired in SA-induced stomatal closure and ROS production but were not affected in these responses after ABA treatment (Figure 7). Therefore, the signaling cascade involving AHK3, ARR2, PRX33, and PRX34 may preferentially regulate the CK and SA signaling pathways in an ABA-independent manner.
Figure 7.
CK Signaling Components Are Required for Stomatal Responses Induced by SA but Not by ABA.
(A) and (B) Stomatal apertures in Col-0 wild-type, ahk3-3, and arr2-4 mutants (A), and prx33-3 and prx34-2 mutants (B) after 2 h of incubation with control solution, 10 µM SA, or 1 µM ABA. Values are means ± se (n ≥ 100).
(C) and (D) ROS production detected by H2DCF-DA fluorescence in guard cells of Col-0 wild-type, ahk3-3, and arr2-4 mutants (C), and prx33-3 and prx34-2 mutants (D) 30 min after treatment with control solution, 1 µM ABA, or 10 µM SA. Values are means ± se (n ≥ 60). Different letters indicate significant differences at P < 0.001 based on a Tukey’s HSD test (see ANOVA tables; Supplemental Data Set 1).
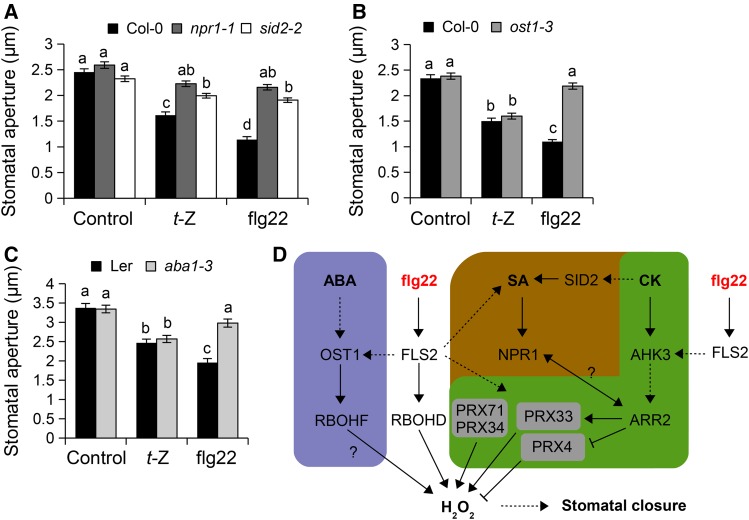
The SA-signaling regulator NPR1 is required for SA-induced stomatal closure, and both NPR1 and SID2 are known to be involved in stomatal immunity but are not required for ABA-mediated stomatal closure (Melotto et al., 2006; Zeng and He, 2010; Montillet et al., 2013). npr1-1 and sid2-2 mutants, which as expected were impaired in flg22-triggered ROS production and stomatal closure, did not close stomata or accumulate H2O2 after t-Z treatment (Figure 8A; Supplemental Figure 7A). In ARR2 and IPT3 OE lines, SA-mediated ROS production was more pronounced as compared with the wild-type Col-0 (Supplemental Figure 7B). It was previously shown that ARR2 positively regulates the SA signaling pathway downstream of NPR1 by direct interaction with the transcription factor TGA3 (Choi et al., 2010). Surprisingly, t-Z- and flg22-mediated stomatal closure was not affected in the tga3-3 mutant (Supplemental Figure 7C). Moreover, the tga3-3/ARR2 OE line showed constitutive stomatal closure as in ARR2 OE plants (Supplemental Figure 7D), suggesting that TGA3 is not required for stomatal immunity and ARR2-mediated stomatal closure. Nevertheless, these results indicate that SA signaling via NPR1 is necessary for CK-mediated ROS production and stomatal closure and that CK may activate SA signaling in guard cells.
Figure 8.
CK-Mediated Stomatal Closure Is Mechanistically Linked to the SA Pathway.
(A) to (C) Stomatal apertures in Col-0 wild-type, npr1-1, and sid2-2 plants (A), Col-0 wild type and ost1-3 (B), and Landsberg erecta wild type and aba1-3 (C) after 2 h of incubation with control solution, 1 µM t-Z, or 5 µM flg22. Values are means ± se (n ≥ 100). Different letters indicate significant differences at P < 0.001 based on a Tukey’s HSD test (see ANOVA tables; Supplemental Data Set 1).
(D) Hypothetical model for the regulation of stomatal immunity by cytokinins. This model is based on the information provided in this study and references cited in the Discussion. In guard cells, perception of the PAMP flg22 by FLS2 is mechanistically linked to ABA and SA signaling pathways, illustrated by blue and brown shading, respectively. PRX33, PRX34, and PRX71 activate (arrows), while PRX4 represses (T-bars), PAMP-triggered ROS production. Independently of ABA and through a signaling cascade involving AHK3, ARR2, PRX33, and PRX34, CK (green shading) together with SA induces ROS production and stomatal closure to promote preinvasive defense responses. Dashed lines and question marks indicate indirect and uncertain connections, respectively.
To further clarify hormonal crosstalk in PAMP-mediated stomatal closure, we analyzed stomatal movements after t-Z treatment in the ost1-3 mutant defective in ABA-mediated ROS production and stomatal closure (Mustilli et al., 2002) and in the ABA-deficient mutant aba1-3. Importantly, ost1-3 and aba1-3 mutants, which were defective in flg22-triggered stomatal closure (Melotto et al., 2006; Guzel Deger et al., 2015), showed wild-type stomatal closure after t-Z treatment (Figures 8B and 8C). Moreover, the t-Z-mediated increase in ROS production was not affected in the ost1-3 mutant (Supplemental Figure 7E). These results suggest that the canonical ABA signaling pathway is not required for CK-mediated stomatal closure.
DISCUSSION
Cytokinins have been reported to play a positive or negative role in plant-microbe interactions depending on pathogen lifestyle (Choi et al., 2011). In this study, we uncover a novel and crucial function of CKs in promoting stomatal defenses and plant resistance to surface-inoculated Pst DC3000 bacteria. In concert with the SA pathway, CK signaling regulates PRX-mediated ROS production in guard cells for the control of stomatal movements upon PAMP perception. Thus, our results reveal the multifaceted roles of CKs in plant immunity, not only in postinvasive defense responses in the apoplastic space (Choi et al., 2010; Grosskinsky et al., 2011; Argueso et al., 2012; Hann et al., 2014), but also in the activation of preinvasive defense responses at the stomata level.
A Plant Growth-Promoting Hormone Induces Stomatal Closure
Cytokinins are well-known growth hormones involved in shoot formation, cell division, nutrient mobilization, and leaf longevity (Hwang et al., 2012). Although it has been reported since the early 1970s that CKs tend to promote stomatal opening, the stomatal response to exogenous application of CK depends on plant species and on the concentration and type of CK used (Acharya and Assmann, 2009). Here, we show that in Arabidopsis, exogenous application of the biologically active t-Z induces stomatal closure in a dose-dependent manner. A significant stomatal closure was observed from 10 nM with an optimal effect at 1 µM. These concentrations are physiologically relevant as AHK3 has a high affinity for t-Z with a Kd of 4.3 nM (Lomin et al., 2015). Moreover, we found that, within the CK receptors, only AHK3 participates in t-Z-mediated stomatal responses. Consistently, AHK3, but not AHK2 and AHK4, activates the CK-sensitive pARR5:GUS reporter gene in guard cells (Stolz et al., 2011), and CK-induced ARR2 phosphorylation is specifically mediated by AHK3 (Kim et al., 2006). We also observed that CK-mediated stomatal closure was correlated with an increase in ROS accumulation in guard cells and the ARR2 overexpression line consistently exhibited constitutive stomatal closure due to high ROS levels. Accordingly, it was shown recently that an increase in endogenous CK levels by induction of IPT gene expression in tobacco (Nicotiana tabacum) induces ROS accumulation and a decrease in stomatal conductance (Novák et al., 2013). The observation that a growth promoting hormone induces stomatal closure is somehow counterintuitive. Although ARR2 overexpression results in smaller stomatal opening, it may be sufficient for CO2 influx, photosynthesis, and growth. Indeed, the ARR2-overexpressing line consistently exhibited larger rosette leaves and increased biomass (Supplemental Figure 8), suggesting that constitutive stomatal closure is not limiting for plant growth, but could prime stomatal immunity.
ARR2-Mediated Transcriptional Activation of PRXs Regulates ROS Homeostasis in Guard Cells
Two recent studies highlighted the importance of transcriptional repression of PRX genes in Arabidopsis for the regulation of ROS homeostasis in root or leaf development (Tsukagoshi et al., 2010; Lu et al., 2014). In rice (Oryza sativa), the drought and salt tolerance transcription factor negatively regulates stomatal closure by activating the expression of genes encoding ROS-scavenging enzymes including peroxidases (Huang et al., 2009). However, drought and salt tolerance homologs were identified in other cereals but not in Arabidopsis. Here, we found that the transcription factor ARR2 can regulate the expression of a subset of PAMP-induced PRX genes in leaves, particularly PRX4, PRX33, PRX34, and PRX71, which are direct ARR2 targets. Interestingly, ARR2 activates PRX33 expression but represses PRX4 expression in guard cells. Moreover, the cell wall peroxidases PRX4, PRX33, PRX34, and PRX71, but not the NADPH oxidases RBOHD and RBOHF, play an important role in CK-mediated stomatal closure. Thus, the CK-signaling component ARR2 is a novel transcriptional regulator of PRX genes for the control of ROS homeostasis in guard cells.
PRXs function in the plant defense response by consuming H2O2 through the peroxidase cycle for cell wall cross-linking or lignification to block pathogen ingress or by producing ROS through the oxidative cycle during the PAMP-triggered oxidative burst (Bolwell et al., 2002; O’Brien et al., 2012a). PRXs can directly generate H2O2 from O2 in the presence of a still unknown reductant and upon extracellular alkalinization, which typically occurs upon PAMP perception. It is known that SA-induced ROS production and subsequent stomatal closure are inhibited by the PRX inhibitor SHAM and are independent of NADPH oxidases, particularly RBOHD/F (Khokon et al., 2011). Similar observations were made using the fungal PAMP chitosan or yeast elicitor extracts (Khokon et al., 2010a, 2010b). However, the identity of these PRXs involved in PAMP- and SA-mediated stomatal closure has remained puzzling. Here, we provide genetic evidence that at least PRX33 and PRX34 positively regulate SA- and PAMP-mediated stomatal closure through the production of ROS. By contrast, PRX4 may inhibit flg22-mediated stomatal closure probably through the consumption of H2O2 to avoid detrimental effects of ROS excess.
Relative Contribution of PRXs and NADPH Oxidases to Preinvasive Immunity
During postinvasive immunity, PAMP-triggered oxidative burst is dependent on both the plasma membrane NADPH oxidase RBOHD and cell wall peroxidases PRX33 and PRX34 (O’Brien et al., 2012a). However, PRX33/34 may have a more important role than RBOHD in downstream defense responses such as callose deposition and expression of PTI marker genes, suggesting that ROS produced by RBOHD and PRXs are not functionally equivalent in apoplastic defense responses (Daudi et al., 2012). By contrast, at the guard cell level, previous work clearly indicated that RBOHD, as part of the FLS2/BIK1 complex, is required for PAMP- and bacteria-induced stomatal closure (Kadota et al., 2014; Li et al., 2014). Here, we show that at least PRX4, PRX33, PRX34, and PRX71 also function in preinvasive immunity. Surprisingly, the disruption of one gene is not compensated by other functional PRX genes, especially for the tandem duplicated PRX33 and PRX34 genes, which share a high level of sequence similarity (94% at the protein level). However, although the basal expression level of PRX34 is higher than that of PRX33, our genetic analysis suggests that ARR2-mediated activation of PRX33 expression may compensate for the loss of PRX34 function in the ARR2 OE/prx34-2 line. In addition, the constitutive stomatal closure observed in the ARR2 OE line may also be caused by the ARR2-mediated transcriptional repression of PRX4, which negatively regulates ROS accumulation in guard cells.
It was previously observed that mutations in either PRX33 or PRX34 downregulate the expression of both genes and that RBOHD expression is dependent on the expression of PRX33 and PRX34 (Daudi et al., 2012; O’Brien et al., 2012b). However, in our genetic backgrounds, the expression of PRX33 in the prx34-2 mutant and PRX34 in the prx33-3 mutant is not affected (Supplemental Figure 6). Similarly, RBOHD and RBOHF expression is not regulated by PRX33 or PRX34 (Supplemental Figure 9). Therefore, the observation that plants defective in PRX33 or PRX34 but with functional RBOHD are impaired in PAMP-mediated stomatal responses suggests that plasma membrane NADPH oxidases and cell wall PRXs act cooperatively to produce ROS during PAMP-triggered stomatal closure, perhaps in the same signaling cascade. Interestingly, a recent study indicates a concerted action of the NADPH oxidase RBOHF and PRXs that colocalize at the Casparian strips for localized lignin deposition (Lee et al., 2013). A similar mechanism may occur for ROS production in stomatal defense.
CK together with SA Activates Stomatal Immunity
A previous study demonstrated that CK activates the expression of the SA biosynthesis gene SID2/ICS1 and induces an increase in SA level in plants (Choi et al., 2010). Recently, it was shown that repression of ICS1 expression and SA accumulation by NAC transcription factors is important for COR-mediated stomatal reopening (Zheng et al., 2012; Du et al., 2014). Interestingly, COR-mediated stomatal reopening was compromised by CKs or in the ARR2 OE line. Moreover, the overexpression of IPT3 or ARR2 enhanced flg22- and SA-triggered ROS production, indicating that CKs may overactivate stomatal immunity through ARR2. Importantly, SID2 and NPR1 are required for CK-mediated stomatal closure and conversely AHK3 and ARR2 regulate the SA pathway in guard cells. As observed before in mesophyll cells (Choi et al., 2010), it is plausible that CK functions together with SA in guard cells and converges on ARR2, which acts as a hub that connects the two signaling pathways. We propose that a signaling cascade involving AHK3, ARR2, PRX33, and PRX34 activates stomatal immunity through the SA pathway in an ABA-independent manner (Figure 8D). Still, our results indicate that ABA signaling positively regulates stomatal immunity as observed before (Melotto et al., 2006; Zeng and He, 2010; Du et al., 2014; Guzel Deger et al., 2015). Thus, our study highlights the existence of two separate branches involving the ABA and the CK/SA pathways, which both contribute to stomatal defense.
Our results raise an intriguing question on the function of CKs in PAMP- or bacteria-triggered immunity as an endogenous or exogenous signal. A reduction in endogenous CKs (Naseem et al., 2012) and a decrease in the expression of IPT3 and CK-induced type-A ARR genes has been reported in the late stages, 1 to 3 d after Pst DC3000 inoculation (Thilmony et al., 2006). Because type-A ARRs are negative regulators of CK signaling (Hwang et al., 2012), a reduction of their expression during Pst infection may also derepress CK signaling. By contrast, the Pst DC3000 effector HopQ1 was shown to induce an increase in CK levels and an upregulation of type-A ARRs (Hann et al., 2014). Our data indicate that CK content increases transiently in leaves soon after Pst DC3000 infection (1 h), likely due to PAMP perception. By contrast, the expression of CK-responsive ARR5 and ARR6 genes in guard cells was not affected by flg22 treatment (Figure 4). Alternatively, the CK signaling components AHK3 and ARR2 could be activated by biotic stresses in a CK-independent manner, which is supported by the observation that flg22 induces AHK3 and ARR2 expression in guard cells. Although P. syringae has not been reported to produce CKs, many pathogenic bacteria, such as Agrobacterium tumefaciens and other Pseudomonas strains, produce CKs or induce CK biosynthesis to increase sink activity of the colonized tissues and reallocate nutrients toward the pathogen (Akiyoshi et al., 1987; Choi et al., 2011). We speculate that during evolution, plants developed a specific signaling pathway in guard cells to promote preinvasive immunity against CK-secreting pathogens.
METHODS
All experiments reported here were repeated at least three times with similar results. Statistically significant groups were determined by Student’s t tests or ANOVAs followed by Tukey’s honestly significant difference (HSD) posthoc test using R software.
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) mutant lines in the Col-0 background, except aba1-3 in the Landsberg erecta background, were obtained from the Arabidopsis Biological Resource Center at Ohio State University and the European Arabidopsis Stock Centre and are listed in Supplemental Table 5. All T-DNA insertion mutants were confirmed by genotyping prior to usage using PCR primers (Supplemental Table 6). T-DNA insertion sites for the prx mutants were determined by sequencing. The IPT1, IPT3, and ARR2 overexpression lines were previously described (Kim et al., 2006; Choi et al., 2010). F3 plants of the double transgenic lines ARR2 OE/tga3-3, ARR2 OE/prx33-3, and ARR2 OE/prx34-2 were generated by crossing homozygous parental lines. The progeny were selected on appropriate antibiotics, and genotyping was performed by PCR. Four- to five-week-old plants grown on soil in a growth chamber at 21 to 23°C, 40 to 60% humidity, and illuminated with fluorescent tubes at a light intensity of 100 µmol m–2 s–1 light intensity under short-day (10 h light/14 h dark) conditions were used for all the experiments.
Bacterial Infection Assay
Bacterial strains Pst DC3000 and Pst DC3000 COR− (DB4G3) (Brooks et al., 2004) were cultivated overnight at 28°C in King’s B medium supplemented with appropriate antibiotics. Bacteria were collected by centrifugation at 3000g for 5 min at room temperature and washed twice in 10 mM MgCl2. Plants were surface inoculated by spraying with a bacterial solution of 108 colony-forming units (cfu)/mL in 10 mM MgCl2 containing 0.02% Silwet L-77, and plants were covered to maintain high humidity until disease symptoms developed. Alternatively, rosette leaves were syringe infiltrated with a bacterial solution of 106 cfu/mL in 10 mM MgCl2. After 3 d, bacterial growth in the apoplast of three leaves per plant and four plants per genotype was determined as previously described (Katagiri et al., 2002).
Measurements of Stomatal Aperture
Stomatal experiments were conducted as previously described (Desclos-Theveniau et al., 2012). Leaf peels were collected from the abaxial side of young fully expanded leaves and floated in stomatal buffer (10 mM MES-KOH and 30 mM KCl, pH 6.15) for 2.5 h under light (100 µmol m–2 s–1) to ensure that most stomata were opened before treatments. Solutions of chemicals or bacterial suspension at 108 cfu/mL−1 in 10 mM MgCl2 were directly added in the stomatal buffer. Purified chemicals, except flg22 peptide (AnyGen), were purchased from Sigma-Aldrich and used at the following concentrations: 5 µM flg22, 1 µM t-Z, 1 µM ABA, 10 µM SA, 1.6 µM (0.5 ng/µL) COR, 1 mM ASC, 2 mM SHAM, 20 µM DPI, and 1 µM sodium azide. Control solutions were stomatal buffer containing 0.01% ethanol for ABA and SA, 1% ethanol for SHAM, 0.1% DMSO for DPI, and 10 µM NaOH for t-Z, and water for COR, sodium azide, and ASC. After treatments, epidermal peels were further incubated under light for 2 h and observed under a light microscope (Carl Zeiss; Axioplan 2). Stomatal apertures of at least 100 stomata in random areas were measured using ImageJ 1.42 software.
Monitoring ROS in Guard Cells
The fluorescent dye H2DCF-DA (Sigma-Aldrich) was used to measure H2O2 levels in guard cells. After 2.5 h incubation in stomatal buffer, epidermal peels were incubated with 50 µM H2DCF-DA in 0.1% DMSO for 15 min. Excess H2DCF-DA was then removed by washing three times for 20 min with stomatal buffer. Then, 5 µM flg22, 1 µM t-Z, 1 µM ABA, 10 µM SA, or control solutions were added. After 30 min incubation, H2DCF-DA fluorescence was observed with a fluorescence microscope (Axioplan 2), and fluorescence intensity of at least 60 stomata was analyzed using ImageJ software.
Quantification of Endogenous Cytokinins
Young fully expanded rosette leaves were syringe inoculated with 108 cfu/mL Pst DC3000 bacteria or mock solution (10 mM MgCl2) and collected at 1 and 3 h postinoculation. For each biological replicate, two infiltrated leaves from three different plants were pooled and freeze-dried. Extraction and quantification of cytokinins was performed as described previously (Kojima et al., 2009) with an UPLC-ESI-qMS/MS (AQUITY UPLC system/Xevo-TQS; Waters) with an ODS column (AQUITY UPLC BEH C18; 1.7 µm, 2.1 × 100 mm; Waters).
Analysis of Transcriptome Databases
The transcriptome data sets related to biotic stress response were downloaded from AtGenExpress (TAIR) and include time-course experiments on the leaves of 5-week-old Arabidopsis plants collected 2, 6, and 24 h after inoculation with the bacterial strains Pst DC3000, Pst avrRpm1, Pst DC3000 hrcC−, and Pseudomonas syringae pv phaseolicola (ME00331; F. Brunner and T. Nürnberger, unpublished data), or 1 and 4 h after treatment with HrpZ, NPP1, flg22, and LPS elicitors (ME00332; F. Brunner and T. Nürnberger, unpublished data). The data sets for leaves and guard cells (E-GEOD-19520; Pandey et al., 2010) and for guard cells and mesophyll cells in which transcriptional inhibitors (actinomycin D and cordycepin) were added or not during protoplast isolation (E-MEXP-1443; Yang et al., 2008) were downloaded from ArrayExpress (EMBL-EBI). For each profile, the intensities of probes were log2 transformed and then normalized using the GCRMA method (Wu et al., 2004). Fold changes were calculated as log2 median differences between the normalized values in control and biotic stress-treated conditions. The heat maps were generated by hierarchical clustering with euclidean distance and complete linkage.
Isolation of Guard Cell Protoplasts
For each condition, ∼50 young fully expanded leaves were directly collected or immerged for 2 h in stomatal buffer containing 0.02% (v/v) Silwet-L77 with or without 1 µM flg22. Guard cell protoplasts were isolated as previously described (Obulareddy et al., 2013) in the presence of transcriptional inhibitors 0.01% (w/v) cordycepin and 0.0033% (w/v) actinomycin D. For each sample, ∼106 guard cell protoplasts were obtained for RNA extraction and their purity was above 98%.
Gene Expression Analysis
Three fully expanded rosette leaves were either not treated or syringe infiltrated with 1 µM flg22 or control solution (10 mM MgSO4) and plants were left for 2 h in a growth room before assessment. For leaf samples, total RNA was extracted using Trizol reagent (Invitrogen), and genomic DNA was removed by digestion with DNase I (DNA-free; Ambion) according to the manufacturer's protocol. For guard cells, RNA was extracted using the RNeasy Plant Mini Kit with in-column DNase I digestion (Qiagen). One microgram (leaves) or 200 ng (guard cells) of total RNA was reverse transcribed using 500 ng of oligo(dT)15 and the ImProm-II reverse transcription system following the manufacturer’s instructions (Promega). cDNA was diluted 2-fold before quantitative PCR or PCR. PCR amplifications were done with 2 μL of the first-strand cDNA as template, 0.025 units of Ex Taq DNA polymerase (Takara), 250 µM dNTP, and 0.5 µM of primers in a total volume of 20 µL. The cycling conditions were 95°C for 5 min for one initial step followed by 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min, for 25 to 45 cycles. The PCR was terminated with one extra step at 72°C for 10 min. Quantitative real-time PCR reactions were performed on a Light Cycler 2 (Roche) using 10 μL SYBR Premix Ex Taq (Takara), 2 μL of cDNA, and 0.5 µM of primers in a total volume of 20 μL per reaction. The cycling conditions were composed of an initial 20-s denaturation step at 95°C, followed by 45 cycles of 95°C for 7 s, 60°C for 10 s, and 72°C for 13 s. A melting curve was run from 65 to 95°C to ensure the specificity of the products. Data were analyzed with the delta Ct method using Light Cycler 2.0 software. Elongation factor 1α (EF1) and ubiquitin 1 (UBQ1) were used as reference genes for normalization of gene expression levels. In Figure 4 and Supplemental Tables 2 and 3, Col-0 wild type without any treatment or control treatment were considered as controls (expression level = 1). RT-PCR and qRT-PCR primer sequences are listed in Supplemental Table 7.
ChIP
ChIP was performed essentially as described (Gendrel et al., 2005) with minor modifications. Four rosette leaves from at least 18 plants (1.5 to 2 g) of 35S:ARR2-HA (Choi et al., 2010) or wild-type Col-0 (used as a negative control) were cross-linked under vacuum for 10 min in a solution containing 0.4 M sucrose, 10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 1 mM PMSF, and 1% formaldehyde. Glycine was added to a final concentration of 125 mM to stop the cross-linking. Leaves were ground in liquid nitrogen, resuspended with 25 mL of extraction buffer 1 (0.4 M sucrose, 10 mM Tris-HCl, pH 8.0, 10 mM MgCl2, 5 mM β-mercaptoethanol, 0.1 mM PMSF, and 1× protease inhibitor cocktail [Roche]), and filtered through four layers of Miracloth (Calbiochem). The filtrate was centrifuged at 3000g and 4°C for 20 min. The pellet was resuspended in 1 mL of extraction buffer 2 (0.25 M sucrose, 10 mM Tris-HCl, pH 8.0, 10 mM MgCl2, 1% Triton X-100, 5 mM β-mercaptoethanol, 0.1 mM PMSF, and 1× protease inhibitor cocktail) and centrifuged at 12,000g and 4°C for 10 min. The pellet was resuspended in 300 μL of extraction buffer 3 (1.7 M sucrose, 10 mM Tris-HCl, pH 8.0, 0.15% Triton X-100, 2 mM MgCl2, 5 mM β-mercaptoethanol, 0.1 mM PMSF, and 1× protease inhibitor cocktail), loaded on top of 1.5 mL of extraction buffer 3, and centrifuged at 16,000g and 4°C for 1 h. The nuclear pellet was resuspended in 500 μL nuclei lysis buffer (50 mM Tris-HCl, pH 8.0, 10 mM EDTA, 1% SDS, and 1× protease inhibitor cocktail) and sonicated five times (15 s each) to yield DNA fragments of 0.5 to 1 kb. After centrifugation at 12,000g and 4°C for 5 min, the supernatant was diluted 10-fold with ChIP dilution buffer (16.7 mM Tris-HCl, pH 8.0, 1.2 mM EDTA, 167 mM NaCl, and 1.1% Triton X-100) and precleared with preequilibrated salmon sperm DNA/protein G agarose beads (Millipore) for 1 h at 4°C. Then, 1 mL of the precleared chromatin solution was immunoprecipitated with 5 μL of an anti-HA antibody (Abcam; catalog no. ab9110) overnight at 4°C and extracted by incubating with 60 μL of preequilibrated salmon sperm DNA/protein G agarose beads for 2 h at 4°C. The beads were successively washed with 1 mL of washing buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, and 20 mM Tris-HCl, pH 8.0) containing 150 mM NaCl, 1 mL of washing buffer containing 500 mM NaCl, and twice with TE buffer (10 mM Tris-HCl, pH 8.0, and 1 mM EDTA). The immunocomplexes were eluted twice from the beads with 250 μL of elution buffer (1% SDS and 0.1 M NaHCO3) and then reverse cross-linked overnight with a final concentration of 200 mM NaCl at 65°C. After removing proteins with proteinase K, DNA was purified by phenol-chloroform extraction and ethanol precipitation. Precipitated DNA was resuspended with 50 μL of TE buffer and 2 μL was used as a template for quantitative real-time PCR analysis. PCR conditions were the same as for qRT-PCR and the sequences of primers are listed in Supplemental Table 8. A 10-μL aliquot of sonicated chromatin was reverse cross-linked as an input control. Input samples were first used to normalize the results. Fold difference was then calculated by calculating the ratios between normalized results from wild-type Col-0 plants and from ARR2-HA plants. Finally, the fold enrichment was calculated as the ratio between the probes and EF1 as a negative control.
Accession Numbers
All sequence data from this article can be found in the Arabidopsis Genome Initiative data library, and accession numbers are listed in Supplemental Table 9.
Supplemental Data
Supplemental Figure 1. ARR2 participates in t-Z-mediated stomatal closure.
Supplemental Figure 2. Cytokinin inhibits the COR-dependent reopening of stomata.
Supplemental Figure 3. CK-mediated stomatal closure is dependent on apoplastic peroxidases.
Supplemental Figure 4. The expression of a set of PRX genes is induced by bacteria or PAMPs in leaves.
Supplemental Figure 5. Molecular characterization of prx mutants.
Supplemental Figure 6. PRX33 and PRX34 expression in ARR2 OE/prx33-3 and ARR2 OE/prx34-2 lines.
Supplemental Figure 7. CK functions with SA in guard cells independently of the ABA pathway.
Supplemental Figure 8. ARR2 overexpression enhances plant growth.
Supplemental Figure 9. RBOHD and RBOHF expression is not affected in prx33-3 and prx34-2 mutants.
Supplemental Table 1. Cytokinin concentrations in Col-0 (WT) leaves at 1 and 3 h after inoculation with Pst DC3000 bacteria.
Supplemental Table 2. Expression levels of PRX genes in guard cell protoplasts of Col-0 (WT) and ARR2-overexpressing line (ARR2 OE) and in Col-0 after flg22 treatment.
Supplemental Table 3. Expression levels of PRX genes in whole leaves of Col-0 (WT), ARR2- and IPT3-overexpressing lines (ARR2 OE and IPT3 OE), and in Col-0 after control or flg22 treatments.
Supplemental Table 4. Positions of putative B-type ARR binding sites (AGATT) in PRX4, PRX33, PRX34, and PRX71 promoters.
Supplemental Table 5. Description of the mutant lines used in the study.
Supplemental Table 6. PCR primers used for genotyping the mutant lines.
Supplemental Table 7. Primers used for RT-PCR and quantitative RT-PCR.
Supplemental Table 8. Primers used for ChIP qPCR.
Supplemental Table 9. Gene sequences used in this article and the corresponding accession numbers.
Supplemental Data Set 1. ANOVA tables.
Acknowledgments
We thank Jean-Luc Montillet for critical reading of the manuscript and helpful discussions. We thank Tatsuo Kakimoto for ahk2-2tk seeds and Ming-Che Shih for prx4-2 seeds. D.A. was funded by the European Union’s Seventh Framework Programme for research, technological development, and demonstration under Grant GA-2010-267243–PLANT FELLOWS. I.H. was supported by the Next-Generation BioGreen 21 Program (PJ01184402), Rural Development Administration, Republic of Korea and Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT, and future planning (2014R1A2A1A10052592).
AUTHOR CONTRIBUTIONS
D.A. conducted the experiments. S.L. contributed to the ChIP experiments and bacterial infection assay. S.L. and D.S. analyzed the transcriptome databases. J.C. contributed plant materials and assisted with the stomatal assays. Y.T. and H.S. analyzed the cytokinin levels. D.A., S.L., and I.H. analyzed the data. D.A. and I.H. designed the research and wrote the manuscript with comments from all authors.
Glossary
- PAMP
pathogen-associated molecular pattern
- COR
coronatine
- ROS
reactive oxygen species
- CK
cytokinin
- ABA
abscisic acid
- SA
salicylic acid
- PRX
peroxidase
- ASC
ascorbate
- DPI
diphenylene iodium chloride
- SHAM
salicylhydroxamic acid
- ChIP
chromatin immunoprecipitation
- HSD
honestly significant difference
- cfu
colony-forming units
Footnotes
Articles can be viewed without a subscription.
References
- Acharya B.R., Assmann S.M. (2009). Hormone interactions in stomatal function. Plant Mol. Biol. 69: 451–462. [DOI] [PubMed] [Google Scholar]
- Akiyoshi D.E., Regier D.A., Gordon M.P. (1987). Cytokinin production by Agrobacterium and Pseudomonas spp. J. Bacteriol. 169: 4242–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso C.T., Ferreira F.J., Epple P., To J.P.C., Hutchison C.E., Schaller G.E., Dangl J.L., Kieber J.J. (2012). Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity. PLoS Genet. 8: e1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud D., Hwang I. (2015). A sophisticated network of signaling pathways regulates stomatal defenses to bacterial pathogens. Mol. Plant 8: 566–581. [DOI] [PubMed] [Google Scholar]
- Bolwell G.P., Bindschedler L.V., Blee K.A., Butt V.S., Davies D.R., Gardner S.L., Gerrish C., Minibayeva F. (2002). The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J. Exp. Bot. 53: 1367–1376. [PubMed] [Google Scholar]
- Brooks D.M., Hernández-Guzmán G., Kloek A.P., Alarcón-Chaidez F., Sreedharan A., Rangaswamy V., Peñaloza-Vázquez A., Bender C.L., Kunkel B.N. (2004). Identification and characterization of a well-defined series of coronatine biosynthetic mutants of Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe Interact. 17: 162–174. [DOI] [PubMed] [Google Scholar]
- Choi J., Choi D., Lee S., Ryu C.-M., Hwang I. (2011). Cytokinins and plant immunity: old foes or new friends? Trends Plant Sci. 16: 388–394. [DOI] [PubMed] [Google Scholar]
- Choi J., Huh S.U., Kojima M., Sakakibara H., Paek K.-H., Hwang I. (2010). The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in Arabidopsis. Dev. Cell 19: 284–295. [DOI] [PubMed] [Google Scholar]
- Daudi A., Cheng Z., O’Brien J.A., Mammarella N., Khan S., Ausubel F.M., Bolwell G.P. (2012). The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclos-Theveniau M., Arnaud D., Huang T.-Y., Lin G.J.-C., Chen W.-Y., Lin Y.-C., Zimmerli L. (2012). The Arabidopsis lectin receptor kinase LecRK-V.5 represses stomatal immunity induced by Pseudomonas syringae pv. tomato DC3000. PLoS Pathog. 8: e1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M., et al. (2014). Closely related NAC transcription factors of tomato differentially regulate stomatal closure and reopening during pathogen attack. Plant Cell 26: 3167–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel A.-V., Lippman Z., Martienssen R., Colot V. (2005). Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods 2: 213–218. [DOI] [PubMed] [Google Scholar]
- Grosskinsky D.K., et al. (2011). Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling. Plant Physiol. 157: 815–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzel Deger A., Scherzer S., Nuhkat M., Kedzierska J., Kollist H., Brosché M., Unyayar S., Boudsocq M., Hedrich R., Roelfsema M.R.G. (2015). Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure. New Phytol. 208: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann D.R., Domínguez-Ferreras A., Motyka V., Dobrev P.I., Schornack S., Jehle A., Felix G., Chinchilla D., Rathjen J.P., Boller T. (2014). The Pseudomonas type III effector HopQ1 activates cytokinin signaling and interferes with plant innate immunity. New Phytol. 201: 585–598. [DOI] [PubMed] [Google Scholar]
- Huang X.-Y., Chao D.-Y., Gao J.-P., Zhu M.-Z., Shi M., Lin H.-X. (2009). A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev. 23: 1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I., Sheen J., Müller B. (2012). Cytokinin signaling networks. Annu. Rev. Plant Biol. 63: 353–380. [DOI] [PubMed] [Google Scholar]
- Issak M., Okuma E., Munemasa S., Nakamura Y., Mori I.C., Murata Y. (2013). Neither endogenous abscisic acid nor endogenous jasmonate is involved in salicylic acid-, yeast elicitor-, or chitosan-induced stomatal closure in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 77: 1111–1113. [DOI] [PubMed] [Google Scholar]
- Kadota Y., Sklenar J., Derbyshire P., Stransfeld L., Asai S., Ntoukakis V., Jones J.D., Shirasu K., Menke F., Jones A., Zipfel C. (2014). Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 54: 43–55. [DOI] [PubMed] [Google Scholar]
- Katagiri F., Thilmony R., He S.Y. (2002). The Arabidopsis thaliana-pseudomonas syringae interaction. The Arabidopsis Book 1: e0039, doi/10.1199/tab.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokon M.A.R., Hossain M.A., Munemasa S., Uraji M., Nakamura Y., Mori I.C., Murata Y. (2010a). Yeast elicitor-induced stomatal closure and peroxidase-mediated ROS production in Arabidopsis. Plant Cell Physiol. 51: 1915–1921. [DOI] [PubMed] [Google Scholar]
- Khokon M.A.R., Uraji M., Munemasa S., Okuma E., Nakamura Y., Mori I.C., Murata Y. (2010b). Chitosan-induced stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis. Biosci. Biotechnol. Biochem. 74: 2313–2315. [DOI] [PubMed] [Google Scholar]
- Khokon A.R., Okuma E., Hossain M.A., Munemasa S., Uraji M., Nakamura Y., Mori I.C., Murata Y. (2011). Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 34: 434–443. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Ryu H., Hong S.H., Woo H.R., Lim P.O., Lee I.C., Sheen J., Nam H.G., Hwang I. (2006). Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc. Natl. Acad. Sci. USA 103: 814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Kamada-Nobusada T., Komatsu H., Takei K., Kuroha T., Mizutani M., Ashikari M., Ueguchi-Tanaka M., Matsuoka M., Suzuki K., Sakakibara H. (2009). Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography-tandem mass spectrometry: an application for hormone profiling in Oryza sativa. Plant Cell Physiol. 50: 1201–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J.M., Mori I.C., Pei Z.-M., Leonhardt N., Torres M.A., Dangl J.L., Bloom R.E., Bodde S., Jones J.D.G., Schroeder J.I. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22: 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Choi H., Suh S., Doo I.-S., Oh K.-Y., Choi E.J., Schroeder Taylor A.T., Low P.S., Lee Y. (1999). Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 121: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Rubio M.C., Alassimone J., Geldner N. (2013). A mechanism for localized lignin deposition in the endodermis. Cell 153: 402–412. [DOI] [PubMed] [Google Scholar]
- Li L., Li M., Yu L., Zhou Z., Liang X., Liu Z., Cai G., Gao L., Zhang X., Wang Y., Chen S., Zhou J.-M. (2014). The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15: 329–338. [DOI] [PubMed] [Google Scholar]
- Lomin S.N., Krivosheev D.M., Steklov M.Y., Arkhipov D.V., Osolodkin D.I., Schmülling T., Romanov G.A. (2015). Plant membrane assays with cytokinin receptors underpin the unique role of free cytokinin bases as biologically active ligands. J. Exp. Bot. 66: 1851–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Wang T., Persson S., Mueller-Roeber B., Schippers J.H.M. (2014). Transcriptional control of ROS homeostasis by KUODA1 regulates cell expansion during leaf development. Nat. Commun. 5: 3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R., Stiller J., Powell J., Rusu A., Manners J.M., Kazan K. (2015). Fusarium oxysporum triggers tissue-specific transcriptional reprogramming in Arabidopsis thaliana. PLoS One 10: e0121902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M., Underwood W., He S.Y. (2008). Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 46: 101–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M., Underwood W., Koczan J., Nomura K., He S.Y. (2006). Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980. [DOI] [PubMed] [Google Scholar]
- Mersmann S., Bourdais G., Rietz S., Robatzek S. (2010). Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol. 154: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montillet J.-L., et al. (2013). An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol. 11: e1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli A.-C., Merlot S., Vavasseur A., Fenzi F., Giraudat J. (2002). Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseem M., Philippi N., Hussain A., Wangorsch G., Ahmed N., Dandekar T. (2012). Integrated systems view on networking by hormones in Arabidopsis immunity reveals multiple crosstalk for cytokinin. Plant Cell 24: 1793–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák J., Pavlů J., Novák O., Nožková-Hlaváčková V., Špundová M., Hlavinka J., Koukalová Š., Skalák J., Černý M., Brzobohatý B. (2013). High cytokinin levels induce a hypersensitive-like response in tobacco. Ann. Bot. (Lond.) 112: 41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J.A., Daudi A., Butt V.S., Bolwell G.P. (2012a). Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236: 765–779. [DOI] [PubMed] [Google Scholar]
- O’Brien J.A., Daudi A., Finch P., Butt V.S., Whitelegge J.P., Souda P., Ausubel F.M., Bolwell G.P. (2012b). A peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defense. Plant Physiol. 158: 2013–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obulareddy N., Panchal S., Melotto M. (2013). Guard cell purification and RNA isolation suitable for high-throughput transcriptional analysis of cell-type responses to biotic stresses. Mol. Plant Microbe Interact. 26: 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S., Wang R.S., Wilson L., Li S., Zhao Z., Gookin T.E., Assmann S.M., Albert R. (2010). Boolean modeling of transcriptome data reveals novel modes of heterotrimeric G-protein action. Mol. Syst. Biol. 6: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z.-M., Murata Y., Benning G., Thomine S., Klüsener B., Allen G.J., Grill E., Schroeder J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734. [DOI] [PubMed] [Google Scholar]
- Sakai H., Aoyama T., Oka A. (2000). Arabidopsis ARR1 and ARR2 response regulators operate as transcriptional activators. Plant J. 24: 703–711. [DOI] [PubMed] [Google Scholar]
- Sirichandra C., Wasilewska A., Vlad F., Valon C., Leung J. (2009). The guard cell as a single-cell model towards understanding drought tolerance and abscisic acid action. J. Exp. Bot. 60: 1439–1463. [DOI] [PubMed] [Google Scholar]
- Stolz A., Riefler M., Lomin S.N., Achazi K., Romanov G.A., Schmülling T. (2011). The specificity of cytokinin signalling in Arabidopsis thaliana is mediated by differing ligand affinities and expression profiles of the receptors. Plant J. 67: 157–168. [DOI] [PubMed] [Google Scholar]
- Swanson S.J., Choi W.-G., Chanoca A., Gilroy S. (2011). In vivo imaging of Ca2+, pH, and reactive oxygen species using fluorescent probes in plants. Annu. Rev. Plant Biol. 62: 273–297. [DOI] [PubMed] [Google Scholar]
- Thilmony R., Underwood W., He S.Y. (2006). Genome-wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J. 46: 34–53. [DOI] [PubMed] [Google Scholar]
- Torres M.A. (2010). ROS in biotic interactions. Physiol. Plant. 138: 414–429. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H., Busch W., Benfey P.N. (2010). Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143: 606–616. [DOI] [PubMed] [Google Scholar]
- Valério L., De Meyer M., Penel C., Dunand C. (2004). Expression analysis of the Arabidopsis peroxidase multigenic family. Phytochemistry 65: 1331–1342. [DOI] [PubMed] [Google Scholar]
- Wu Z., Irizarry R.A., Gentleman R., Martinez-Murillo F., Spencer F. (2004). A model-based background adjustment for oligonucleotide expression arrays. J. Am. Stat. Assoc. 99: 909–917. [Google Scholar]
- Yang Y., Costa A., Leonhardt N., Siegel R.S., Schroeder J.I. (2008). Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W., He S.Y. (2010). A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol. 153: 1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X.-Y., Spivey N.W., Zeng W., Liu P.-P., Fu Z.Q., Klessig D.F., He S.Y., Dong X. (2012). Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 11: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]