Abstract
Conditioning small groups of root pericycle cells for future lateral root formation has a major impact on overall plant root architecture. This priming of lateral roots occurs rhythmically, involving temporal oscillations in auxin response in the root tip. During growth, this process generates a spatial pattern of prebranch sites, an early stage in lateral root formation characterized by a stably maintained high auxin response. To date, the molecular mechanism behind this rhythmicity has remained elusive. Some data implicate a cell-autonomous oscillation in gene expression, while others strongly support the importance of tissue-level modulations in auxin fluxes. Here, we summarize the experimental data on periodic lateral root priming. We present a theoretical framework that distinguishes between a priming signal and its subsequent memorization and show how major roles for auxin fluxes and gene expression naturally emerge from this framework. We then discuss three mechanisms that could potentially induce oscillations of auxin response: cell-autonomous oscillations, Turing-type patterning, and tissue-level oscillations in auxin fluxes, along with specific properties of lateral root priming that may be used to discern which type of mechanism is most likely to drive lateral root patterning. We conclude with suggestions for future experiments and modeling studies.
PERSPECTIVE
INTRODUCTION
The root system is often referred to as plant’s hidden half (Eshel and Beeckman, 2013), and optimizing this considerably less exploited part of the plant may be one of the most tractable ways to generate major improvements in crop yield. The architecture of the root system is determined by the extent and positioning of root branching (Figure 1A). Thus, our capacity to improve the root architecture of crop species will benefit from an improved understanding of the mechanisms determining root system architecture, particularly the initiation, formation, and outgrowth of lateral roots (Den Herder et al., 2010; Ghanem et al., 2011; Kong et al., 2014; Kochian, 2016).
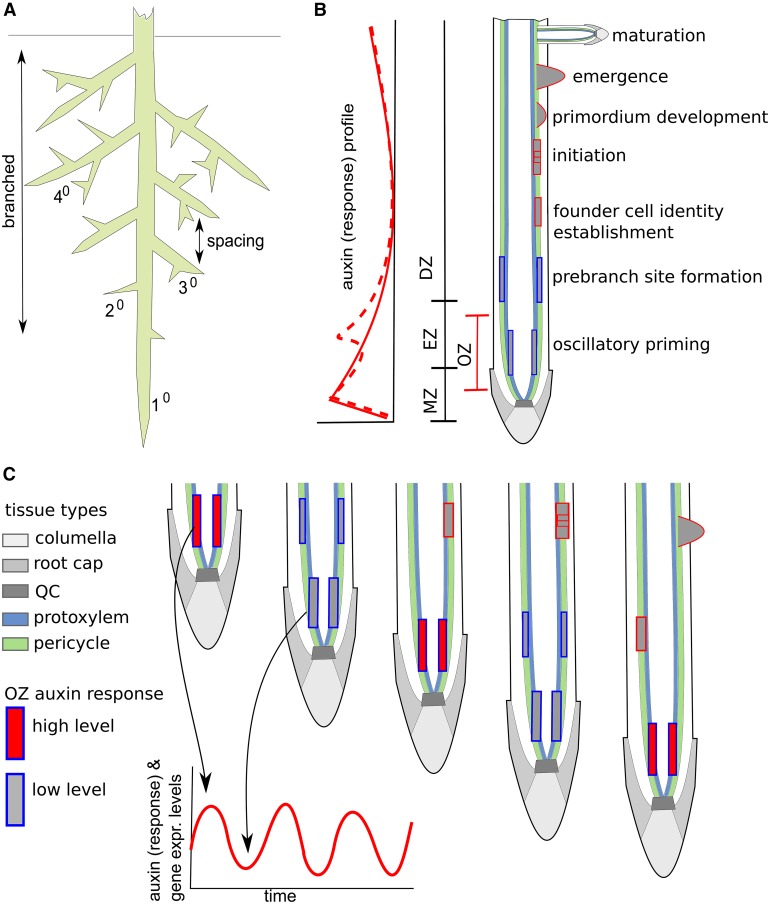
Figure 1.
Root System Architecture and the Dynamics of Lateral Root Development.
(A) Schematic overview of the major determinants of root system architecture.
(B) Left: Schematic overview of the root tip longitudinal auxin (response) profile with oscillation induced transient elevations indicated with a dashed line. Right: Formation of a single lateral root goes through the indicated characteristic sequence of stages as it progresses through the different longitudinal zones of the root. MZ, meristematic zone; EZ, elongation zone; DZ, differentiation zone; OZ, oscillation zone. Color coding as shown for (C).
(C) In protoxylem cells of the basal meristem, cells undergo temporal priming oscillations in auxin concentration and/or auxin response levels as well as oscillations in the expression of many other genes that lead these cells to pass on a priming signal to the overlying pericycle cells. Throughout root growth, these primed cells become displaced, translating into a spatial pattern of sites competent for forming a lateral root. An additional mechanism is responsible for the left-right symmetry breaking that ensures lateral root formation on one side of the root. Given that it is currently not known at which stage this symmetry breaking occurs, we did not indicate it in the sequence of events shown here.
The plant hormone auxin is a major regulator of root system architecture. In the main root, an auxin maximum determines the location of the stem cell niche that maintains root growth (Sabatini et al., 1999), while an auxin gradient extending from this maximum influences growth rate and the subdivision of the main root into zones (e.g., the meristematic zone, elongation zone, and differentiation zone) (Mähönen et al., 2014). Lateral root formation progresses through a regular series of stages that occur as cells traverse these different zones of the main root (Malamy and Benfey, 1997; Péret et al., 2009a; Benková and Bielach, 2010; see Figure 1C and Table 1 for an explanation of terms).
Table 1. Definition of Terms.
| Term | Definition |
|---|---|
| Meristem | Region of the root where cell division occurs, encompassing the stem cell niche with slowly dividing cells as well as the rest of the meristem with rapidly dividing cells. |
| Basal meristem | Shootward portion of the meristem. |
| Transition zone | Boundary region between the meristem and elongation zone where cell division ceases and cell elongation sets in. |
| Elongation zone | Region of the root shootward of the meristem in which no further cell divisions occur and rapid cell elongation occurs. |
| Differentiation zone | Region of the root shootward of the elongation zone where no further cell elongation occurs and cells fully differentiate. |
| Oscillation zone | Region of the root in which auxin (response) and gene expression oscillations are observed, overlapping with the basal meristem and the rootward part of the elongation zone. |
| Priming signal | Initial signal leading toward specification of a small group of pericycle cells to form lateral root founder cells. |
| Prebranch site | Subset of cells that underwent a priming event, maintain a stable auxin response, and that will later form a lateral root. |
| Priming | Signaling in a subset of protoxylem cells that, when successful, leads to the formation of prebranch sites in the overlaying pericycle cells. |
| Founder cells | A subset of pericycle cells, usually at one of the two xylem poles, progressing from prebranch site state toward lateral root primordium development. |
| Initiation | The initial, asymmetric divisions that demarcate the start of lateral root development. |
The earliest, and least understood, step in lateral root formation is called priming. Priming refers to a periodic signaling process that seems to involve changes in auxin (response) levels and/or gene expression that lead to the specification of cells competent for future lateral root formation (De Smet et al., 2007; Moreno-Risueno et al., 2010). Successful priming leads to the production of patches of pericycle cells called prebranch sites from which lateral roots may form. Although stably maintained staining of the auxin-responsive DR5:Luciferase(LUC) reporter (used to define prebranch sites; Moreno-Risueno et al., 2010; Xuan et al., 2015, 2016) lacks cellular resolution, DR5:GUS reporter staining has been observed in protoxylem strands of the basal meristem (De Smet et al. 2007). Based on the latter observation, it was proposed that the priming signal occurs only in the protoxylem and somehow is transmitted to the overlaying pericycle cells to obtain the potential to form prebranch sites (De Smet et al., 2007). However, an alternative explanation is that priming occurs in a broader spatial domain and that limited DR5:GUS staining arises from differential auxin sensitivities. As the primed cells progress through the differentiation zone, some attain founder cell identity and undergo asymmetric division, a defining moment in lateral root formation termed initiation (Malamy and Benfey, 1997). Additional divisions and differential cell expansion give rise to a lateral root primordium that subsequently emerges from the main root.
Auxin synthesis, transport, and response play key roles in many of these stages, including the transition to lateral root initiation (DeSmet et al., 2010; Ditengou et al., 2008; Dubrovsky et al., 2008; Goh et al., 2012; Laskowski et al., 2008; Lavenus et al., 2013; Marhavý et al., 2013, 2014, 2016; Pérez-Torres et al., 2008). Increases in auxin response activate expression of genes including the lateral root-inducing transcription factor GATA23 (De Rybel et al., 2010) and enable the onset of pericycle cell divisions (Vermeer et al., 2014; Marhavý et al., 2016). In later stages of lateral root development, cell wall loosening is attributed to auxin export from the primordium into the overlying tissue (Péret et al., 2009b; Kim and Lee, 2013). Together with water loss in overlying cells (Péret et al., 2012), this enables emergence of the lateral root.
In this review, we examine the earliest steps of lateral root formation that lead to periodic prebranch site formation and the role of auxin in them. We start with a description of the available data on lateral root priming, showing how certain studies suggest that oscillations in auxin level drive priming, while others point to the involvement of gene expression oscillations. We suggest a theoretical framework for lateral root priming that distinguishes between an initial priming signal and subsequent memory formation, a distinction that allows us to resolve this apparent paradox by suggesting critical roles for both auxin and gene expression dynamics. We describe possible mechanisms that may generate periodic formation of primed sites and the distinct requirements and limitations of these mechanisms. Ultimately, this should lead to experimentally testable predictions enabling us to determine the mechanism driving repetitive priming.
EXPERIMENTAL DATA ON PRIMING
The earliest report of oscillatory lateral root priming was made by De Smet et al. (2007). They measured the DR5:GUS reporter for the cell’s transcriptional response to auxin at 7.5-h intervals and observed fluctuations in the percentage of plants that showed localized expression in patches of protoxylem cells located ∼200 μm from the root tip (DeSmet et al., 2007). This suggested that fluctuations in auxin response occur in this region with a periodicity of 15 h. To test whether these peaks of auxin response lead to lateral root formation, the authors marked epidermal cells in the region of DR5:GUS expression. Because DR5:GUS staining kills plants, this was done using two populations of plants: The location of DR5:GUS expression was observed in one set, and marks were placed in the corresponding location in a second set. The fraction of these marks that correctly predicted the sites of lateral root formation varied in a temporally oscillatory pattern that matched the observed DR5:GUS pattern (DeSmet et al., 2007), supporting an inductive role for oscillations in auxin response in lateral root formation. Additionally, it was shown that these oscillations and lateral root formation could be influenced by gravitropic stimulation, which is known to modulate auxin distribution in the root tip (Friml et al., 2002; Ottenschläger et al., 2003; Band et al., 2012). Together, these results were taken to imply that oscillations in the levels of auxin prime the specification of lateral root founder cells and that gravitropic bending may cause these auxin-level fluctuations.
To further investigate these oscillatory events in real time, Moreno-Risueno et al. (2010) constructed a DR5:LUC reporter and observed the resulting response dynamics along the root over time. Fluctuations in DR5:LUC expression were observed in a region roughly corresponding to the shootward part of the meristem and the elongation zone, which was termed the oscillation zone. Over time, some subsets of cells stably maintained a high level of DR5:LUC expression. These subsets predicted sites where lateral root primordia formed and so were called prebranch sites. Oscillations in DR5:LUC expression were present even when roots were grown inside the agar, a condition that results in relatively straight roots. This indicated that while gravitropic curvature may modulate the timing of priming oscillations, it does not drive them (Moreno-Risueno et al., 2010). Surprisingly, pIAA19:Luciferase, a reporter with similar auxin inducibility though potentially different additional regulation, did not oscillate. This raised the question as to what extent oscillatory DR5:LUC expression reflected oscillations in auxin levels (Moreno-Risueno et al., 2010). The authors then measured gene expression dynamics in the oscillation zone. This was done by measuring expression in 40 roots and ordering them using the level of DR5:GUS expression in the oscillation zone as a proxy for the phase of the oscillation. This analysis identified a group of about 2000 genes that oscillated in-phase with the level of DR5:GUS expression and ∼1500 genes that oscillated in antiphase. Together, these results were taken to suggest that fluctuations in auxin levels alone may be insufficient for priming and instead may require oscillations in gene expression.
In a subsequent study, Xuan et al. (2015) further investigated under which conditions the temporal fluctuations in DR5:LUC expression that Moreno-Risueno et al. (2010) defined as priming lateral root initiation lead to successful prebranch site formation. The authors demonstrated that loss-of-function mutations in TIR1 and AFB2, genes involved in auxin perception that are predominantly expressed in the oscillation zone, inhibited the formation of prebranch sites that stably express DR5:LUC. Mutations in genes implicated in the conversion of indole-3-butyric acid to the active, endogenous auxin indole-3-acetic acid (IAA), IBR1, IBR3, and IBR10, had a limited effect on priming frequency yet substantially reduced the amplitude of priming oscillations and the number of prebranch sites. A loss-of-function mutant in ibr3 could be rescued by expressing the IBR3 gene in the outer layer of the root cap that encases the primary root, a group of cells called the lateral root cap. Together, these results indicate that the root cap is an important source of auxin for lateral root priming and that auxin produced in this region needs to be perceived in the oscillation zone in order to result in subsequent prebranch site formation (Xuan et al., 2015).
In a follow-up study, Xuan et al. (2016) further focused on auxin dynamics in the lateral root cap and abutting regions. They observed the regular disappearance of DR5rev:VENUS-N7 expression in cells of the lateral root cap when these cells reach the transition between the meristem and the elongation zone, just prior to their apoptosis. When the location in which this DR5rev:VENUS-N7 expression disappears in the root cap is marked, lateral roots are later seen to arise from pericycle cells in the main root close to the location of the fixed marker. In the smb mutant, in which delayed apoptosis of the root cap is associated with an abnormally long root cap, formation of prebranch sites is significantly slower and less regular. From these data, it was concluded that periodic death of cells in the root cap influences the auxin fluxes that lead to formation of prebranch sites (Xuan et al., 2016).
Formation of prebranch sites has also been shown to be influenced by an as yet uncharacterized carotenoid-derived molecule (Van Norman et al. 20,014). Interestingly, this carotenoid-derived signal is generated in the differentiation zone just shootward of the oscillatory region, implying a non-cell-autonomous action. More recently, strigolactone signaling, known to modulate auxin fluxes, was reported to influence prebranch site formation (Jiang et al., 2016). The data from Jiang et al. (2016) further support the importance for auxin fluxes in lateral root priming.
THEORETICAL FRAMEWORK FOR LATERAL ROOT PRIMING AND INITIATION
Here, we present a theoretical framework for lateral root priming and initiation in the hopes that it can inform our search for the underlying patterning mechanisms.
Function of Priming
Priming is a term used in a wide variety of biological disciplines that generally implies a start-up event or signal that serves as a necessary prerequisite for another event to take place, in this case, lateral root formation. It also generally means that the system is only sensitive to this startup signal within a limited developmental window. Finally, the term priming implies that, if successful, a memory is formed of the startup signal, in this case formation of a stable prebranch site, that is subsequently acted upon by a sequence of events leading to lateral root initiation. While several auxin-signaling modules involved in later stages of lateral root development have been described, the molecular underpinnings of the initial priming signal and its subsequent memorization have remained elusive (DeSmet et a., 2010; Goh et al., 2012; Lavenus et al. 2013).
Lateral roots develop in the differentiation zone (Figure 1C), thus requiring cell division in a region in which most cells have lost this potential. Recent findings suggest that mechanical interactions between the pericycle and overlaying endodermis prevent general, widespread pericycle cell cycle activation and that an elevated auxin response is required to overcome these constraints and enable lateral root initiation (Vermeer et al., 2014; Marhavý et al., 2016). In addition, an elevated auxin response is necessary for reorientation of subsequent divisions to generate correct lateral root primordia development (Marhavý et al., 2016). Because lateral root initiation takes place in a region of the root that has a low level of auxin response (Dubrovsky et al., 2006, 2011), it has been suggested that the primary function of lateral root priming is to produce regularly inter spaced patches of cells with an enhanced capacity to respond to auxin, thereby conditioning these cells to respond to auxin levels to which surrounding pericycle cells are insensitive (Van Norman et al., 2013) and enabling them to start the divisions necessary for lateral root initiation. Consistent with this, application of large amounts of exogenous auxin bypasses the need for priming and can induce lateral root formation along the entire root (Laskowski et al., 1995).
Priming Signal versus Memory Formation
In the case of lateral root initiation, we thus expect that the priming signal should induce an enhanced auxin response, while the priming event (memory formation) should maintain that response, thus leading to the formation of stable DR5:LUC-expressing prebranch sites. Indeed, the distinction between a successful priming event and an unsuccessful one is the retention of the elevated auxin response. The observed intermittent decrease in auxin response suggests that initiation and maintenance of this response involve two distinct events (Xuan et al., 2015). Because the level of auxin response in a cell is a function of both auxin concentration and auxin sensitivity, an elevated auxin response may result either from elevated auxin levels, upregulation of auxin response genes, or a combination of both.
Modeling studies have shown that maintenance of a localized maximum in auxin level is difficult due to high rates of auxin transport through the plant (Deinum et al., 2012). Indeed, successful formation of an auxin maximum during lateral root initiation critically depends on changes in AUX1, PIN1, and PIN3 increasing local retention, influx, and reflux of auxin, respectively (Laskowski et al., 2008; Ditengou et al., 2008, Marhavý et al., 2013, 2014). Unless similar, as yet unidentified processes occur at the site of priming, a transient elevation in auxin level will rapidly dissolve, as demonstrated in recent simulations (Xuan et al., 2016). Thus, local increases in auxin are unlikely to be sufficient for memory formation within the plant tissue. Still, this leaves open the possibility that a transient elevation of auxin levels constitutes the initial priming signal. Recent data from the Beeckman group demonstrates the importance of auxin synthesized in the lateral root cap for oscillation amplitude (Xuan et al., 2015) and suggests that apoptosis-dependent auxin fluxes from the root cap impact oscillation frequency (Xuan et al., 2016). Together, these data suggest that changes in auxin levels may constitute or at least significantly impact the priming signal. Furthermore, these studies suggest that auxin from the root cap is a major determinant of priming (Xuan et al., 2015, 2016). This view is consistent with the observation that auxin response increases in the endodermis prior to the pericycle cells (Marhavý et al., 2013). Intriguingly, earlier studies suggested that the priming signal may move outward from the protoxylem to pericycle cells (De Smet et al., 2007). More detailed mapping of auxin fluxes in the oscillation zone and meristem may help resolve these apparently conflicting observations.
Independent of whether auxin itself constitutes the priming signal, subsequent stable memory formation is likely to involve a change in gene expression. A gene regulatory motif that can be switched from one state to another upon receipt of the signal, e.g., a motif that allows for bistability, is ideally suited for persistent memory formation (Thomas, 1981; Thomas and Kaufman, 2001a, 2001b). An example of a bistable switch is the BDL/IAA12-MP/ARF5 auxin response module that induces degradation of BDL, thus derepressing MP (Lau et al., 2011), with MP subsequently inducing transcription of both BDL and MP. This motif allows a sufficiently large, transient auxin elevation to become transformed into persistent MP activation, while smaller auxin elevations have no persistent effect (Lau et al., 2011). While the BDL-MP module has thus far been reported to only play a role in later stages of lateral root development (De Smet et al., 2010), similar bistability motifs could provide a mechanism for memorizing the priming signal. Alternatively, induction of genes encoding stable mRNAs or proteins could induce long-lasting, but eventually declining, memory.
Lateral Root Positioning
While priming produces an elevated auxin response in a broad region of cells, subsequent lateral root initiation normally occurs on only one xylem pole and from a subset of pericycle cells. Data from roots that were bent at different frequencies indicate that lateral root priming frequency and positioning are regulated by distinct mechanisms (Kircher and Schopfer, 2016). Certain aspects of lateral root positioning appear to resemble phyllotaxis (Taylor-Teeples et al., 2016). For example, both the auxin antagonist cytokinin and the PLETHORA transcription factors that affect auxin transport and biosynthesis affect phyllotaxis and lateral root spacing (Bielach et al., 2012; Chang et al., 2015; Hofhuis et al., 2013). Such a similarity suggests that, like phyllotaxis, lateral root spacing involves a competition for auxin. Modeling further supports the idea that a competition for auxin may help determine on which xylem pole a lateral root develops (el-Showk et al., 2015). The auxin response modules acting early during lateral root formation, such as the one active prior to the acquisition of founder cell identity that leads to expression of GATA23 and a later one that leads to induction of ACR4 that confines division to a limited number of cells (De Rybel et al., 2010; De Smet et al., 2007), are activated by high levels of auxin and thus depend on this competition for auxin. On the other hand, many genes involved in auxin transport and biosynthesis are downstream of these modules (Lavenus et al., 2015). Thus, these auxin response modules both depend on and shape the competition for auxin. Environmental conditions such as moisture that impact lateral root positioning and affect auxin distribution patterns likely feed into these control networks (Bao et al., 2014).
Interestingly, lateral root positioning also appears to depend on auxin-independent processes. Kircher and Schopfer (2016) demonstrated that changing the frequency of root growth curvature influenced left-right positioning but not the frequency of lateral root formation, with lateral roots predominantly positioned on outer curves. Furthermore, application of auxin was unable to override this curvature-dependent positioning. The authors suggest that the microtubule organization arising at the concave side of the root may locally prevent the divisions necessary for lateral root initiation (Kircher and Schopfer, 2016), while an earlier study by Richter et al. (2009) points to a role for bending-induced calcium signaling.
THEORETICAL MECHANISMS FOR PERIODIC PATTERNING
Oscillatory dynamics can be generated through several different mechanisms. In a biological context, we often distinguish between oscillatory dynamics that arise cell-autonomously and those that emerge from the collective properties of multiple cells. In addition, we distinguish between true oscillations, in which temporal variations occur within individual cells, and apparent oscillations, in which temporal variations occur at a constant position but not within individual cells. Mechanisms that can generate true oscillations include a cell-autonomous clock that would drive changes in gene expression, Turing-type patterning under parameter conditions generating temporal oscillations, and auxin flux through tissue that could cause emergent alternations in auxin level. Apparent oscillations are generated by combining a Turing mechanism generating temporally stable spot or stripe patterns with tissue growth. In the following sections, we discuss these potential mechanisms, specific hypotheses that have been based on them, and the distinct requirements and limitations of each. Ultimately, this should lead to experimentally testable predictions enabling us to determine the mechanism driving repetitive formation of prebranch sites.
Cell-Autonomous Clocks
A classic example of a cell-autonomous oscillator is a gene whose protein product functions as a repressor of that gene’s expression (Thomas, 1981). This simple system leads to production of the protein followed by a decline due to its own repression, after which repression itself decreases and protein levels can increase again. For oscillations to arise, a temporal delay determined by transcription, translation, and degradation rates is needed. This type of oscillation underlies circadian oscillations and the somitogenesis clock patterning the vertebrate body axis (Hardin et al., 1990; Palmeirim et al., 1997). An example of a more complex system in which oscillations arise is in the pacemaker cells of the heart (Noble, 1962) that rhythmically send out waves of electricity ensuring the coordinated contraction of the heart. Here, the different voltage dependencies of several ion channels together cause the continuous generation of action potentials.
In recent years, several hypotheses have been put forward for interactions capable of generating cell-autonomous oscillations. Middleton et al. (2010) proposed that the auxin response network itself could generate oscillations. When auxin binds to its receptor, it results in degradation of transcriptional repressors of the AUX/IAA family. Degradation of these AUX/IAA proteins derepresses the auxin response factors (ARFs) to which they bind, thereby inducing transcription of downstream genes (Chapman and Estelle, 2009). Because the AUX/IAA proteins are themselves targets of the ARFs, a negative feedback loop arises that results in oscillations under certain parameter conditions (Figure 2A). A weakness of this hypothesis is that it requires AUX/IAA transcription rates that are considerably faster than are supported by current experimental data (Mellor et al., 2016a).
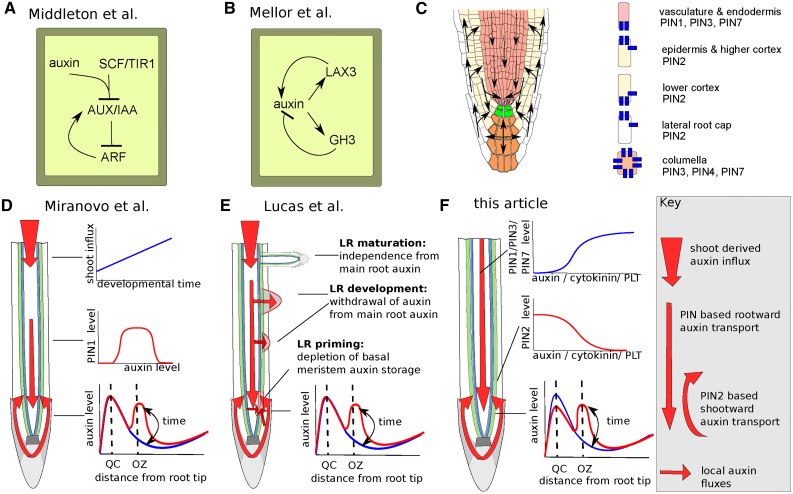
Figure 2.
Alternative Hypotheses for Lateral Root Priming Oscillations.
(A) Middleton et al. (2010) showed that the induction of AUX/IAA expression by ARF constitutes a negative feedback capable of generating cell-autonomous oscillations in auxin response (ARF) and auxin concentration levels.
(B) Mellor et al. (2016a) proposed a combination of positive feedback between auxin and LAX3 and a negative feedback between auxin and GH3 to result in cell-autonomous auxin level oscillations.
(C) Left: PIN-driven root tip auxin reflux, with PIN, PIN3, and PIN7 mediated rootward flux in the vascular tissues, apolar outward flux in the columella dependent on PIN3, PIN4, and PIN7, and PIN2-dominated shootward and inward flux in the outer tissues (Blilou et al., 2005). Right: Characteristic cell-level PIN patterns for the different cell types present in the root.
(D) to (F) Flux-based tissue-level mechanisms. Relevant auxin fluxes are indicated by red arrows as described in the key to the right of (F); assumptions underlying the models are indicated with black arrows pointing toward the root, and the resulting spatiotemporal auxin (response) dynamics are indicated with black arrows pointing away from the root.
(D) In the Mironova et al. (2010) model, auxin-dependent regulation of PIN1 is suggested to generate oscillations in auxin levels in the basal meristem. Lower graph: Blue and red lines depict the distinct oscillation phases when auxin (response) levels in the oscillation zone are low or high, respectively. These phases arise as a result of shoot-dependent auxin influx that increases over developmental time (upper blue graph) combined with a dependence of vascular PIN1 levels on auxin levels that follows an optimum curve (middle red graph).
(E) Lucas et al. (2008a) showed that if priming occurs when auxin in the basal meristem exceeds a certain threshold level (thus depleting the pool of auxin in this region) and refilling occurs gradually from the shoot-derived auxin influx and local root tip auxin reflux, oscillations in basal meristem auxin level arise. Developing (but not mature) lateral roots could contribute to this oscillation by consuming auxin flowing from the shoot to the root tip, making lateral root development and priming interdependent.
(F) In the new hypothesis proposed here, we assume an opposite dependence of PINs in the vasculature cells and PIN2 in the outer cells on auxin, cytokinin, and/or PLETHORA. This contrasting regulation is assumed to give rise to out-of-phase oscillations in PIN-mediated rootward auxin fluxes and PIN2-mediated shootward and inward auxin fluxes. Together, this is hypothesized to give rise to transient elevations in auxin levels that travel around the root tip reflux root, causing oscillations in the basal meristem but also possibly modulating the quiescent center auxin maximum.
The auxin response downstream of ARFs can affect the amount of auxin present in a cell, generating additional possibilities for oscillations. Mellor et al. (2016a) recently demonstrated that combining the auxin-inducible expression of the auxin importer LAX3, which generates a positive feedback loop, with auxin-inducible expression of the auxin-degrading enzyme GH3, generating a negative feedback, constitute an additional mechanism for cell-autonomous oscillations (Figure 2B). The potential impact of auxin degradation was further highlighted by modeling the auxin-inducible expression of DIOXYGENASE FOR AUXIN OXIDATION1 (DAO1) that reduces auxin levels through oxidation (Mellor et al., 2016b; Porco et al., 2016). Combining auxin-induced DAO1 and GH3 expression can transform what would otherwise be a stable 2-fold increase in auxin level to a single, sharp peak with a temporal duration of ∼1 to 2 h (Mellor et al., 2016b), thus potentially overcoming the earlier problem of oscillations being too slow to underlie lateral root priming. Interestingly, both the pure AUX/IAA auxin-signaling model and the GH3/LAX3 model generate oscillations in auxin levels. In case of the GH3/LAX3 oscillator, these logically result from variations in auxin turnover and import, whereas in the case of the pure AUX/IAA oscillator, these result from variations in AUX/IAA levels and, hence, the amount of bound and free auxin.
Finally, cell-autonomous, membrane transport-dependent oscillations in calcium and proton levels in the oscillation zone have been suggested to drive gene expression oscillations (Baluška and Mancuso, 2013). However, the periods, which are in the minute rather than hour range, as well as mutant phenotypes appear more consistent with a role in root circumnutation (Shabala and Newman, 1997) and in encoding environmental information (Shabala et al., 2006) than the considerably slower process of lateral root priming.
Turing-Type Patterning
In contrast to cell-autonomous oscillations, periodic fluctuations in auxin response may also arise as an emergent result of tissue-level interactions. Such mechanisms imply that cells in the tissue behave in a coordinated manner. In the case of prebranch site formation, the oscillation in question is one of auxin response as well as in the expression of many genes. Because of the observed oscillation in auxin response, and because auxin plays a major role in coordinating plant cells, it is likely that tissue-level, emergent mechanisms revolve around modulations in auxin levels. One mechanism for generating oscillations in a tissue-level manner involves a system capable of generating a Turing pattern (Turing, 1952). Turing patterns arise from the interactions between two basic elements: an activator, which diffuses slowly, and an inhibitor, which can diffuse more rapidly. In this system, the activator induces production of itself and the inhibitor represses its own production. In addition, one more positive and one more negative regulation are present in a Turing system. The activator can induce the inhibitor and the inhibitor represses the activator, thus causing the activator to induce its own repressor, or the activator can repress the inhibitor while the inhibitor induces the activator, causing the activator to repress its own inducer. In both cases, this results in a combination of positive and negative feedback. While a temporally static spatial array of spots or stripes is a common outcome if feedbacks are fast, in case of slow, delayed feedbacks true temporal oscillations arise. A fundamental difference between this and the cell-autonomous mechanism described above is that the Turing system requires multiple coupled cells.
Turing systems can also generate apparent oscillations—temporally stable spots or stripes—that arise in the context of a growing tissue. In Turing systems, the spots or stripes are spaced at a specific, parameter-dependent wavelength, and the patterns can be described as a standing wave (Turing, 1952). When tissue with a Turing pattern grows, spots or stripes are drawn apart, and new, intermediary spots or stripes are generated once the distance exceeds a certain wavelength-dependent threshold. Thus, at a constant position, growth leads to apparent oscillatory dynamics as old spots or stripes are displaced and new spots or stripes are subsequently formed. In contrast to the mechanisms described above, true oscillations do not occur within a specific cell. An example of this type of patterning has been described for phyllotaxis, where local amplification of auxin levels (activation) and global competition for auxin (inhibition) take place on a growing field of cells, resulting in the continuous generation of new auxin maxima that pattern leaf primordia at the shoot meristem (Reinhardt et al., 2003).
Thus far, no concrete hypotheses have been put forward for Turing-based priming generating either true or apparent oscillations. Still, the idea of growth-based periodic patterning deserves further study given that the region of the root in which changes in DR5:LUC are seen, the oscillation zone, is the portion of the root where rapid cell elongation takes place (Moreno-Risueno et al., 2010; Xuan et al., 2015, 2016). If such a mechanism were to be involved in lateral root priming or positioning, then, in parallel with phyllotaxis, it might involve feedbacks between tissue-level auxin fluxes that generate spatially periodic elevations in auxin level. A potential candidate mechanism could be the interplay between auxin and cytokinin signaling that are well known for their antagonistic interactions.
Tissue-Level Alterations in Auxin Fluxes
Besides Turing mechanisms, other mechanisms exist that generate periodicity in a tissue-level emergent manner. An example of this is found in certain cardiac arrhythmias. Heart tissue is electrically excitable and responds to stimulation by generating an action potential. As a consequence, heart cells neighboring a cell generating an action potential become activated and will generate an action potential themselves, thus allowing for the transmission of action potentials across the heart. If an anatomical abnormality results in a circular circuit in the heart, action potentials keep circling this circuit and hence reactivate the same series of cells over and over again (Allessie et al., 1972; Nomura and Glass, 1996). For an individual cell, this periodic reactivation causes oscillatory dynamics. In a similar way, local modulations in auxin flux could be passed on to neighboring cells, causing oscillations in auxin levels. Several hypotheses for such emergent, tissue-level oscillatory priming have been proposed. To understand these mechanisms, we need to first consider the different auxin fluxes that affect auxin patterning in the root.
During the early stages of forming a lateral root primordium, development depends on auxin derived from the shoot (Wightman and Thimann, 1980; Hinchee and Rost, 1986; Reed et al., 1998; Bhalerao et al., 2002) and main root (Benková et al., 2003; Casimiro et al., 2001; Ditengou et al., 2008; Laskowski et al., 2008; Lewis et al., 2011; Marhavý et al., 2013, 2014). Slightly later, the lateral root establishes an independent auxin maximum at its apex (Benková et al., 2003), essentially recreating the auxin landscape present in the main root tip. In both the main and lateral root, auxin gradients arise from a specific pattern of auxin transport that efficiently recycles auxin back to the root tip, thus locally focusing the maximum of the auxin gradient (Blilou et al., 2005; Grieneisen et al., 2007). Underlying these so-called reflux loops are tissue-specific proteins (PIN proteins) that export auxin out of the cell. Because PIN proteins are orientated in a polar manner on one or more sides of a cell, their location establishes directional auxin fluxes (Blilou et al., 2005; Grieneisen et al., 2007) (Figure 2C). Different hypotheses for emergent, tissue-level oscillations involve various aspects of these auxin fluxes.
In a simulation study, Mironova et al. (2010) demonstrated that a combined positive feedback of intermediate auxin levels and negative feedback of high auxin levels on vascular PIN1 levels generates a localized auxin maximum a short distance from the border of the root tip, consistent with the location of the quiescent center. If these feedbacks are combined with the assumption that shoot-derived auxin influx increases during development, an instability arises in this auxin maximum resulting in spatiotemporal oscillations in the auxin level in the basal meristem (Figure 2D). An important question regarding this hypothesis is how it fits with the observation that the relative contribution of shoot-derived auxin decreases as roots age (Bhalerao et al., 2002).
Lucas et al. (2008a) proposed that the onset of priming requires a certain threshold level of auxin in the basal meristem that priming subsequently depletes and that the level of auxin in the basal meristem gradually recovers as a result of auxin influx from the shoot and from the reflux loop in the root tip. This mechanism implies that oscillations are a result rather than a cause of lateral root priming. This view is supported by experimental evidence showing that the frequency of lateral root initiation occurring in response to an inductive gravitropic stimulus varies depending on the time elapsed between such stimuli (Lucas et al., 2008b). Furthermore, Lucas et al. (2008a) assume that all developing (but not mature) lateral roots consume auxin from the shoot tip auxin flux, causing competition for auxin between developing lateral roots and lateral root priming (Figure 2E). A limitation of this hypothesis is that there are currently no data that show significant oscillations in the total amount of auxin in the root meristem as a result of priming. However, in the context of recent data on the importance of lateral root cap auxin production for lateral root priming (Xuan et al., 2015, 2016), the model might be reformulated with lateral root cap cell number and auxin content being the source that is exhausted and requires time to regenerate (Figure 2E).
In addition to these published models, we propose a new hypothesis for emergent lateral root priming in which self-organized fluctuations in auxin level both result in and arise from changes in auxin transporters, somewhat similar to the recircling of an action potential along a closed circuit. Imagine, for example, that the PIN proteins that promote movement of auxin toward the root tip are upregulated while those that promote auxin movement from the root tip toward the oscillation zone and shoot are downregulated by the same factor. In such a case, expression of the transcription factor would increase auxin flow toward the root tip and decrease flow toward the oscillation zone, a joint action that would reduce the amount of auxin present in the oscillation zone. If our hypothetical transcription factor were auxin-inducible, then the decreased level of auxin in the oscillation zone would lower its expression. This would increase net transport of auxin toward the oscillation zone, thus allowing the auxin levels to become reestablished. The potential for this sort of oscillatory loop is supported by the fact that PIN expression levels are not only dependent on levels of auxin and cytokinin but also on the auxin-inducible transcription factors of the PLETHORA family (Santuari et al., 2016; Vieten et al., 2005; Ruzicka et al., 2009; Šimášková et al., 2015; Blilou et al., 2005) (Figure 2F). Whether the actual regulation of PINs produces oscillations in the relative strength of the auxin fluxes directed toward the root tip and the shoot remains to be investigated. A major difference between this mechanism and the mechanism proposed by Mironova et al. (2010) is that this one involves different types of PINs and, hence, the entire reflux loop. Another difference is that oscillations are proposed to arise independently of increases in shoot-derived auxin influx.
The hypotheses described above implicitly assume that an oscillation in auxin level or response would give rise to oscillations in the expression of many other genes because their expression is either directly regulated by auxin response factors or indirectly via other, auxin-dependent genes. A cell-autonomous oscillator would be expected to result in many genes oscillating, as downstream targets of core clock genes.
DISTIGUISHING THE LIKELIHOOD OF ALTERNATIVE MECHANISMS
Distinguishing between the three mechanisms described above is difficult in part because observations of auxin response or gene expression oscillations do not distinguish between cause and effect. In addition, it is possible that all three of the theoretical mechanisms may be involved in different stages of lateral root priming. As an example, the elevated auxin response that serves as the priming signal could induce auxin degradation via GH3 and DAO1, which may contribute to the downward phase of these oscillations. Spatial restriction of the elevated auxin responses may be facilitated by auxin-induced changes in auxin transport, for example, in AUX1, and by auxin-cytokinin interactions. It is therefore important to distinguish between a core mechanism that drives the oscillation of the priming signal, subsidiary mechanisms that contribute to the precision and robustness of oscillations but are not essential for their occurrence, and downstream processes that occur as a consequence of lateral root priming. Given the importance of lateral roots for plant survival, the core oscillatory mechanism is likely to be highly robust and redundant. As a consequence, abolishing individual parts may not eliminate the oscillations, obscuring their identification as part of the core oscillator. Thus, an important question is how we can determine the nature of the oscillator driving lateral root priming.
Spatial Restriction of Oscillatory Dynamics
Discovering what spatially restricts the oscillations in DR5 expression to the oscillation zone could help discern whether oscillations arise from a cell-autonomous clock, an emergent flux-based oscillation, or Turing-type patterning. Finding that the oscillations critically depend on factors expressed specifically in cells of the oscillation zone, such as transcription factors, cofactors, small RNAs, or peptides would align with a cell-autonomous oscillator. By contrast, finding that region-specific organization and activity of auxin transporters confers the spatial restriction of oscillatory DR5 expression would align with an emergent flux-based oscillation. Noteworthy in this respect is that the oscillation zone is centered in the region where the lateral root cap terminates (Xuan et al., 2015), polarity of PIN2 in the cortical cell files changes from basal to apical orientation (Grieneisen et al., 2007), and vascular PIN1 becomes increasingly restricted to the rootward face of cells (Omelyanchuk et al., 2016), possibly giving this location a distinct flux profile that is more prone to oscillations in auxin level. In addition, while in this case auxin oscillations are expected to traverse the entire root tip reflux loop, locally elevated expression of auxin response genes may amplify the response to auxin levels in a region specific manner. The carotenoid-derived signal that is produced in the differentiation zone yet affects priming in the oscillation zone may fulfill such a function (Van Norman et al., 2014). Finally, while a tissue-level mechanism depending on Turing-type patterning and growth would automatically constrain periodic fluctuations to the region of growth, it would require additional constraints to limit these oscillations specifically to the protoxylem cell files.
Environmental and Developmental Impacts on Priming Frequency
The ability to flexibly adapt to varying environmental conditions is one of the hallmarks of plant development and of lateral root spacing in particular. A large part of this variation is likely to arise independently of priming frequency, affecting lateral root formation at later developmental stages. As an example, the variation in lateral root density resulting from differences in phosphate and nitrate levels arises from altered levels of TIR1/AFB family auxin receptors. This type of change would be expected to affect the rate with which priming results in prebranch site formation, not the priming frequency itself (Pérez-Torres et al., 2008; Vidal et al., 2010). In addition, environmental factors such as moisture and root curvature influence lateral root positioning more strongly than lateral root density (Bao et al., 2014; Kircher and Schopfer, 2016). Still, it seems that environmental conditions could also impinge on the earliest stage of lateral root patterning, the priming process itself.
Moreno-Risueno et al. (2010) reported priming frequency to be largely independent of a series of different applied growth conditions. Indeed, final root length attained 6 d after germination varied considerably more with growth conditions than the number of prebranch sites that were formed. However, it is well known that the rate of root growth is not constant. Rather, plants go through a phase of strongly accelerating growth during the first 10 to 14 d after germination, after which root elongation plateaus at a constant rate (Beemster and Baskin, 1998; Moubayidin et al., 2010). Therefore, small changes in growth rate result in considerably larger changes in final root length. As an example, naively assuming simple exponential growth, the ∼40% difference in final root length at day 6 observed when increasing temperature from 18°C to 24°C can be computed to arise from a difference in growth rate of only 6%. This growth rate difference is comparable in size to the observed increase in prebranch sites from 24 to 26 (8%). This suggests that for the applied conditions growth rate and priming frequency may be correlated.
To further investigate this, we compared lateral root priming periods reported in different experimental studies alongside the auxin response reporter, the developmental age of plants, and growth conditions used in these studies (Table 1) (DeSmet et al., 2007; Moreno-Risueno et al., 2010; Xuan et al., 2015, 2016; Kircher et al., 2016). The reported lateral root priming periods range from ∼4 to 15 h, and a general trend between increasing lateral root priming frequency (decreasing priming period) and increasing plant age can be seen. This correlation is further supported by studies showing that the distance between lateral roots does not increase with increasing root growth rate (Dubrovsky et al., 2006). Rather, root length and lateral root numbers increase in a similar manner (Julkowska et al., 2014). Indeed, it was recently shown that the priming period increases significantly as plants progress through the early stages of development, corresponding to the time window when root growth rates are known to also increase substantially (Beemster and Baskin, 1998; Moubayidin et al., 2010) (Supplemental Figure 1A in Xuan et al., 2015). This suggests that environmental conditions that significantly affect root growth rate may automatically affect priming frequency. In addition, environmental conditions may also affect priming rate independently of growth rate. Consistent with this, recent studies demonstrated the importance of auxin fluxes derived from the root cap, a major sensory organ of the root, for priming efficiency and frequency (Xuan et al., 2015, 2016).
Thus, the extent to which a proposed priming mechanism is capable of simulating variable priming rates may serve as an additional test of its feasibility. As an example, a mechanism that combines Turing-type patterning and growth would automatically result in a coupling between growth rate and priming frequency, making this dependency an inherent part of the process. By contrast, to influence priming independently of growth rate, environmental conditions should somehow influence the wavelength of the Turing pattern. Similarly, tissue-level flux modulations are likely to automatically depend on meristem size and, hence, root growth rate, while growth rate-independent influence of environmental conditions will impose additional demands on the mechanism. Finally, for a cell-autonomous mechanism to depend on root growth rate and environmental conditions, the timescales governing gene expression oscillations should be dependent on plant hormone levels that correlate with these changes. Thus far, models of gene regulatory networks that are based on parameter settings within the range of experimental measurement have failed to produce periodicities similar to those that are observed (Middleton et al., 2010; Mellor et al., 2016a), while proposed tissue-level mechanisms have not yet attempted to explain this particular aspect of priming (Table 2).
Table 2. Overview of Priming Frequencies and Experimental Conditions Reported in Different Studies.
| Study |
Priming Period |
DR5 Reporter |
Developmental Age |
Growth Conditions |
| De Smet et al. (2007) | ∼15 h | GUS | 11–55 h PG | 22°C; vertical, CL; atop agar |
| Moreno-Risueno et al. (2010) | ∼6-7 h | LUC | 6 d PG | 22°C; vertical, 16 h light; atop agar |
| Xuan et al. (2015) | ∼4 h | LUC | 8 d PG | 22°C; vertical, 16 h light; atop agar |
| Xuan et al. (2016) | ∼4 h | LUC | 3 d PG | 22°C; vertical, CL, atop agar |
| Kircher and Schopfer (2016) | ∼4 h | LUC | 5–10 d PG | 25°C; vertical, CL; inside agar |
PG, postgermination; CL, continuous light.
SUMMARY AND FUTURE PROSPECTS
The repetitive priming of sites forming future lateral roots is a major determinant of root system architecture. Still, it remains unclear whether this periodic priming arises from a cell-autonomous clock generating oscillations in auxin response and gene expression, from tissue-level modulations in auxin fluxes, or Turing-type periodic patterning. To discern the mechanism underlying repetitive lateral root priming, spatiotemporally detailed measurements of root tip auxin fluxes using recently proposed sensitive auxin response reporters (Brunoud et al., 2012; Liao et al., 2015) are necessary to investigate whether priming oscillations are indeed highly localized or travel through the root tip reflux loop.
In addition, more data are needed on how root clock period changes in different conditions and whether and how this correlates with root tip auxin dynamics. We also need more genetic information, aimed at teasing apart genes strongly correlated with lateral root priming from genes that correlate more strongly with the circadian cycle (Voß et al., 2015), and at differentiating between genes that constitute the core oscillator and their downstream effectors.
Finally, we recommend that this data be used to develop new models simulating cell-autonomous or emergent, tissue-level oscillatory dynamics. The explicit goals of this modeling effort should be to determine what conditions are necessary for the different mechanisms to give rise to priming-type oscillations and to generate experimentally testable predictions that distinguish between the hypothesized mechanisms. Together, these efforts will be invaluable in determining the actual mechanism underlying lateral root priming.
Acknowledgments
K.H.t.T. is supported by VIDI Grant 864.14.003 of the Netherlands Scientific Organization and M.L. by Oberlin College. We thank Ben Scheres for helpful discussions.
AUTHOR CONTRIBUTIONS
K.H.t.T. designed the major setup and wrote the manuscript. M.L. contributed to the writing.
Footnotes
Articles can be viewed without a subscription.
References
- Allessie M.A., Bonke F.I.M., Schopman F.J.G. (1972). Circus movement in atrial muscle as a mechanism of supraventricular tachycardia. J. Physiol. (Paris) 65 (suppl.): 324A. [PubMed] [Google Scholar]
- Baluška F., Mancuso S. (2013). Root apex transition zone as oscillatory zone. Front. Plant Sci. 4: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band L.R., et al. (2012). Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc. Natl. Acad. Sci. USA 109: 4668–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y., et al. (2014). Plant roots use a patterning mechanism to position lateral root branches toward available water. Proc. Natl. Acad. Sci. USA 111: 9319–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster G.T., Baskin T.I. (1998). Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol. 116: 1515–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E., Bielach A. (2010). Lateral root organogenesis—from cell to organ. Curr. Opin. Plant Biol. 13: 677–683. [DOI] [PubMed] [Google Scholar]
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. [DOI] [PubMed] [Google Scholar]
- Bhalerao R.P., Eklöf J., Ljung K., Marchant A., Bennett M., Sandberg G. (2002). Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 29: 325–332. [DOI] [PubMed] [Google Scholar]
- Bielach A., Podlesáková K., Marhavy P., Duclercq J., Cuesta C., Müller B., Grunewald W., Tarkowski P., Benková E. (2012). Spatiotemporal regulation of lateral root organogenesis in Arabidopsis by cytokinin. Plant Cell 24: 3967–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I., Xu J., Wildwater M., Willemsen V., Paponov I., Friml J., Heidstra R., Aida M., Palme K., Scheres B. (2005). The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44. [DOI] [PubMed] [Google Scholar]
- Brunoud G., Wells D.M., Oliva M., Larrieu A., Mirabet V., Burrow A.H., Beeckman T., Kepinski S., Traas J., Bennett M.J., Vernoux T. (2012). A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 482: 103–106. [DOI] [PubMed] [Google Scholar]
- Casimiro I., Marchant A., Bhalerao R.P., Beeckman T., Dhooge S., Swarup R., Graham N., Inzé D., Sandberg G., Casero P.J., Bennett M. (2001). Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Ramireddy E., Schmülling T. (2015). Cytokinin as a positional cue regulating lateral root spacing in Arabidopsis. J. Exp. Bot. 66: 4759–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E.J., Estelle M. (2009). Mechanism of auxin-regulated gene expression in plants. Annu. Rev. Genet. 43: 265–285. [DOI] [PubMed] [Google Scholar]
- Deinum E.E., Geurts R., Bisseling T., Mulder B.M. (2012). Modeling a cortical auxin maximum for nodulation: different signatures of potential strategies. Front. Plant Sci. 3: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Herder G., Van Isterdael G., Beeckman T., De Smet I. (2010). The roots of a new green revolution. Trends Plant Sci. 15: 600–607. [DOI] [PubMed] [Google Scholar]
- De Rybel B., et al. (2010). A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr. Biol. 20: 1697–1706. [DOI] [PubMed] [Google Scholar]
- De Smet I., et al. (2007). Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681–690. [DOI] [PubMed] [Google Scholar]
- De Smet I., et al. (2010). Bimodular auxin response controls organogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 107: 2705–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditengou F.A., Teale W.D., Kochersperger P., Flittner K.A., Kneuper I., van der Graaff E., Nziengui H., Pinosa F., Li X., Nitschke R., Laux T., Palme K. (2008). Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 105: 18818–18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky J.G., Gambetta G.A., Hernández-Barrera A., Shishkova S., González I. (2006). Lateral root initiation in Arabidopsis: developmental window, spatial patterning, density and predictability. Ann. Bot. (Lond.) 97: 903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky J.G., Sauer M., Napsucialy-Mendivil S., Ivanchenko M.G., Friml J., Shishkova S., Celenza J., Benková E. (2008). Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl. Acad. Sci. USA 105: 8790–8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky J.G., Napsucialy-Mendivil S., Duclercq J., Cheng Y., Shishkova S., Ivanchenko M.G., Friml J., Murphy A.S., Benková E. (2011). Auxin minimum defines a developmental window for lateral root initiation. New Phytol. 191: 970–983. [DOI] [PubMed] [Google Scholar]
- el-Showk S., Help-Rinta-Rahko H., Blomster T., Siligato R., Marée A.F., Mähönen A.P., Grieneisen V.A. (2015). Parsimonious model of vascular patterning links transverse hormone fluxes to lateral root initiation: auxin leads the way, while cytokinin levels out. PLOS Comput. Biol. 11: e1004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel A., Beeckman T. (2013). Plant Roots: The Hidden Half, 4th ed. (Boca Raton, FL: CRC Press). [Google Scholar]
- Friml J., Wiśniewska J., Benková E., Mendgen K., Palme K. (2002). Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809. [DOI] [PubMed] [Google Scholar]
- Ghanem M.E., Hichri I., Smigocki A.C., Albacete A., Fauconnier M.L., Diatloff E., Martinez-Andujar C., Lutts S., Dodd I.C., Pérez-Alfocea F. (2011). Root-targeted biotechnology to mediate hormonal signalling and improve crop stress tolerance. Plant Cell Rep. 30: 807–823. [DOI] [PubMed] [Google Scholar]
- Goh T., Kasahara H., Mimura T., Kamiya Y., Fukaki H. (2012). Multiple AUX/IAA-ARF modules regulate lateral root formation: the role of Arabidopsis SHY2/IAA3-mediated auxin signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367: 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieneisen V.A., Xu J., Marée A.F., Hogeweg P., Scheres B. (2007). Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449: 1008–1013. [DOI] [PubMed] [Google Scholar]
- Hardin P.E., Hall J.C., Rosbash M. (1990). Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343: 536–540. [DOI] [PubMed] [Google Scholar]
- Hofhuis H., Laskowski M., Du Y., Prasad K., Grigg S., Pinon V., Scheres B. (2013). Phyllotaxis and rhizotaxis in Arabidopsis are modified by three PLETHORA transcription factors. Curr. Biol. 23: 956–962. [DOI] [PubMed] [Google Scholar]
- Hinchee M.A.W., Rost T.L. (1986). The development of lateral roots in cultured pea seedlings I The role of seedling organs and growth regulators. Bot. Gaz. 147: 137–147. [Google Scholar]
- Jiang L., Matthys C., Marquez-Garcia B., De Cuyper C., Smet L., De Keyser A., Boyer F.D., Beeckman T., Depuydt S., Goormachtig S. (2016). Strigolactones spatially influence lateral root development through the cytokinin signaling network. J. Exp. Bot. 67: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julkowska M.M., Hoefsloot H.C., Mol S., Feron R., de Boer G.J., Haring M.A., Testerink C. (2014). Capturing Arabidopsis root architecture dynamics with ROOT-FIT reveals diversity in responses to salinity. Plant Physiol. 166: 1387–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lee H.W. (2013). Direct activation of EXPANSIN14 by LBD18 in the gene regulatory network of lateral root formation in Arabidopsis. Plant Signal. Behav. 8: e22979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S., Schopfer P. (2016). Priming and positioning of lateral roots in Arabidopsis. An approach for an integrating concept. J. Exp. Bot. 67: 1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L.V. (2016). Root architecture. J. Integr. Plant Biol. 58: 190–192. [DOI] [PubMed] [Google Scholar]
- Kong X., Zhang M., De Smet I., Ding Z. (2014). Designer crops: optimal root system architecture for nutrient acquisition. Trends Biotechnol. 32: 597–598. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Grieneisen V.A., Hofhuis H., Hove C.A., Hogeweg P., Marée A.F., Scheres B. (2008). Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 6: e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M.J., Williams M.E., Nusbaum H.C., Sussex I.M. (1995). Formation of lateral root meristems is a two-stage process. Development 121: 3303–3310. [DOI] [PubMed] [Google Scholar]
- Lau S., De Smet I., Kolb M., Meinhardt H., Jürgens G. (2011). Auxin triggers a genetic switch. Nat. Cell Biol. 13: 611–615. [DOI] [PubMed] [Google Scholar]
- Lavenus J., Goh T., Roberts I., Guyomarc’h S., Lucas M., De Smet I., Fukaki H., Beeckman T., Bennett M., Laplaze L. (2013). Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci. 18: 450–458. [DOI] [PubMed] [Google Scholar]
- Lavenus J., et al. (2015). Inference of the Arabidopsis lateral root gene regulatory network suggests a bifurcation mechanism that defines primordia flanking and central zones. Plant Cell 27: 1368–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.R., Negi S., Sukumar P., Muday G.K. (2011). Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 138: 3485–3495. [DOI] [PubMed] [Google Scholar]
- Liao C.Y., Smet W., Brunoud G., Yoshida S., Vernoux T., Weijers D. (2015). Reporters for sensitive and quantitative measurement of auxin response. Nat. Methods 12: 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M., Guédon Y., Jay-Allemand C., Godin C., Laplaze L. (2008a). An auxin transport-based model of root branching in Arabidopsis thaliana. PLoS One 3: e3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M., Godin C., Jay-Allemand C., Laplaze L. (2008b). Auxin fluxes in the root apex co-regulate gravitropism and lateral root initiation. J. Exp. Bot. 59: 55–66. [DOI] [PubMed] [Google Scholar]
- Mähönen A.P., ten Tusscher K., Siligato R., Smetana O., Díaz-Triviño S., Salojärvi J., Wachsman G., Prasad K., Heidstra R., Scheres B. (2014). PLETHORA gradient formation mechanism separates auxin responses. Nature 515: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J.E., Benfey P.N. (1997). Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44. [DOI] [PubMed] [Google Scholar]
- Marhavý P., Vanstraelen M., De Rybel B., Zhaojun D., Bennett M.J., Beeckman T., Benková E. (2013). Auxin reflux between the endodermis and pericycle promotes lateral root initiation. EMBO J. 32: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhavý P., Duclercq J., Weller B., Feraru E., Bielach A., Offringa R., Friml J., Schwechheimer C., Murphy A., Benková E. (2014). Cytokinin controls polarity of PIN1-dependent auxin transport during lateral root organogenesis. Curr. Biol. 24: 1031–1037. [DOI] [PubMed] [Google Scholar]
- Marhavý P., Montesinos J.C., Abuzeineh A., Van Damme D., Vermeer J.E., Duclercq J., Rakusová H., Nováková P., Friml J., Geldner N., Benková E. (2016). Targeted cell elimination reveals an auxin-guided biphasic mode of lateral root initiation. Genes Dev. 30: 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor N., Bennett M.J., King J.R. (2016a). GH3-mediated auxin conjugation can result in either transient or oscillatory transcriptional auxin responses. Bull. Math. Biol. 78: 210–234. [DOI] [PubMed] [Google Scholar]
- Mellor N., et al. (2016b). Dynamic regulation of auxin oxidase and conjugating enzymes AtDAO1 and GH3 modulates auxin homeostasis. Proc. Natl. Acad. Sci. USA 113: 11022–11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton A.M., King J.R., Bennett M.J., Owen M.R. (2010). Mathematical modelling of the Aux/IAA negative feedback loop. Bull. Math. Biol. 72: 1383–1407. [DOI] [PubMed] [Google Scholar]
- Mironova V.V., Omelyanchuk N.A., Yosiphon G., Fadeev S.I., Kolchanov N.A., Mjolsness E., Likhoshvai V.A. (2010). A plausible mechanism for auxin patterning along the developing root. BMC Syst. Biol. 4: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Risueno M.A., Van Norman J.M., Moreno A., Zhang J., Ahnert S.E., Benfey P.N. (2010). Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 329: 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moubayidin L., Perilli S., Dello Ioio R., Di Mambro R., Costantino P., Sabatini S. (2010). The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr. Biol. 20: 1138–1143. [DOI] [PubMed] [Google Scholar]
- Noble D. (1962). A modification of the Hodgkin-Huxley equations applicable to Purkinje fibre action and pace-maker potentials. J. Physiol. 160: 317–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., Glass L. (1996). Entrainment and termination of reentrant wave propagation in a periodically stimulated ring of excitable media. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics 53: 6353–6360. [DOI] [PubMed] [Google Scholar]
- Omelyanchuk N.A., Kovrizhnykh V.V., Oshchepkova E.A., Pasternak T., Palme K., Mironova V.V. (2016). A detailed expression map of the PIN1 auxin transporter in Arabidopsis thaliana root. BMC Plant Biol. 16 (suppl 1.): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenschläger I., Wolff P., Wolverton C., Bhalerao R.P., Sandberg G., Ishikawa H., Evans M., Palme K. (2003). Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc. Natl. Acad. Sci. USA 100: 2987–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B., De Rybel B., Casimiro I., Benková E., Swarup R., Laplaze L., Beeckman T., Bennett M.J. (2009a). Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 14: 399–408. [DOI] [PubMed] [Google Scholar]
- Péret B., Larrieu A., Bennett M.J. (2009b). Lateral root emergence: a difficult birth. J. Exp. Bot. 60: 3637–3643. [DOI] [PubMed] [Google Scholar]
- Péret B., et al. (2012). Auxin regulates aquaporin function to facilitate lateral root emergence. Nat. Cell Biol. 14: 991–998. [DOI] [PubMed] [Google Scholar]
- Pérez-Torres C.A., López-Bucio J., Cruz-Ramírez A., Ibarra-Laclette E., Dharmasiri S., Estelle M., Herrera-Estrella L. (2008). Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20: 3258–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porco S., et al. (2016). Dioxygenase-encoding AtDAO1 gene controls IAA oxidation and homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 113: 11016–11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R.C., Brady S.R., Muday G.K. (1998). Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 118: 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D., Pesce E.R., Stieger P., Mandel T., Baltensperger K., Bennett M., Traas J., Friml J., Kuhlemeier C. (2003). Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260. [DOI] [PubMed] [Google Scholar]
- Richter G.L., Monshausen G.B., Krol A., Gilroy S. (2009). Mechanical stimuli modulate lateral root organogenesis. Plant Physiol. 151: 1855–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka K., Simásková M., Duclercq J., Petrásek J., Zazímalová E., Simon S., Friml J., Van Montagu M.C., Benková E. (2009). Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc. Natl. Acad. Sci. USA 106: 4284–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S., Beis D., Wolkenfelt H., Murfett J., Guilfoyle T., Malamy J., Benfey P., Leyser O., Bechtold N., Weisbeek P., Scheres B. (1999). An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472. [DOI] [PubMed] [Google Scholar]
- Santuari L., et al. (2016). The PLETHORA gene regulatory network guides growth and cell differentiation in Arabidopsis. Plant Cell 28: 2937–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S., Shabala N., Gradman D., Chen Z., Newman I., Mancuso S. (2006). Oscillations in plant membrane transport: model predictions, experimental validation, and physiological implications. J. Exp. Bot. 57: 171–184. [DOI] [PubMed] [Google Scholar]
- Shabala S.N., Newman I.A. (1997). Proton and calcium flux oscillations in the elongation zone correlate with root nutation. Physiol. Plant. 100: 917–926. [PubMed] [Google Scholar]
- Šimášková M., et al. (2015). Cytokinin response factors regulate PIN-FORMED auxin transporters. Nat. Commun. 6: 8717. [DOI] [PubMed] [Google Scholar]
- Taylor-Teeples M., Lanctot A., Nemhauser J.L. (2016). As above, so below: Auxin's role in lateral organ development. Dev Biol. 419: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. (1981). On the relation between the logical structure of systems and their ability to generate multiple steady states or sustained oscillations. In Numerical Methods in the Study of Critical Phenomena, Springer Series in Synergetics, Vol. 9, J. Della Dora, J. Demongeot, and B. Lacolle, eds (Berlin, Heidelberg, Germany: Springer), pp. 180–193.
- Thomas R., Kaufman M. (2001a). Multistationarity, the basis of cell differentiation and memory. i. Structural conditions of multistationarity and other nontrivial behavior. Chaos 11: 165–179. [DOI] [PubMed] [Google Scholar]
- Thomas R., Kaufman M. (2001b). Multistationarity, the basis of cell differentiation and memory. ii. Logical analysis of regulatory networks in terms of feedback circuits. Chaos 11: 180–195. [DOI] [PubMed] [Google Scholar]
- Turing A.M. (1952). The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 237: 37–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Norman J.M., Xuan W., Beeckman T., Benfey P.N. (2013). To branch or not to branch: the role of pre-patterning in lateral root formation. Development 140: 4301–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Norman J.M., Zhang J., Cazzonelli C.I., Pogson B.J., Harrison P.J., Bugg T.D., Chan K.X., Thompson A.J., Benfey P.N. (2014). Periodic root branching in Arabidopsis requires synthesis of an uncharacterized carotenoid derivative. Proc. Natl. Acad. Sci. USA 111: E1300–E1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer J.E., von Wangenheim D., Barberon M., Lee Y., Stelzer E.H., Maizel A., Gelner N. (2014). A spatial accommodation by neighboring cells is required for organ initiation in Arabidopsis. Science 343: 178–183. [DOI] [PubMed] [Google Scholar]
- Vidal E.A., Araus V., Lu C., Parry G., Green P.J., Coruzzi G.M., Gutiérrez R.A. (2010). Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 107: 4477–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieten A., Vanneste S., Wisniewska J., Benková E., Benjamins R., Beeckman T., Luschnig C., Friml J. (2005). Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132: 4521–4531. [DOI] [PubMed] [Google Scholar]
- Voß U., et al. (2015). The circadian clock rephases during lateral root organ initiation in Arabidopsis thaliana. Nat. Commun. 6: 7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman F., Thimann K.V. (1980). Hormonal factors controlling the initiation and development of lateral roots. I. Sources of primordia-inducing substances in the primary root of pea seedlings. Physiol. Plant. 49: 13–20. [Google Scholar]
- Xuan W., Audenaert D., Parizot B., Möller B.K., Njo M.F., De Rybel B., De Rop G., Van Isterdael G., Mähönen A.P., Vanneste S., Beeckman T. (2015). Root cap-derived auxin pre-patterns the longitudinal axis of the Arabidopsis root. Curr. Biol. 25: 1381–1388. [DOI] [PubMed] [Google Scholar]
- Xuan W., et al. (2016). Cyclic programmed cell death stimulates hormone signaling and root development in Arabidopsis. Science 351: 384–387. [DOI] [PubMed] [Google Scholar]