Abstract
Background/Objective
studies on the association of dementia with specific body composition (BC) components are scarce. Our aim was to investigate associations of BC measures with different levels of cognitive function in late-life.
Methods
we studied 5,169 participants (mean age 76 years, 42.9% men) in the AGES-Reykjavik Study of whom 485 (9.4%) were diagnosed with mild cognitive impairment (MCI) and 307 (5.9%) with dementia. Visceral fat, abdominal and thigh subcutaneous fat, and thigh muscle were assessed by computed tomography. MCI and dementia were based on clinical assessment and a consensus meeting; those without MCI or dementia were categorised as normal. Multinomial regression models assessed the associations stratified by sex and in additional analyses by midlife body mass index (BMI).
Results
among women, there was a decreased likelihood of dementia per SD increase in abdominal subcutaneous fat (OR 0.72; 95% CI: 0.59–0.88), thigh subcutaneous fat (0.81; 0.67–0.98) and thigh muscle (0.63; 0.52–0.76), but not visceral fat, adjusting for demographics, vascular risk factors, stroke and depression. Inverse associations of fat with dementia were attenuated by weight change from midlife and were strongest in women with midlife BMI <25. In men, one SD increase in thigh muscle was associated with a decreased likelihood of dementia (0.75; 0.61–0.92). BC was not associated with MCI in men or women.
Conclusion
a higher amount of abdominal and thigh subcutaneous fat were associated with a lower likelihood of dementia in women only, while more thigh muscle was associated with a lower likelihood of dementia in men and women.
Keywords: body composition, dementia, cognition, weight loss, ageing, older people
Background
Obesity in midlife has been associated with greater cognitive decline and increased risk of dementia [1, 2]. However, in late-life obesity has been associated with a decreased likelihood of having dementia [3–5], possibly due to weight loss in the prodromal phase of dementia [6]. Most studies examining the relationship between obesity and dementia infer an association of adiposity with dementia, but use indirect measurements, such as body mass index (BMI) and waist circumference. However, ageing is associated with an increase in fat mass and a decrease in lean mass, making BMI a less suitable measurement for total body adiposity in older persons. In addition, BMI does not provide a measure of muscle [7] or of the specific fat components of visceral and subcutaneous fat, which are related to different metabolic profiles. For example, compared to subcutaneous fat, visceral fat has been shown to be more metabolically active and is more strongly associated with insulin resistance [8].
Studies on the association between specific body composition (BC) components and dementia are scarce and did not use BC measures that separate visceral from subcutaneous fat [9]. Identifying critical changes in BC associated with different levels of cognitive function can help understand underlying metabolic changes accompanying dementia, and help in clinical care of older persons.
Here, we examine in a large well-characterised cohort followed from mid to late-life the association of the amount of abdominal fat (visceral and subcutaneous), thigh subcutaneous fat and thigh lean muscle tissue with mild cognitive impairment (MCI), considered an intermediary stage to dementia and dementia in older persons. We also investigate whether these associations are different by midlife BMI.
Methods
Subjects
The Age Gene/Environment Susceptibility (AGES)-Reykjavik Study, previously described [10], is a population-based cohort study of 5,764 Icelandic men and women aged 66–96 years. The study extends the Reykjavik Study established in 1967 by the Icelandic Heart Association to prospectively study cardiovascular disease in Iceland. Informed consent was obtained from all participants. The study was approved by the National Bioethics Committee in Iceland, which acts as the Institutional Review Board for the IHA (VSN-00-063), and by the National Institute on Aging Intramural Institutional Review Board.
Measures
Cognitive status (normal-MCI-dementia)
Case finding was based on a three-step procedure, previously described [10, 11]. Dementia and MCI were diagnosed by a consensus conference following the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [12]. MCI was defined by cognitive performance (scoring <−1.5 SDs below a cut-point determined from the distribution of scores in a cohort subsample) on memory or two other domains, which is not severe enough to cross the threshold for dementia [13].
Body composition
CT imaging of the mid-thigh and abdomen at the L4/L5 vertebrae was performed with a four-row detector system (Sensation; Siemens Medical Systems, Erlangen, Germany) and has been described previously [14]. Abdominal visceral fat and subcutaneous fat were estimated from a single 10 mm thick trans-axial section. Abdominal visceral fat was distinguished from subcutaneous fat by tracing along the facial plane defining the internal abdominal wall. Adipose areas were calculated by multiplying the number of pixels by the pixel area using specialised software (University of California, San Francisco). Thigh subcutaneous fat and muscle were estimated from a single 10 mm thick axial section as described previously [15]. Total thigh subcutaneous fat and muscle were determined from the right and left thigh.
BMI was calculated as measured weight in kilograms divided by measured height in metres squared. Midlife BMI was categorised as low to normal BMI (<25.0 kg/m2) and overweight to obese (≥25.0 kg/m2) [4]. Percentage weight change from midlife was calculated as: ((late-life weight − midlife weight)/midlife weight) × 100.
Co-variates
We corrected for potential confounders: age, education (primary, secondary or higher than secondary), depressive symptoms assessed with the 15-item Geriatric Depression Scale (GDS) (high depression symptomatology was classified as a score ≥6) [16] and smoking history.
Potential confounders or mediators we adjusted for were: triglyceride/high-density lipoprotein (TG/HDL) ratio (level of triglycerides divided by level of HDL cholesterol), high-sensitivity C-reactive protein (hs-CRP), presence of type 2 diabetes, hypertension and prevalent stroke. Presence (ε2/4, ε3/4 and ε4/4 genotype) or absence (ε2/2, ε2/3 and ε 3/3 genotype) of an apolipoprotein E (APOE) ε4 allele was included as a potential moderator. See Appendix 1 (Supplementary data are available in Age and Ageing online) for detailed methodology.
Analytical sample
Of the 5,764 participants, we excluded 252 missing data on cognitive status, additionally 296 with missing CT-scan data and additionally 47 with missing data on various confounders, giving a final sample of 5,169. Participants excluded due to missing data (n = 595), were significantly older, were more likely to be female, to have less formal education, to have diabetes, to have had a stroke, to report depressive symptoms, less likely to report a history of smoking, had higher hs-CRP levels, a higher TG/HDL ratio and gained less weight from midlife.
Furthermore, of the 5,169 participants, we coded the 264 participants with missing data for the 15-point GDS score into a third category for missing (≥6, considered to have depression symptomatology, <6 having no significant symptomatology and missing).
Statistical analyses
Because of known differences in BC between men and women, all analyses were stratified by sex. Sex-specific z-scores were calculated for each individual (individual value for the BC measure minus the mean for their sex and divided by the SD for their sex). Differences in baseline characteristics were analysed with ANOVA or Chi-square tests.
Multinomial regression techniques were used to analyse the association between continuous Z-score values of abdominal fat, thigh subcutaneous fat, and thigh muscle (independent variables) and cognitive status (normal, MCI and demented). We adjusted models of adiposity, muscle and cognitive status for age (model 0), plus other potential confounders (Model 1; educational level, smoking status and depressive symptoms). For Model 2, we added to Model 1, potential mediators (diabetes, hypertension, stroke, TG/HDL ratio, hs-CRP), and in Model 3 we added to Model 2, change in weight from midlife. In addition, we investigated the potential moderating effect of having an APOE ε4 allele by testing the statistical significance of the interaction between APOE ε4 and BC measures (APOE ε4 status × BC variable) on cognitive status in Model 2. As there were generally no significant interactions, we do not report further on this.
Finally, we performed the analyses stratified by midlife BMI category (low-normal: BMI <25; overweight-obese: BMI ≥25) and additionally tested the interaction between midlife BMI category and BC measures (midlife BMI category × BC variable) on cognitive status in Model 2.
Analyses were conducted using IBM SPSS Statistics for Macintosh, version 20 (IBM Corp., Armonk, NY, USA). A P-value of <0.05 was considered statistically significant.
Results
Baseline characteristics
Of the 5,169 participants, 2,217 were men (42.9%), of whom 249 (11.2%) had MCI and 146 (6.6%) had dementia (Table 1). Mean age of the total sample was 76.4 years (SD = 5.5). Of the 2,952 woman (57.1%), 236 (8.0%) had MCI and 161 (5.5%) had dementia. Mean age of midlife weight and BMI assessment was 49.9 years (SD = 6.1) for men and 52.2 years (SD = 7.0) for women. Further descriptions of the cohort are found in Table 1. Men had more visceral fat and muscle tissue than women, while women had more abdominal and thigh subcutaneous fat than men (Appendix 2, Supplementary data are available in Age and Ageing online).
Table 1.
Characteristics of study group (N = 5,169), stratified by cognitive status and sexa
| Men, N = 2,217 | P-value | Women, N = 2,952 | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Normal | MCI | Dementia | Normal | MCI | Dementia | |||
| N (%) | 1,822 (82.2) | 249 (11.2) | 146 (6.6) | 2,555 (86.6) | 236 (8.0) | 161 (5.5) | ||
| Age, mean (SD) | 75.8 (5.1) | 79.7 (5.6) | 80.2 (5.2) | <0.001 | 75.7 (5.3) | 80.3 (5.5) | 81.4 (5.4) | <0.001 |
| Educational level, N (%) | <0.001 | <0.001 | ||||||
| Low | 219 (12.0) | 96 (38.6) | 47 (32.2) | 654 (25.6) | 126 (53.4) | 81 (50.3) | ||
| Middle | 967 (53.1) | 130 (52.2) | 78 (53.4) | 1,254 (49.1) | 94 (39.8) | 62 (38.5) | ||
| High | 636 (34.9) | 23 (9.2) | 21 (14.4) | 647 (25.3) | 16 (6.8) | 18 (11.2) | ||
| Type 2 diabetes, N (%) | 275 (15.1) | 36 (14.5) | 29 (19.9) | 0.28 | 237 (9.3) | 26 (11.0) | 15 (9.3) | 0.68 |
| Depression, N (%) | <0.001 | <0.001 | ||||||
| No | 1,665 (91.4) | 205 (82.3) | 97 (66.4) | 2,268 (88.8) | 200 (84.7) | 121 (75.2) | ||
| Yes | 78 (4.3) | 31 (12.4) | 23 (15.8) | 169 (6.6) | 24 (10.2) | 24 (14.9) | ||
| Unknown | 79 (4.3) | 13 (5.2) | 26 (17.8) | 118 (4.6) | 12 (5.1) | 16 (9.9) | ||
| Late-life BMI (kg/m2), mean (SD) | 26.9 (3.8) | 26.6 (4.0) | 26.5 (3.7) | 0.36 | 27.3 (4.8) | 27.0 (4.8) | 26.0 (4.7) | 0.004 |
| Smoking, N (%) | 0.02 | 0.57 | ||||||
| Never | 497 (27.3) | 89 (35.7) | 51 (34.9) | 1,344 (52.6) | 130 (55.1) | 84 (52.2) | ||
| Former | 1,118 (61.4) | 132 (53.0) | 78 (53.4) | 893 (35.0) | 72 (30.5) | 53 (32.9) | ||
| Current | 207 (11.4) | 28 (11.2) | 17 (11.6) | 318 (12.4) | 34 (14.4) | 24 (14.9) | ||
| Hypertension, N (%) | 1,448 (79.5) | 212 (85.1) | 118 (80.8) | 0.11 | 2,059 (80.6) | 205 (86.9) | 137 (85.1) | 0.03 |
| HDLb (mg/dl), median (IQR) | 52.51 (44.40–62.55) | 50.58 (43.05–61.00) | 50.58 (43.24–65.06) | 0.06 | 64.86 (54.83–76.83) | 63.90 (52.51–75.29) | 61.78 (50.97–72.78) | 0.05 |
| Triglyceridesb (mg/dl), median (IQR) | 88.50 (66.37–123.01) | 95.58 (69.03–137.17) | 93.36 (69.03–123.89) | 0.10 | 93.81 (70.80–130.09) | 98.67 (72.79–128.98) | 97.35 (71.24–134.96) | 0.44 |
| TG/HDL ratiob, median (IQR) | 1.71 (1.11–2.62) | 1.79 (1.21–2.82) | 1.74 (1.13–2.76) | 0.04 | 1.47 (0.98–2.24) | 1.62 (0.97–2.35) | 1.50 (1.02–2.50) | 0.16 |
| Hs-CRPb (mg/l), median (IQR) | 1.80 (0.90–3.50) | 1.80 (0.90–3.55) | 1.90 (1.10–4.30) | 0.07 | 2.00 (1.00–4.00) | 1.80 (1.00–4.38) | 2.00 (1.00–3.90) | 0.65 |
| Change in weight (%) from midlife, mean (SD) | 2.77 (11.13) | 1.26 (11.41) | −0.97 (11.44) | <0.001 | 5.95 (13.55) | 1.49 (15.56) | −3.15 (14.66) | <0.001 |
| Midlife BMI, N (%) | 0.37 | 0.01 | ||||||
| Low-normal (<25 kg/m2) | 827 (45.4) | 108 (43.4) | 58 (39.7) | 1,499 (58.7) | 121 (51.5) | 78 (48.4) | ||
| Overweight-obese (≥25 kg/m2) | 995 (54.6) | 141 (56.6) | 88 (60.3) | 1,056 (41.3) | 115 (48.5) | 83 (51.6) | ||
| Stroke, N (%) | 128 (7.0) | 23 (9.2) | 26 (17.8) | <0.001 | 151 (5.9) | 27 (11.4) | 17 (10.6) | 0.001 |
| APOE ε4 allele carriers, N (%) | 532 (29.2) | 66 (26.5) | 69 (47.3) | <0.001 | 673 (26.3) | 70 (29.7) | 55 (34.2) | 0.06 |
Abbreviations: APOE ε4, apolipoprotein E epsilon 4; hs-CRP, high-sensitivity C-reactive protein; TG/HDL, triglyceride/HDL cholesterol ratio.
aDifferences between groups were tested by using ANOVA for continuous variables or Chi-square tests for categorical variables.
bP-values are derived from analysis with log-transformed outcomes.
BC and cognitive status
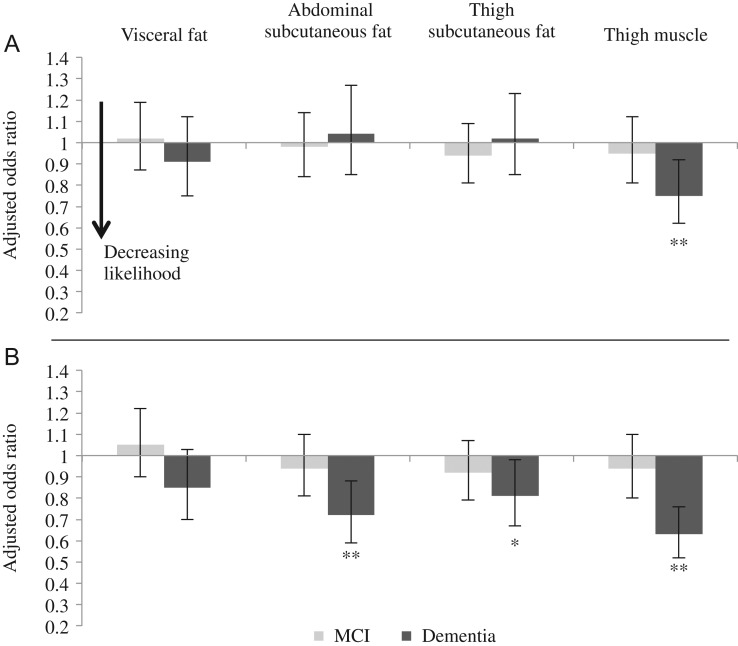
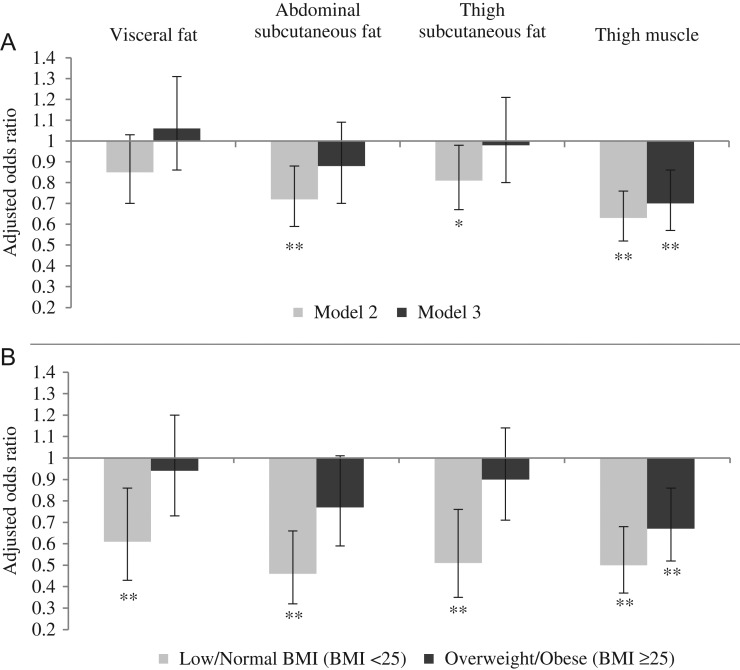
In women, visceral fat was not significantly associated with dementia (Figure 1B; Appendix 3, Supplementary data are available in Age and Ageing online). In contrast, abdominal subcutaneous fat was significantly associated with dementia, such that an increment of one SD in abdominal subcutaneous fat was associated with a 28% decreased likelihood of dementia in women (Figure 1B; Appendix 3, Model 2, Supplementary data are available in Age and Ageing online). After further adjustment for weight change from midlife, which was inversely associated with dementia (less weight loss/more weight gain was association with a decreased likelihood of dementia), this association attenuated and became non-significant (Figure 2A; Appendix 3, Model 3, Supplementary data are available in Age and Ageing online). Thigh subcutaneous fat was also significantly associated with a decreased likelihood of dementia in Models 0–2 (Appendix 3, Supplementary data are available in Age and Ageing online). One SD increase in subcutaneous fat was associated with a 19% decreased likelihood of dementia in women (Figure 1B; Appendix 3, Model 2, Supplementary data are available in Age and Ageing online). This association was attenuated and became non-significant after further adjustment for weight change from midlife (Figure 2A; Appendix 3, Model 3, Supplementary data are available in Age and Ageing online). Finally, in women, thigh muscle was significantly associated with a decreased likelihood of dementia, whereby an increment of one SD in thigh muscle was associated with a 37% decreased likelihood of dementia (Figure 1B; Appendix 3, Model 2, Supplementary data are available in Age and Ageing online). This association was attenuated slightly after adjustment for weight change from midlife, but remained statistically significant (Figure 2A; Appendix 3, Model 3, Supplementary data are available in Age and Ageing online).
Figure 1.
Associations between BC and cognitive status in men (Panel A) and women (Panel B) adjusted for potential confounders or mediators (Model 2). Model 2: Adjustment for age, educational level, smoking status, depressive symptoms, type 2 diabetes, hypertension, stroke, triglyceride/HDL ratio and hs-CRP. Bars are odds ratios per SD increase in BC measures and error bars are 95% confidence intervals. Reference group is the normal cognitive status group. *P-value <0.05, **P-value <0.01.
Figure 2.
Panel A shows associations between BC and cognitive status in women before (Model 2) and after (Model 3) additional adjustment for percentage change in weight from midlife and Panel B shows the associations of Model 2 stratified by midlife BMI category. Model 2: Adjustment for age, educational level, smoking status, depressive symptoms, type 2 diabetes, hypertension, stroke, triglyceride/HDL ratio and hs-CRP; Model 3: Model 2 + adjustment for % change in weight from midlife. Bars are odds ratios for dementia per SD increase in BC measures and error bars are 95% confidence intervals. Reference group is the normal cognitive status group and odds ratios for MCI are not shown. *P-value <0.05, **P-value <0.01.
In women, BC measures were not significantly associated with MCI; however, the ORs for abdominal, thigh subcutaneous fat and muscle tended to be intermediate to the ORs of those who were cognitively normal and those with dementia (Figure 1B; Appendix 3, Models 0–2, Supplementary data are available in Age and Ageing online). In Model 3, these associations attenuated somewhat (Appendix 3, Model 3, Supplementary data are available in Age and Ageing online).
In men, fat measures were not associated with cognitive status (Figure 1A; Appendix 3, Models 0–2, Supplementary data are available in Age and Ageing online). In contrast, an increment of one SD in thigh muscle was significantly associated with a 25% decreased likelihood of dementia (Figure 1A, Appendix 3, Model 2, Supplementary data are available in Age and Ageing online). Adjusting for weight change from midlife did not change the association (Appendix 3, Model 3, Supplementary data are available in Age and Ageing online). BC measures were not associated with MCI in men (Figure 1A, Appendix 3, Models 0–2, Supplementary data are available in Age and Ageing online).
Since vascular dementia may be a part of the residuals from stroke, we performed a sensitivity analysis to examine whether our results (Appendix 3, Model 2, Supplementary data are available in Age and Ageing online) would change after excluding participants with stroke. Excluding these participants did not change the associations (data shown in Appendix 4, Supplementary data are available in Age and Ageing online).
In addition, we performed a sensitivity analysis excluding participants with missing data for depressive symptoms. After excluding these participants, the P-value of the association between thigh muscle and dementia in men increased slightly and became non-significant (P = 0.06). Other results did not change (data not shown).
Analyses stratified by BMI at midlife
In women with a low-normal midlife BMI (midlife BMI <25), the likelihood of dementia significantly decreased per SD increase in visceral fat, abdominal and thigh subcutaneous fat. Associations in the overweight-obese group (midlife BMI ≥25) were attenuated towards the null for the subcutaneous fat and visceral fat body components (Figure 2B; Appendix 5, Model 2, Supplementary data are available in Age and Ageing online) (Pinteraction visceral fat = 0.10; Pinteraction abdominal subcutaneous fat = 0.07; Pinteraction thigh subcutaneous fat = 0.03). Per SD increase in thigh muscle the likelihood of dementia significantly decreased in both women with midlife BMI <25 and midlife BMI ≥25 (Figure 2B; Appendix 5, Model 2, Supplementary data are available in Age and Ageing online) (Pinteraction muscle = 0.19). There were no significant associations between BC measures and MCI in women with a BMI <25 or BMI ≥25 (Appendix 5, Models 0–2, Supplementary data are available in Age and Ageing online).
In men, measures of fat were not associated with dementia in adults with midlife BMI <25 or BMI ≥ 25 (Appendix 5, Models 0–2, Supplementary data are available in Age and Ageing online). However, an increase of one SD in thigh muscle was associated with a decreased likelihood of dementia in men with a midlife BMI ≥25, but not with a midlife BMI <25 (Appendix 5, Model 2, Supplementary data are available in Age and Ageing online) (Pinteraction muscle = 0.03, Model 2).
Excluding those with midlife BMI <18.5 (N for men = 13, N for women = 39) did not change the results.
Discussion
In this cross-sectional study of a large community-based cohort of older adults, we found that higher amounts of abdominal subcutaneous fat, subcutaneous thigh fat and thigh muscle were associated with a decreased likelihood of dementia in women; the association of visceral fat with dementia was not statistically significant. These associations were independent of demographics, vascular risk factors, hs-CRP, depression and stroke and were not moderated by APOE ε4 status. Associations between fat and dementia were related to weight change from midlife (less weight loss/more weight gain was association with a decreased likelihood of dementia in women), while thigh muscle was associated with dementia independent of the weight change from midlife. The associations of BC, particularly thigh subcutaneous fat, with the likelihood of dementia were stronger in women with a BMI <25 compared with a BMI ≥25. In men, only an increase in thigh muscle was associated with a decreased likelihood of dementia, independent of weight change.
Our results are in line with previous studies showing that a decline in body weight and a low late-life BMI are associated with dementia [5, 6, 17]. Comparison to other studies of the association between BC and cognition is difficult because previous studies did not include the combination of measures that was included in this study, i.e. multiple measures of cognitive status, BC and change in weight from midlife [18–20].
The inverse associations between fat and dementia in women may reflect processes related to the regulation of female hormones. Adipose tissue is the major source of endogenous oestrogens in postmenopausal women [21, 22]. Oestrogens may affect cognition by binding to oestrogen receptors that are located throughout the brain, especially in regions of the hippocampus and amygdala, which are involved in learning and memory [23]. Indeed, studies have shown that endogenous estradiol is associated with a decreased risk of cognitive impairment [18, 24]. Another potential mechanistic pathway may involve the hormone leptin. Leptin is produced by adipose tissue, particularly by subcutaneous fat [25], and has been associated with learning and memory processes and with lower dementia risk [4]. Furthermore, some studies have suggested a possible beneficial role for thigh subcutaneous fat as more gluteofemoral fat tissue (fat in thigh and buttocks) has been associated with lower glucose and lipid levels and as loss of subcutaneous fat, observed in patients with lipodystrophic syndromes, has been associated with an increased risk for insulin resistance, diabetes and dyslipidemia [26]. However, in our study adjustment for TG/HDL cholesterol ratio, an indicator of insulin resistance [27] and predictor of coronary heart disease [28], and diabetes did not change the association between thigh subcutaneous fat and dementia when entered separately in the model. This makes this explanation less likely.
Although hormonal and metabolic processes may be involved, our findings indicate that associations between adiposity and dementia in women are not independent of weight loss, which can precede diagnosis of dementia by several years [6] and may be caused by neurodegenerative changes [29]. Several factors directly related to neurodegeneration could lead to unintended weight loss in dementia, including cognitive and psychiatric problems, altered olfaction and gustation, and pathology of energy homoeostatic centres in the brain, particularly the hypothalamus and autonomic centres [30]. Dementia patients may forget to eat due to their memory problems, or may be less motivated to eat due to loss of initiative and/or due to loss in sense of smell that can accompany dementia [31, 32]. Potential mediators including vascular risk factors, stroke and hs-CRP did not explain associations of BC with dementia, which again may indicate that neurodegenerative changes are involved. Furthermore, in our sample, women with dementia lost more weight from midlife than men with dementia (−3.15% vs. −0.97%, respectively), which may explain why we only found associations between adiposity and dementia in women.
Reduced thigh muscle tissue was independently associated with the likelihood of dementia in both men and women. This is in line with previous research showing that lean mass was reduced in individuals with early AD and was associated with poorer cognition and brain atrophy [9]. In contrast to fat, associations between low thigh muscle and dementia persisted even after adjustment for weight change. This suggests that the composition of weight loss from midlife was predominately fat, which is consistent with research showing that a decline in muscle mass is not always reflected in a decrease in body weight [33]. The association between reduced muscle tissue and dementia may be explained by a decreased production of sex hormones and/or an increased production of inflammatory factors, which have both been associated with loss of muscle mass [34] and dementia [29, 35]. This may suggest that muscle loss and dementia share common mechanisms.
In this study, BC measures were not significantly associated with MCI. Previous studies have shown inverse associations of fat and muscle mass with cognitive impairment [18, 19] but these studies did not use clinical outcomes and did not consider dementia patients as a separate group. Loss of fat and muscle tissue may be the result of neurodegenerative changes associated with dementia and it is possible that we did not find associations between BC and MCI, because not all MCI patients develop dementia.
Data on midlife BMI in this study uniquely allowed us to stratify associations between BC measures and dementia by midlife BMI. We found that the association between more fat, particularly subcutaneous fat, and a decreased likelihood of dementia was stronger in women with a low to normal midlife BMI than in women with a high midlife BMI. This may indicate that women with a high midlife BMI follow a different trajectory towards old age than women with a low to normal midlife BMI. In addition, midlife BMI seems to influence cross-sectional associations between BC and cognitive status at late-life. However, our results should be regarded as hypotheses-generating research and more research is needed to examine the longitudinal relationship between changes in BC and cognitive status.
In conclusion, our findings highlight the importance of monitoring changes in weight in older persons with poor cognition with the aim of reducing the negative consequences of loss of fat and muscle, such as increased mortality, morbidity and progressive disability [36]. It will, therefore, be important to develop strategies to prevent weight and muscle loss. However, in addition to meeting the challenges of developing such programmes, it is also important to better understand whether the BC changes reflect neurodegenerative pathways, which are in general less tractable to change with standard interventions, like dietary interventions and interventions to increase physical activity.
Key points.
Late-life obesity has been associated with a decreased likelihood of having dementia.
We examined associations of visceral fat, subcutaneous fat and thigh muscle with cognitive status at old age.
A higher amount of abdominal and thigh subcutaneous fat were associated with a lower likelihood of dementia in woman.
More thigh muscle was associated with a lower likelihood of dementia in both men and women.
Findings suggest that it is important to monitor changes in weight when individuals, particularly women, enter old age.
Supplementary Material
Supplementary data
Supplementary data mentioned in the text are available to subscribers in Age and Ageing online.
Conflicts of interest
None declared.
Funding
This work was supported by the ‘National Institutes of Health’ contract N01-AG-12100, the ‘National Institute on Aging Intramural Research Program’, ‘Hjartavernd’ (the Icelandic Heart Association) and the ‘Althingi’ (the Icelandic Parliament). PJJS received funds from ‘Internationale Stichting Alzheimer Onderzoek’ to undertake this study.
References
- 1. Luchsinger JA, Gustafson DR. Adiposity and Alzheimer's disease. Curr Opin Clin Nutr Metab Care 2009; 12: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cournot M, Marquie JC, Ansiau D et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology 2006; 67: 1208–14. [DOI] [PubMed] [Google Scholar]
- 3. Power BD, Alfonso H, Flicker L, Hankey GJ, Yeap BB, Almeida OP. Body adiposity in later life and the incidence of dementia: the health in men study. PLoS One 2011; 6: e17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gustafson DR. Adiposity and cognitive decline: underlying mechanisms. J Alzheimers Dis 2012; 30(Suppl 2): S97–112. [DOI] [PubMed] [Google Scholar]
- 5. Fitzpatrick AL, Kuller LH, Lopez OL et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol 2009; 66: 336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stewart R, Masaki K, Xue QL et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol 2005; 62: 55–60. [DOI] [PubMed] [Google Scholar]
- 7. Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups. Am J Epidemiol 1996; 143: 228–39. [DOI] [PubMed] [Google Scholar]
- 8. Carr DB, Utzschneider KM, Hull RL et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 2004; 53: 2087–94. [DOI] [PubMed] [Google Scholar]
- 9. Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol 2010; 67: 428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris TB, Launer LJ, Eiriksdottir G et al. Age, gene/environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007; 165: 1076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang M, Jonsson PV, Snaedal J et al. The effect of midlife physical activity on cognitive function among older adults: AGES-Reykjavik Study. J Gerontol A Biol Sci Med Sci 2010; 65: 1369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
- 13. Lopez OL, Becker JT, Jagust WJ et al. Neuropsychological characteristics of mild cognitive impairment subgroups. J Neurol Neurosurg Psychiatry 2006; 77: 159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murphy RA, Nalls MA, Keller M et al. Candidate gene association study of BMI-related loci, weight, and adiposity in old age. J Gerontol A Biol Sci Med Sci 2013; 68: 661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johannesdottir F, Aspelund T, Siggeirsdottir K et al. Mid-thigh cortical bone structural parameters, muscle mass and strength, and association with lower limb fractures in older men and women (AGES-Reykjavik Study). Calcif Tissue Int 2012; 90: 354–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yesavage JA, Brink TL, Rose TL et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17: 37–49. [DOI] [PubMed] [Google Scholar]
- 17. Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology 2007; 69: 739–46. [DOI] [PubMed] [Google Scholar]
- 18. Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. The implications of body fat mass and fat distribution for cognitive function in elderly women. Obes Res 2004; 12: 1519–26. [DOI] [PubMed] [Google Scholar]
- 19. Nourhashemi F, Andrieu S, Gillette-Guyonnet S et al. Is there a relationship between fat-free soft tissue mass and low cognitive function? Results from a study of 7,105 women. J Am Geriatr Soc 2002; 50: 1796–801. [DOI] [PubMed] [Google Scholar]
- 20. Kanaya AM, Lindquist K, Harris TB et al. Total and regional adiposity and cognitive change in older adults: the Health, Aging and Body Composition (ABC) study. Arch Neurol 2009; 66: 329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Szymczak J, Milewicz A, Thijssen JH, Blankenstein MA, Daroszewski J. Concentration of sex steroids in adipose tissue after menopause. Steroids 1998; 63: 319–21. [DOI] [PubMed] [Google Scholar]
- 22. Newton CJ, Samuel DL, James VH. Aromatase activity and concentrations of cortisol, progesterone and testosterone in breast and abdominal adipose tissue. J Steroid Biochem 1986; 24: 1033–9. [DOI] [PubMed] [Google Scholar]
- 23. McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci 2012; 126: 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yaffe K, Lui LY, Grady D, Cauley J, Kramer J, Cummings SR. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. Lancet 2000; 356: 708–12. [DOI] [PubMed] [Google Scholar]
- 25. Van Harmelen V, Reynisdottir S, Eriksson P et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1998; 47: 913–7. [DOI] [PubMed] [Google Scholar]
- 26. Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010; 34: 949–59. [DOI] [PubMed] [Google Scholar]
- 27. McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med 2003; 139: 802–9. [DOI] [PubMed] [Google Scholar]
- 28. Gaziano JM, Hennekens CH, O'Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation 1997; 96: 2520–5. [DOI] [PubMed] [Google Scholar]
- 29. Mrak RE, Griffin WS. Interleukin-1, neuroinflammation, and Alzheimer's disease. Neurobiol Aging 2001; 22: 903–8. [DOI] [PubMed] [Google Scholar]
- 30. Aziz NA, van der Marck MA, Pijl H, Olde Rikkert MG, Bloem BR, Roos RA. Weight loss in neurodegenerative disorders. J Neurol 2008; 255: 1872–80. [DOI] [PubMed] [Google Scholar]
- 31. Berger AK, Fratiglioni L, Forsell Y, Winblad B, Backman L. The occurrence of depressive symptoms in the preclinical phase of AD: a population-based study. Neurology 1999; 53: 1998–2002. [DOI] [PubMed] [Google Scholar]
- 32. Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol 1998; 55: 84–90. [DOI] [PubMed] [Google Scholar]
- 33. Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr 2007; 26: 389–99. [DOI] [PubMed] [Google Scholar]
- 34. Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol (1985) 2003; 95: 1717–27. [DOI] [PubMed] [Google Scholar]
- 35. Barron AM, Pike CJ. Sex hormones, aging, and Alzheimer's disease. Front Biosci (Elite Ed) 2012; 4: 976–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alibhai SM, Greenwood C, Payette H. An approach to the management of unintentional weight loss in elderly people. CMAJ 2005; 172: 773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.