Abstract
Hand-held point-of-care devices are increasingly used in wildlife settings because they are simple, portable, cheap and use small amounts of blood. However, our data show low accuracy of a glucose meter across different ages and physiological states in grey seals. Glucometers need rigorous testing before use in wildlife species.
Keywords: Glucose, glucometer, phocid, pinniped, point-of-care, validation
Abstract
Glucose is an important metabolic fuel and circulating levels are tightly regulated in most mammals, but can drop when body fuel reserves become critically low. Glucose is mobilized rapidly from liver and muscle during stress in response to increased circulating cortisol. Blood glucose levels can thus be of value in conservation as an indicator of nutritional status and may be a useful, rapid assessment marker for acute or chronic stress. However, seals show unusual glucose regulation: circulating levels are high and insulin sensitivity is limited. Accurate blood glucose measurement is therefore vital to enable meaningful health and physiological assessments in captive, wild or rehabilitated seals and to explore its utility as a marker of conservation relevance in these animals. Point-of-care devices are simple, portable, relatively cheap and use less blood compared with traditional sampling approaches, making them useful in conservation-related monitoring. We investigated the accuracy of a hand-held glucometer for ‘instant’ field measurement of blood glucose, compared with blood drawing followed by laboratory testing, in wild grey seals (Halichoerus grypus), a species used as an indicator for Good Environmental Status in European waters. The glucometer showed high precision, but low accuracy, relative to laboratory measurements, and was least accurate at extreme values. It did not provide a reliable alternative to plasma analysis. Poor correlation between methods may be due to suboptimal field conditions, greater and more variable haematocrit, faster erythrocyte settling rate and/or lipaemia in seals. Glucometers must therefore be rigorously tested before use in new species and demographic groups. Sampling, processing and glucose determination methods have major implications for conclusions regarding glucose regulation, and health assessment in seals generally, which is important in species of conservation concern and in development of circulating glucose as a marker of stress or nutritional state for use in management and monitoring.
Introduction
Development of rapid, field accessible and informative markers of stress and nutritional state are useful for conservation efforts in vertebrate species. Glucose is a vital metabolic fuel for glycolytic tissues (Winkler et al., 2003; Grundy, 2004; Mergenthaler et al., 2013). In most mammals, glucose is tightly regulated through modulation of endogenous glucose production (EGP), and glucose uptake and oxidation. Significant deviations in blood glucose levels can cause serious acute and chronic metabolic disturbance and cell death (e.g. Cryer et al., 2003; Kawahito et al., 2009). Rapid glucose mobilization is essential for acute stress responses, which confer a survival advantage. In vertebrates, chronically elevated glucose levels may result from persistent, repeated stress or cortisol exposure (Barton et al., 1987; Khani and Tayek, 2001), whereas fasting and depletion of body energy reserves can lower circulating glucose (Cherel et al., 1988). Glucose levels correlate with habitat quality, reproductive state and/or body condition in many bird species (Fairbrother et al., 1990; Ruiz et al., 2002; Lill, 2011; Minias and Kaczmarek, 2013; Kaliński et al., 2014), and can therefore be useful as an indicator of stress, condition or nutritional status in a conservation context.
Stable or increasing seal populations in UK waters is one of the indicators of Good Environmental Status (GES) in the European Marine Framework Strategy Directive (MFSD). Grey seals (Halichoerus grypus) are the most abundant phocids in UK waters (SCOS, 2015). Ensuring the effectiveness of MFSD policy requires knowledge of the drivers of grey seal population change, which include stress and energy balance (Hall et al., 2001, 2002). Glucose levels may be useful as physiological ecology markers for conservation efforts in seals globally if blood glucose can be measured more rapidly and cheaply than other stress markers, such as glucocorticoids. Seals typically have high postprandial and fasting glucose levels and EGP compared with similar sized terrestrial animals (Schweigert, 1993; Hall, 1998; Keith and Ortiz, 2006; Houser et al., 2012, 2013; Schermerhorn, 2013), which can make interpretation of their glucose measurements challenging.
Whilst determination of glucose levels from processed plasma is considered the gold standard (Haanestad and Lundblad, 1997), the ability to measure blood glucose in real time in wild seals is of great appeal because it would reduce volumes of blood taken, remove the need for centrifugation (Haeckel et al., 2002), abrogate processing delays that result in glycolysis (Lin et al., 1976; Chan et al., 1989; Fobker, 2014) and avoid addition of preservatives that interfere with enzymes used in glucose determination (Waring et al., 2007; Tonyushkina and Nichols, 2009). Additives can also render the remaining sample useless for determination of other analytes, which is problematic when sample number or volume is limited for ethical or logistical reasons.
Glucometers are a vital point-of-care (POC) technology used in humans to manage conditions that result in hyper- or hypoglycaemia (Tonyushkina and Nichols, 2009). Glucometer performance relative to laboratory clinical analysis has been tested for humans (Frishman et al., 1992; Brunner et al., 1998) and companion animals (Cohn et al., 2000; Weiss and Reusch, 2000; Cohen et al., 2009; Johnson et al., 2009; Summa et al., 2014). POCs designed for humans have been used in field conditions for measurements in wildlife species (reviewed by Stoot et al., 2014). However, it is important to define their technical accuracy against standard laboratory methods (Lindholm and Altimiras, 2016), a practice that has often been overlooked in non-domesticated animals. Validations of glucometers in wild mammals are scarce (Stoot et al., 2014).
We sampled wild, healthy grey seals to determine (i) whether field glucometer readings correlate with plasma measurements in matched samples to assess the performance of the device for future work; (ii) the effect of time delays on sampling and processing of the blood samples on laboratory determination of glucose and (iii) effects of age, physiological and development state and time of day on glucose measurements, which can inform evaluation and interpretation of normal glucose values and appropriate sampling regimes for health assessment, management and conservation efforts.
Materials and methods
Animal handling and sampling protocol
Grey seals were sampled on the Isle of May breeding site, Scotland (56° 12’ N, 2° 32’ W) during October–December 2012. Capture and handling complied with the Animal (Scientific Procedures) Act 1986 and were performed by licenced personnel under Home Office Licence 60/4009. Adult females were observed daily throughout lactation, from when they were first identified from either brands or flipper tags. Pup date of birth was recorded where possible. Mother–pup pairs (n = 20) were sampled early (Day 5) and late (Day 15) in the suckling period. Pups were assumed to have weaned when the female was not seen in attendance for a full day (Bennett et al., 2007, 2015). After weaning, pups were located on the colony and sampled again early (Day 5 postweaning; n = 17) and late (Day 15 postweaning; n = 13) in the fasting period (Bennett et al., 2015).
Each time they were captured, adult females were anaesthetized using a 1 ml 100 kg−1 intramuscular dose of Zoletil100TM (Virbac, Cedex, France) delivered using a pressurized dart. Length, axillary girth and mass of mothers and pups were recorded as previously described (Pomeroy et al., 1999; Bennett et al., 2007). On first capture, pups were tagged in the interdigital webbing (Rototag; Dalton ID Systems, Henley on Thames, Oxon, UK) to allow subsequent identification (Fedak and Anderson, 1982). A plasma sample and glucometer readings were taken at each capture. Blood was drawn from the epidural sinus using either 19 gauge, 2 inch needles or 18 gauge 3.5 inch spinal needles, whichever was most appropriate for animal size, to fill a sterile 10 ml potassium ethylenediaminetetraacetic acid (K3 EDTA) Vacutainer (Becton Dickinson, Oxon, UK) as previously described (Bennett et al., 2015). Tubes were kept on cold packs until processing. After the final draw, and within 2 min of the blood sample collection, one glucometer strip (One Touch® Ultra®; Lifescan, Miliptas, CA) was removed from the sealed packet. A spot of the venous blood from the needle was transferred to the reaction site and the strip placed immediately into the glucometer (One Touch® Ultra®) according to the manufacturers’ instructions. This was repeated once more to determine precision. Time of sample draw and glucometer reading were recorded in each case.
Blood processing
Vacutainers were processed immediately on return to the laboratory by centrifugation at 2000 g for 15 min. The plasma portion was drawn from the clot using a glass Pasteur pipette and frozen in 500 μl aliquots at −20°C until processing. Time taken to remove plasma from the clot and to freeze it were recorded.
Glucose measurement
Plasma samples (200 μl) were deproteinized, centrifuged and assayed for glucose in a microplate format using a VERSAmaxTM plate reader (Molecular Devices, 73 Sunnyvale, CA, USA) at 410 nm using the glucose oxidase method according to Webster (1996). Visibly haemolysed samples were not analysed.
Statistical analysis
Statistical analyses were performed in RStudio (Version 0.99.893—© 2009–2016; RStudio Team, 2015). We investigated the relationship between the glucose estimates from the glucometer and the laboratory measurement of plasma within each age/developmental state (i.e. mother or pup, and early or late in suckling or postweaning fast) of the animals using Pearson's correlation. Linear mixed effect models (LMEs) were used to investigate whether the discrepancy between plasma glucose and glucometer reading could be explained by plasma glucose concentration, when in the season the sample was taken (an index of operator experience), and time elapsed between sampling and removing plasma (to establish the effect of sample processing time). An LME was then performed to investigate the effect of developmental stage (mother or pup), physiological state (early or late in suckling, and early or late in fasting), time of day and, where appropriate, when in the season the sample was taken, time from sampling to removal from clot and time from removal from clot to freezing on glucose measurements from both the glucometer and laboratory method. Since time of day is a circular value (Fisher, 1993; Bennett et al., 2001), local time was transformed to both cosine and sine values (cosToD = cos(h/23) × 360 and sinToD = sin(h/23) × 360, respectively). Developmental stage, physiological state, time elapsed between sampling and removing the plasma from the cellular portion, and the time taken between removal from the cellular portion and freezing (Clark et al., 1990; Morris et al., 2002) and cosToD and sinToD were included as fixed effects. All LMEs were fitted using maximum likelihood and included individual as a random effect to control for repeated measures. Model selection was performed using forward stepwise regression and models were compared using the ‘anova’ function in R (Bennett et al., 2015).
Results
Performance of glucometer relative to a laboratory method
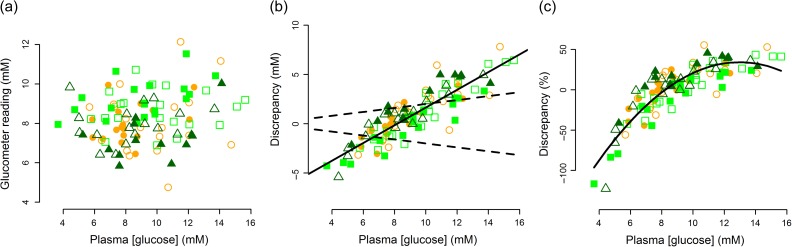
Characteristics of the mother pup pairs, glucometer readings and corresponding laboratory values and their correlations are shown in Table 1. There was a weak, positive correlation between plasma glucose and glucometer readings in pups late in the suckling period (Fig. 1a). No correlations existed between the two methods in the other age or developmental/physiological state categories. The co-efficient of variation for the glucometer was 4.83 ± 4.76%. There was no significant relationship between when in the season the sample was taken and precision of the glucometer measurement (LME: T = 0.32; P = 0.751; df = 57; Akaike's Information Criterion (AIC) = 632.83; n (obs) = 105; n (individuals) = 43).
Table 1:
Mean ( ± sd) mass; length; girth; glucose measured immediately in the field using a hand held glucometer (One Touch® Ultra®) (glucometer reading); and glucose levels in plasma (Plasma glucose) of grey seal mothers and pups early and late in the suckling period and pups early and late in the fast after weaning
| Adult females | Pups | |||||
|---|---|---|---|---|---|---|
| Early suckling | Late suckling | Early suckling | Late suckling | Early fast | Late fast | |
| Mass (kg) | 169.07 (16.72) | 135.11 (11.18) | 25.51 (3.21) | 41.97 (4.86) | 40.79 (3.80) | 37.62 (4.29) |
| Length (cm) | 175.63 (4.95) | 170 (5.35) | 95.92 (3.45) | 104.05 (4.66) | 102.65 (4.50) | 102.08 (5.99) |
| Girth (cm) | 146.26 (13.27) | 132.29 (9.94) | 77.69 (4.52) | 97.89 (6.26) | 96.00 (4.72) | 91.92 (3.95) |
| Glucometer reading (mM) | 8.15a,d (1.75) | 8.01a,c,e (0.95) | 8.62b,d,e (0.95) | 9.21b (0.96) | 7.96a,c (0.96) | 7.25c (1.09) |
| Plasma glucose (mM) | 9.69a,c (2.51) | 8.21b (1.82) | 9.74a,c (2.4) | 8.95a,b,c (2.69) | 8.34a,b,c (2.51) | 9.89c (2.56) |
| Correlation | 0.17 | 0.36 | 0.05 | 0.51 | 0.15 | 0.43 |
| T | 0.48 | 1.47 | 0.21 | 2.41 | 0.57 | 1.57 |
| df | 17 | 15 | 18 | 17 | 15 | 11 |
| P | 0.636 | 0.161 | 0.840 | 0.027 | 0.576 | 0.145 |
| Difference | 1.54a (2.89) | 0.19a (1.73) | 1.12a,b (2.73) | −0.25b (2.36) | 0.38a (2.55) | 2.34c (2.32) |
| Sampling–separation (min) | 89.74a,b,c (24.49) | 101.25a (29.05) | 89.25a,b (23.57) | 102.05a (28.13) | 74.65b,c (18.33) | 72.46c (20.74) |
| Separation–freezing (min) | 81.84a,b (116.56) | 75.35a (52.75) | 46.15b (58.99) | 73.58a (51.01) | 18.12c (16.78) | 20.08b,c (5.93) |
Correlation between the two glucose measurements, T, degrees of freedom and P, the mean absolute difference between the two glucose measures, time elapsed between sample collection and removal from the cellular portion; and time taken from separation to freezing. Bold font indicates a significant positive correlation between the glucometer and plasma glucose readings (Pearson's correlation co-efficient). Within the glucometer, plasma glucose, difference, time between sampling and separation, and time between separation and freezing rows, cells that share at least one same letter superscript are not significantly different from each other (LME: P < 0.05).
Figure 1:
Relationship between plasma glucose concentration measured in the laboratory using the glucose oxidase method and (a) the glucometer venous blood glucose reading, (b) absolute discrepancy and (c) percentage discrepancy of the glucometer venous blood glucose reading. Plasma samples were obtained at the same time as venous glucometer readings from adult female grey seals (circles) during early (Day 5; n = 19; open symbol) and late lactation (Day 15; n = 17; closed symbol); their pups (squares) during early (n = 20; open symbol) and late suckling (n = 19; closed symbol); and the same pups (triangles) early (Day 5; n = 17; open symbol) and late (Day 15; n = 13; closed symbol) in the postweaning fast. Solid trendlines drawn for illustrative purposes. In (b) dashed lines represent 20% glucometer accuracy across the concentration range, which is deemed acceptable for clinical purposes in humans.
The range of accuracy of the glucometer relative to our plasma measurement was 0.5–122%. Absolute and percentage discrepancy of the venous glucometer reading relative to the plasma glucose concentration is shown in Fig. 1b and c. There was a significant positive relationship between the plasma glucose concentration and the absolute discrepancy between the laboratory and glucometer measurements (Plasma-glucometer: LME: T = 20.26; P < 0.0001; df = 56; AIC = 334.29; n (obs) = 105; n (individuals) = 43), such that the glucometer overestimates values below ~ 8 mM and underestimates at values above 8 mM. The percentage inaccuracy was much greater at lower measured plasma glucose values. The degree of accuracy of the glucometer compared with the plasma measurement differed between age/stage categories. The glucometer discrepancy compared with laboratory measurements was smallest in the late suckling pups, and tended to overestimate values in these animals. The discrepancy was greatest and the glucometer tended to overestimate glucose in late weaned pups. The discrepancy between the glucometer reading and laboratory measurement was similar between adults early and late in lactation, pups early in lactation and pups early in the postweaning fast. The glucometer underestimated the true plasma value in these age categories (Table 1; Fig. 1b and c).
Effects of sample processing, age stage and time of day on glucose measurements
Plasma glucose levels were best explained by the developmental/physiological state of the animal and the time elapsed between sampling and removal of plasma from the cellular portion of the blood. Samples from weaned pups were processed significantly faster than those from pups and females late in suckling (Table 1; LME: df = 57; n (obs) = 105; n (individuals) = 43). However, developmental/physiological differences in laboratory measured glucose were not an artefact of processing time because the model specifically accounted for effects of time elapsed from sampling to removal from clot, and weaned pups did not have the lowest glucose levels. There was a significant decrease (by 1.77 ± 0.79 mM) in plasma glucose in adult females from early (9.69 ± 2.51 mM) to late (8.21 ± 1.82 mM) lactation (LME: T = 2.17; P = 0.034; df = 56; AIC = 498.92; n (obs) = 105; n (individuals) = 43). The late lactation values for females were also lower than those in their pups early in the suckling period (LME: T = 2.56; P = 0.013; df = 56) and late in the postweaning fast (LME: T = 2.19; P = 0.033; df = 56). There were no other differences in glucose levels between the different developmental/physiological states.
Given the differences in plasma glucose measured in the laboratory between developmental/physiological states described above, there was a small increase in measured plasma glucose with time elapsed between sampling and separation of the plasma from the clot across all developmental/physiological states (LME: glucose = 0.024 (time elapsed) + 5.81; T = 2.34; P = 0.022; df = 56). Time taken from removal from clot to freezing did not account for any additional variation in plasma glucose levels (ANOVA: L ratio = 0.130; P = 0.288). Glucose levels were not related to time of day (sinToD: LME: T = 0.15; P = 0.880; cosToD: LME: T = 0.09; P = 0.282). Inclusion of time of day with age/stage category and time elapsed between sampling and separation of the plasma from the clot did not improve the model fit (sinTOD: ANOVA: L ratio = 0.145; P = 0.699; cosTOD: ANOVA: L ratio = 1.02; P = 0.314). In addition, the time elapsed between sampling and removal from the clot did not contribute to the discrepancy between the glucometer and plasma measurements (LME: T = 1.03; P = 0.307; df = 55; AIC = 506.57; n (obs) = 105; n (individuals) = 43).
Discussion
Performance of glucometer relative to a laboratory method
We chose the One Touch® Ultra® (Lifescan, Miliptas, CA) device because it was widely available at the time of the study, affordable, can tolerate a wide range of haematocrit (Hct), temperature and humidity and performs well in humans with hyperglycaemia (Brunner et al., 1998), which is similar to the normal blood glucose range for grey seals (Bennett et al., 2013; Hall, 1998; Schweigert, 1993). Glucometer precision was extremely high. The device was easy to use and gave reproducible readings, even when the operator was inexpert. This is a clear benefit when field teams change or include inexperienced volunteers. However, glucometer accuracy was very poor relative to the laboratory method. The American Diabetes Association recommends readings from glucometers should be within 15% of laboratory-derived values to be usable (Stahl et al., 2001). Many organizations allow an accuracy of 20%. Only 32% of our glucometer readings were within 15% of the laboratory glucose measurement and less than half (47%) fell within 20% accuracy. Accuracy was highest between 8 and 10 mM, which is the typical range of blood glucose measurements in grey seals (Schweigert, 1993; Hall, 1998; Bennett et al., 2013). In dogs, some glucometers show high levels of inaccuracy that are concentration dependent, similar to our findings (Cohen et al., 2009). In elephant seals (Mirounga angustirostris), the iSTAT, a device that performs multiple clinical chemistry readings simultaneously, is inaccurate for glucose measurements (Larsen et al., 2002). Other glucometers intended for use in humans, dogs or cats also perform poorly in wild mammals, such as deer (Burdick et al., 2012) and prairie dogs (Higbie et al., 2015). In ferrets (Mustela putorius furo) canine, but not human glucometers, perform satisfactorily (Peritz et al., 2013; Summa et al., 2014). Unfortunately, there is no way to distinguish the accurate from inaccurate readings from the glucometer alone, and therefore a correction factor cannot be applied.
A number of possibilities may explain poor glucometer performance in seals. Firstly, we used venous whole blood, whereas glucometers are designed for capillary whole blood. However, our approach matched the sample type (i.e. both from the venous compartment) and therefore should show a closer relationship between measurements (Haeckel et al., 2002). We used the venous compartment to avoid multiple sampling sites on the same animal. In addition, capillary sampling in seals may not represent whole body glucose levels because capillaries often constrict during handling stress or anaesthesia.
Plasma or serum typically has 11–12% higher glucose content than whole blood at an Hct of 45% (Tonyushkina and Nichols, 2009). Glucometer measurements depend on plasma proteins and number of cells present. The One Touch® Ultra contains a 12% offset to account for the difference, and this constant may not be appropriate for seals (Castellini and Castellini, 1989). For human blood, the One Touch® Ultra is accurate for Hct between 30 and 55% and high Hct can reduce the glucose measurement. Grey seal Hct is 40–52% (Hall, 1998; Noren et al., 2008) and therefore should be within the device tolerance. However, Hct in seals increases rapidly to over 60% during breath holding or diving (Castellini et al., 1996), during epinephrine induced splenic contraction (Hurford et al., 1996; Cabanac et al., 1997), and changes with age (Hall, 1998; Noren et al., 2008). Variation in breathing rate and splenic contraction during handling stress or anaesthesia in grey seals may produce large Hct fluctuations, and alter pH and oxygenation, which can affect glucometer readings (Tang et al., 2001).
Glucometers are calibrated for erythrocyte settling rate, which is 1–4 mm h−1 in grey seal pups (Hall, 1998) compared with 12–23 mm h−1 in humans (Wetteland et al., 1996). Seal haemoglobin (Hb) concentration is also higher (Noren et al., 2008). Differences in any or all of these conditions in seal blood compared with normal human blood could prevent correlation between the glucometer readings and laboratory analysis. While it may be possible to calibrate the glucometer by taking Hct, settling rate and/or water content into consideration, because these measurements can be performed in the field in some circumstances, the need to obtain plasma samples for calibration would negate the value of the glucometer for minimizing sample volume, and another POC or an Hct centrifuge would be required in the field. Glucometers that are intended for use in companion animals (Weiss and Reusch, 2000; Zini et al., 2009), which have more similar Hct and settling rates to seals (Stephens, 1938), or those that measure Hct simultaneously (e.g. Rao et al., 2005), may perform better for species with high and variable Hct. Interestingly, accounting for Hct, red blood cell count, Hb, total protein levels or creatinine did not improve the glucometer estimates in ferret venous blood (Summa et al., 2014), suggesting that additional factors affect device performance.
We saw a weak correlation between plasma and glucometer readings in pups only late in the suckling period, which suggests that this age/developmental category has the lowest circulating levels of interfering substances. This is surprising given that suckling pups are likely to have the highest levels of circulating fats (Schweigert, 1993), which can interfere with glucometer performance at high levels (Heinemann, 2010; Lindholm and Altimiras, 2016). Reported triglyceride levels in grey seals should be well within the tolerance of the device we used (<34.2 mM: Heinemann, 2010), whereas cholesterol levels in postweaned pups could approach levels that cause interference (<18.1 mM: Schweigert, 1993; Mazzarro et al., 2003).
Glucometers and test strips are sensitive to temperature and moisture changes (King et al., 1995; Tonyushkina and Nichols, 2009). Our device and strips were kept dry until use, field conditions fell within the 10–90% humidity range recommended and we avoided sampling during rain. Inaccuracy may be introduced by other field conditions, such as contact of needle with fur, skin and fat, or presence of dirt and sea spray.
Stability of blood glucose
Glucose concentration falls by 5–7% per hour in human blood when serum or plasma is in contact with erythrocytes (Tonyushkina and Nichols, 2009), dropping by 25% within 4 h and 75% within 24 h of sampling (Lin et al., 1976; Chan et al., 1989; Fobker, 2014). Here, chilling avoided rapid glycolysis, which is an advantage in field situations in which substantial delays between sampling and processing can occur.
We saw a small but significant increase in glucose with time elapsed between sampling and removal of the plasma. Glucose production by the cellular portion is unlikely (Bennett and Burchell, 2013; Jun et al., 2012). Hb, a leading cause of interference in clinical chemistry assays (Grafmeyer et al., 1995; Lippi et al., 2008), was removed by deproteinization. However, our assay detects hydrogen peroxide (H2O2), which can be produced by deproteinization of Hb (Galleman and Eyer, 1990). H2O2 may then interfere in proportion to haemolysis in the original sample. EDTA and cold storage produce greater haemolysis (Muro et al., 1998; Mafuvadze and Erlwanger, 2007; Walencik and Witseka, 2007). Sodium fluoride and sodium citrate in Vacutainers minimize glycolysis and avoid the need to chill samples. However, these inhibitors take up to 4 h to take effect (Cavalier et al., 2008; Mikesh and Bruns, 2008; Gambino et al., 2009) or interfere with enzymes used in glucose determination (Waring et al., 2007). Although not an issue in large animals such as seals, draw volume from small mammals and birds can be limited, which may lead to under-dilution of stabilizers. Based on the small but statistically significant increase in blood glucose with time on the clot, we recommend plasma be removed from the cellular fraction as soon as possible, irrespective of chilling, and that direct contact with ice packs is avoided.
Impact of method on conclusions regarding blood glucose levels in seals
Our plasma glucose measurements show no change in grey seal pups, either during suckling or after weaning, similar to Hall (1998) and Schweigert (1993). Circulating glucose in females fell from early to late in lactation. Group differences were not a result of differences in time taken to process the samples. Fasting, lactating adult female elephant seals show lower glucose levels (6.96–8.16 mM) than those reported here, and either no change or an increase in glucose levels during lactation (Houser et al., 2007; Fowler et al., 2008). Typically, elevated EGP is required in other mammals to support milk production (Mohammad et al., 2009). Glucose may thus be higher in grey seals earlier in lactation to support high milk output (Mellish et al., 1999; Mellish and Iverson, 2000). The reduction in circulating glucose later in lactation may reflect decreased EGP to protect protein reserves. In contrast to the plasma values, the glucometer suggested glucose was stable in mothers during lactation, and fell from suckling to fasting in pups. Conclusions based on the glucometer would thus be inappropriate for health or physiological assessment.
Glucose levels show diurnal fluctuations in humans (Sennels et al., 2015), but we saw no time of day effect. Since diurnal glucose changes were too small to be detected it appears less critical to standardize time of day, which can be difficult in field conditions, compared with the need to process samples rapidly.
Conclusion
In other species with high glucose and Hct (e.g. penguins: Pygoscelis sp., Ibaňez et al., 2015), glucometer readings have been assumed to be usable based on similarities to values seen in prior studies (e.g. Campos Medeiros et al., 2011). Although the glucometer readings here were similar to glucose concentrations found elsewhere in grey seals (Schweigert, 1993; Hall, 1998; Bennett et al., 2013), they differ markedly from the plasma glucose results in matched samples. The discrepancy between the two methods produces different conclusions about typical changes that occur in mothers and pups during suckling and fasting. In species of conservation concern (e.g. Rea et al., 1998; Reif et al., 2004) such differences could result in use of inappropriate management criteria. Our data highlight that assumptions about glucometer accuracy based on similarities in the range of values obtained are not sufficient, and underscore the need for standardization and care in blood sample collection and handling to minimize artefactual effects on laboratory glucose measurements. A glucometer for use in species or situations when high and fluctuating Hct and lipids are expected, and variable temperature and humidity are likely to occur, would present significant advantages for use in wildlife medicine, conservation and management, and basic research.
Acknowledgements
The authors would like to thank Andy Atfield for use of the glucometer and Vaida Surviliene for help in the field. We would like to thank Scottish Natural Heritage for the permit to work on the Isle of May.
Funding
This work was supported in part by National Capability funding to Sea Mammal Research Unit, University of St Andrews (Grant No. SMRU 1001) from the UK Natural Environment Research Council. L.M.T. was supported by internal funding from Plymouth University.
Author contributions
K.A.B., S.E.W.M., A.J.H. and L.M.T. contributed to the rationale for this work. K.A.B. designed and carried out the field sampling with assistance from S.E.W.M. S.M. processed the blood samples for glucose analysis supported by L.M.T. K.A.B. performed the statistical analysis. K.A.B. and L.M.T. wrote the first draft of this manuscript and all authors contributed to the final write up.
References
- Barton BA, Schreck CB, Barton LD (1987) Effects of chronic cortisol administration and daily acute stress on growth, physiological conditions, and stress responses in juvenile rainbow trout. Dis Aquat Org 2: 173–185. [Google Scholar]
- Bennett KA, Burchell A (2013) von Gierke disease In S Maloy, K Hughes, eds, Brenner's Online Encyclopedia of Genetics, Ed 2 Elsevier, pp 304–307. [Google Scholar]
- Bennett KA, McConnell BJ, Fedak MA (2001) Diurnal and seasonal variations in the duration and depth of the longest dives in southern elephant seals (Mirounga leonina): possible behavioural and physiological constraints. J Exp Biol 204: 649–662. [DOI] [PubMed] [Google Scholar]
- Bennett KA, Speakman JR, Moss SEW, Pomeroy PP, Fedak MA (2007) Effects of mass and body composition on fasting fuel utilisation in grey seal pups (Halichoerus grypus: Fabricius): an experimental study using supplementary feeding. J Exp Biol 210: 3043–3053. [DOI] [PubMed] [Google Scholar]
- Bennett KA, Hammill M, Currie S (2013) Liver glucose-6-phosphatase proteins in suckling and weaned grey seal pups: structural similarities to other mammals and relationship to nutrition, insulin signalling and metabolite levels. J Comp Physiol B 183: 1075–1088. [DOI] [PubMed] [Google Scholar]
- Bennett KA, Hughes J, Stamatas S, Brand S, Foster NL, Moss SEW, Pomeroy PP (2015) Adiponectin and insulin in grey seals during suckling and fasting: relationship with nutritional state and body mass during nursing in mothers and pups. Physiol Biochem Zool 88: 295–310. [DOI] [PubMed] [Google Scholar]
- Brunner GA, Ellmerer M, Sendlhofer G, Wutte A, Trajanoski Z, Schaupp L, Quehenberger F, Wach P, Krejs GJ, Pieber TR (1998) Validation of home blood glucose monitors with respect to clinical and analytical approaches. Diabetes Care 21: 585–590. [DOI] [PubMed] [Google Scholar]
- Burdick S, Mitchell MA, Neil J, Heggem B, Whittington J, Acierno MJ (2012) Evaluation of two point of care meters and a portable clinical chemistry analyser for measurement of blood glucose concentration in juvenile white-tailed deer (Odocoileus virginianus). J Am Vet Med Assoc 240: 596–599. [DOI] [PubMed] [Google Scholar]
- Cabanac A, Folkow LP, Blix AS (1997) Volume capacity and contraction control of the seal spleen. J App Physiol 82: 1989–1994. [DOI] [PubMed] [Google Scholar]
- Campos Medeiros LC, Pereira Mayorga LFS, Campos Behring RC, Chippari Gomes AR, Valdetaro Rangel MC (2011) Avaliação da glicose sérica de Pinguins de Magalhães (Spheniscus magellanicus Forster, 1781) no Espírito Santo, Brasil. Medvep—Revista Científica de Medicina Veterinária—Pequenos Animais e Animais de Estimação 10: 72–73. [Google Scholar]
- Castellini MA, Castellini JM (1989) Influence of hematocrit on whole blood glucose levels: new evidence from marine mammals. Am J Physiol 256: R1220–R1224. [DOI] [PubMed] [Google Scholar]
- Castellini MA, Costa DP, Huntley A (1996) Hematocrit variation during sleep apnea in elephant seal pups. Am J Physiol 251: R429–R431. [DOI] [PubMed] [Google Scholar]
- Cavalier E, Carlisi A, Chapelle J-P, Delanaye P (2008) Stabilisation of blood glucose in blood specimens: mechanisms of delay in fluoride inhibition of glycolysis. Clin Chem 54: 930–932. [DOI] [PubMed] [Google Scholar]
- Chan AY, Swaminathan R, Cockram CS (1989) Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin Chem 35:315–317. [PubMed] [Google Scholar]
- Cherel Y, Robin J-P, Walch O, Karmann P, Netchitailo P, Le Maho Y (1988) Fasting in king penguin 1. Hormonal and metabolic changes during breeding. Am J Physiol R254: 170–177. [DOI] [PubMed] [Google Scholar]
- Clark ML, Humphreys S, Frayn KN (1990) Stability of plasma glucose during storage. Ann Clin Biochem 27: 373–377. [DOI] [PubMed] [Google Scholar]
- Cohen TA, Nelson RW, Kass PH, Christopher MM, Feldman EC (2009) Evaluation of six portable blood glucose meters for measuring blood glucose concentration in dogs. J Am Vet Med Assoc. 235: 276–280. [DOI] [PubMed] [Google Scholar]
- Cohn LA, McCaw DL, Tate DJ, Johnson JC (2000) Assessment of five portable glucose meters, a point of care analyser, and colour test strips for measuring blood glucose concentration in dogs. J Am Vet Med Assoc 216: 198–202. [DOI] [PubMed] [Google Scholar]
- Cryer PE, Davis SN, Shamoon H (2003) Hypoglycemia in diabetes. Diabetes Care 26: 1902–1912. [DOI] [PubMed] [Google Scholar]
- Fairbrother A, Craig MA, Walker K, O'Loughlin D (1990) Changes in mallard (Anas platyrhynchos) serum chemistry due to age, sex, and reproductive condition. J Wild Dis 26: 67–77. [DOI] [PubMed] [Google Scholar]
- Fedak MA, Anderson SS (1982) The energetics of lactation: accurate measurements from a large wild mammal, the grey seal (Halichoerus grypus) J Zool Lond 198: 473–479. [Google Scholar]
- Fisher NI. (1993) Statistical Analysis of Circular Data. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Fobker M. (2014) Stability of glucose in plasma with different anticoagulants. Clin Chem Lab Med 52: 1057–1060. [DOI] [PubMed] [Google Scholar]
- Fowler MA, Champagne CD, Houser DS, Crocker DE (2008) Hormonal regulation of glucose clearance in lactating northern elephant seals (Mirounga angustirostris). J Exp Biol 211: 2943–2949. [DOI] [PubMed] [Google Scholar]
- Frishman D, Ardito DM, Graham SM Jr (1992) Performance of glucose monitors. Lab Med 23:179–184. [Google Scholar]
- Galleman D, Eyer P (1990) On the mechanism of hydrogen peroxide formation during precipitation of haemoglobin with perchloric acid. Biol Chem 371: 881–887. [DOI] [PubMed] [Google Scholar]
- Gambino R, Piscitelli J, Ackattupathil TA, Theriault JL, Andrin RD, Sanfilippo ML, Etienne M (2009) Acidification of blood is superior to sodium fluoride alone as an inhibitor of glycolysis. Clin Chem 55:1019–1021. [DOI] [PubMed] [Google Scholar]
- Grafmeyer D, Bondon M, Manchon M, Levillain P (1995) The influence of bilirubin, haemolysis and turbidity on 20 analytical tests performed on automatic analysers. Eur J Clin Biochem 33: 31–52. [DOI] [PubMed] [Google Scholar]
- Grundy JC. (2004) Aspects of blood cell biochemistry: erythrocytes, platelets, and stem cells In Storey KB. ed, Functional Metabolism: Regulation and Adaptation. John Wiley, New Jersey, pp 505–528. [Google Scholar]
- Haanestad U, Lundblad A (1997) Accurate and precise isotope dilution mass spectrometry method for determining glucose in whole blood. Clin Chem. 43: 5794–8000. [PubMed] [Google Scholar]
- Haeckel R, Brinck U, Colic D, Janka HU, Püntmann I, Schneider J, Viebrock C (2002) Comparability of blood glucose concentrations measured in different sample systems for detecting glucose intolerance. Clin Chem 48: 936–939. [PubMed] [Google Scholar]
- Hall AJ. (1998) Blood chemistry and haematology of gray seal (Halichoerus grypus) pups from birth to postweaning. J Zoo Wildlife Med 29: 401–407. [PubMed] [Google Scholar]
- Hall AJ, McConnell BJ, Barker RJ (2001) Factors affecting first-year survival in grey seals and their implications for life history strategy. J Anim Ecol 70: 138–149. [Google Scholar]
- Hall AJ, McConnell BJ, Barker RJ (2002) The effect of total immunoglobulin levels, mass and condition on the first-year survival of grey seal pups. Funct Ecol 16: 462–474. [Google Scholar]
- Heinemann L. (2010) Quality of glucose measurement with blood glucose meters at the point of care: relevance of interfering factors. Diabetes Tech Therapeut 12: 847–857. [DOI] [PubMed] [Google Scholar]
- Higbie CT, Eshar D, Bello NM (2015) Evaluation of three point-of-care meters and a portable veterinary chemistry analyzer for measurement of blood glucose concentrations in black-tailed prairie dogs (Cynomys ludovicianus). Am J Vet Res 76: 532–539. [DOI] [PubMed] [Google Scholar]
- Houser DS, Champagne CD, Crocker DE (2007) Lipolysis and glycerol gluconeogenesis in simultaneously fasting and lactating northern elephant seals. Am J Physiol Regul Integr Comp Physiol 293: R2376–R2381. [DOI] [PubMed] [Google Scholar]
- Houser DS, Crocker DE, Tift MS, Champagne CD (2012) Glucose oxidation and non-oxidative glucose disposal during prolonged fasts of the northern elephant seal pup (Mirounga angustirostris). Am J Physiol 303: R562–R570. [DOI] [PubMed] [Google Scholar]
- Houser DS, Champagne CD, Crocker DE (2013) A non-traditional model of the metabolic syndrome: the adaptive significance of insulin resistance in fasting adapted seals. Front Endocrinol 4:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurford WE, Hochachka PW, Schnieder RC, Guyton GP, Stanek KS, Zapol DG, Liggins GC, Zapol WM (1996) Splenic contraction, catecholamine release, and blood volume redistribution during diving in the Weddell seal. J App Physiol 80: 298–306. [DOI] [PubMed] [Google Scholar]
- Ibaňez AE, Najle R, Larsen K, Pari M, Figueroa A, Montalti D (2015) Haematological values of three Antacrctic penguins: Gentoo (Pygoscelis papua), Ádelie (P. adeliae) and chinstrap (P. antarcticus). Polar Res 34: 25718. [Google Scholar]
- Johnson BM, Fry MM, Flatland B, Kirk CA (2009) Comparison of a human portable blood glucose meter, veterinary portable glucose meter and automated chemistry analyser for measurement of blood glucose concentrations in dogs. J Am Vet Med Assoc 235: 1309–1313. [DOI] [PubMed] [Google Scholar]
- Jun HS, Cheung YY, Lee YM, Mansfield BC, Chou JY (2012) Glucose-phosphatase-β, implicated in a congenital neutropenia syndrome, is essential for macrophage energy homeostasis and functionality. Blood 119: 4047–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliński A, Bańbura M, Glądalski M, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Cyżewska I, Bańbura J (2014) landscape patterns of variation in blood glucose concentration of nestling blue tits (Cyanistes caeruleus). Landsc Ecol 29: 1521–1530. [Google Scholar]
- Kawahito S, Kitahata H, Oshita S (2009) Problems associate with glucose toxicity: role of hyperglycemia-induced oxidative stress. World J Gastroenterol 15: 4137–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith EO, Ortiz CL (2006) Glucose kinetics in neonatal elephant seals during postweaning aphagia. Mar Mamm Sci 5: 99–115. [Google Scholar]
- Khani S, Tayek JA (2001) Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome. Clin Sci 101: 739–747. [DOI] [PubMed] [Google Scholar]
- King JM, Eigenmann CA, Colaguiri S (1995) Effect of ambient temperature and humidity on performance of blood glucose meters. Diabet Med 12: 337–340. [DOI] [PubMed] [Google Scholar]
- Larsen RS, Haulena M, Grindem CB, Gulland FMD (2002) Blood values of juvenile northern elephant seals (Mirounga angustirostris) obtained using a portable clinical chemistry analyser. Vet Clin Pathol 31: 106–110. [DOI] [PubMed] [Google Scholar]
- Lill A. (2011) Sources of variation in blood glucose concentrations of free-living birds. Avian Biol Res 4: 78–86. [Google Scholar]
- Lin YL, Smith CH, Dietzler DN (1976) Stabilization of blood glucose by cooling with ice: an effective procedure for preservation of samples from adults and newborns. Clin Chem 22: 2031–2033. [PubMed] [Google Scholar]
- Lindholm C, Altimiras J (2016) point of care devices for physiological measurements in field conditions. A smorgasbord of instruments and validation procedures. Comp Biochem Physiol A 202: 99–111. [DOI] [PubMed] [Google Scholar]
- Lippi G, Blanckaert N, Bonini P, Green S, Kitchen S, Palicka V, Vassault VJ, Plebani M (2008) Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin Chem Lab Med 46: 764–772. [DOI] [PubMed] [Google Scholar]
- Mafuvadze B, Erlwanger KH (2007) The effect of EDTA, heparin and storage on the erythrocyte osmotic fragility, plasma osmolality and haematocrit of adult ostriches (Struthio camelus). Veterinarski Arhiv 77: 427–434. [Google Scholar]
- Mazzarro LM, Dunn LJ, Furr HC, Clark RM (2003) Serum retinol, alpha tocopherol, and lipids in four species of adult captive pinnipeds. Zoo Biol 22: 83–96. [Google Scholar]
- Mellish JE, Iverson SJ (2000) Blood metabolites as indicators of nutrient utilisation in fasting, lactating phocid seals: does depletion of nutrient reserves terminate lactation. Can J Zool 79: 303–311. [Google Scholar]
- Mellish JE, Iverson SJ, Bowen WD (1999) Variation in milk production and lactation performance in grey seals and consequences for pup growth and weaning characteristics. Physiol Biochem Zool 72: 677–690. [DOI] [PubMed] [Google Scholar]
- Mergenthaler P, Lindauer U, Dienel GA, Meisel A (2013) Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikesh LM, Bruns DE (2008) Stabilization of glucose in blood specimens: mechanism of delay in fluoride inhibition of glycolysis. Clin Chem 54:930–932. [DOI] [PubMed] [Google Scholar]
- Minias P, Kaczmarek K (2013) Concentrations of plasma metabolites as predictors of nestling condition in the Great Cormorant Phalacrocorax carbo sinensis. Ornis Fenn 90: 142–150. [Google Scholar]
- Mohammad MA, Sunehag AL, Chacko SK, Pontius AS, Maningat PD, Haymond MW (2009) Mechanisms to conserve glucose in lactating women during a 42 h fast. Am J Physiol Endocrinol Metab 297: E879–E888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JD, Fernandez JM, Chapa AM, Gentry LR, Thorn KE, Weick TM (2002) Effects of sample handling, processing, storage and hemolysis on measurements of key energy metabolites in ovine blood. Small Ruminant Res 43: 157–166. [Google Scholar]
- Muro J, Cuenca R, Pastor J, Vinas L, Lavin S (1998) Effects of lithium heparin and tripotassium EDTA on hemtalogic values of Hermann's tortoises (Testudo hermanni). J Zoo Wildlife Med 29: 40–44. [PubMed] [Google Scholar]
- Noren SR, Iverson SJ, Boness DJ (2008) Development of the blood and muscle oxygen stores in gray seals (Halichoerus grypus): implications for juvenile diving capacity and the implications for the necessity of a terrestrial postweaning fast. Physiol Biochem Zool 78: 482–490. [DOI] [PubMed] [Google Scholar]
- Peritz OA, Antinoff N, Chen S, Kass PH, Paul-Murphy JR (2013) Evaluation of portable blood glucose meters for measurement of blood glucose concentration in ferrets (Mustela putorius furo). J Am Vet Med Assoc 242: 350–354. [DOI] [PubMed] [Google Scholar]
- Pomeroy PP, Fedak MA, Rothery P, Anderson SS (1999) Consequences of maternal size for reproductive expenditure and pupping success of grey seals at North Rona, Scotland. J App Ecol 68: 235–253. [Google Scholar]
- Rao LV, Jakubiak F, Sidwell JS, Winkelman JW, Snyder ML (2005) Accuracy evaluation of a new glucometer with automated hematocrit measurement and correction. Clin Chim Acta 356: 178–183. [DOI] [PubMed] [Google Scholar]
- Rea LD, Castellini MA, Fadely BS, Loughlin TR (1998) Health status of young Alaska Steller sea lion pups (Eumetopias jubatus) as indicated by blood chemistry and haematology. Comp Biochem Physiol A 120: 617–623. [DOI] [PubMed] [Google Scholar]
- Reif JS, Bachand A, Aguirre AA, Borjesson DL, Kashinsky L, Braun R, Antonelis G (2004) Morphometry, hematology, and serum chemistry in the Hawaiian monk seal (Monachus schauinslandi). Mar Mamm Sci 20: 851–860. [Google Scholar]
- RStudio Team (2015) RStudio: Integrated Development for R. RStudio, Inc., Boston, MA: http://www.rstudio.com/. [Google Scholar]
- Ruiz G, Rosenmann M, Novoa FF, Sabat P (2002) Hematological parameters and stress index in rufous-collared sparrows dwelling in urban environments. Condor 104:162–166. [Google Scholar]
- Schermerhorn T. (2013) Normal glucose metabolism in carnivores overlaps with diabetes pathology in non-carnivores. Front Endocrinol 4: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigert FJ. (1993) Effects of energy mobilisation during fasting and lactation on plasma metabolites in the grey seal (Halichoerus grypus) Comp Biochem Physiol 105A: 347–352. [DOI] [PubMed] [Google Scholar]
- SCOS (2015) Scientific advice on matters related to the management of seal populations: 2015. http://www.smru.st-andrews.ac.uk/files/2016/08/SCOS-2015.pdf.
- Sennels HP, Jøregnesen HL, Fahrenkrug J (2015) Diurnal changes of metabolic markers in healthy young males—the Bjispeberg study of diurnal variations. Scan J Clin Lab invest 75: 686–692. [DOI] [PubMed] [Google Scholar]
- Stahl M, Jörgensen LGM, Hylthoft Petersen P, Brandslund I, De Fine Olivarius N, Borch-Johnsen K (2001) Optimization of preanalytical conditions and analysis of plasma glucose. 1. Impact of the new WHO and ADA recommendations on diagnosis of diabetes mellitus. Scand J Clin Invest 61:169–180. [DOI] [PubMed] [Google Scholar]
- Stephens JG. (1938) Concentration and sedimentation rates of blood from the splenic artery and vein. J Physiol 94: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoot LJ, Cairns NA, Cull F, Taylor JJ, Jeffrey JD, Morin F, Mandelman JW, Clark TD, Cooke SJ (2014) Use of portable blood physiology point-of-care devices for basic and applied research on vertebrates: a review. Conserv Physiol 2 doi:10.1093/conphys/cou011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summa NM, Eshar D, Lee-Chow B, Larrat S, Brown DC (2014) comprise of a human portable glucometer and an automated clinical chemistry analyser for measurement of blood glucose concentration in pet ferrets (Mustela putorius furo). Can Vet J 55: 865–869. [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Louie RF, Lee JH, Lee DM, Miller EE, Kost GJ (2001) Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med 29: 1062–1070. [DOI] [PubMed] [Google Scholar]
- Tonyushkina K, Nichols JH (2009) Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol 3: 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walencik J, Witeska M (2007) The effects of anticoagulants on hematological indices and blood cell morphology of common carp (Cyprinus carpio L.). Comp Biochem Physiol C 146: 331–335. [DOI] [PubMed] [Google Scholar]
- Waring WS, Evans LE, Kirkpatrick CT (2007) Glycolysis inhibitors negatively bias blood glucose measurements: potential impact on the reported prevalence of diabetes mellitus. J Clin Pathol 60: 820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster S. (1996) Measurement of crustacean hyperglycemic hormone levels in the edible crab Cancer pagurus during emersion stress. J Exp Biol 199: 1579–1585. [DOI] [PubMed] [Google Scholar]
- Weiss G, Reusch C (2000) Evaluation of five portable glucose meters for use in dogs. J Am Vet Med Assoc 216: 203–209. [DOI] [PubMed] [Google Scholar]
- Wetteland P, Røger M, Solberg HE, Iversen OH (1996) Population-based erythrocyte sedimentation rates in 3910 subjectively healthy Norwegian adults: a statistical study based on men and women from the Oslo area. J Int Med 240: 125–131. [DOI] [PubMed] [Google Scholar]
- Winkler BS, Pourcho RG, Starnes C, Slocum J, Slocum N (2003) Metabolic mapping in mammalian retina: a biochemical and 3H-2-deoxyglucose autoradiographic study. Exp Eye Res 77: 327–337. [DOI] [PubMed] [Google Scholar]
- Zini E, Moretti S, Tschuor F, Reusch CE (2009) Evaluation of a new portable glucose meter designed for use in cats. Schweiz Arch Tierheilkd 151: 448–451. [DOI] [PubMed] [Google Scholar]