Abstract
The nature of molybdenum cofactor in the bacterial enzyme dimethyl sulfoxide reductase has been investigated by application of alkylation conditions that convert the molybdenum cofactor in chicken liver sulfite oxidase and milk xanthine oxidase to the stable, well-characterized derivative [di(carboxamidomethyl)]molybdopterin. The alkylated pterin obtained from dimethyl sulfoxide reductase was shown to be a modified form of alkylated molybdopterin with increased absorption in the 250-nm region of the spectrum and altered chromatographic behavior. The complex alkylated pterin was resolved into two components by treatment with nucleotide pyrophosphatase. These were identified as di(carboxamidomethyl)molybdopterin and GMP by their absorption spectra, coelution with standard compounds, and by further degradation by alkaline phosphatase to dephospho [di(carboxamidomethyl)]molybdopterin and guanosine. The GMP moiety was sensitive to periodate, identifying it as the 5' isomer. Chemical analysis of the intact alkylated pterin showed the presence of two phosphate residues per pterin. These results established that the pterin isolated from dimethyl sulfoxide reductase contains the phosphoric anhydride of molybdopterin and 5'-GMP, which is designated molybdopterin guanine dinucleotide.
Full text
PDF
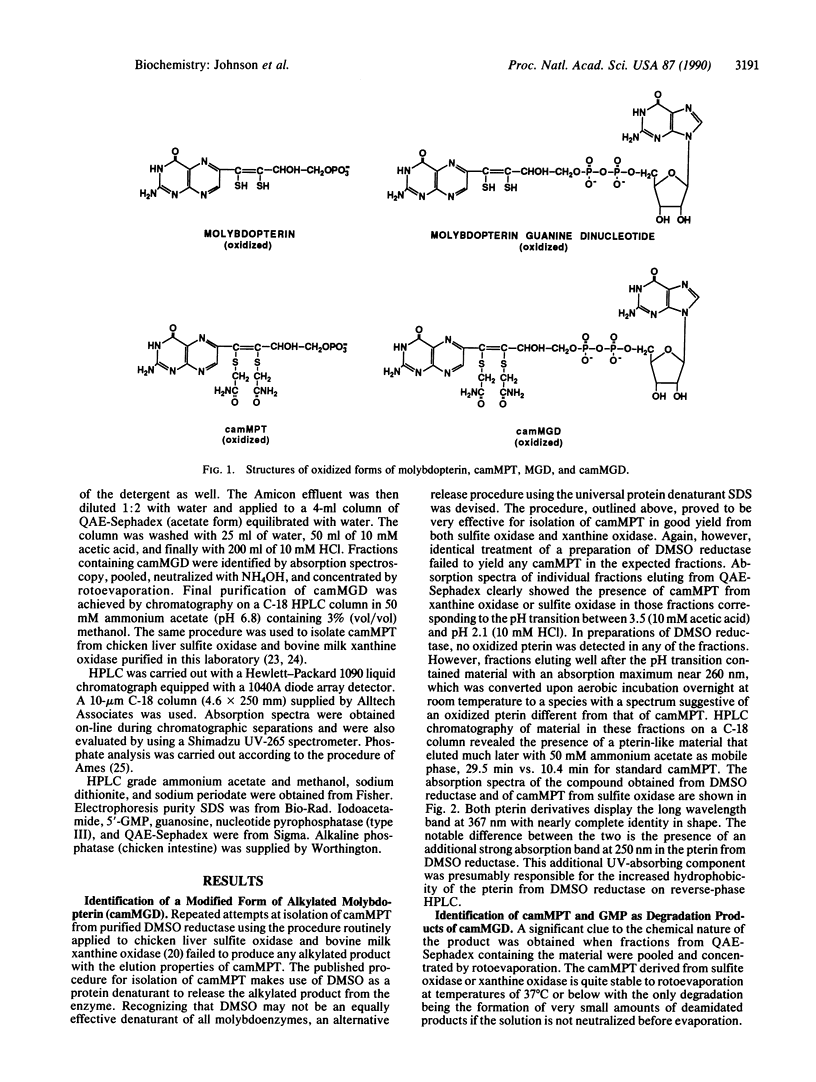
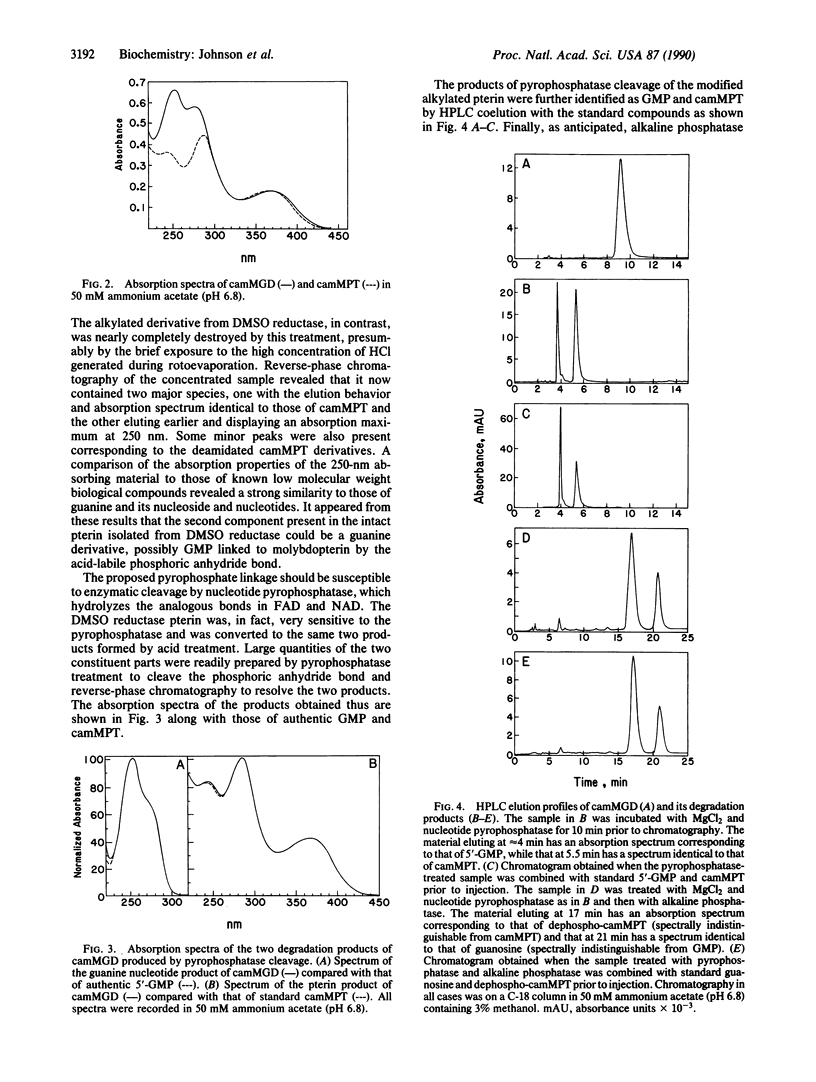


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deistung J., Bray R. C. Isolation, in the intact state, of the pterin molybdenum cofactor from xanthine oxidase. Biochem J. 1989 Oct 15;263(2):477–483. doi: 10.1042/bj2630477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth G. L. Occurrence of molybdenum in the nicotinic acid hydroxylase from Clostridium barkeri. Arch Biochem Biophys. 1983 Mar;221(2):565–569. doi: 10.1016/0003-9861(83)90176-5. [DOI] [PubMed] [Google Scholar]
- Fukuoka M., Fukumori Y., Yamanaka T. Nitrobacter winogradskyi cytochrome a1c1 is an iron-sulfur molybdoenzyme having hemes a and c. J Biochem. 1987 Sep;102(3):525–530. doi: 10.1093/oxfordjournals.jbchem.a122084. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Hainline B. E., Rajagopalan K. V., Arison B. H. The pterin component of the molybdenum cofactor. Structural characterization of two fluorescent derivatives. J Biol Chem. 1984 May 10;259(9):5414–5422. [PubMed] [Google Scholar]
- Johnson J. L., Hainline B. E., Rajagopalan K. V. Characterization of the molybdenum cofactor of sulfite oxidase, xanthine, oxidase, and nitrate reductase. Identification of a pteridine as a structural component. J Biol Chem. 1980 Mar 10;255(5):1783–1786. [PubMed] [Google Scholar]
- Johnson J. L., Rajagopalan K. V. Structural and metabolic relationship between the molybdenum cofactor and urothione. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6856–6860. doi: 10.1073/pnas.79.22.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D. L., Rajagopalan K. V. Purification and properties of sulfite oxidase from chicken liver. Presence of molybdenum in sulfite oxidase from diverse sources. J Biol Chem. 1972 Oct 25;247(20):6566–6573. [PubMed] [Google Scholar]
- Kramer S. P., Johnson J. L., Ribeiro A. A., Millington D. S., Rajagopalan K. V. The structure of the molybdenum cofactor. Characterization of di-(carboxamidomethyl)molybdopterin from sulfite oxidase and xanthine oxidase. J Biol Chem. 1987 Dec 5;262(34):16357–16363. [PubMed] [Google Scholar]
- Krüger B., Meyer O. Structural elements of bactopterin from Pseudomonas carboxydoflava carbon monoxide dehydrogenase. Biochim Biophys Acta. 1987 Apr 30;912(3):357–364. doi: 10.1016/0167-4838(87)90040-9. [DOI] [PubMed] [Google Scholar]
- Krüger B., Meyer O. The pterin (bactopterin) of carbon monoxide dehydrogenase from Pseudomonas carboxydoflava. Eur J Biochem. 1986 May 15;157(1):121–128. doi: 10.1111/j.1432-1033.1986.tb09647.x. [DOI] [PubMed] [Google Scholar]
- Liu C. L., Mortenson L. E. Formate dehydrogenase of Clostridium pasteurianum. J Bacteriol. 1984 Jul;159(1):375–380. doi: 10.1128/jb.159.1.375-380.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O., Rajagopalan K. V. Molybdopterin in carbon monoxide oxidase from carboxydotrophic bacteria. J Bacteriol. 1984 Feb;157(2):643–648. doi: 10.1128/jb.157.2.643-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason A., Lee K. Y., Pan S. S., Ketchum P. A., Lamberti A., DeVries J. Invitro formation of assimilatory reduced nicotinamide adenine dinucleotide phosphate: nitrate reductase from a Neurospora mutant and a component of molybdenum-enzymes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3242–3246. doi: 10.1073/pnas.68.12.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATEMAN J. A., COVE D. J., REVER B. M., ROBERTS D. B. A COMMON CO-FACTOR FOR NITRATE REDUCTASE AND XANTHINE DEHYDROGENASE WHICH ALSO REGULATES THE SYNTHESIS OF NITRATE REDUCTASE. Nature. 1964 Jan 4;201:58–60. doi: 10.1038/201058a0. [DOI] [PubMed] [Google Scholar]
- Pienkos P. T., Shah V. K., Brill W. J. Molybdenum cofactors from molybdoenzymes and in vitro reconstitution of nitrogenase and nitrate reductase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5468–5471. doi: 10.1073/pnas.74.12.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh M. G., Campbell W. H. Quaternary structure and composition of squash NADH:nitrate reductase. J Biol Chem. 1985 Mar 25;260(6):3380–3385. [PubMed] [Google Scholar]
- Satoh T., Kurihara F. N. Purification and properties of dimethylsulfoxide reductase containing a molybdenum cofactor from a photodenitrifier, Rhodopseudomonas sphaeroides f.s. denitrificans. J Biochem. 1987 Jul;102(1):191–197. doi: 10.1093/oxfordjournals.jbchem.a122032. [DOI] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Properties of formate dehydrogenase in Methanobacterium formicicum. J Bacteriol. 1982 Apr;150(1):1–7. doi: 10.1128/jb.150.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Hattori Y., Hasegawa T., Haraguchi H., Ishimoto M. Studies on nitrate reductase of Clostridium perfringens. IV. Identification of metals, molybdenum cofactor, and iron-sulfur cluster. J Biochem. 1987 Feb;101(2):503–509. doi: 10.1093/oxfordjournals.jbchem.a121937. [DOI] [PubMed] [Google Scholar]
- Siefermann-Harms D., Fritz B., Ninnemann H. Evidence for a pterin-derivative associated with the molybdenum cofactor of Neurospora crassa nitrate reductase. Photochem Photobiol. 1985 Dec;42(6):771–778. doi: 10.1111/j.1751-1097.1985.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Weiner J. H., MacIsaac D. P., Bishop R. E., Bilous P. T. Purification and properties of Escherichia coli dimethyl sulfoxide reductase, an iron-sulfur molybdoenzyme with broad substrate specificity. J Bacteriol. 1988 Apr;170(4):1505–1510. doi: 10.1128/jb.170.4.1505-1510.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto I., Okubo N., Ishimoto M. Further characterization of trimethylamine N-oxide reductase from Escherichia coli, a molybdoprotein. J Biochem. 1986 Jun;99(6):1773–1779. doi: 10.1093/oxfordjournals.jbchem.a135655. [DOI] [PubMed] [Google Scholar]
- Yamamoto I., Saiki T., Liu S. M., Ljungdahl L. G. Purification and properties of NADP-dependent formate dehydrogenase from Clostridium thermoaceticum, a tungsten-selenium-iron protein. J Biol Chem. 1983 Feb 10;258(3):1826–1832. [PubMed] [Google Scholar]
- Yamamoto I., Shimizu H., Tsuji T., Ishimoto M. Purification and properties of nitrate reductase from Mitsuokella multiacidus. J Biochem. 1986 Mar;99(3):961–969. doi: 10.1093/oxfordjournals.jbchem.a135559. [DOI] [PubMed] [Google Scholar]



