Abstract
Background
Chong-Myung-Tang (CMT) extract is widely used in Korea as a traditional herbal tonic for increasing memory capacity in high-school students and also for numerous body ailments since centuries. The use of CMT to improve the learning capacity has been attributed to various plant constituents, especially black ginseng, in it. Therefore, in this study, we have first investigated whether black ginseng-enriched CMT extracts affected spatial learning using the Morris water maze (MWM) test. Their molecular mechanism of action underlying improvement of learning and memory was examined in vitro.
Methods
We used two types of black ginseng-enriched CMT extracts, designated as CM-1 and CM-2, and evaluated their efficacy in the MWM test for spatial learning behavior and their anti-inflammatory effects in BV2 microglial cells.
Results
Our results show that both black ginseng-enriched CMT extracts improved the learning behavior in scopolamine-induced impairment in the water maze test. Moreover, these extracts also inhibited nitric oxide production in BV2 cells, with significant suppression of expression of proinflammatory cytokines, especially inducible nitric oxide synthase, cyclooxygenase-2, and interleukin-1β. The protein expression of mitogen-activated protein kinase and nuclear factor-κB pathway factors was also diminished by black ginseng-enriched CMT extracts, indicating that it not only improves the memory impairment, but also acts a potent anti-inflammatory agent for neuroinflammatory diseases.
Conclusion
Our research for the first time provides the scientific evidence that consumption of black ginseng-enriched CMT extract as a brain tonic improves memory impairment. Thus, our study results can be taken as a reference for future neurobehavioral studies.
Keywords: anti-inflammation, black ginseng, BV2 cells, cytokines, learning
1. Introduction
Today's competitive and modern world requires an extra dose of strength both mentally and physically. This strength is necessary to function properly in every field of life, from studies to some kind of heavy physical activity. Keeping this requirement in mind, many food supplements and tonics are designed to provide energy boost, which the routine diet cannot provide to the required level. Korea can be reckoned as the country rich in producing vitalizing Oriental herbal tonics for almost all the minor and major ailments of body without any side effects. Chong-Myung-Tang (CMT) is one such kind of herbal tonic. CMT is a mixture of almost three to five kinds of various plants and herbs [1], and is widely used as a memory-enhancing supplement in Korea since centuries, especially for the high-school students. The common conception behind using this tonic is that it enhances the learning retention in young adult students especially at the time of their academic tests.
CMT, which is classically an Oriental Korean medicine, has been mentioned in the Authentic Korean Oriental medicine book Dongui Bogam (Principles and Practices of Eastern Medicine). Its major constituents are Acorus gramineus Soland, Polygala tenuifolia Willdenow, and Poria cocos Wolf [1], [2]. This book is listed in the United Nations Educational, Scientific and Cultural Organization's Memory of the World Programme as it is considered the most oldest and unique book, which acts as a bridge between the Oriental and modern medicine [3]. The herbal tonic CMT is prepared in accordance with guidelines specified in this book for different herbal products so as to utilize their maximum effect, but with some modifications. Botanically, CMT is composed of various herbs and plants including roots of A. gramineus Soland, sclerotium of P. cocos Wolf, roots of P. tenuifolia Willdenow, black ginseng, roots of Angelica gigas Nakai, seeds of Ziziphus jujuba Miller, roots of Liriope platyphylla Wang et Tang, roots of Glycyrrhiza uralensis Fisch, Schisandra chinensis, fruit of Lycium chinense Mill., Artemisia capillaris Thunberg, and Zingiber officinale (ginger). All of these herbs contribute to the memory-enhancing effects of CMT. Among them, the most major component of CMT is the black ginseng extract. Black ginseng extract has been shown to increase learning in scopolamine-induced amnesic mice. Moreover, black ginseng has also been studied widely for various other purposes including its efficacy in treating hypercholesterolemia and its antioxidant and antiaging effects [4], [5], [6].
The other components of CMT are A. gramineus, which is a medicinal herb that is being used in Korean medicine for the treatment of digestive disorders, convulsion, neurodegenerative diseases. It is also an antibacterial agent [7]. Polysaccharides from the sclerotium of P. cocos Wolf are shown to elicit immune-stimulatory properties by increasing the production of nitric oxide (NO), nuclear factor-κB (NF-κB) induction, and B-lymphocytes production [8]. Roots of P. tenuifolia Willdenow have been reported to possess protective effects against learning and memory impairment in scopolamine-induced amnesia model of rats [9]. A. gigas Nakai roots are traditionally used for the treatment of anemia, common colds, and female reproductive system disorders as well as an analgesic, anti-inflammatory, antitumor, and neuroprotective agent [10], [11]. Seeds of Z. jujuba Miller are shown to possess anxiolytic and antioxidant effects [12]. Roots of L. platyphylla Wang et Tang show antidiabetic and anticardiovascular disease effects [13]. Roots of G. uralensis Fisch have been studied for their antiulcer, anti-inflammatory, and antioxidant activities [14], [15]. Increased working capacity, mental performance, and endurance are manifested by S. chinensis, besides the fact that it also possesses anticancerous, anti-inflammatory, and immune-stimulating properties [16], [17]. Fruits of L. chinense Mill. have been used in energy-boosting drinks and for vitalizing the mood and body. It also has antiatherosclerotic properties [18]. A. capillaris Thunberg extract has been reported to show anxiolytic effects [19]. Z. officinale is a treasure box containing many valuable health-promoting effects. It is especially used in digestive disorders such as bloating and gastric infections. It is also very commonly used to treat common cold and cough [20].
Altogether, by summing up the individual effects of each component of CMT, especially black ginseng, it can be considered that the formulation possesses memory increasing and anxiety-relieving properties, both of which are biggest factors for success in academic examinations. We used the traditional Morris water maze (MWM) test to evaluate the memory-enhancing effects of two types of CMT extracts, namely, CM-1 and CM-2. Our results showed that both extracts increased the latency time for MWM, indicating that CMT is indeed effective as a nervine tonic. Our in vitro results on BV2 microglial cells indicate that CMT is also a very potent anti-inflammatory agent, which is attributed to the individual anti-inflammatory activities of its components.
2. Materials and methods
2.1. Materials
Dulbecco's modified Eagle's medium (DMEM; Welgene Co., Gyeongsangbuk-do, Korea), fetal bovine serum (FBS; Welgene Co.), streptomycin and penicillin (Lonza, Walkersville, MD, USA), TRIzol reagent (Invitrogen, Carlsbad, CA, USA), oligo-dT (Bioneer Co., Daejeon, Korea), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β primers were obtained from Bioneer Co. Lipopolysaccharide (LPS; Escherichia coli 055:B5) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO, USA). Specific antibodies used against phosphorylated extracellular signal-regulated kinase (ERK) and/or total form of ERK, Jun amino-terminal kinases (JNK), p38, IκB kinase (IKK) α/β, IκB, NF-κB p65, iNOS, COX-2, β-actin, and secondary antibody rabbit horseradish peroxidase-linked antibody were purchased from Cell Signaling Technology (Beverly, MA, USA). All other reagents and chemicals were obtained from Sigma Aldrich.
2.2. Preparation of CMT extracts
The herbal components in black ginseng-enriched CM-1 extract are roots of A. gramineus Soland, sclerotium of P. cocos Wolf, roots of P. tenuifolia Willdenow, black ginseng, roots of A. gigas Nakai, seeds of Z. jujuba Miller, roots of L. platyphylla Wang et Tang, roots of G. uralensis Fisch, and S. chinensis (percentage composition: 1:1:1:1:1:0.75:0.75:1.5:1.5, w/w, respectively), whereas those in the CM-2 extract are black ginseng, roots of P. tenuifolia Willdenow, roots of A. gramineus Soland, seeds of Z. jujuba Miller, roots of A. gigas Nakai, fruits of L. chinense Mill., A. capillaris Thunberg, Z. officinale, and sclerotium of P. cocos Wolf (percentage composition: 1.2:1:1:1:1:0.8:0.5:0.3:1, w/w, respectively). The percentage of black ginseng in CM-2 is more than that in CM-1 and this had some additional effects, which will be discussed in a later section. All of these herbal components were boiled in hot water, then cooled, and strained to obtain the extracts.
2.3. Animal experiments
Male C57BL/6 mice (age, 6 mo; weight, 26–29 g), were purchased from Charles River–Orient Biotechnology (Gyeonggi-do, Korea). Each experimental group included 10 mice. The mice were housed in a specific pathogen-free barrier facility at 21 ± 2°C with a relative humidity of 60 ± 10% under a 12-h light and dark cycle. Feed and water were provided ad libitum. All animal care and experimental procedures were approved by the Animal Care Committee of the Daejeon University.
2.4. Morris water maze test
The MWM test was applied for the assessment of learning and memory retention [21]. For this purpose, we conducted the place navigation test. First, the mice were given pretraining once every day for 2 wk in such a way that they were placed into the maze in the northeast, southeast, northwest, and southwest directions from the selected platform and then manually directed to the platform so they can memorize the location. After 2 wk, all mice received scopolamine intraperitoneally and then galantamine and black ginseng-enriched CMT extracts orally, which served as a positive control and CMT extract, respectively. After 2 wk, mice were observed again for their ability to locate and reach the platform without any manual assistance (memory retention evaluation). The distance and time taken by mice to reach to platform were recorded as time through latency and distance through latency, respectively. In addition, the pathway taken by mice to reach the platform/probe was also recorded by image photography.
2.5. Cell culture
Murine microglial cell line BV2 (American Type Culture Collection) was cultured in DMEM supplemented with 8% FBS (Welgene Co) and 100 IU/mL penicillin and 100 μg/mL streptomycin sulfate (Lonza). The incubating conditions were humidified 5% CO2 at 37°C.
2.6. Nitric oxide assay
NO was measured according to the method based on Griess reaction assay. Briefly BV2 cells were seeded in 24-well plates and incubated with or without LPS (0.1 μg/mL) in the absence or presence of CMT extracts at indicated concentrations overnight. The cell culture supernatants (100 μL) were then mixed with Griess reagent (0.2% naphthylethylenediamine dihydrochloride and 2% sulphanilamide in 5% phosphoric acid) in double-distilled water at equal volumes and incubated for 10 min at room temperature. The absorbance in each well was then analyzed at 540 nm in a microplate reader (VersaMax, Molecular devices, CA, USA).
2.7. Cell viability (MTT) assay
To determine the cytotoxic effects of black ginseng-enriched CMT extracts, cell viability assay was performed using the MTT reagent, which was added to the culture medium at a final concentration of 0.1 mg/mL. After 4 h of incubation at 37°C in 5% CO2, the resulting violet-colored crystals were dissolved in dimethyl sulfoxide (100 μL/well) and absorbance values were measured at 560 nm.
2.8. RNA extraction and quantitative reverse transcription-polymerase chain reaction
BV2 cells were pretreated with or without black ginseng-enriched CMT extracts at indicated concentrations for 30 min and then stimulated with LPS (0.1 μg/mL) for 18 h. Total RNA was extracted using TRIzol reagent (Invitrogen) following the manufacturer's instructions. The later procedure was followed in accordance with our previous research [22] (Table 1).
Table 1.
Sequence of primers used in this study
| Gene | Primer | oligonucleotide sequence (5′→3′) |
|---|---|---|
| GAPDH | F | 5′-CAATGAATACGGCTACAGCAAC-3′ |
| R | 5′-AGGGAGATGCTCAGTGTTGG-3′ | |
| iNOS | F | 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ |
| R | 5′-GGCTGTCAGAGCCTCGTGGCTTTGG-3′ | |
| COX-2 | F | 5′-TCTCAGCACCCACCCGCTCA-3′ |
| R | 5′-GCCCCGTAGACCCTGCTCGA-3′ | |
| IL-1β | F | 5′-CAGGGTGGGTGTGCCGTCTTTC-3′ |
| R | 5′-TGCTTCCAAACCTTTGACCTGGGC-3′ | |
| TNF-ɑ | F | 5′-TTGACCTCAGCGCTGAGTTG-3′ |
| R | 5′-CCTGTAGCCCACGTCGTAGC-3′ | |
| IL-6 | F | 5′-GTACTCCAGAAGACCAGAGG-3′ |
| R | 5′-TGCTGGTGACAACCACGGCC-3′ |
COX-2, cyclooxygenase-2; F, forward; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IL, interleukin; iNOS, inducible nitric oxide synthase; R, reverse; TNF-α, tumor necrosis factor-α
2.9. Western blot analysis
BV2 cells were treated or left untreated with black ginseng-enriched CMT extracts in the presence or absence of LPS (0.1 μg/mL). Proteins were then measured using the PRO-MEASURE assay kit (PRO-PREP; iNtRON Biotechnology, Seongnam, Korea). Proteins were then separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membrane (Millipore, Immobilon-P, Billerica MA, USA). Nonspecific binding on the nitrocellulose filter paper was minimized by adding a blocking buffer containing 5% nonfat dry milk and 0.1% Tween-20 in Tris-buffered saline. The membranes were then incubated with specific primary antibodies overnight at 4°C followed by a 2-h incubation with horseradish peroxidase-conjugated antirabbit antibody (1:3,000 dilution; Cell Signaling, Beverly, MA, USA). Bound antibodies were visualized using enhanced chemiluminescence (Supex) and images were analyzed using ImageJ software. β-Actin was taken as internal control.
2.10. Statistical analysis
Data were presented as mean ± standard deviation. One-way analysis of variance and Dunnett test were used for the statistical evaluation of data. SAS 9.3 (SAS Institute Inc., Cary, NC, USA) was used for performing the analysis. All p values less than 0.01 were considered significant.
3. Results
3.1. Black ginseng-enriched CMT extracts supplementation improves scopolamine-induced memory and learning impairment
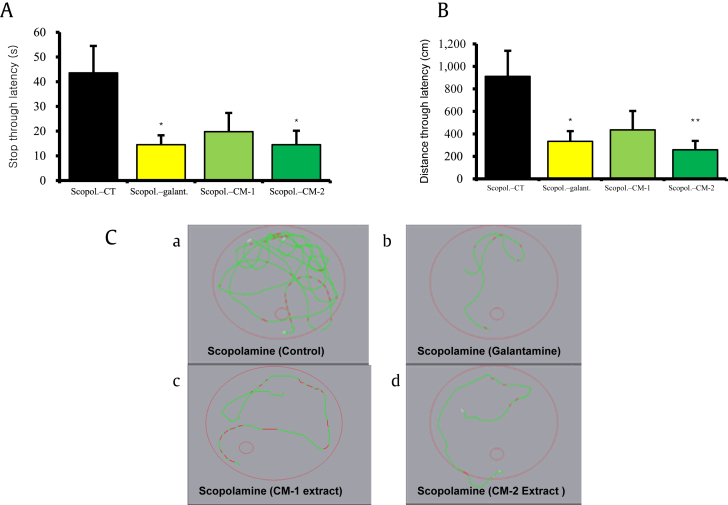
To evaluate the effects of black ginseng-enriched CMT extracts on learning impairments caused by scopolamine. Time through and distance through latency are the measurable factors in MWM that shows the extent of hippocampal memory retention. As can be seen in Figs. 1A and 1B, only scopolamine-treated mice showed increased time to reach the platform, indicating that scopolamine strongly impaired the targeted region for learning in mice brain. By contrast, mice treated with black ginseng-enriched CMT extracts showed reduced distance and time through latency; in particular, mice treated with the CM-2 extract having a high proportion of black ginseng had a remarkable statistical significance. This indicates that the proportion of black ginseng in CM-2 extract has improved the scopolamine-induced amnesia in mice. Furthermore, in Fig. 1C, it is clearly indicated that CM-2-extract-treated mice took less number of crossings to reach the platform/probe, which is in sharp contrast to scopolamine-induced amnesic mice that took numerous crossings to locate the platform. Therefore, the MWM test potently supports our hypothesis that both black ginseng-enriched CMT extracts are efficacious for memory impairment and retention.
Fig. 1.
Effects of black ginseng-enriched Chong-Myung-Tang extract on the scopolamine-induced impairment of learning and memory in the stop-through-type Morris water maze test. (A and B) Escape latency in terms of time and distance taken to find the visible/nonvisible platform. *p < 0.01, **p < 0.005 compared to scopolamine control (Scopol-CT) were considered statistically significant. (C) Visible tracking movement of mice to reach to platform in the probe test. Galant., galantamine; Scopol., scopolamine.
3.2. Black ginseng-enriched CMT extracts suppressed LPS-induced inflammation in a dose-dependent manner
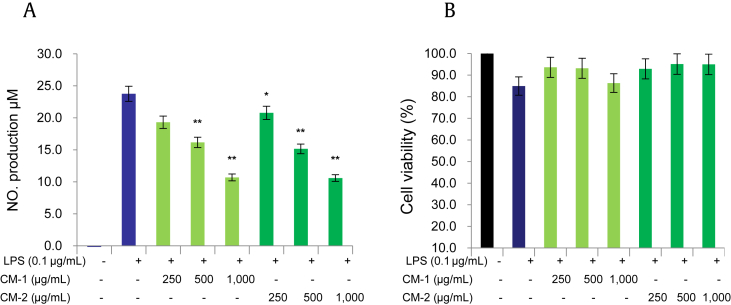
LPS is a well-known stimulator of NO gas production. Moreover, LPS also acts as an immunostimulatory factor for Gram-negative bacteria and can cause systemic inflammation if excessively stimulated. Therefore, we sought to check whether black ginseng-enriched CMT extracts affected LPS-induced NO production in BV2 cells. As shown in Fig. 2A, CMT extracts sharply attenuated LPS-induced NO production when injected to culture media 30 min after the sample injection. Furthermore, the extracts did not show any cytotoxic effect over this concentration range (Fig. 2B).
Fig. 2.
Black ginseng-enriched Chong-Myung-Tang (CMT) extracts suppressed lipopolysaccharide (LPS)-mediated nitric oxide (NO) release without showing cytotoxicity. (A) BV2 cells were seeded in 24-well plates overnight and then preincubated with CMT extracts (250–1,000 μg/mL) for 30 min and stimulated with LPS (0.1 μg/mL) for 18 h. The cell supernatant was then transferred to 96-well plates and homogenized with equal amounts of Griess reagent, and NO production was measured at 540 nm. (B) Effects of CMT extracts on cell viability determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and absorbance measurement at 560 nm (B). Values in bar graph are mean ± standard deviation of at least three independent experiments. *p < 0.005 and **p < 0.001 compared with LPS only.
3.3. Inhibitory effects of black ginseng-enriched CMT extracts on the expression of proinflammatory cytokines in BV2 cells
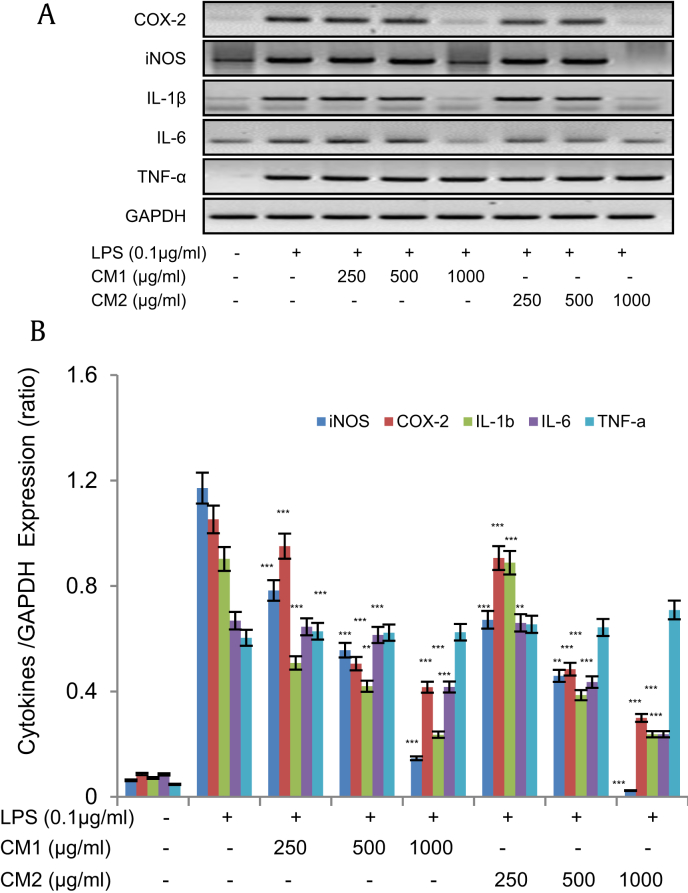
The production of proinflammatory cytokines is a basic biological response toward inflammatory insult in cells. Therefore, we evaluated whether black ginseng-enriched CMT extracts can affect the proinflammatory cytokine expression in BV2 cells. As it is evident from Figs. 3A and 3B, black ginseng-enriched CMT extracts significantly abolished the expressions of iNOS, COX-2, IL-1β, and IL-6 messenger RNA in a dose-dependent manner with the CM-2 extract again showing more potent suppression than the CM-1 extract. However, both the extracts did not affect the expression of TNF-α.
Fig. 3.
Black ginseng-enriched Chong-Myung-Tang (CMT) extracts inhibited messenger RNA (mRNA) expressions of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), interleukin-1β (IL-1β), IL-6 and tumor necrosis factor-α (TNF-α) in BV2 cells. (A and B) BV2 cells were pretreated with black ginseng-enriched CMT extracts (250–1,000 μg/mL) for 30 min and then stimulated with lipopolysaccharide (LPS; 0.1 μg/mL) overnight. Total RNA was extracted by TRIzol RNA extraction reagent and mRNA expression of iNOS, COX-2, IL-1β, IL-6, and TNF-α was determined by reverse transcription-polymerase chain reaction. GAPDH was used as the housekeeping gene. Images are representative of three independent experiments. Values in bar graph are mean ± standard deviation of four independent experiments (B). *p < 0.01, **p < 0.005, and ***p < 0.001 compared with LPS only. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
3.4. Black ginseng-enriched CMT extracts reduce protein expression levels of iNOS and COX-2
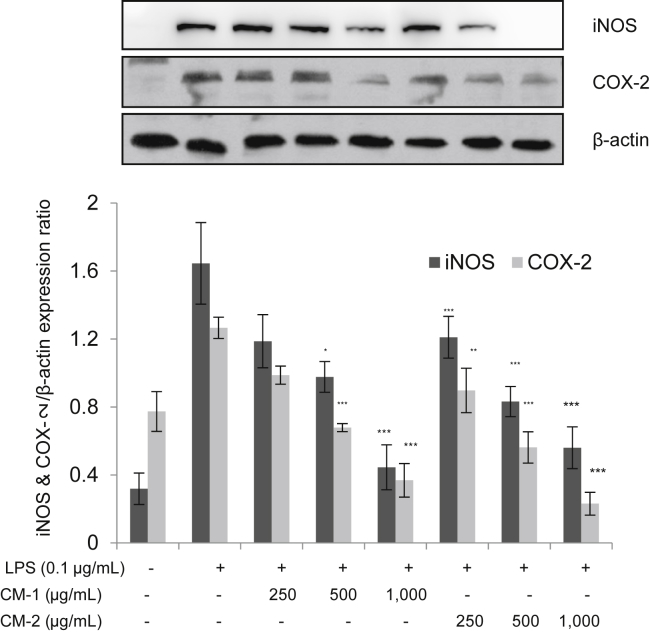
To confirm the translational inhibition of iNOS and COX-2, an immunoblotting study was performed to evaluate the expressions of these two proinflammatory cytokines. As shown in Fig. 4, black ginseng-enriched CMT extracts, especially CM-2, inhibited the protein expression of both iNOS and COX-2 in a concentration-dependent manner, confirming that the extracts are effective at the translational levels of these proinflammatory cytokine proteins.
Fig. 4.
Reduction in the protein expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) by Chong-Myung-Tang (CMT) extracts. Western blot images are representative of three independent experiments. Bar values of * p < 0.01, **p < 0.005, and ***p < 0.001, compared with LPS, were considered statistically significant.
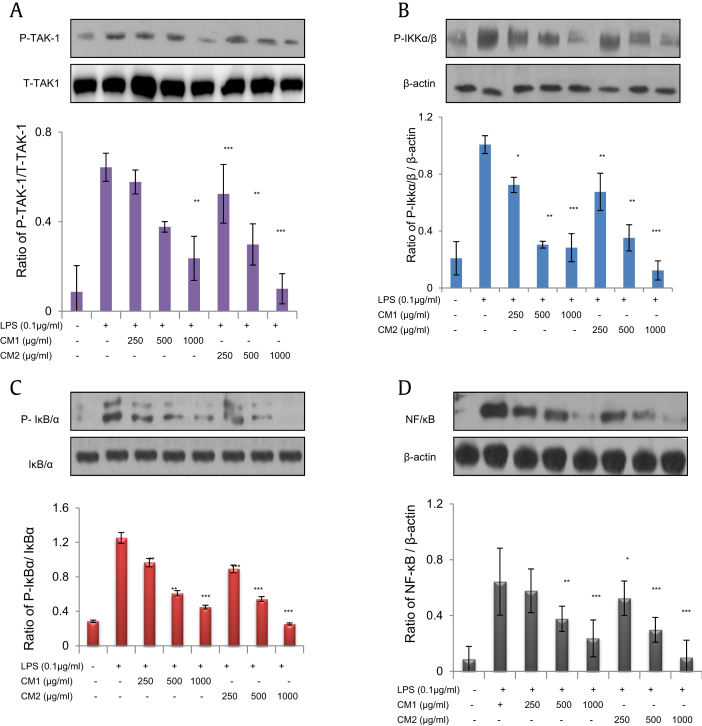
3.5. Diminution of NF-κB pathway by black ginseng-enriched CMT extracts
NF-κB is one of the major transcription factors for inflammatory pathways [23], [24]. This pathway comes into play when LPS binds to its Toll-like receptor 4 (TLR4). This receptor–ligand association activates a series of factors (i.e., transforming growth factor beta-activated kinase 1, IKK α/β, IκB/α, and NF-κB). As shown in Figs. 5A–5D, black ginseng-enriched CMT extracts have suppressed the phosphorylation of every factor in this pathway, with CM-2 again exhibiting increased suppression in phosphorylation than CM-1.
Fig. 5.
Diminution of nuclear factor-κB (NF-κB) pathway by black ginseng-enriched Chong-Myung-Tang (CMT) extracts. BV2 cells were pretreated with CMT extracts and then stimulated with or without lipopolysaccharide (LPS; 0.1 μg/mL) overnight. Nuclear and cytosolic proteins were separated using the NE-PER extraction kit. Data are mean ± standard deviation (n = 3); *p < 0.01, **p < 0.005, ***p < 0.001 compared with LPS only. (A) Phosphorylation of transforming growth factor beta-activated kinase 1 (P-TAK-1) compared with total (T-TAK1). (B) Phosphorylation of IκB kinase (P-IKK) α/β compared with β-actin. (C) Phosphorylation of P-IκBα compared with total IκBα. (D) Phosphorylation of NF-κB compared with β-actin.
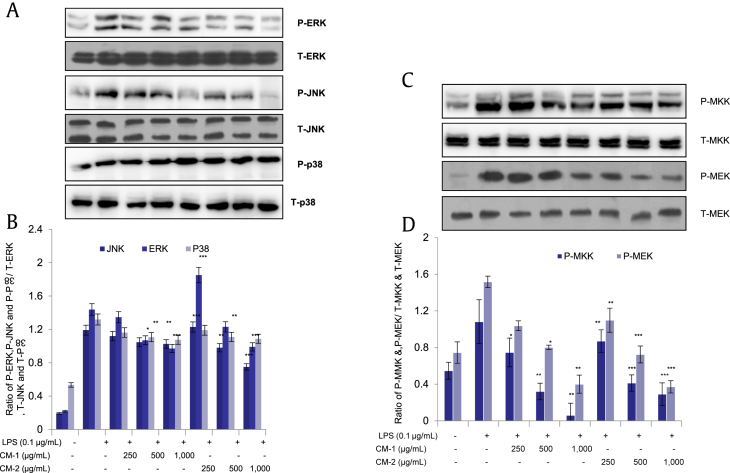
3.6. Black ginseng-enriched CMT extracts depress c-Jun N-terminal kinase/mitogen-activated protein kinase pathway
Mitogen-activated protein kinase (MAPK) pathway is a well-known inflammatory mediation pathway. We pursued to evaluate the effects of black ginseng-enriched CMT extracts on activation of MAPKs (ERK, JNK, and p38). Black ginseng-enriched CMT extracts showed a dose-dependent inhibitory effect on MAPK pathway, inhibiting the phosphorylation of ERK and JNK, but it did not show any effect on p38 phosphorylation as shown in Figs. 6A and 6B. However, the upstream regulators for JNK (i.e., MKK) and ERK (i.e., MEK) were also shown to be suppressed dose dependently (Figs. 6C and 6D). In this case also, CM-2 demonstrated more potent inhibitory phosphorylating effects. These results indicate that CMT extracts have potent memory improvement, memory retention, and inflammatory alleviation properties.
Fig. 6.
Depression of mitogen-activated protein kinase (MAPK) pathway by black ginseng-enriched Chong-Myung-Tang (CMT) extracts. Protein extraction, running, transferring, and analyzation were carried out according to the procedure followed for the nuclear factor-κB (NF-κB) pathway. Images are representative of at least three independent experiments. Data are mean ± standard deviation (n = 3); *p < 0.01, **p < 0.005, ***p < 0.001 compared with lipopolysaccharide only. (A and B) Phosphorylation of extracellular signal-regulated kinase (P-ERK), Jun amino-terminal kinase (P-JNK), and p38 (P-p38) compared with (Total form of extracellular signal regulated kinase) (T-ERK), Total form of c-Jun-n-terminal kinase (T-JNK), and Total form of p38 (T-p38), respectively. (C and D) Phosphorylation of MKK (P-MKK) compared with T-MKK. Phosphorylation of MEK (P-MEK) compared with T-MEK. MEK/MKK, mitogen-activated protein kinase kinase.
4. Discussion
The connection between memory impairment and inflammation is deep rooted. Perhaps, inflammation plays a major role in serious pathologies related to neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, and even human immunodeficiency virus-induced dementia [25], [26]. Hippocampus, especially the CA1 area of the dorsal hippocampus, is involved in the formation and retention of memory. This region also has anatomical connections with various other regions of the forebrain, especially the medial septum through its ascending and descending fibers. Acetylcholine is an important neurotransmitter that is responsible for the memory, cognitive learning, and attention process. Some previous studies have suggested that cholinergic projections from the medial septum to the hippocampal region are necessary for memory retention [27], [28]. Scopolamine is an anticholinergic drug that acts as an antagonist for muscarinic M1 receptors. Acetylcholine-induced memory formation and retention can be reduced by scopolamine treatment, and so scopolamine can be used as an amnestic agent for studying the effects of various drugs or extracts on memory retention. N-Methyl-d-aspartate (NMDA) receptor subtype of glutamate receptor plays a major role in neural physiology, synaptic plasticity, and behavioral learning and memory process [29]. Glutamate is a type of excitatory neurotransmitter that is abundant in the hippocampal region of mammalian brain. The possible connection between memory retention and inflammation has been poorly understood. However, one hypothesis suggests that reduction in NMDA neurotransmitters occurs as a result of systemic inflammation due to which there is increased production of NO and proinflammatory cytokines, especially ILs [29], [30], [31].
Considering the cholinergic and glutaminergic involvement of neurotransmitters in memory retention and inflammation, we hypothesize a mechanism via which scopolamine treatment in vivo and LPS stimulation of BV2 microglial cells in vitro would reduce the levels of acetylcholine and NMDA receptors in the hippocampal region of mice brain, thereby causing impairments in memory retention and activation of inflammatory response. Our results show that CMT treatment has potently reduced the distance through latency and time through latency in mice with scopolamine-induced amnesia through the MWM test (Fig. 1).
Microglial cells are the resident macrophage cells responsible for immune surveillance in the central nervous system. They are particularly important for inflammatory conditions in the brain resulting from any antigen. The role of these cells in serious neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, dementia, etc. is inevitable. When these cells are activated by any foreign insult, they release proinflammatory cytokines that manifest the inflammatory response [32]. Pathologically excessive secretion of these proinflammatory cytokines can pose an irreversible damage to major organs [33]. Therefore, their levels should be maintained at ranges that do not create any serious anomaly in the brain. In our research, we found that black ginseng-enriched CMT extracts not only inhibited the NO synthesis, which is produced due to bacterial toxin challenge (i.e., LPS) but also inhibited the levels of all major proinflammatory cytokines, including iNOS, COX-2, IL-1β, and IL-6, respectively (Fig. 2, Fig. 3).
NO is a free radical gas that is generated when LPS binds to its TLR4, which causes the oxidative conversion of l-arginine to l-citrulline. The production of this gas is the primary response of cell toward foreign infiltration. However, the physiological safety that is posed by NO release is only beneficial to some extent, as its uncontrolled overproduction leads to chronic inflammatory diseases [34]. Therefore, in simple terms, the production of NO should be kept in control using various anti-inflammatory regimens. In our study, we have shown that black ginseng-enriched CMT extracts have dose dependently suppressed the levels of NO, indicating that it possesses strong anti-inflammatory characteristics without any cytotoxicity over the given dosage, as determined by the MTT cell viability assay (Fig. 2).
Proinflammatory cytokines are one of the prime products that are secreted by microglial cells in response to a foreign pathogen. Previous experimental data have shown that TNF-α, IL-1β, IL-6, etc. levels are highly increased in Alzheimer's disease brain [35]. This ultimately leads to neuronal loss and hyperpolarization of neurons. Basically, these proinflammatory cytokines are effective in fighting against the foreign invader, but similar to NO production, if their release is not kept in check, they can lead to serious neuronal inflammation that predisposes the individual to almost noncurable diseases such as dementia, Alzheimer's disease. In our study, CMT extracts have potently inhibited the levels of IL-1β, IL-6, iNOS, and COX-2 in BV2 microglial cells not only at the transcriptional but also at the translational levels that were stimulated by LPS (Fig. 3, Fig. 4). This shows that black ginseng-enriched CMT extract could potentially be useful for treating neuroinflammation.
The inflammatory signaling is basically a combination of several signaling pathways that become activated when a foreign pathogen gains entry into a cell. Among these pathways the most classical one is the NF-κB pathway. This pathway is triggered by TNF-α and other IL cytokines, which results in the activation of myeloid differentiation factor 88 (MyD88)-dependent IKK. The result is the nuclear translocation of NF-κB that causes the release of more proinflammatory cytokines [36], [37], [38]. This pathway is actually the mainstay of any compound or extract that has to be termed as an anti-inflammatory agent. Black ginseng-enriched CMT extracts have suppressed the phosphorylation of these factors involved in this pathway, thereby supporting their efficiency as a strong antineuroinflammatory agent (Fig. 5).
The MAPK is another inflammatory pathway that is most commonly studied for cellular inflammatory signaling. This pathway is always activated when there is some extracellular or intracellular stress to cell. The components of this pathway can modulate the intracellular proteins by addition of phosphate groups to their serine/threonine amino acid terminals [39]. LPS was used as an inflammation mediator in this study, and this induced cytokine stress in the cell that ultimately activated the MAPK pathway. It essentially starts from the level of MAP3K (MAPK kinase kinase and MAP kinase kinase kinase), then mitogen-activated protein kinase kinase (MKK 1–7 and MEK), and finally MAPK (JNK, ERK, and p38). The interaction between these three different sets of MAPK factors serves to create an inflammatory output [40]. Our results show that black ginseng-enriched CMT extracts have dosed dependently inhibited the phosphorylation of JNK and ERK and their upstream factors (i.e., MKK and MEK) in this pathway. However, we did not observe any effect on p38 phosphorylation (Fig. 6). The NF-κB and MAPK pathways are considered as the “Holy Grail” for any plant or nonplant material due to their anti-inflammatory property. Therefore, our results clearly demonstrate that CMT extracts, especially the CM2 extract, have this marvelous property.
4.1. Conclusion
This study is the first and one of its kind to create a connection between the in vivo scopolamine-induced amnesia in mice and inflammatory suppression in vitro in BV2 microglial cells. We have shown that the inflammatory damage, which resulted in memory impairment and proinflammatory cytokine production by scopolamine and LPS, has been potently attenuated by black ginseng-enriched CMT extracts, with the CM-2 extract exhibiting significantly greater effects. Our research for the first time provides the scientific evidence that consumption of black ginseng-enriched CMT extract as a brain tonic improves memory impairment and the results can be taken as a reference for future neurobehavioral studies.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the Regional Technology Development (R&D) Program for MOTIE (Ministry of Trade, industry and Energy), Republic of Korea in 2014.
Contributor Information
Hyun-Kyoung Kim, Email: kimhk4@seowon.ac.kr, kimhk4@empas.com.
Man-Hee Rhee, Email: rheemh@knu.ac.kr.
References
- 1.Oh Y.J., Kim B.K. A study of ChongMyungTang (CMT) and HyangbujaChongMyungTang (HCMT) on dementia—extract & nano powder drug types. J Orien Neuropsychiatry. 2006;17:79–105. [Google Scholar]
- 2.Lee M.R., Yun B.S., Park S.Y., Ly S.Y., Kim S.N., Han B.H., Sung C.K. Anti-amnesic effect of Chong-Myung-Tang on scopolamine-induced memory impairments in mice. J Ethnopharmacol. 2010;132:70–74. doi: 10.1016/j.jep.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 3.Jeon J., Jo C. Editing style of Imwon-Gyeongjeji, Inje-ji and inclusion of the medicinal knowledge of the late period of Joseon — Comparing mainly with Dongui-Bogam. Uisahak. 2012;21:403–448. [Article in Korean] [PubMed] [Google Scholar]
- 4.Gonzalez-Burgos E., Fernandez-Moriano C., Gomez-Serranillos M.P. Potential neuroprotective activity of Ginseng in Parkinson's disease: a review. J Neuroimmune Pharmacol. 2015;10:14–29. doi: 10.1007/s11481-014-9569-6. [DOI] [PubMed] [Google Scholar]
- 5.Nitta H., Matsumoto K., Shimizu M., Ni X.H., Watanabe H. Panax ginseng extract improves the scopolamine-induced disruption of 8-arm radial maze performance in rats. Biol Pharm Bull. 1995;18:1439–1442. doi: 10.1248/bpb.18.1439. [DOI] [PubMed] [Google Scholar]
- 6.Ong W.Y., Farooqui T., Koh H.L., Farooqui A.A., Ling E.A. Protective effects of ginseng on neurological disorders. Front Aging Neurosci. 2015;7:129. doi: 10.3389/fnagi.2015.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryuk J.A., Kim Y.S., Lee H.W., Ko B.S. Identification of Acorus gramineus, A. calamus, and A. tatarinowii using sequence characterized amplified regions (SCAR) primers for monitoring of Acori graminei rhizoma in Korean markets. Int J Clin Exp Med. 2014;7:2488–2496. [PMC free article] [PubMed] [Google Scholar]
- 8.Lee K.Y., Jeon Y.J. Polysaccharide isolated from Poria cocos sclerotium induces NF-kappaB/Rel activation and iNOS expression in murine macrophages. Int Immunopharmacol. 2003;3:1353–1362. doi: 10.1016/S1567-5769(03)00113-9. [DOI] [PubMed] [Google Scholar]
- 9.Park C.H., Choi S.H., Koo J.W., Seo J.H., Kim H.S., Jeong S.J., Suh Y.H. Novel cognitive improving and neuroprotective activities of Polygala tenuifolia Willdenow extract, BT-11. J Neurosci Res. 2002;70:484–492. doi: 10.1002/jnr.10429. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J., Li L., Jiang C., Xing C., Kim S.H., Lu J. Anti-cancer and other bioactivities of Korean Angelica gigas Nakai (AGN) and its major pyranocoumarin compounds. Anticancer Agents Med Chem. 2012;12:1239–1254. doi: 10.2174/187152012803833071. [DOI] [PubMed] [Google Scholar]
- 11.Oh T.W., Park K.H., Jung H.W., Park Y.K. Neuroprotective effect of the hairy root extract of Angelica gigas NAKAI on transient focal cerebral ischemia in rats through the regulation of angiogenesis. BMC Complement Altern Med. 2015;15:101. doi: 10.1186/s12906-015-0589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng W.H., Hsieh M.T., Lee Y.S., Lin Y.C., Liao J. Anxiolytic effect of seed of Ziziphus jujuba in mouse models of anxiety. J Ethnopharmacol. 2000;72:435–441. doi: 10.1016/s0378-8741(00)00255-5. [DOI] [PubMed] [Google Scholar]
- 13.Choi S.B., Wha J.D., Park S. The insulin sensitizing effect of homoisoflavone-enriched fraction in Liriope platyphylla Wang et Tang via PI3-kinase pathway. Life Sci. 2004;75:2653–2664. doi: 10.1016/j.lfs.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 14.Jo E.H., Hong H.D., Ahn N.C., Jung J.W., Yang S.R., Park J.S., Kim S.H., Lee Y.S., Kang K.S. Modulations of the Bcl-2/Bax family were involved in the chemopreventive effects of licorice root (Glycyrrhiza uralensis Fisch) in MCF-7 human breast cancer cell. J Agric Food Chem. 2004;52:1715–1719. doi: 10.1021/jf035012t. [DOI] [PubMed] [Google Scholar]
- 15.Chung W.T., Lee S.H., Kim J.D., Sung N.S., Hwang B., Lee S.Y., Yu C.Y., Lee H.Y. Effect of the extracts from Glycyrrhiza uralensis Fisch on the growth characteristics of human cell lines: anti-tumor and immune activation activities. Cytotechnology. 2001;37:55–64. doi: 10.1023/A:1016111713056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee K.P., Kang S., Park S.J., Kim J.M., Lee J.M., Lee A.Y., Chung H.Y., Choi Y.W., Lee Y.G., Im D.S. Anti-allergic effect of alpha-cubebenoate isolated from Schisandra chinensis using in vivo and in vitro experiments. J Ethnopharmacol. 2015;173:361–369. doi: 10.1016/j.jep.2015.07.049. [DOI] [PubMed] [Google Scholar]
- 17.Panossian A., Wikman G. Pharmacology of Schisandra chinensis Bail.: an overview of Russian research and uses in medicine. J Ethnopharmacol. 2008;118:183–212. doi: 10.1016/j.jep.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Yeh Y.C., Hahm T.S., Sabliov C.M., Lo Y.M. Effects of Chinese wolfberry (Lycium chinense P. Mill.) leaf hydrolysates on the growth of Pediococcus acidilactici. Bioresour Technol. 2008;99:1383–1393. doi: 10.1016/j.biortech.2007.01.058. [DOI] [PubMed] [Google Scholar]
- 19.dela Pena I.J., Hong E., Kim H.J., de la Pena J.B., Woo T.S., Lee Y.S., Cheong J.H. Artemisia capillaris Thunberg produces sedative-hypnotic effects in mice, which are probably mediated through potentiation of the GABAA receptor. Am J Chin Med. 2015;43:667–669. doi: 10.1142/S0192415X1550041X. [DOI] [PubMed] [Google Scholar]
- 20.Mahady G.B., Pendland S.L., Yun G.S., Lu Z.Z., Stoia A. Ginger (Zingiber officinale Roscoe) and the gingerols inhibit the growth of Cag A+ strains of Helicobacter pylori. Anticancer Res. 2003;23:3699–3702. [PMC free article] [PubMed] [Google Scholar]
- 21.Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saba E., Jeon B.R., Jeong D.H., Lee K., Goo Y.K., Kwak D., Kim S., Roh S.S., Kim S.D., Nah S.Y. A novel Korean Red Ginseng compound gintonin inhibited inflammation by MAPK and NF-B pathways and recovered the levels of MIR-34a and MIR-93 in RAW 264.7 cells. Evid Based Complement Alternat Med. 2015;2015:624132. doi: 10.1155/2015/624132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moynagh P.N. The NF-kappaB pathway. J Cell Sci. 2005;118:4589–4592. doi: 10.1242/jcs.02579. [DOI] [PubMed] [Google Scholar]
- 25.Wang D.S., Zurek A.A., Lecker I., Yu J., Abramian A.M., Avramescu S., Davies P.A., Moss S.J., Lu W.Y., Orser B.A. Memory deficits induced by inflammation are regulated by alpha5-subunit-containing GABAA receptors. Cell Rep. 2012;2:488–496. doi: 10.1016/j.celrep.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khakpai F., Nasehi M., Haeri-Rohani A., Eidi A., Zarrindast M.R. Scopolamine induced memory impairment; possible involvement of NMDA receptor mechanisms of dorsal hippocampus and/or septum. Behav Brain Res. 2012;231:1–10. doi: 10.1016/j.bbr.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 28.Portero-Tresserra M., Del Olmo N., Marti-Nicolovius M., Guillazo-Blanch G., Vale-Martinez A. d-Cycloserine prevents relational memory deficits and suppression of long-term potentiation induced by scopolamine in the hippocampus. Eur Neuropsychopharmacol. 2014;24:1798–1807. doi: 10.1016/j.euroneuro.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Stellwagen D., Beattie E.C., Seo J.Y., Malenka R.C. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yirmiya R., Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Wilson C.J., Finch C.E., Cohen H.J. Cytokines and cognition—the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 32.Pickering M., Cumiskey D., O'Connor J.J. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90:663–670. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- 33.Aravalli R.N., Hu S., Rowen T.N., Palmquist J.M., Lokensgard J.R. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol. 2005;175:4189–4193. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- 34.Jang S., Dilger R.N., Johnson R.W. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr. 2010;140:1892–1898. doi: 10.3945/jn.110.123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben Menachem-Zidon O., Goshen I., Kreisel T., Ben Menahem Y., Reinhartz E., Ben Hur T., Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–2262. doi: 10.1038/sj.npp.1301606. [DOI] [PubMed] [Google Scholar]
- 36.Kang G., Kong P.J., Yuh Y.J., Lim S.Y., Yim S.V., Chun W., Kim S.S. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappab bindings in BV2 microglial cells. J Pharmacol Sci. 2004;94:325–328. doi: 10.1254/jphs.94.325. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sethi G., Sung B., Aggarwal B.B. Nuclear factor-kappaB activation: from bench to bedside. Exp Biol Med (Maywood) 2008;233:21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- 39.Hommes D.W., Peppelenbosch M.P., van Deventer S.J. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut. 2003;52:144–151. doi: 10.1136/gut.52.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arthur J.S., Ley S.C. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]