Abstract
A dose-response meta-analysis was conducted to assess the association of vitamin C, D, E with risk of bladder cancer. Pertinent studies were identified in PubMed and Embase. The random-effect model was used. The relative risk (95% confidence interval) of bladder cancer was 0.99 (0.95–1.03) for every 100 IU/day increment in vitamin D from diet plus supplement and 0.95 (0.90–1.00) for every 10 nmol/L increment in circulating vitamin D. The effect for every 10 mg/day increment was 0.96 (0.90–1.02) for vitamin E from diet plus supplement, 0.83 (0.72–0.95) from diet and 0.88 (0.67–1.15) from supplement, and the effect was 0.84 (0.76–0.94) for every 1 mg/dL increment in circulating α-Tocopherol and 1.22 (1.00–1.49) for every 0.1 mg/dL increment in circulating γ-Tocopherol. The observed association for vitamin D and vitamin E was significant among smokers but not among non-smokers. No significant association was found between vitamin C and risk of bladder cancer in the dose-response analysis. Based on the dose-response analysis, the risk of bladder cancer might be inversely associated with vitamin D and E (especially α-Tocopherol), but positively associated with γ-Tocopherol.
Worldwide, 386,300 new cases and 150,200 deaths from bladder cancer were estimated to occur in 2008, and the majority of bladder cancer occurs in males1. The American Cancer Society presents 4 recommendations to reduce cancer risk, including maintain a healthy weight, adopt a physically active lifestyle, consume a healthy diet with an emphasis on plant foods, and limit alcoholic consumption2. Smoking and occupational exposures are the major risk factors of bladder cancer in Western countries, whereas chronic infection with Schistosoma hematobium in developing countries1. Vitamin D receptors have been detected in superficial transitional cell carcinoma of the human bladder3, and vitamin D inhibits proliferation and induces apoptosis in human bladder tumor cells in vitro4. Vitamin C and vitamin E are also supposed to have a protective effect against bladder development through their actions as an antioxidant and free radical scavenger5. Many epidemiology studies have been conducted to assess the association of Vitamin C6,7,8,9,10,11,12,13,14,15,16,17,18,19,20, D7,16,21,22,23,24,25 and E7,8,9,10,12,13,14,15,16,17,18,19,26,27,28,29 with risk of bladder risk, but the results are not consistent. Therefore, we performed a dose-response meta-analysis to quantitatively summarize the evidence from epidemiological studies on the association of Vitamin C, vitamin D and vitamin E with risk of bladder cancer.
Results
Literature search and study characteristics
The flow chart for study inclusion is shown in supplementary Figure 1. For vitamin C from diet plus supplement, 8 studies in 7 articles7,8,10,15,16,17,20 (2 studies in 1 article20 by sex) were included involving 2,021 cases among 194,443 participants. For vitamin C from diet, 14 studies in 12 articles6,8,9,11,12,14,15,17,18,19,20,30 (2 studies in 2 articles20,30 by sex) were included involving 5,765 cases among 292,002 participants. For vitamin C from supplement, 9 studies from 8 articles8,10,13,14,16,17,18,20 (2 studies in 1 article20 by sex) were included involving 3,331 cases among 1,199,984 participants. For circulating vitamin C, only 1 article6 was identified.
For vitamin D from diet plus supplement, 3 articles7,16,25 were included involving 842 cases among 49,156 participants. For circulating vitamin D, 4 articles21,22,23,24 were included involving 1,737 cases among 12,944 participants. No articles were identified for vitamin D from diet only or supplement only.
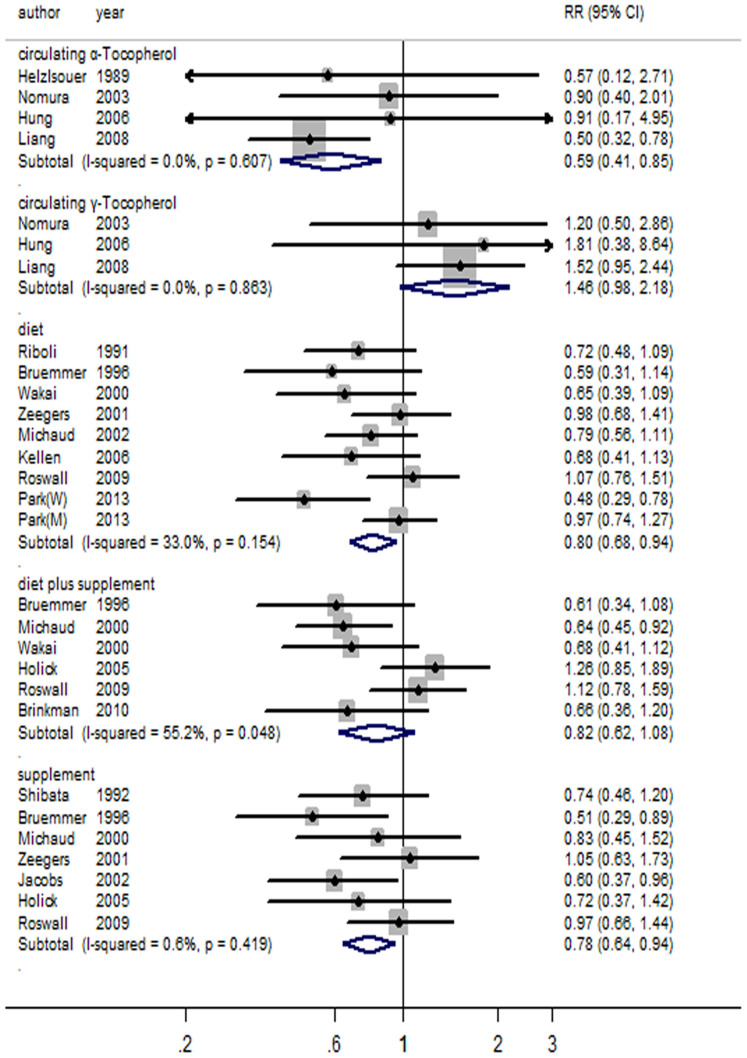
For vitamin E from diet plus supplement, 6 articles7,8,10,15,16,17 were included involving 1,760 cases among 194,182 participants. For vitamin E from diet, 9 studies from 8 articles8,9,12,14,15,17,19,30 were included involving 2,985 cases among 275,265 participants. For vitamin E form supplement, 7 articles8,10,13,14,16,17,18 were included involving 3,070 cases among 1,199,723 participants. For circulating α-Tocopherol, 4 articles26,27,28,29 were included involving 614 cases among 1,256 participants. For circulating γ-Tocopherol, 3 articles26,27,28 were included involving 579 cases among 1,151 participants. The detailed characteristics of the included studies are shown win supplementary table 1.
Quantitative Synthesis
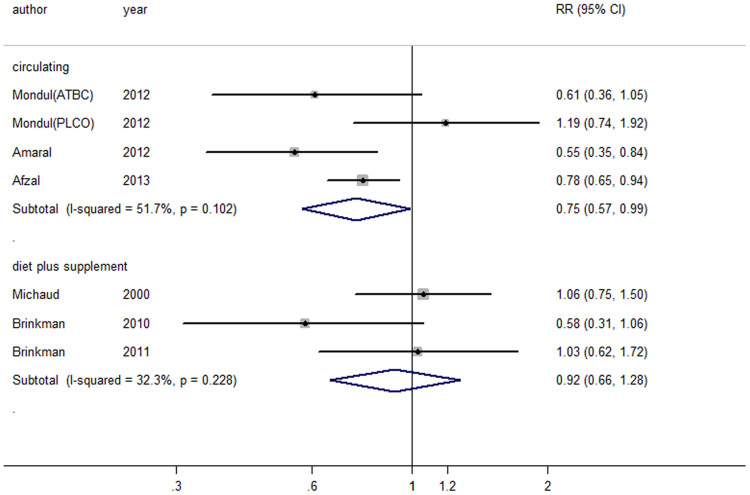
Vitamin D and bladder cancer (Table 1 and Figure 2–3)
Although no association was found between bladder cancer and vitamin D from diet plus supplement, an inverse association was found or indicated with circulating vitamin D overall [0.75 (0.57–0.99), I2 = 51.7%], in cohort studies [0.82 (0.61–1.11), I2 = 46.3%] and case-control studies [0.55 (0.36–0.85), n = 1]. No publication bias was found for vitamin D from diet plus supplement (P = 0.41) and for circulating vitamin D (P = 0.87), and no individual study had an excessive influence in sensitivity analysis, respectively. Because only 3 articles for vitamin D from diet plus supplement and 4 articles for circulating vitamin D were included, subgroup analysis was not conducted further.
Figure 2. Forest plot for vitamin D and risk of bladder cancer.
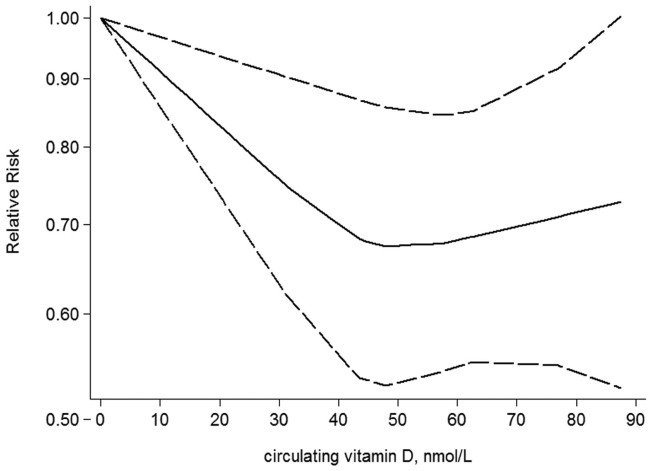
The risk of bladder cancer was 0.99 (0.95–1.03), Pfor nonlinearity = 0.78 for every 100 IU/day increment of vitamin D from diet and supplement, and 0.95 (0.90–1.00), Pfor nonlinearity = 0.10 for every 10 nmol/L increment of circulating vitamin D (Figure 3) (Table 1).
Figure 3. Dose-response analysis for circulating vitamin D and risk of bladder cancer.
Table 1. Dose-response analysis on vitamin C, vitamin D and vitamin E with risk of bladder cancer.
| Na | N (cases) | Dose increment | RR (95% CI) | Pfor nonlinearity | |
|---|---|---|---|---|---|
| Vitamin C (diet plus supplement) | 7 | 1617 | every 100 mg/day | 0.96 (0.90–1.02) | 0.95 |
| Vitamin C (diet) | 8 | 3757 | every 100 mg/day | 0.93 (0.82–1.05) | 0.13 |
| Vitamin C (supplement) | 4 | 843 | every 100 mg/day | 0.94 (0.85–1.03) | 0.51 |
| Vitamin C (circulating) | 1 | 856 | every 10 umol/L | 0.96 (0.91–1.02) | 0.76 |
| Vitamin D (diet plus supplement) | 3 | 840 | every 100 IU/day | 0.99 (0.95–1.03) | 0.78 |
| Vitamin D (circulating) | 3 | 1739 | every 10 nmol/L | 0.95 (0.90–1.00) | 0.10 |
| Vitamin E (diet plus supplement) | 5 | 1401 | every 10 mg/day | 0.96 (0.90–1.02) | 0.73 |
| Vitamin E (diet) | 4 | 1057 | every 10 mg/day | 0.83 (0.72–0.95) | 0.31 |
| Vitamin E (supplement) | 2 | 582 | every 10 mg/day | 0.88 (0.67–1.15) | 0.26 |
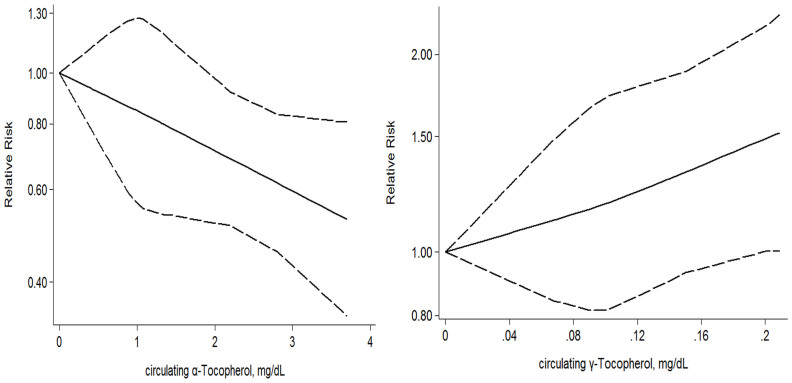
| α-Tocopherol (circulating) | 4 | 614 | every 1 mg/dl | 0.84 (0.76–0.94) | 0.97 |
| γ-Tocopherol (circulating) | 3 | 579 | every 0.1 mg/dL | 1.22 (1.00–1.49) | 0.85 |
a: number of studies.
Discussion
To our knowledge, this is the first dose-response meta-analysis to quantitatively summarize the evidence on vitamin C, vitamin D and vitamin E and risk of bladder cancer. In this meta-analysis, vitamin D and vitamin E (especially α-Tocopherol) were found inversely associated with risk of bladder in a linear dose-response manner, and the association was stronger among smokers. Vitamin C might not be associated with risk of bladder cancer. However, γ-Tocopherol might be positively associated with risk of bladder cancer.
Categories of the three micronutrients (vitamin C, vitamin D, and vitamin E) levels differed between the included studies, which might complicate the interpretation of the pooled results across study populations with different categories (i.e. pooling the results comparing highest vs. lowest categories31,32). In this respect, a dose–response meta-analysis with restricted cubic spline functions provides a solution to this problem from which the dose-response relationship (linear or non-linear) was first assessed and then a summary risk estimate can be derived for a standardized increase of the micronutrients levels33. We also assessed the associations between circulating vitamin C levels, circulating α-Tocopherol levels, circulating γ-Tocopherol, diet and supplement vitamin D intakes and risk of bladder cancer, which are not assessed in the available meta-analysis31,32. In addition, this meta-analysis found that the observed association for vitamin D and vitamin E was significant among smokers but not among non-smokers, which was also not assessed in the available meta-analysis31,32.
Several plausible mechanisms have been proposed by which vitamin E and vitamin C may delay various steps in carcinogenesis through its actions as an antioxidant and free radical scavenger5,34,35, including limiting free radical induced DNA damage and the formation of carcinogens such as N-nitroso compounds, decreasing the concentration of the bladder carcinogen 3-hydroxanthranilic acid, inhibiting the carcinogenic effect of saccharin and cancer cell growth, and stimulating immune function and apoptosis. Vitamin D is not an antioxidant, but its activity in preventing cancer development involves regulation of adherence and signaling, inhibition of proliferation, differentiation, cell cycle stabilization, promotion of apoptosis, and anti-neoangiogenesis36. In this meta-analysis, the observed association was more prominent in smokers. This finding is in line with the hypothesis that smokers may benefit more from vitamin D and vitamin E intake than nonsmokers, because the high antioxidant content and anticancer effects of vitamins may reduce the oxidative damage caused by cigarette smoking37, and current smokers have significantly lower concentrations of serum vitamin E than nonsmokers38. There was a 32% reduction in prostate cancer incidence and a 41% reduction in prostate cancer mortality after 6.1 years of treatment of α-Tocopherol in the Alpha-Tocopherol, Beta Carotene Cancer Prevention Trial including 29,133 male smokers39, although this benefit was attenuated during posttrial follow-up40. However, the benefit on prostate cancer with vitamin E supplementation was not observed in the Physicians' Health Study II Trial41,42 and the Selenium and Vitamin E Cancer Prevention Trial43,44, and both trials involved participants of very low levels of smoking. These findings on prostate cancer could also help understand the finding that vitamin E selectively protected against bladder cancer in smokers in this meta-analysis. In this meta-analysis, γ-Tocopherol might increase the risk of bladder. Although the mechanism has yet to be determined, the residual confounding by smoking cannot be ruled out, because smoking decreases some plasma antioxidants but increases γ-tocopherol levels45,46.
Although no association was found for vitamin C from diet plus supplement and vitamin C from supplement, an inverse association was found for vitamin C from diet. However, the inverse association was mainly caused by the results from case-control studies, and no association was indicated in cohort studies [1.02 (0.85–1.23)], and this was also the case for vitamin E. The distinction between the results from case-control and cohort studies might be attributed to the differences in study design considering prospective cohort studies do not suffer from recall bias and are anticipated to be less likely to have selection bias relative to case-control studies and are also believed to provide better evidence for causality in which vitamins intake precedes bladder incidence compared with case-control studies. For vitamin D, although no association was found with vitamin D from diet plus supplement, an inverse association was found with circulating vitamin D. This discrepancy might arise from the fact that self-reported dietary intake of vitamin D is a less accurate measure of vitamin D status than are circulating concentrations, because vitamin D is produced endogenously in response to sun exposure. Among the 4 articles included for circulating vitamin D, an inverse association was found or indicated in 3 articles21,23,24, but an increase (although not significant) risk of bladder associated with higher circulating vitamin D levels was found in 1 article22. As explained in the article22, this difference may be explained by the inclusion of women and nonsmokers in the current analysis, as a modest inverse association was also found when restricting the analysis to male smokers22. In addition, although the departure from a non-linearity was not significant (Pfor nonlinearity = 0.78) that might be caused by the relatively small number of studies, the shape of the dose-response analysis suggested that the increased risk of bladder cancer was most pronounced at levels of circulating vitamin D less than 50 nmol/L. Importantly, the distribution of circulating vitamin D levels was higher in the PLCO study22, and few participants (about 43%) had circulating vitamin D levels < 50 nmol/L than those in the other studies (73%24 and 74%23). Therefore, the different distributions of circulating vitamin D levels across studies may make comparison between studies difficult and this could also contribute to the between-study heterogeneity. For vitamin E, although the observed association was mainly evident in case-control studies, circulating α-Tocopherol was also found inversely associated with risk of bladder cancer, and no heterogeneity (I2 = 0.00%) was found across studies.
Other factors that might influence the observed association should be considered. The inverse association between circulating vitamin D and risk of bladder cancer was stronger among men with lower vitamin D binding protein (DBP) [low DBP: 0.47 (0.23–1.00), high DBP: 0.83 (0.40–1.75)]23, and among low-FGFR3 expressers24. In addition, season of blood collection, physical activity, α-Tocopherol supplementation and time from blood collection to case diagnosis were also found to influence the association22,24. No association was found between circulating vitamin C and bladder cancer by prognostic subgroups (aggressive and nonaggressive)6. The association between circulating α-Tocopherol and bladder cancer risk differed by vitamin E supplement [users: 0.48 (0.28–0.83), non-users: 1.76 (0.71–4.35)]; however, the effect of total vitamin E on bladder cancer risk was 0.49 (0.22–1.07) among non-users and 0.84 (0.44–1.59) among users of α-Tocopherol and beta carotene supplement in another study12. The limited data precluded a more robust assessment in this meta-analysis.
Other limitations should also be of concern. First, although we extracted the RRs that reflected the greatest degree of control for potential confounders, the extent to which they were adjusted and the possibility that the observed association was due to unmeasured or residual confounding should be considered. Therefore, the observed association for α-Tocopherol, γ-tocopherol, and vitamin D should be confirmed by randomized controlled trials. One randomized controlled trial42 (the results were updated41) was identified, and no significant association was found between vitamin E supplement [bladder cancer death: 0.79 (0.33–1.88), bladder cancer: 1.21 (0.76–1.94)] and vitamin C supplement [bladder cancer death: 0.92 (0.39–2.17), bladder cancer: 0.85 (0.53–1.36)]. Second, misclassification and inaccurate measurement of vitamins intake should be of concern in observational studies. Third, although no publication bias was found, or the results did not change with the trim and fill method, validity of publication bias test should be questioned because of small number of studies included, especially for vitamin D, α-Tocopherol and γ-tocopherol.
In conclusion, vitamin D and vitamin E (especially α-Tocopherol) might be inversely associated with bladder cancer risk, while γ-tocopherol might be positively associated with bladder cancer risk. These results need to be confirmed in randomized controlled trials.
Methods
Literature search and selection
We performed a literature search up to September 2014 using the databases of Pubmed and Embase, using the following search terms (bladder cancer) AND (((((((((Ascorbic Acid) OR vitamin C) OR Vitamin E) OR Tocopherol) OR vitamin D) OR 1,25-dihydroxyvitamin D) OR 25-hydroxyvitamin D) OR 25-hydroxyvitamin D3) OR 25-hydroxyvitamin D2) without restrictions. We also reviewed the reference lists from retrieved articles to search for further relevant studies.
For inclusion, studies must fulfill the following criteria: (1) exposure of interest was Vitamin C, vitamin D or vitamin E; (2) outcome of interest was bladder cancer; (3) relative risk (RR) or odds ratio with 95% confidence interval (CI) was provided (we presented all results with RR for simplicity), or data available to calculate them; (4) conducted in humans; (5) for dose–response analysis, the number of cases and participants or person-years for each category of vitamins must also be provided (or data available to calculate them). The most recent study was included for duplicate publications.
Data extraction
The following data were extracted: first author, study design, cohort name and follow-up duration for cohort studies, publication year, country where the study was conducted, participants' mean age, number of cases and total participants, methods for the measurement of vitamins intake, RR (95% CI) and adjusted covariates. For dose–response analysis, the number of cases and participants (or person-years) and RR (95% CI) for each category of vitamins were also extracted. We assigned the median level of vitamins for each category to each corresponding RR estimate. If the highest category of the studies was open-ended, we assumed that the boundary had the same amplitude as the adjacent category.
Statistical Analysis
We first pooled the study-specific logarithms of RR for the highest versus lowest level of vitamins levels, using a random-effects model. Between studies heterogeneity was evaluated using I2 statistic, and I2 values of 25%, 50%, and 75% represent low, moderate, and high heterogeneity47, respectively. To explore sources of heterogeneity among studies, we conducted meta-regression analysis. A sensitivity analysis was conducted by omitting one study at each turn and recalculating the pooled estimates for the remainder of the studies to evaluate whether the results could have been affected markedly by a single study. Publication bias was assessed by Egger test, and the trim and fill method48 was used to incorporate theoretical missing studies when publication bias was detected.
We then conducted a two stage random effects dose response meta-analysis49. First, a restricted cubic spline model was estimated with a generalised least squares regression with 3 knots at percentiles 25%, 50%, and 75% of the distribution of vitamins levels, then the estimated study-specific 2 regression coefficients (3 knots minus 1) were combined in a multivariate random-effects meta-analysis. A test for a non-linear relation was calculated by making the coefficient of the second spline equal to zero. We used STATA version 12.0 (StataCorp LP, College Station, TX) to analyse the data, and P < 0.05 was considered statistically significant.
Supplementary Material
Supplementary Information
Figure 1. Forest plot for vitamin C and risk of bladder cancer.
Figure 4. Forest plot for vitamin E and risk of bladder cancer.
Figure 5. Dose-response analysis for circulating α-Tocopherol and circulating γ-Tocopherol risk of bladder cancer.
Footnotes
The authors declare no competing financial interests.
Author Contributions F.C., Q.L. and Y.Q. designed the study. F.C. and Q.L. conducted the literature search. F.C., Y.Y. and W.Y. conducted the data extraction and statistical analysis. F.C., Q.L. and F.S. wrote the manuscript. Y.Q. and Q.L. interpreted the results. All authors reviewed the manuscript.
References
- Jemal A. et al. Global cancer statistics. CA Cancer J Clin. 61, 69–90 (2011). [DOI] [PubMed] [Google Scholar]
- Kushi L. H. et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 62, 30–67 (2012). [DOI] [PubMed] [Google Scholar]
- Sahin M. O. et al. 1,25 Dihydroxyvitamin D(3) receptor expression in superficial transitional cell carcinoma of the bladder: a possible prognostic factor? Eur Urol. 47, 52–57 (2005). [DOI] [PubMed] [Google Scholar]
- Konety B. R. et al. Effects of vitamin D (calcitriol) on transitional cell carcinoma of the bladder in vitro and in vivo. J Urol. 165, 253–258 (2001). [DOI] [PubMed] [Google Scholar]
- Leppert J. T. et al. Prevention of bladder cancer: a review. Eur Urol. 49, 226–234 (2006). [DOI] [PubMed] [Google Scholar]
- Ros M. M. et al. Plasma carotenoids and vitamin C concentrations and risk of urothelial cell carcinoma in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 96, 902–910 (2012). [DOI] [PubMed] [Google Scholar]
- Brinkman M. T. et al. Minerals and vitamins and the risk of bladder cancer: results from the New Hampshire Study. Cancer Causes Control. 21, 609–619 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roswall N. et al. Micronutrient intake and risk of urothelial carcinoma in a prospective Danish cohort. Eur Urol. 56, 764–770 (2009). [DOI] [PubMed] [Google Scholar]
- Kellen E., Zeegers M. & Buntinx F. Selenium is inversely associated with bladder cancer risk: a report from the Belgian case-control study on bladder cancer. Int J Urol. 13, 1180–1184 (2006). [DOI] [PubMed] [Google Scholar]
- Holick C. N. et al. Intake of fruits and vegetables, carotenoids, folate, and vitamins A, C, E and risk of bladder cancer among women (United States). Cancer Causes Control. 16, 1135–1145 (2005). [DOI] [PubMed] [Google Scholar]
- Castelao J. E. et al. Carotenoids/vitamin C and smoking-related bladder cancer. Int J Cancer 110, 417–423 (2004). [DOI] [PubMed] [Google Scholar]
- Michaud D. S. et al. Intakes of fruits and vegetables, carotenoids and vitamins A, E, C in relation to the risk of bladder cancer in the ATBC cohort study. Br J Cancer 87, 960–965 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E. J. et al. Vitamin C and vitamin E supplement use and bladder cancer mortality in a large cohort of US men and women. Am J Epidemiol. 156, 1002–1010 (2002). [DOI] [PubMed] [Google Scholar]
- Zeegers M. P., Goldbohm R. A. & van den Brandt P. A. Are retinol, vitamin C, vitamin E, folate and carotenoids intake associated with bladder cancer risk? Results from the Netherlands Cohort Study. Br J Cancer 85, 977–983 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai K. et al. Foods and nutrients in relation to bladder cancer risk: a case-control study in Aichi Prefecture, Central Japan. Nutr Cancer 38, 13–22 (2000). [DOI] [PubMed] [Google Scholar]
- Michaud D. S. et al. Prospective study of dietary supplements, macronutrients, micronutrients, and risk of bladder cancer in US men. Am J Epidemiol. 152, 1145–1153 (2000). [DOI] [PubMed] [Google Scholar]
- Bruemmer B., White E., Vaughan T. L. & Cheney C. L. Nutrient intake in relation to bladder cancer among middle-aged men and women. Am J Epidemiol. 144, 485–495 (1996). [DOI] [PubMed] [Google Scholar]
- Shibata A., Paganini-Hill A., Ross R. K. & Henderson B. E. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: a prospective study. Br J Cancer 66, 673–679 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboli E. et al. Diet and bladder cancer in Spain: a multi-centre case-control study. Int J Cancer 49, 214–219 (1991). [DOI] [PubMed] [Google Scholar]
- Nomura A. M., Kolonel L. N., Hankin J. H. & Yoshizawa C. N. Dietary factors in cancer of the lower urinary tract. Int J Cancer 48, 199–205 (1991). [DOI] [PubMed] [Google Scholar]
- Afzal S., Bojesen S. E. & Nordestgaard B. G. Low plasma 25-hydroxyvitamin D and risk of tobacco-related cancer. Clin Chem. 59, 771–780 (2013). [DOI] [PubMed] [Google Scholar]
- Mondul A. M., Weinstein S. J., Horst R. L., Purdue M. & Albanes D. Serum vitamin D and risk of bladder cancer in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening trial. Cancer Epidemiol Biomarkers Prev. 21, 1222–1225 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondul A. M., Weinstein S. J., Virtamo J. & Albanes D. Influence of vitamin D binding protein on the association between circulating vitamin D and risk of bladder cancer. Br J Cancer 107, 1589–1594 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral A. F. et al. Plasma 25-hydroxyvitamin D(3) and bladder cancer risk according to tumor stage and FGFR3 status: a mechanism-based epidemiological study. J Natl Cancer Inst. 104, 1897–1904 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman M. T. et al. Dietary intake of micronutrients and the risk of developing bladder cancer: results from the Belgian case-control study on bladder cancer risk. Cancer Causes Control 22, 469–478 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D. et al. Plasma vitamins E and A and risk of bladder cancer: a case-control analysis. Cancer Causes Control 19, 981–992 (2008). [DOI] [PubMed] [Google Scholar]
- Hung R. J. et al. Protective effects of plasma carotenoids on the risk of bladder cancer. J Urol. 176, 1192–1197 (2006). [DOI] [PubMed] [Google Scholar]
- Nomura A. M., Lee J., Stemmermann G. N. & Franke A. A. Serum vitamins and the subsequent risk of bladder cancer. J Urol. 170, 1146–1150 (2003). [DOI] [PubMed] [Google Scholar]
- Helzlsouer K. J., Comstock G. W. & Morris J. S. Selenium, lycopene, alpha-tocopherol, beta-carotene, retinol, and subsequent bladder cancer. Cancer Res. 49, 6144–6148 (1989). [PubMed] [Google Scholar]
- Park S. Y. et al. Fruit and vegetable intakes are associated with lower risk of bladder cancer among women in the Multiethnic Cohort Study. J Nutr. 143, 1283–1292 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Y., Wang X. L. & Yu Z. J. Vitamin C and E intake and risk of bladder cancer: a meta-analysis of observational studies. Int J Clin Exp Med. 7, 4154–4164 (2014). [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Huang J. L., Qiu M. X. & Ma Z. W. Impact of serum vitamin D level on risk of bladder cancer: a systemic review and meta-analysis. Tumour Biol. (2014). [DOI] [PubMed] [Google Scholar]
- Desquilbet L. & Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 29, 1037–1057 (2010). [DOI] [PubMed] [Google Scholar]
- Dusinska M. et al. Nutritional supplementation with antioxidants decreases chromosomal damage in humans. Mutagenesis 18, 371–376 (2003). [DOI] [PubMed] [Google Scholar]
- Federico A., Morgillo F., Tuccillo C., Ciardiello F. & Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer 121, 2381–2386 (2007). [DOI] [PubMed] [Google Scholar]
- Garland C. F., Gorham E. D., Mohr S. B. & Garland F. C. Vitamin D for cancer prevention: global perspective. Ann Epidemiol. 19, 468–483 (2009). [DOI] [PubMed] [Google Scholar]
- Valko M., Rhodes C. J., Moncol J., Izakovic M. & Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 160, 1–40 (2006). [DOI] [PubMed] [Google Scholar]
- Galan P. et al. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J Clin Nutr. 59, 1181–1190 (2005). [DOI] [PubMed] [Google Scholar]
- Heinonen O. P. et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 90, 440–446 (1998). [DOI] [PubMed] [Google Scholar]
- Virtamo J. et al. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA. 290, 476–485 (2003). [DOI] [PubMed] [Google Scholar]
- Wang L. et al. Vitamin E and C supplementation and risk of cancer in men: posttrial follow-up in the Physicians' Health Study II randomized trial. Am J Clin Nutr. 100, 915–923 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaziano J. M. et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 301, 52–62 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E. A. et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 306, 1549–1556 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman S. M. et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 301, 39–51 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich M. et al. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am J Clin Nutr. 77, 160–166 (2003). [DOI] [PubMed] [Google Scholar]
- Gabriel H. E. et al. A comparison of carotenoids, retinoids, and tocopherols in the serum and buccal mucosa of chronic cigarette smokers versus nonsmokers. Cancer Epidemiol Biomarkers Prev. 15, 993–999 (2006). [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ. 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S. & Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 56, 455–463 (2000). [DOI] [PubMed] [Google Scholar]
- Orsini N., Li R., Wolk A., Khudyakov P. & Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 175, 66–73 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information