Abstract
Although most studies have reported that high serum lactate dehydrogenase (LDH) levels are associated with poor prognosis in several malignancies, the consistency and magnitude of the impact of LDH are unclear. We conducted the first comprehensive meta-analysis of the prognostic relevance of LDH in solid tumors. Overall survival (OS) was the primary outcome; progression-free survival (PFS) and disease-free survival (DFS) were secondary outcomes. We identified a total of 68 eligible studies that included 31,857 patients. High LDH was associated with a HR for OS of 1.48 (95% CI = 1.43 to 1.53; P < 0.00001; I2 = 93%), an effect observed in all disease subgroups, sites, stages and cutoff of LDH. HRs for PFS and DFS were 1.70 (95% CI = 1.44 to 2.01; P < 0.00001; I2 = 13%) and 1.86(95% CI = 1.15 to 3.01; P = 0.01; I2 = 88%), respectively. Analysis of LDH as a continuous variable showed poorer OS with increasing LDH (HR 2.11; 95% CI = 1.35 to 3.28). Sensitivity analyses showed there was no association between LDH cutoff and reported HR for OS. High LDH is associated with an adverse prognosis in many solid tumors and its additional prognostic and predictive value for clinical decision-making warrants further investigation.
Cancer is the leading cause of death in economically developed countries and the second leading cause of death in developing countries1. In the United States, a total of 1,660,290 new cancer cases and 580,350 cancer deaths were projected to occur in 20132. In Europe, there were an estimated 3.45 million new cases of cancer (excluding non-melanoma skin cancer) and 1.75 million deaths from cancer in 20123. Furthermore, the global burden of cancer continues to increase, largely because of population growth and increased life-expectancy3. Invasion and metastasis are two important hallmarks of cancer and are responsible for the majority of cancer deaths4. Although much effort has been devoted to the diagnosis and therapy of cancers, the overall prognosis is still unsatisfactory. A lack of knowledge of molecular biomarkers in cancer has limited the development of personalized therapies and improvements in survival. Therefore, there is an urgent need for universal, effective, readily available and inexpensive biomarkers in solid tumors to identify patients with a poor prognosis so that novel treatments can be initiated earlier.
The metabolism of cancer cells differs from that of normal cells. This is largely because cancer cells exhibit metabolic alterations that are frequently associated with reprogramming. Unlike normal cells, cancer cells preferentially metabolize glucose by glycolysis to generate sufficient energy for the demands of rapid proliferation, even in the presence of adequate oxygen5.This phenomenon is known as the Warburg effect and is one of the predominant metabolicalterations that occur during malignant transformation. In this process, transcriptional programs regulated by oncogenes stabilize hypoxia-inducible factor 1 alpha (HIF-1α). HIF-1α contributes to the upregulation of most enzymes involved in the glycolytic pathway, including lactate dehydrogenase (LDH).In the final step of aerobic glycolysis, LDH converts pyruvate tolactate, which is coupled with the oxidation of NADH to NAD+. These metabolic changes are reflected by an elevated serum LDH level6(hereinafter LDH).
Elevated LDH has been recognized as a poor prognostic indicator in cancer for many years7,8,9,10. LDH has also been incorporated in prognostic scores for several types of cancer11. However, the consistency and magnitude of the prognostic impact of LDH are unclear12,13,14. The aim of this study was to review published studies and use standard meta-analytic techniques to quantify the prognostic value of LDH in various solid tumors.
Methods
Data sources and searches
This analysis was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines15. PubMed was searched for studies evaluating the LDH and survival in solid tumors from 1978 to 2014. We used various medical subject heading terms, including “l-lactate dehydrogenase”, “prognosis”, “multivariate analysis” and “proportional hazard model”. Title/abstract words included “lactate dehydrogenase”, “LDH”, “prognosis”, “prognose”, “prognostic”, “multivariate analysis”, “proportional hazard model”, “COX proportional hazard model” and “COX models”. The full search strategy is described in the Supplementary Methods (available online).
Study selection
Inclusion criteria for the primary analysis were as follows: 1) studies of people with solid tumors reporting on the prognostic impact of LDH; 2) prospective or retrospective cohort design with a clearly defined source population and justifications for all excluded eligible cases; 3) sample size greater than 200; 4)statistical analysis using multivariate proportional hazards modeling that adjusted for clinical prognostic factors; and 5) reporting of the resultant adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) or a P value for overall survival (OS). For the secondary analyses, studies providing a HR for cancer-specific survival (CSS), progression-free survival (PFS), disease-free survival (DFS), or recurrence-free survival (RFS) were included as well.
Data extraction
OS was the primary outcome of interest. CSS, PFS, and DFS were secondary outcomes. Two authors (J.Z. and H.W.) independently extracted information using predefined data abstraction forms. The following details were extracted: name of first author, year of publication, number of patients included in analysis, disease site, disease stage (non-metastatic, metastatic, mixed [both non-metastatic and metastatic]), study type (prospective or retrospective), cutoff defining high LDH, and HRs and associated 95% confidence intervals for OS, PFS, DFS, or RFS as applicable. HRs were extracted preferentially from multivariate analyses where available. Where several HR values were given in an article, the value adjusted for most confounders was used.
Data synthesis
The meta-analysis was conducted initially for all included studies for each of the endpoints of interest. Subgroup analyses were conducted for predefined parameters such as disease site, disease stage and LDH cutoff, and all data were limited to multivariate analyses. Disease site subgroups were generated if at least three studies on that site were available; the remaining studies were pooled in a subgroup termed “other.” LDH cutoff subgroups were < 250 U/L, 250–300 U/L, 301–400 U/L, and >400 U/L. In three studies, the effect of LDH was reported as a continuous variable; we pooled those studies separately. Univariate meta-regression model analysis was performed to evaluate the relationship between covariates (LDH cutoff) and the HR for OS.
Statistical analyses
The meta-analysis was performed with RevMan 5.2 analysis software (Cochrane Collaboration, Copenhagen, Denmark). Estimates of HRs were weighted and pooled using the generic inverse-variance and random-effect model16. Analyses were conducted for all studies, and differences between the subgroups were assessed using methods described by Deeks et al.17. Publication bias was assessed by visual inspection of the funnel plot. Heterogeneity was assessed using Cochran Q and I2 statistics. Meta-regression analysis was conducted using Stata12.0 software. All statistical tests were two-sided, and statistical significance was defined as P less than 0.05. No correction was made for multiple testing.
Results
Description of studies
Sixty-eight studies were included in the meta-analysis. The selection process for the systematic review is shown in Figure S1 and the characteristics of the included studies are shown in Table 1. A total of 31,857 patients were included and the median trial sample size was 363.
Table 1. Baseline Characteristics of Included Studies.
| No | Fist Author | Year | Sample Size | LDH (High/Low) | Site | Stage | Cutoff (UI/L) | Outcome | Study type | Follow-up Time(mo) | Risk of Bias | Adjusted Variable |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Laurie41 | 2007 | 210 | 109/47 | SCLC | N | ULN | OS | P | NA | L | Gender, ECOG PS, Anemia grade |
| 2 | Motzer7 | 2013 | 1059 | NA | RCC | M | 1.5ULN | PFS/OS | R | NA | L | Ethnic origin, ECOG PS, Time from diagnosis to treatment, Bone metastases, Hb, Ca, Neutrophils, Cytokine |
| 3 | Polee32 | 2003 | 350 | 296/54 | Esophageal cancer | M + N | ULN | OS | R | NA | L | WHO Performance, Extent of disease, Paclitaxel |
| 4 | Han31 | 2003 | 383 | 232/151 | Many kinds of cancer | M + N | ULN | OS | R | NA | H | PS(WHO), White blood count, Hb, Number of sites of metastases |
| 5 | Atzpodien30 | 2003 | 425 | 330/95 | RCC | M | 220 | OS | R | 20 + | L | Neutrophil counts, CRP, Time from diagnosis of tumour to metastatic disease, Number of metastatic sites, Bone metastases |
| 6 | Bidard56 | 2012 | 267 | 121/99 | Breast cancer | M | ULN | PFS | P | 14.9 | L | Triple negative, PS, Number of metastatic sites, CTC, CA15-3, CYFRA 21-1, CEA,ALP, C-INDEX |
| 7 | Culp47 | 2010 | 566 | 107/366 | RCC | M | 618 | OS | P | 20 | L | Albumin, ALP, Hb, Metastasectomy at any time, Liver metastasis, Clinical tumor classification, Fuhrman nuclear grade, No. of metastatic sites at CN, Sarcomatoid dedifferentiation, Clear cell histology, treatment |
| 8 | Pierga28 | 2001 | 1336 | 1039/297 | Breast cance | M | ULN | OS | P | NA | L | Karnofsky index, Disease free interval, No. of metastatic sites, Liver involvement, Adjuvant chemotherapy |
| 9 | Cook37 | 2006 | 635 | 566/69 | HRPC | M | 454 | OS | R | NA | L | Age, PSA, Hb, Albumin, Analgesics, ECOG, NTx, BAP |
| 10 | Wan8 | 2013 | 400 | 367/33 | Nasopharyngeal carcinoma | N | 245 | DFS/OS | R | NA | L | Age, Tumor stage, Node stage |
| 11 | Mekenkamp9 | 2012 | 1010 | 637/365 | Colorectal cancer | M | ULN | OS | R | NA | L | Diameter, Invasion depth, Lymph node status, Number lymph nodes, Number positive lymph nodes, MMR status, KRAS mutation status,BRAF mutation status |
| 12 | Sougioultzis54 | 2011 | 311 | 137/173 | Gastric carcinoma | M | 225 | OS | R | NA | L | Palliative gastrectomy, Chemotherapy, Liver metastasis, Abdominal/Peritoneal metastasis, Histological grade, CA72−4, Weight loss, Blood transfusions |
| 13 | Zhou61 | 2012 | 465 | 424/31 | Nasopharyngeal carcinoma | M + N | 245 | DFS/OS | R | 44.7 | L | N category, T category, Age |
| 14 | Lagerwaard23 | 1999 | 1292 | 1081/211 | Many kinds of cancer | M | ULN | OS | R | NA | L | PS, Number and distribution of brain metastases, Site of primary tumor, Histology, Interval between primary tumor and brain metastases, Systemic tumor activity, Response to steroid treatment, Treatment modality |
| 15 | Aoe35 | 2005 | 309 | 448/157 | Lung cancer | M + N | 450 | OS | R | NA | H | Anemia, TNM stage ECOG PS, Sex, Histologic type, Age |
| 16 | Bacci38 | 2007 | 742 | 464/278 | Ewing’s sarcoma | M + N | ULN | OS | R | NA | L | Pelvis, Other sites, Interval symptoms to diagnosis, Fever |
| 17 | Armstrong55 | 2012 | 404 | 264/140 | RCC | M | ULN | OS | R | NA | H | Treatment, Interaction term, KPS, Prior nephrectomy, No. of metastatic sites, Corrected calcium, Hb |
| 18 | Gripp40 | 2007 | 205 | 130/75 | Many kinds of cancer | M + N | 240 | OS | P | NA | L | WBC, Dyspnea,Morphine, KPS, Brain metastasis, Colorectal, Breast |
| 19 | Giaccone36 | 2005 | 216 | NA | SCLC | N | ULN | OS | P | NA | H | Sex, Chest radiotherapy, PCI, Platelets |
| 20 | Motzer29 | 2002 | 463 | NA | RCC | M + N | 1.5 ULN | OS | R | 46 | L | Karnofsky PS, Hb, Calcium, Time from initial RCC diagnosis to start of interferon-alpha therapy |
| 21 | Bacci26 | 2000 | 357 | 238/121 | Ewing’s sarcoma | N | ULN | OS | R | 126 | L | Sex, Age, Fever, Anemia, Axial location, Radiation therapy only for local control, Type of chemotherapy regimen, Chemotherapy-induced necrosis |
| 22 | Motzer24 | 1999 | 670 | NA | RCC | M + N | 1.5ULN | OS | R | 33 | L | KPS, Hb, Ca, Prior nephrectomy. |
| 23 | Feliu | 2011 | 406 | NA | Many kinds of cancer | M + N | NA | OS | P | NA | H | ECOG PS, TTD, Albumin, Lymphocytes |
| 24 | Scher25 | 1999 | 254 | 164/90 | CRPC | M + N | 230 | OS | R | NA | H | No 50% decline within 12 wk, Hb, Age |
| 25 | Escudier39 | 2007 | 300 | 222/52 | RCC | M | 1.5ULN | OS | P | NA | L | ECOG PS, Number of metastatic sites, Time from nephrectomy to metastatic disease, ALP, Ca |
| 26 | Kawahara19 | 1997 | 284 | 147/137 | SCLC | M + N | ULN | OS | R | NA | H | PS, Stage, ALP, CEA, Sex |
| 27 | Chibaudel52 | 2011 | 535 | 283/252 | Colorectal Cancer | M | ULN | OS | R | NA | L | Age, Sex, PS, No.sites, Liver involvement, Primitive tumor, Time to metastasis, Adjuvant CT, ALP, CEA |
| 28 | Kim48 | 2010 | 257 | NA | NSCLC | M + N | ULN | OS | R | NA | H | ECOG PS, Skin rash |
| 29 | Hashimoto46 | 2009 | 326 | NA | Pancreatic cancer | M + N | 220 | OS | R | NA | H | Recurrence vs. metastasis, KPS, Liver metastasis, Peritoneal metastasis, ALP, CRP |
| 30 | Tanrikulu50 | 2010 | 363 | NA | Pleural mesothelioma | M + N | 500 | OS | R | NA | H | KPS, Pleural fluid glucose level, CRP, Pleural effusion, Pleural thickening on chest CT, Platelet count |
| 31 | Aoe33 | 2004 | 611 | NA | Lung Cancer | M + N | 450 | OS | R | NA | H | Platelet count, TNM stage, ECOG PS, Sex, Histologic type, Age |
| 32 | Giroux10 | 2012 | 245 | 177/45 | NSCLC | M + N | 500 | OS | R | NA | H | Number of treatment lines, PS, Surgery, Maintenance therapy, Time to first progression of tumour |
| 33 | Suh49 | 2010 | 209 | 94/115 | Many kinds of cancer | M + N | 502 | OS | R | NA | H | Anorexia, Resting dyspnea, ECOG, Leukocytosis, Bilirubin, Creatinine |
| 34 | Bacci34 | 2004 | 1421 | 1116/305 | Osteosarcoma | M + N | 240 | OS | R | NA | L | Other sites, Interval symptoms to diagnosis, Treatment |
| 35 | Saito42 | 2007 | 241 | NA | Prostate Cancer | M | 400 | OS | R | 31 | L | Age, performance status, clinical presentation, disease localization, pathologic findings, PSA, PSA/PAP ratio, CEA, ALP,CRP |
| 36 | Hannisdal18 | 1993 | 202 | NA | Bladder Cancer | M + N | 400 | OS | R | NA | H | Erythrocyte sedimentation rate, Hb, ALP, GGT, Creatinine, Albumin |
| 37 | Tonini20 | 1997 | 246 | 162/106 | Neuroblastoma | M + N | 1000 | OS | R | NA | L | MYCN oncogene amplification, Abdominal tumor, Stage, Vanillylmandelic (VMA) urinary excretion, Ferritin, Neuron-specific enolase (NSE) |
| 38 | Li58 | 2012 | 533 | NA | Nasopharyngeal carcinoma | M + N | 240 | OS | R | NA | H | AJCC T category, AJCC N category, Age |
| 39 | Jin65 | 2013 | 689 | 379/310 | Nasopharyngeal carcinoma | M | 245 | OS | R | NA | L | Sex, Age, Metastasis at presentation, Lung metastasis, Post-treatment S-LDH level, Drug number of chemotherapy, Number of involved sites, Liver metastasis, Bone metastasis |
| 40 | Wei75 | 2014 | 601 | NA | Nasopharyngeal carcinoma | N | 225 | DFS/OS | R | 51.5 | L | Age, T classification, N classification |
| 41 | Sau14 | 2013 | 329 | 154/175 | NSCLC | M + N | ULN | OS | R | NA | L | Age, Sex, PS, Histopathology, smoking status, Response after 1-line CT, First-line CT, PFS after 1-line CT, Second-line CT |
| 42 | Wang74 | 2014 | 499 | 75/39 | SCLC | M + N | 240 | OS | R | NA | L | ECOG-PS, Extensive disease, NLR |
| 43 | Yamaguchi76 | 2014 | 206 | NA | Neuroendocrine carcinoma of the digestive system | M + N | ULN | OS | R | NA | H | Age, Sex, PS, Primary site, Liver metastasis, First-line chemotherapy, Prior surgery |
| 44 | Halabi70 | 2014 | 1050 | 565/482 | CRPC | M | ULN | OS | R | NA | L | ECOG PS, Disease site, Opioid analgesic use, Albumin, Hb, PSA,ALP |
| 45 | Templeton73 | 2014 | 357 | NA | CRPC | M | 1.2 ULN | OS | R | NA | H | Age, ECOG PS, Number of comorbidities, Gleason sum score, Lymph node metastatic only, Bone metastasis, Visceral metastasis, Liver metastasis, Hb, Albumin, ALP, PSA, PSA-doubling time, NLR |
| 46 | Du62 | 2013 | 286 | 197/89 | RCC | M + N | 1.5 ULN | DFS/OS | R | NA | L | Fibrinogen, Hb, Ca, T stage, Fuhrman grade, Tumor size |
| 47 | Shinohara67 | 2013 | 473 | 388/34 | RCC | M | 1.5 ULN | OS | R | NA | L | Time from initial diagnosis to metastasis, Hb, Ca, CRP, Liver metastasis, Bone metastasis, Lymph node metastasis |
| 48 | Poprach72 | 2014 | 319 | 285/34 | RCC | M | 1.5 ULN | PFS/OS | R | 15 | L | Time from diagnosis to TKI, Neutrophils, ECOG PS |
| 49 | Powles66 | 2013 | 204 | 52/55 | Seminoma | M + N | 1.5 ULN | PFS | R | NA | H | Age, IPFSG score |
| 50 | van Kessel68 | 2013 | 290 | 152/138 | Colorectal Cancer | M | ULN | OS | R | NA | L | Gender, Age, Number of first line cycles, Metastases, Resection prim. Tumour, Study-arm, Response category |
| 51 | Giessen64 | 2013 | 215 | 270/201 | Colorectal Cancer | M | 250 | OS | R | 55.4 | L | Liver-limited disease, N-stage of primary, KPS, ALP |
| 52 | Weide69 | 2013 | 372 | 263/175 | Melanoma | M | ULN | OS | R | 27 | L | S100B, Cerebral metastases, First systemic therapy |
| 53 | Meckbach71 | 2014 | 215 | 131/63 | Melanoma | M | ULN | OS | R | 46 | L | Brain metastasis |
| 54 | Durnali63 | 2013 | 240 | 101/81 | Osteosarcoma | M + N | ULN | RFS/OS | R | 51 | L | Gender, ALP, Histological subtype, Metastasis at diagnosis, Surgical margins, Tumor necrosis rate, Postoperative chemotherapy, Surgery after recurrence, Chemotherapy after recurrence,, |
| 55 | He13 | 2013 | 239 | 154/82 | Colorectal Cancer | M | ULN | PFS/OS | R | NA | H | Age, Gender, Lines of chemotherapy,CEA,CA19-9, GGT,ALP |
| 56 | Weide60 | 2012 | 855 | 502/228 | Melanoma | M | ULN | OS | R | NA | L | S100B, Time interval between initial diagnosis and stage IV diagnosis, Site of distant metastasis, Number of involved distant sites |
| 57 | Shinohara59 | 2012 | 361 | 299/23 | RCC | M | 1.5ULN | OS | R | 21.5 | L | Time from initial diagnosis to treatment, Hb, Prognostic metastatic group |
| 58 | Jakob57 | 2012 | 677 | 263/97 | Melanoma | M | ULN | OS | R | 12 | L | Age, Gender, M1 Category, Mutation |
| 59 | Bedikian51 | 2011 | 740 | 430/275 | Melanoma | M | ULN | OS | R | NA | L | Age, Chemoresponse, Albumin, M-stage, Location of primary melanoma |
| 60 | Neuman45 | 2008 | 589 | 246/125 | Melanoma | M | 200 | OS | P | NA | L | Sex, Age at diagnosis of stage IV disease, Antecedent stage, DFI, Site of disease, No. of organs involved, No. of metastases |
| 61 | Schmidt43 | 2007 | 363 | 317/46 | Melanoma | M | 2ULN | PFS/OS | R | 50.4 | L | Sex, Site, ECOG PS, Leukocytes, Neutrophils |
| 62 | Bedikian44 | 2008 | 616 | 358/258 | Melanoma | M | 618 | OS | R | NA | L | ECOG PS, Disease stage, Metastatic sites, Visceral metastasis, Albumin, Response to treatment |
| 63 | Viganó27 | 2000 | 227 | 142/85 | Many kinds of cancer | M + N | 618 | OS | R | NA | L | Primary tumor, Liver metastasis, Comorbidity, Weight loss, ECOG PS, Nausea, Clinical estimation of survival, Albumin, Lymphocyte count |
| 64 | Tamura22 | 1998 | 253 | NA | SCLC | M + N | ULN | OS | R | NA | H | Extent of disease, Number of metastatic sites, Albumin, Weight loss |
| 65 | Eton O21 | 1998 | 318 | NA | Melanoma | M | 225 | OS | R | NA | H | Albumin, Soft tissue and/or single visceral organ metastases (especially lung), Sex, Enrollment late in the decade |
| 66 | D’AMICO77 | 2005 | 494 | NA | HRPC | M | 74-2077 | OS | R | 15.6-16.8 | L | Hb, Age, ECOG PS, ALP, Treatment, PSA response duration, PSA |
| 67 | Halabi78 | 2003 | 760 | NA | HRPC | M | 173-437 | OS | R | NA | H | PS, Gleason, ALP, PSA, Visceral disease, Hb |
| 68 | Schellhammer79 | 2013 | 512 | NA | CRPC | M | 84-1662 | OS | P | NA | L | PSA, Hb, ECOG, ALP, Gleason score |
Abbreviations: SCLC: small-cell lung cancer; NSCLC: non-small-cell lung cancer; RCC: renal cell carcinoma; HRPC: hormone-refractory prostate cancer; CRPC: castration refractory prostate cancer; ULN: upper limit of normal; OS: overall survival; PFS: progression-free survival; DFS: disease-free survival; RFS: recurrence-free survival; M: metastatic; N: non-metastatic; M + N: mixed (non-metastatic and metastatic); R: retrospective; P: prospective; L : low risk; High: high risk; NA: not available; PS: performance score; KPS: Karnofsky performance score ; LDH : Lactic dehydrogenas; ALP: alkaline phosphatase; PSA: prostate specific antigen; Hb: hemoglobin; Ca: calcium; PS: Performance Status; ECOG PS: Eastern Cooperative Oncology Group Performance Status ; ALP: alkaline phosphatase; CTC: circulating tumor; NLR: neutrophils / lymphocytes; CRP: C-reaction protein; IPFSG: International Prognostic Factors Study Group; CA19-9: carbohydrate antigen 19-9; CEA: carcinoembryonic antigen; GGT: gamma-glutamyl transpeptidase; DFI: DFI: disease-free interval
Overall survival
Sixty-three studies comprising 29,620 patients reported HRs for OS. All studies analyzed LDH as a dichotomous variable. The studies have clearly shown that upper limit of normal (ULN) remains common for high LDH. The median cutoff for high LDH was 250U/L (range = 200–1000).
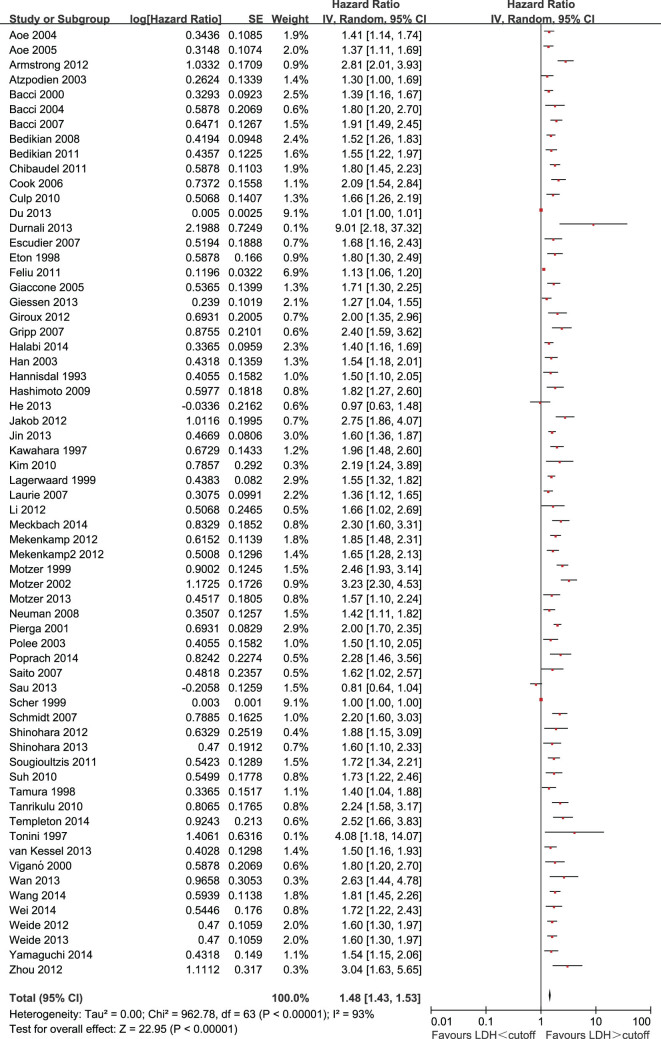
Two of the 63 eligible studies (3.2%) reported a non-statistically significant HR. A forest plot of all studies is presented in Figure 1. Overall, LDH greater than the cutoff was associated with a HR for OS of 1.48 (95% CI = 1.43 to 1.53; P < 0.00001). As the heterogeneity among studies was significant (P < 0.00001; I2 = 93%), a random-effects model was applied. To explore potential sources of heterogeneity, we performed subgroup analysis in the following subgroups: disease site, tumor stage, and LDH subdivided by predefined cutoffs.
Figure 1. Forest plots showing HR for OS for LDH greater than or less than the cutoff.
HRs for each study are represented by the squares, the size of the square represents the weight of the study in the meta-analysis, and the horizontal linecrossing the square represents the 95% confidenceinterval (CI). All statistical tests were two-sided.
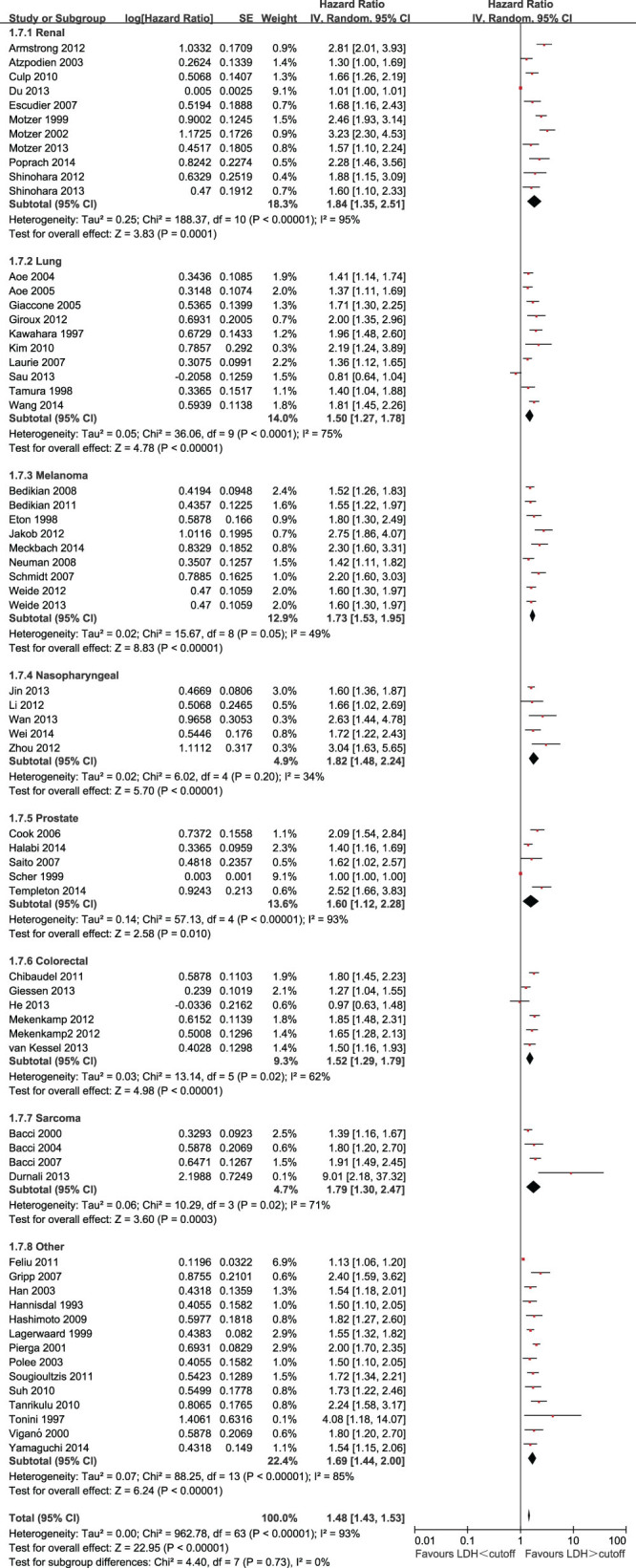
The effect of LDH on OS among disease subgroups is shown in Figure 2. The prognostic effect of LDH was highest in renal cell carcinoma (HR = 1.84, 95% CI = 1.35 to 2.51), followed by nasopharyngeal carcinoma (HR = 1.82, 95% CI = 1.48 to 2.24), sarcoma (HR = 1.79, 95% CI = 1.30 to 2.47), melanoma (HR = 1.76, 95% CI = 1.56 to 1.98), prostate cancer (HR = 1.55, 95% CI = 1.06 to 2.26), colorectal cancer (HR = 1.52, 95% CI = 1.29 to 1.79), and lung cancer (HR = 1.50, 95% CI = 1.27 to 1.78). The HR for the subgroup of other unselected solid tumors was 1.69 (95% CI = 1.44 to 2.00). For the eight disease-site subgroups analyzed, there was statistically significant heterogeneity between disease sites (P < 0.00001), but no significant differences in the prognostic values of LDH between the subgroups (P for subgroup difference = 0.68).
Figure 2. Forest plots showing HRs by disease subgroups.
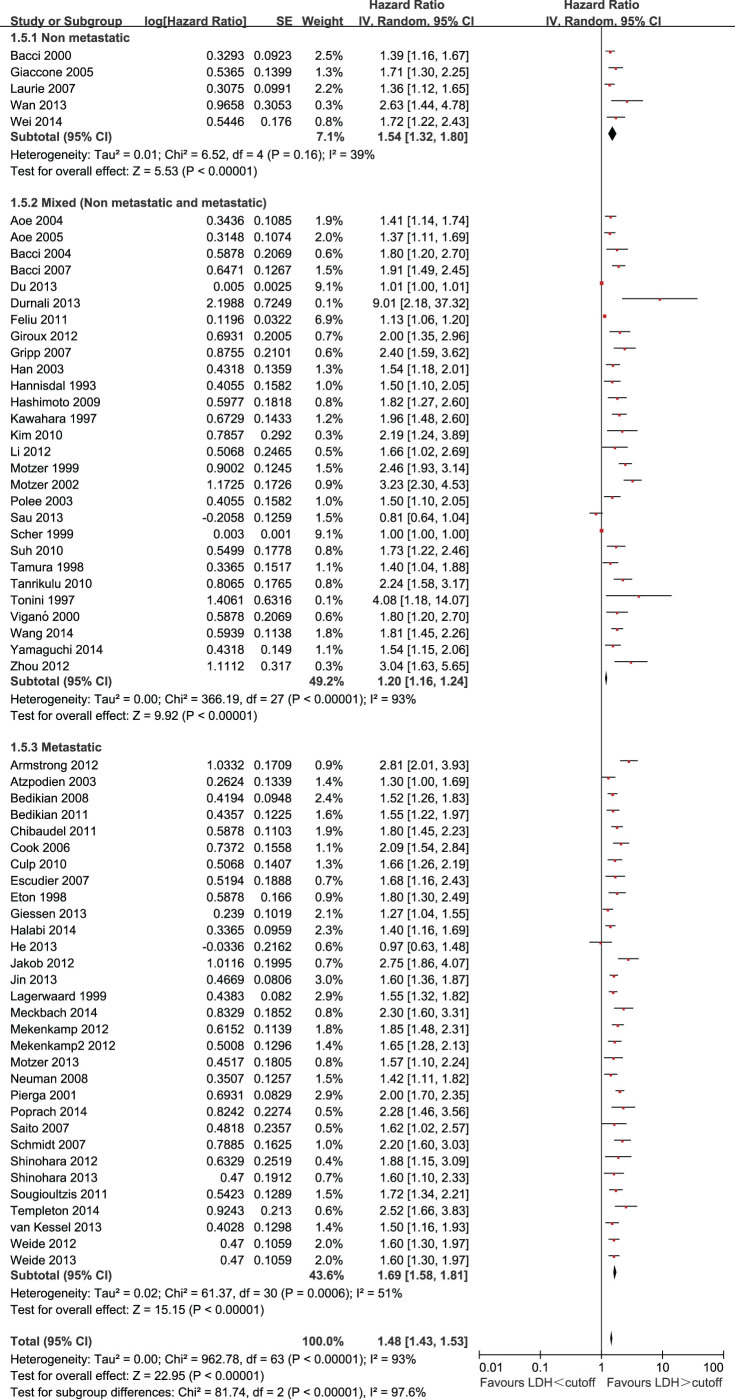
The effect of LDH on OS among different disease stages is shown in Figure 3. The HRs were 1.54 (95% CI = 1.32 to 1.80) for non-metastatic disease, 1.70 (95% CI = 1.59 to 1.82) for metastatic disease, and 1.20 (95% CI = 1.16 to 1.24) for a mixed group consisting of studies that included both metastatic and non-metastatic patients. There was statistically significant heterogeneity between disease stages (P < 0.00001). The prognostic value of LDH also varied significantly between different disease stages (P for subgroup difference < 0.00001).
Figure 3. Forest plots showing HRs by stage subgroups.
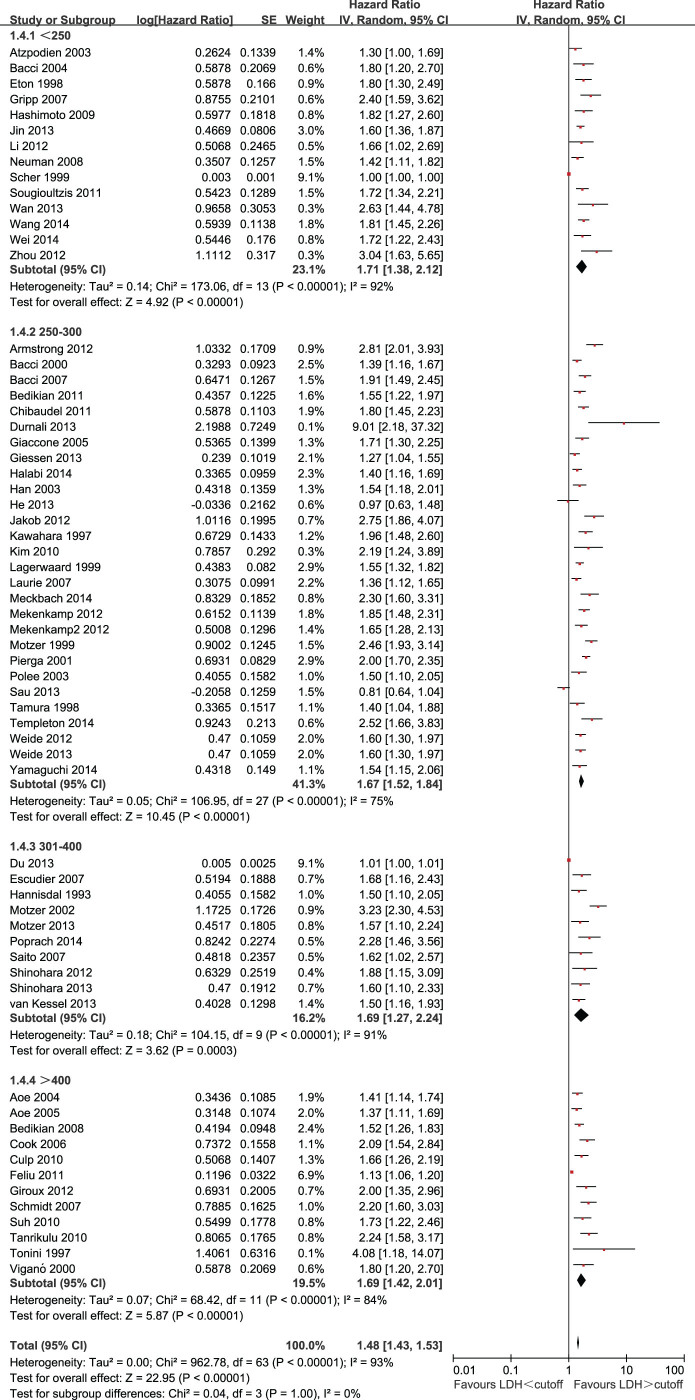
The effect of LDH on OS among different cutoffs for LDH is shown in Figure 4. The HRs were 1.71 (95% CI = 1.38 to 2.12) for LDH cutoff < 250U/L, 1.67(95% CI = 1.52 to 1.84) for LDH cutoff 250 to 300U/L, 1.69 (95% CI = 1.27 to 2.24) for LDH cutoff 301 to 400U/L, and 1.72(95% CI = 1.45 to 2.05)for LDH cutoff > 400 U/L. There was no statistically significant heterogeneity between the different cutoffs for LDH (P for subgroup difference = 0.99).
Figure 4. Forest plots showing HRs by LDH cutoffs.
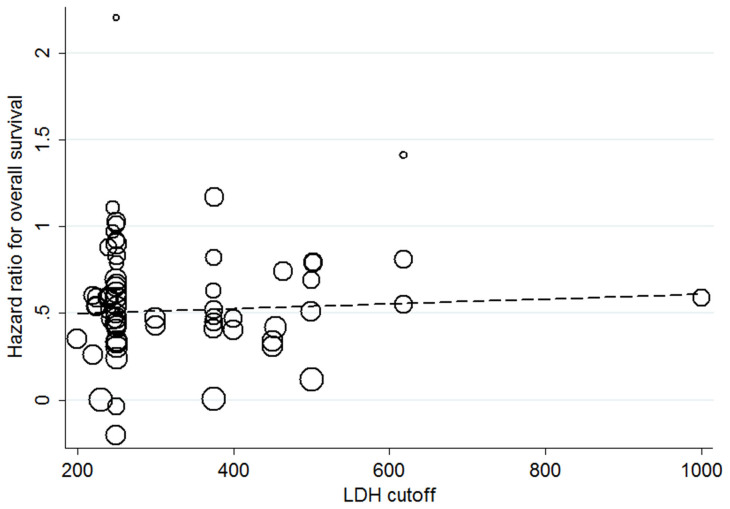
The scatter plot for the univariate meta-regression analysis is shown in Figure 5.A total of 63 studies was included in the meta-regression analysis. Overall, there was no statistically significant association between LDH cutoff and the HR for OS (P = 0.614).
Figure 5. Study-level (i.e., at the individual publication level) association of the cutoff used to define LDH and the HR for overall survival.
Each study is represented by a circle, and the area of the circleis proportional to the number of patients enrolled in each study. The gradient of the dashed line represents the results of the meta-regression (β = 1.000138).
There was evidence of publication bias, with fewer small studies reporting negative results than would be expected (Supplementary Figure S2).
Three studies, comprising 1,766 patients, analyzed LDH as a continuous variable and reported HRs for OS. The pooled summary HR of these studies was 2.11 (95% CI, 1.35–3.28; P = 0.0003; I2 = 84%) per incremental LDH unit (Supplementary Figure S5).
Progression-free survival
Six studies, comprising 2,451 patients, reported HRs for PFS. Overall, LDH greater than the cutoff was associated with a HR for PFS of 1.70 (95% CI = 1.44 to 2.01; P < 0.00001; I2 = 13%). A forest plot is presented as Figure S3.
Disease-free (Recurrence-free) survival
A total of five trials, comprising 1,992 patients, reported HRs for DFS. Overall, LDH greater than the cutoff was associated with a HR for the endpoints of 1.86 (95% CI = 1.15 to 3.01; P = 0.01; I2 = 88%). A forest plot is presented in Figure S4.
Discussion
This is the first comprehensive meta-analysis of the prognostic relevance of LDH in solid tumors and it is based on a large pool of clinical studies (31,857 patients). We found a consistent effect of an elevated LDH on OS (HR = 1.48, 95%CI = 1.43 to 1.53) across all disease subgroups and stages. In addition, there is a trend toward a stronger prognostic value of LDH in metastatic disease compared with non-metastatic disease, which may reflect greater tumor burden. The prognostic impact of LDH on PFS and DFS (or RFS) is also robust. Interestingly, different cutoffs of LDH for different disease sites were reported in the included studies. However, the result of subgroups analysis for LDH cutoff showed that there was no association between LDH cutoff and reported HR for OS. This result was confirmed by meta-regression of LDH cutoff and HR for OS. Moreover, LDH was also related to poor prognosis in solid tumors when analyzed as a continuous variable. Our conclusions are supported by the fact that our selected studies were confined to those that used proportional hazards modeling to adjust for clinical prognostic factors and where the sample size was greater than 200.
There is a good biologic rationale for the use of LDH as a prognostic marker for cancer patients; however, the exact mechanism is not understood. One potential mechanism may be an association between LDH and the well-established phenomenon of oncogenicanaerobic glycolysis, or the Warburg effect5. This metabolic reprogramming is regulated by HIF-1α, as well as myc, through the transcriptional activation of key genes encoding metabolic enzymes; these include LDH, which converts pyruvate to lactate. This process is closely associated with an increased risk of invasion, metastasis, and patient death77.
These analyses have several important implications. First, they show that a high LDH is associated with worse outcome, which suggests that LDH may be a useful biomarker to direct therapeutic selection78,79.This is because LDH is under the translational control of HIF-1α, as well as myc, and thus is regulated by key oncogenic processes, such as the phosphatidylinositol 3-kinase/Akt/TORC1/hypoxia-inducible factor (PI3K/Akt/TORC1/HIF) pathway80,81,82. A recent study has demonstrated that the TORC1 inhibitor, temsirolimus, could provide therapeutic benefit in patients with RCC and high LDH79. Further work to investigate the predictive value of pretreatment LDH in other solid tumors may provide a more general insight into which patients derive benefit from TORC1 inhibition. Second, they show that increased LDH may be interpreted as reflecting high tumor burden or tumor aggressiveness. This suggests that dynamic changes of LDH level may be useful for predicting the prognosis in cancer patients after a primary operation, adjuvant chemotherapy, hormonal therapy, or radiotherapy65. Third, LDH allows the identification of a subgroup of tumors with a worse outcome. It is essential in the treatment of cancer to distinguish between low- and high-risk patients, thereby allowing stratification for standard or intensified treatment protocols. It has been shown that LDH can be used as an effective biomarker to guide the selection of regorafenib in patients with colorectal cancer; patients with high LDH may not be optimal candidates for regorafenib83.To adequately address these issues and dissect the complex relationship between LDH and cancer, future studies should be conducted within tumor- and stage-specific cohorts.
The strengths of this meta-analysis include the large sample size, estimation of HR using multivariate proportional hazards modeling that adjusted for clinical prognostic factors, and analysis of a massive dataset comprising a large pool of clinical studies. LDH is also likely to be a cancer-specific biomarker, given that it is rarely increased in patients without cancer84. Thus, LDH may be a universal prognostic marker in cancer. To improve research in this area, studies with a more specific focus, such as those that address the impact of an individual LDH level on the prognosis of a homogeneous population of cancer patients (i.e., patients with the same cancer stage and subtype), would likely be more informative.
These analyses have limitations. One of the main limitations is the significant heterogeneity between studies, although we used random-effects models when pooling subgroup data. The heterogeneity in these studies could be explained by different patient characteristics or study designs. To facilitate interpretation, we grouped the patients by tumor type and tumor stage. Another limitation is that this is a literature-based analysis. It is compromised by the potential for publication bias, in which there is a tendency for predominantly positive results to have been published, thus inflating our estimate for the association between LDH and outcome. Our strict inclusion criteria (study size greater than 200, the requirement for HRs, and a requirement for a 95% CI or P value) may have introduced selection bias. Most of the included studies were retrospective, which may have introduced reporting bias. Finally, different cutoffs used to assess high LDH level in these studies might also have contributed to the heterogeneity because it is possible that more false-positive cases were obtained with a cutoff of < 300 U/L than with a cutoff of >300 U/L. However, there is no accepted and validated absolute LDH level above which high LDH can be assigned. Instead, we used a cutoff of ULN. This may have introduced substantial heterogeneity, which may not have been fully accounted for by our use of sensitive analyses. The use of ULN is less robust; however, this was the only feasible method with the data available. An internationally accepted and validated LDH cutoff is warranted.
In summary, our data suggest that pretreatment LDH is a simple, cost-effective prognostic factor that can be considered as a criterion to consider patients in different prognostic groups. LDH is also a potential predictive marker to guide individual therapy decisions in solid tumors. Further, adequate, multi-center prospective studies are required to explore the clinical utility of LDH in solid tumors.
Supplementary Material
Supplementary Information
Acknowledgments
None
Footnotes
The authors declare no competing financial interests.
Author Contributions Conception and design: J.Z. and H.W. Collection and checking eligible studies included in the meta-analysis: J.Z. and Y.Y. Acquisition of data: J.Z. and Y.Y. Analysis of data: J.Z., Y.Y., B.L., Q.Y., P.Z. and H.W. Statistical analyses: J.Z., Y.Y. and B.L. Writing of manuscript: J.Z. and H.W. Preparation of tables and figures: B.L., Q.Y. and P.Z. All authors reviewed the manuscript.
References
- Mathers C., Fat D. M. & Boerma J. T. The global burden of disease : 2004 update.1–146 (World Health Organization, Geneva, Switzerland; 2008). [Google Scholar]
- Siegel R., Naishadham D. & Temal A. Cancer statistics, 2013. CA Cancer J Clin 63, 11–30(2013). [DOI] [PubMed] [Google Scholar]
- Ferlay J. et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49, 1374–1403 (2013). [DOI] [PubMed] [Google Scholar]
- Fidler I. J. The pathogenesis of cancer metastasis: the ’seed and soil’ hypothesis revisited. Nat Rev Cancer 3, 453–458 (2003). [DOI] [PubMed] [Google Scholar]
- Hsu P. P. & Sabatini D. M. Cancer cell metabolism: Warburg and beyond. Cell 134, 703–707 (2008). [DOI] [PubMed] [Google Scholar]
- Serganova I. et al. Metabolic imaging: a link between lactate dehydrogenase A, lactate, and tumor phenotype. Clin Cancer Res 17, 6250–6261 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R. J. et al. Prognostic factors for survival in 1059 patients treated with sunitinib for metastatic renal cell carcinoma. Br J Cancer 108, 2470–2477 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X. B. et al. High pretreatment serum lactate dehydrogenase level correlates with disease relapse and predicts an inferior outcome in locally advanced nasopharyngeal carcinoma. Eur J Cancer 49, 2356–2364 (2013). [DOI] [PubMed] [Google Scholar]
- Mekenkamp L. J. et al. Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. Eur J Cancer 48, 501–509 (2012). [DOI] [PubMed] [Google Scholar]
- Giroux L. E. et al. Factors associated with long-term survival of patients with advanced non-small cell lung cancer. Respirology 17, 134–142 (2012). [DOI] [PubMed] [Google Scholar]
- Lorch A. et al. Prognostic factors in patients with metastatic germ cell tumors who experienced treatment failure with cisplatin-based first-line chemotherapy. J Clin Oncol 28, 4906–4911 (2010). [DOI] [PubMed] [Google Scholar]
- Kamiya N. et al. Clinical outcomes by relative docetaxel dose and dose intensity as chemotherapy for Japanese patients with castration-resistant prostate cancer: a retrospective multi-institutional collaborative study. Int J Clin Oncol 19, 157–164 (2014). [DOI] [PubMed] [Google Scholar]
- He W. Z. et al. Gamma-glutamyl transpeptidase level is a novel adverse prognostic indicator in human metastatic colorectal cancer. Colorectal Dis 15, e443–e452 (2013). [DOI] [PubMed] [Google Scholar]
- Sau S., Biswas A., Roy A., Sau S. & Ganguly S. Retrospective analysis of the clinical and demographic variables on the outcomes after second-line treatment in advanced non-small cell lung cancer. Indian J Med Paediatr Oncol 34, 274–279 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6, e1000100 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane Handbook for Systematic Reviews of Interventions. http://www.cochrane.org/training/cochrane-handbook Accessed October. 24, 2014 (2011).
- Higgins J. P. T. & Green S. (ed.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). (The Cochrane Collaboration., 2011).
- Hannisdal E., Fossa S. D. & Host H. Blood tests and prognosis in bladder carcinomas treated with definitive radiotherapy. Radiother Oncol 27, 117–122 (1993). [DOI] [PubMed] [Google Scholar]
- Kawahara M. et al. Prognostic factors and prognostic staging system for small cell lung cancer. Jpn J Clin Oncol 27, 158–165 (1997). [DOI] [PubMed] [Google Scholar]
- Tonini G. P. et al. MYCN oncogene amplification in neuroblastoma is associated with worse prognosis, except in stage 4s: the Italian experience with 295 children. J Clin Oncol 15, 85–93 (1997). [DOI] [PubMed] [Google Scholar]
- Eton O. et al. Prognostic factors for survival of patients treated systemically for disseminated melanoma. J Clin Oncol 16, 1103–1111 (1998). [DOI] [PubMed] [Google Scholar]
- Tamura M. et al. Prognostic factors of small-cell lung cancer in Okayama Lung Cancer Study Group Trials. Acta Med Okayama 52, 105–111 (1998). [DOI] [PubMed] [Google Scholar]
- Lagerwaard F. J. et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys 43, 795–803 (1999). [DOI] [PubMed] [Google Scholar]
- Motzer R. J. et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 17, 2530–2540 (1999). [DOI] [PubMed] [Google Scholar]
- Scher H. I. et al. Post-therapy serum prostate-specific antigen level and survival in patients with androgen-independent prostate cancer. J Natl Cancer Inst 91, 244–251 (1999). [DOI] [PubMed] [Google Scholar]
- Bacci G. et al. Prognostic factors in nonmetastatic Ewing’s sarcoma of bone treated with adjuvant chemotherapy: analysis of 359 patients at the Istituto Ortopedico Rizzoli. J Clin Oncol 18, 4–11 (2000). [DOI] [PubMed] [Google Scholar]
- Vigano A. et al. Clinical survival predictors in patients with advanced cancer. Arch Intern Med 160, 861–868 (2000). [DOI] [PubMed] [Google Scholar]
- Pierga J. Y. et al. Effect of adjuvant chemotherapy on outcome in patients with metastatic breast carcinoma treated with first-line doxorubicin-containing chemotherapy. Cancer 91, 1079–1089 (2001). [DOI] [PubMed] [Google Scholar]
- Motzer R. J., Bacik J., Murphy B. A., Russo P. & Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20, 289–296 (2002). [DOI] [PubMed] [Google Scholar]
- Atzpodien J., Royston P., Wandert T. & Reitz M. Metastatic renal carcinoma comprehensive prognostic system. Br J Cancer 88, 348–353 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C. et al. Comparison of prognostic factors in patients in phase I trials of cytotoxic drugs vs new noncytotoxic agents. Br J Cancer 89, 1166–1171 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polee M. B. et al. Prognostic factors for survival in patients with advanced oesophageal cancer treated with cisplatin-based combination chemotherapy. Br J Cancer 89, 2045–2050 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoe K. et al. Thrombocytosis as a useful prognostic indicator in patients with lung cancer. Respiration 71, 170–173 (2004). [DOI] [PubMed] [Google Scholar]
- Bacci G. et al. Prognostic significance of serum lactate dehydrogenase in osteosarcoma of the extremity: experience at Rizzoli on 1421 patients treated over the last 30 years. Tumori 90, 478–484 (2004). [DOI] [PubMed] [Google Scholar]
- Aoe K. et al. Serum hemoglobin level determined at the first presentation is a poor prognostic indicator in patients with lung cancer. Intern Med 44, 800–804 (2005). [DOI] [PubMed] [Google Scholar]
- Giaccone G. et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971–08971B; Silva Study). J Clin Oncol 23, 6854–6864 (2005). [DOI] [PubMed] [Google Scholar]
- Cook R. J. et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res 12, 3361–3367 (2006). [DOI] [PubMed] [Google Scholar]
- Bacci G. et al. Ewing’s sarcoma family tumours. Differences in clinicopathological characteristics at presentation between localised and metastatic tumours. J Bone Joint Surg Br 89, 1229–1233 (2007). [DOI] [PubMed] [Google Scholar]
- Escudier B. et al. Prognostic factors of metastatic renal cell carcinoma after failure of immunotherapy: new paradigm from a large phase III trial with shark cartilage extract AE 941. J Urol 178, 1901–1905 (2007). [DOI] [PubMed] [Google Scholar]
- Gripp S. et al. Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol 25, 3313–3320 (2007). [DOI] [PubMed] [Google Scholar]
- Laurie S. A. et al. The impact of anemia on outcome of chemoradiation for limited small-cell lung cancer: a combined analysis of studies of the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol 18, 1051–1055 (2007). [DOI] [PubMed] [Google Scholar]
- Saito T., Hara N., Kitamura Y. & Komatsubara S. Prostate-specific antigen/prostatic acid phosphatase ratio is significant prognostic factor in patients with stage IV prostate cancer. Urology 70, 702–705 (2007). [DOI] [PubMed] [Google Scholar]
- Schmidt H. et al. Pretreatment levels of peripheral neutrophils and leukocytes as independent predictors of overall survival in patients with American Joint Committee on Cancer Stage IV Melanoma: results of the EORTC 18951 Biochemotherapy Trial. J Clin Oncol 25, 1562–1569 (2007). [DOI] [PubMed] [Google Scholar]
- Bedikian A. Y. et al. Prognostic factors that determine the long-term survival of patients with unresectable metastatic melanoma. Cancer Invest 26, 624–633 (2008). [DOI] [PubMed] [Google Scholar]
- Neuman H. B. et al. A single-institution validation of the AJCC staging system for stage IV melanoma. Ann Surg Oncol 15, 2034–2041 (2008). [DOI] [PubMed] [Google Scholar]
- Hashimoto K. et al. Do recurrent and metastatic pancreatic cancer patients have the same outcomes with gemcitabine treatment? Oncology-Basel 77, 217–223 (2009). [DOI] [PubMed] [Google Scholar]
- Culp S. H. et al. Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer 116, 3378–3388 (2010). [DOI] [PubMed] [Google Scholar]
- Kim S. T. et al. Prognostic model to predict outcomes in non-small cell lung cancer patients with erlotinib as salvage treatment. Oncology-Basel 79, 78–84 (2010). [DOI] [PubMed] [Google Scholar]
- Suh S. Y. et al. Construction of a new, objective prognostic score for terminally ill cancer patients: a multicenter study. Support Care Cancer 18, 151–157 (2010). [DOI] [PubMed] [Google Scholar]
- Tanrikulu A. C. et al. A clinical, radiographic and laboratory evaluation of prognostic factors in 363 patients with malignant pleural mesothelioma. Respiration 80, 480–487 (2010). [DOI] [PubMed] [Google Scholar]
- Bedikian A. Y. et al. Predictive factors for the development of brain metastasis in advanced unresectable metastatic melanoma. Am J Clin Oncol 34, 603–610 (2011). [DOI] [PubMed] [Google Scholar]
- Chibaudel B. et al. Simplified prognostic model in patients with oxaliplatin-based or irinotecan-based first-line chemotherapy for metastatic colorectal cancer: a GERCOR study. Oncologist 16, 1228–1238 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliu J. et al. Development and validation of a prognostic nomogram for terminally ill cancer patients. J Natl Cancer Inst 103, 1613–1620 (2011). [DOI] [PubMed] [Google Scholar]
- Sougioultzis S. et al. Palliative gastrectomy and other factors affecting overall survival in stage IV gastric adenocarcinoma patients receiving chemotherapy: a retrospective analysis. Eur J Surg Oncol 37, 312–318 (2011). [DOI] [PubMed] [Google Scholar]
- Armstrong A. J., George D. J. & Halabi S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol 30, 3402–3407 (2012). [DOI] [PubMed] [Google Scholar]
- Bidard F. C. et al. Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: a prospective observational study. Breast Cancer Res 14, R29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob J. A. et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 118, 4014–4023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. et al. Increased pretreatment levels of serum LDH and ALP as poor prognostic factors for nasopharyngeal carcinoma. Chin J Cancer 31, 197–206 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N. et al. A new prognostic classification for overall survival in Asian patients with previously untreated metastatic renal cell carcinoma. Cancer Sci 103, 1695–1700 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide B. et al. Serum markers lactate dehydrogenase and S100B predict independently disease outcome in melanoma patients with distant metastasis. Br J Cancer 107, 422–428 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G. Q. et al. Baseline serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol Phys 82, e359–e365 (2012). [DOI] [PubMed] [Google Scholar]
- Du J. et al. High preoperative plasma fibrinogen is an independent predictor of distant metastasis and poor prognosis in renal cell carcinoma. Int J Clin Oncol 18, 517–523 (2013). [DOI] [PubMed] [Google Scholar]
- Durnali A. et al. Prognostic factors for teenage and adult patients with high-grade osteosarcoma: an analysis of 240 patients. Med Oncol 30, 624 (2013). [DOI] [PubMed] [Google Scholar]
- Giessen C. et al. Evaluation of prognostic factors in liver-limited metastatic colorectal cancer: a preplanned analysis of the FIRE-1 trial. Br J Cancer 109, 1428–1436 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. et al. Serum lactic dehydrogenase strongly predicts survival in metastatic nasopharyngeal carcinoma treated with palliative chemotherapy. Eur J Cancer 49, 1619–1626 (2013). [DOI] [PubMed] [Google Scholar]
- Powles T., Bascoul-Mollevi C., Kramar A., Lorch A. & Beyer J. Prognostic impact of LDH levels in patients with relapsed/refractory seminoma. J Cancer Res Clin Oncol 139, 1311–1316 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N. et al. Is Memorial Sloan-Kettering Cancer Center risk classification appropriate for Japanese patients with metastatic renal cell carcinoma in the cytokine era? Urol Oncol 31, 1276–1282 (2013). [DOI] [PubMed] [Google Scholar]
- van Kessel C. S. et al. Radiological heterogeneity in response to chemotherapy is associated with poor survival in patients with colorectal liver metastases. Eur J Cancer 49, 2486–2493 (2013). [DOI] [PubMed] [Google Scholar]
- Weide B. et al. Serum S100B, lactate dehydrogenase and brain metastasis are prognostic factors in patients with distant melanoma metastasis and systemic therapy. PLoS One 8, e81624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabi S. et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol 32, 671–677 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckbach D. et al. BRAF-V600 mutations have no prognostic impact in stage IV melanoma patients treated with monochemotherapy. PLoS One 9, e89218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poprach A. et al. Clinical and laboratory prognostic factors in patients with metastatic renal cell carcinoma treated with sunitinib and sorafenib after progression on cytokines. Urol Oncol 32, 488–495 (2014). [DOI] [PubMed] [Google Scholar]
- Templeton A. J. et al. Simple prognostic score for metastatic castration-resistant prostate cancer with incorporation of neutrophil-to-lymphocyte ratio. Cancer 120, 3346–3352 (2014). [DOI] [PubMed] [Google Scholar]
- Wang X., Jiang R. & Li K. Prognostic significance of pretreatment laboratory parameters in combined small-cell lung cancer. Cell Biochem Biophys 69, 633–640 (2014). [DOI] [PubMed] [Google Scholar]
- Wei Z., Zeng X., Xu J., Duan X. & Xie Y. Prognostic value of pretreatment serum levels of lactate dehydrogenase in nonmetastatic nasopharyngeal carcinoma: single-site analysis of 601 patients in a highly endemic area. Onco Targets Ther 7, 739–749 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T. et al. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci 105,1176–1181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabi S. et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol 21,1232–1237 (2003). [DOI] [PubMed] [Google Scholar]
- Schellhammer P.F. et al. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology 81,1297–1302 (2013). [DOI] [PubMed] [Google Scholar]
- D'Amico A.V. Chen M.H. Cox M.C. Dahut W. & Figg W.D. Prostate-specific antigen response duration and risk of death for patients with hormone-refractory metastatic prostate cancer. Urology 66,571–576 (2005). [DOI] [PubMed] [Google Scholar]
- Vaupel P. & Mayer A. Hypoxia in tumors: pathogenesis-related classification, characterization of hypoxia subtypes, and associated biological and clinical implications. Adv Exp Med Biol 812, 19–24 (2014). [DOI] [PubMed] [Google Scholar]
- Scartozzi M. et al. Pre-treatment lactate dehydrogenase levels as predictor of efficacy of first-line bevacizumab-based therapy in metastatic colorectal cancer patients. Br J Cancer 106, 799–804 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong A. J., George D. J. & Halabi S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol 30, 3402–3407 (2012). [DOI] [PubMed] [Google Scholar]
- Kim J. W. & Dang C. V. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci 30, 142–150 (2005). [DOI] [PubMed] [Google Scholar]
- Kim J. W. & Dang C. V. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res 66, 8927–8930 (2006). [DOI] [PubMed] [Google Scholar]
- Majumder P. K. et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med 10, 594–601 (2004). [DOI] [PubMed] [Google Scholar]
- Del Prete M. et al. LDH serum levels as a predictive factor for global outcome in pretreated colorectal cancer patients receiving regorafenib: Implications for clinical management. J Clin Oncol 32, 2318 (2014).24934786 [Google Scholar]
- Harrison D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information