Abstract
Background
Panax vietnamensis Ha et Grushv. or Vietnamese ginseng (VG) is a recently discovered ginseng species. Studies on its chemical constituents have shown that VG is remarkably rich in ginseng saponins, particularly ocotillol saponins. However, the psychopharmacological effects of VG have not been characterized. Thus, in the present study we screened the psychopharmacological activities of VG in mice.
Methods
VG extract (VGE) was orally administered to mice at various dosages to evaluate its psychomotor (open-field and rota-rod tests), sedative–hypnotic (pentobarbital-induced sleeping test), antistress (cold swimming test), anxiolytic (elevated plus-maze test), and cognitive (Y-maze and passive-avoidance tests) effects.
Results
VGE treatment increased the spontaneous locomotor activity, enhanced the endurance to stress, reduced the anxiety-like behavior, and ameliorated the scopolamine-induced memory impairments in mice. In addition, VGE treatment did not alter the motor balance and coordination of mice and did not potentiate pentobarbital-induced sleep, indicating that VGE has no sedative-hypnotic effects. The effects of VGE were comparable to those of the Korean Red Ginseng extract.
Conclusion
VG, like other ginseng products, has significant and potentially useful psychopharmacological effects. This includes, but is not limited to, psychomotor stimulation, anxiolytic, antistress, and memory enhancing effects.
Keywords: antifatigue, memory, Panax vietnamensis, psychopharmacological activity, Vietnamese ginseng
1. Introduction
Ginseng (Panax genus of the Araliaceae family) has long been used as a traditional remedy for various health conditions [1]. It is believed to have numerous physical and psychological benefits including energy/endurance boost, mental stimulation, mood improvement, cognitive enhancement, and others [2], [3], [4], [5], [6]. Thus, it is regarded as one of the most important and valuable medicinal herbs in Asian countries such as Korea, China, and Japan [7], [8], [9], [10]. To date, there are 11 identified Panax species; of these, Panax ginseng (Korean ginseng), Panax quinquefolius (American ginseng), and Panax notoginseng (Chinese ginseng) are the most commonly used and widely studied [1], [11].
Vietnamese ginseng (VG, Panax vietnamensis Ha et Grushv.) is a recently discovered ginseng species found in the highlands of Central Vietnam. It is the southernmost growing ginseng known [7], [12]. Similar to other ginseng species, VG has been used in traditional medicine and was reported to have various beneficial effects including, but not limited to, anti-hepatotoxic, antioxidative, and antitumor effects [7], [12], [13]. Interestingly, studies have shown that VG contains higher amounts of ginseng saponins than other Panax species, such as protopanaxadiol-, protopanaxatriol-, and ocotillol- and dammarane-type saponins [10], [14], [15]. These compounds and other active components are thought to underlie the beneficial health effects of ginsengs [16], [17]. Hence, VG is presumed to be one of the most valuable Panax species in the world [14], [18], [19], [20].
Despite this apparent medicinal value, the psychopharmacological effects of VG have not been extensively characterized. Thus, the goal of the present study was to screen the psychopharmacological activities of VG through established animal models. Mice were given VG extract and then were subjected to a battery of tests that evaluate the psychomotor, sedative-hypnotic, antistress, anxiolytic, and cognitive effects of a substance. For comparison, parallel experiments were performed in animals given Panax ginseng or Korean Red Ginseng—the most commonly used ginseng in the world.
2. Materials and methods
2.1. Animals
Male ICR mice (20–25 g), obtained from Hanlim Laboratory Animals Co. (Hwasung, South Korea), were the subjects of this study. Animals were maintained in a standard light-dark cycle, in a temperature- and humidity-controlled animal room (at 22 ± 2°C and 55 ± 5%, respectively), with free access to food and water. To ensure adaptation to the new environment, mice were acclimated to their home cages for at least 7 d prior to behavioral evaluation. The experimental groups, consisting of 8–10 animals per drug, dose, and treatment frequency [acute (1 d) or repeated (7 d)], were chosen by randomized sampling. Animals were fasted the night before the experiments. Animal treatment and maintenance were carried out in accordance with the Principles of Laboratory Animal Care (NIH Publication No. 85-23 revised 1985) and the Animal Care and Use Guidelines of Sahmyook University, Seoul, South Korea.
2.2. Test materials
Vietnamese ginseng was collected from Mount Ngoc Linh, Quang Nam province, Vietnam and identified by one of the authors (M.D.N.). A voucher specimen was deposited in the Herbarium of the College of Pharmacy, Seoul National University, Seoul, Korea (SNUP-2012-A-01). The dried roots and rhizomes of VG (150 g) were refluxed with methanol to yield the total VG extract (VGE; 64 g). HPLC analysis of this extract was previously conducted and reported [10]. VGE was orally given to mice at dosages of 10 mg/kg, 20 mg/kg, 50 mg/kg, or 100 mg/kg. Selection of these dosages was based on the results of our preliminary studies. Korean Red Ginseng extract (KRGE) used was high-value commercial concentrated pure extract prepared from 6-yr-old premium Korean Red Ginseng roots (see http://www.kgcus.com/Concentrated-Extract-50g.html). It was purchased from the Korea Ginseng Corporation (Seoul, South Korea), and given orally to mice at a dose of 100 mg/kg. Scopolamine hydrochloride was obtained from Sigma–Aldrich Co. (St Louis, MO, USA). Pentobarbital sodium was obtained from Hanlim Pharm. Co., Ltd (Seoul, South Korea). Test materials (VGE and KRGE) were dissolved in sterile distilled water and freshly prepared every day. Scopolamine (1.5 mg/kg) and pentobarbital (42 mg/kg) were diluted in physiological saline and were administered intraperitoneally (i.p.). The control group received the vehicle (distilled water) only.
2.3. Behavioral tests
All behavioral experiments were conducted 60 min after the 1st d (acute) and 7th d (repeated) of treatment. Experiments were performed as described in our previous studies [8], [12], [21], [22], [23], but with minor modifications.
2.3.1. Open-field test
The open-field apparatus was a square, Plexiglas, arena measuring 42 cm × 42 cm × 42 cm. To start a session, mice were gently placed in the center of the open-field and were allowed to freely explore the whole arena. VGE, KRGE, or the vehicle was given to mice 60 min before the start of each session. Mice were allowed to habituate to the open-field arena for 2 min to eliminate the bias of novelty. Then, an automated system (Ethovision, Noldus, The Netherlands) recorded the distance moved (cm) and movement duration (s) of each animal for 10 min.
2.3.2. Rota-rod test
The rotating rod or rota-rod apparatus used in this study was purchased from Ugo Basile Corporation (Model 7650; Varese, Italy). It was set at a fixed speed of 36 rotations/min. One day before the actual test, mice were habituated and trained to run on the rotating rod for 3 min. Mice with poor performance were excluded. On the actual day of the experiment, mice were given VGE, KRGE, or vehicle and placed on the rotating rod. Each session lasts for 10 min. Latency time before the first fall (s) and the number of falls (falling frequency) were recorded.
2.3.3. Pentobarbital-induced sleeping test
Experiments were performed in a standard mouse cage, measuring 50 cm × 30 cm × 50 cm, with aspen beddings. Mice were given VGE, KRGE, or vehicle before the start of the test. To start a test and to induce sleep, pentobarbital sodium (42 mg/kg, i.p.) was injected. Immediately after pentobarbital injection, mice were individually placed in cages. The disappearance (onset of sleep) and reappearance (duration of sleep) of the righting reflex was monitored and recorded, for a maximum of 2 h. Animals that did not sleep within 15 min after pentobarbital administration were excluded from the experiment.
2.4. Cold swimming test
The mice were placed in an open tank (50 cm height × 80 cm diameter) filled with cold water (6 ± 2°C) and were allowed to swim for 10 min. Water temperature was monitored regularly throughout the experiment. Mice were administered with VGE, KRGE, or vehicle before placing them into the water. If an animal showed drowning behavior (ceased swimming or barely floating) it was removed from the water and the swimming latency time was recorded. After each session, animals were dried with towels, placed under a warmer, and then returned to their home cages.
2.5. Elevated plus-maze test
To assess anxiety-like behaviors, mice were placed in a plus-maze apparatus made of plastic. The maze has four arms, comprising of two open arms (30 cm × 6 cm) and two closed arms (30 cm × 6 cm), enclosed by 20 cm high walls. Each arm converges to a delimited central area measuring 6 cm × 6 cm. The entire maze was elevated to a height of 50 cm above the floor. Experimental procedures were performed in a stable environment with distraction kept at a minimum. Mice were orally pretreated with VGE, KRGE, or vehicle before placement on the elevated plus-maze (EPM). A session lasts for a maximum of 5 min. The percentage of open arm entries (100 × open/total entries) was calculated for each animal.
2.6. Y-maze test
The Y-maze apparatus consitsts of three identical arms (labeled as A, B, or C), measuring 5 cm × 35 cm × 10 cm, that are proportionally connected to an equilateral triangular center. Mice were placed in one of these arms (arm A) and allowed to freely explore in all three arms. The sequence of arm entries was recorded using the Ethovision System. The alternation behavior (actual alternations) was defined as the consecutive entry into three arms, that is, the combination of three different arms (i.e., ABC, BCA, CAB, BAC), with stepwise combinations in the sequence. The maximum number of alternations was considered as the total number of arms entered minus 2, and the percentage of spontaneous alternation behavior was calculated as:
| (actual alternations/maximum alternations) × 100. | (1) |
To induce memory impairment, scopolamine (1.5 mg/kg, i.p.) was injected 30 min before the test. Test subjects (VGE, KRGE, or vehicle) were administered 60 min before the test. The effects of these drugs on spontaneous alternation behavior of mice were measured for 8 min. The number of arm entries was also recorded and used as an indicator of locomotor activity.
2.7. Passive avoidance test
The passive avoidance apparatus was composed of two identical compartments (light and dark) with grid floor separated by a guillotine door (Gemini Avoidance System; San Diego Instruments, San Diego, CA, USA). A test consisted of two separate trials, an acquisition trial and a retention trial. For the acquisition trial, a mouse was placed in the light compartment and allowed to explore for 10 s. Then, the guillotine door opened allowing access to the dark compartment. When the mouse entered the dark compartment, the door automatically closes and an electrical foot shock (0.5 mA, 3 s) was delivered through the grid floor. The mouse was immediately returned to its home cage. Twenty-four hours later, the retention trial was conducted. The mouse was again placed in the light compartment and the latency to enter into the dark compartment was recorded, for a maximum of 5 min/300 s. To induce memory impairment, scopolamine (1.5 mg/kg, i.p.) was injected to mice 30 min before the acquisition trial. VGE, KRGE, or vehicle was administered 60 min before the acquisition trial.
2.8. Statistical analysis
All data are expressed as mean ± standard deviation. Data were analyzed using one-tailed unpaired t test to compare each group versus the control group. All statistical analyses were conducted using GraphPad Prism version 4.0 software (GraphPad Software, Inc., La Jolla, CA, USA). The accepted level of significance was set at p < 0.05.
3. Results and discussion
3.1. Psychomotor effects of VG on the open-field and rota-rod tests
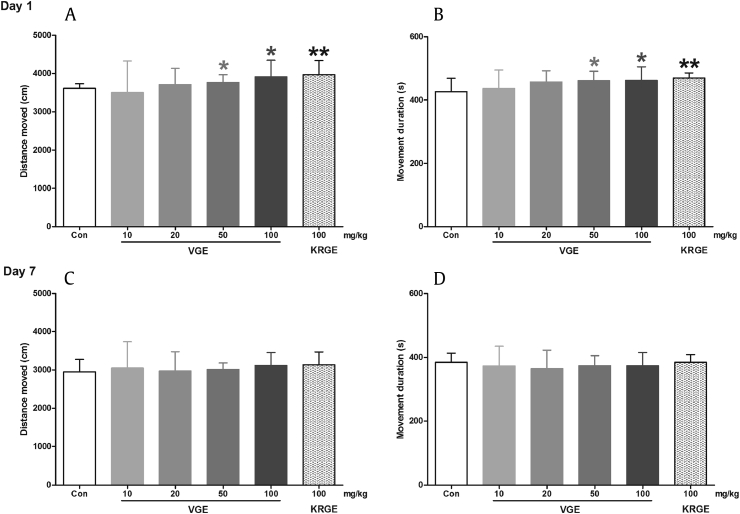
To evaluate the psychomotor effects of VGE, we employed the open-field and rota-rod tests. The open-field test is a widely accepted behavioral tool in measuring the exploratory behavior and spontaneous locomotor activity of rodents. It is commonly used to assess the stimulant, sedative, and/or toxic effects of a substance [24]. Mice acutely treated with VGE demonstrated increased distance moved [50 mg/kg (p < 0.05) and 100 mg/kg (p < 0.05)] and movement duration [50 mg/kg (p < 0.05) and 100 mg/kg (p < 0.05)], as compared to the control group (Figs. 1A, 1B). Mice acutely treated with KRGE (100 mg/kg) also showed increased distance moved (p < 0.01) and movement duration (p < 0.01; Figs. 1A, 1B). These results confirm that VG, like other Panax species, increases the locomotor activity of rodents in the open-field test. Previous studies have also shown that ginseng constituents can also increase locomotor activity. For instance, a study by Tan et al [25] showed that Rb1, a ginsenoside present in both KRGE and VGE, also enhances muscle strength and increases spontaneous locomotor activity in rats. Moreover, an ocotillol-type ginsenoside was also shown to increase the spontaneous locomotor activity of mice and induce neuronal activation [26]. These effects are thought to be mediated through the enhancement of glutamatergic neurotransmission [26]. However, increased locomotor activity was not observed in mice repeatedly treated with the drug for 7 d. This result is consistent with previous studies reporting that long-term treatment of ginseng does not affect locomotor activity, which may be due to the animals' capacity to adapt to the open-field arena and/or tolerance to the locomotor effects of ginseng [9], [27]. Regardless, the results of the open-field test indicate that VG has an enhancing effect on general locomotor activity and does not induce any locomotor impairment.
Fig. 1.
Effects of Vietnamese ginseng extract on the open-field test in mice (n = 8–10). Each bar represents the mean ± standard deviation of the distance moved (A, acute; C, repeated) and movement duration (B, acute; D, repeated) of mice in the open-field arena, for 10 min. * p < 0.05 and ** p < 0.01 significantly different from the control group (unpaired t test). Con, control; KRGE, Korean Red Ginseng extract; VGE, Vietnamese ginseng extract.
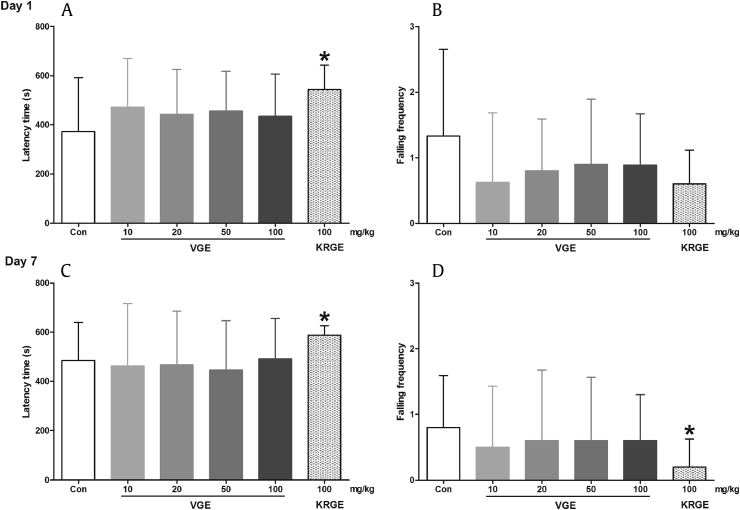
The rota-rod test is a validated tool for evaluating the balance, grip strength, and motor coordination of rodents [28]. The latency to first fall (s) and falling frequency in the rotating rod were used to gauge the motor balance and coordination of each mouse. Although a trend towards increased latency time and decreased falling frequency can be observed, VGE-treated mice, both acute and repeated, failed to show any significant effects on the rota-rod test (Fig. 2). By contrast, KRGE-treated mice showed a significant increase in latency time (p < 0.05) and a decrease in falling frequency (p < 0.05; Fig. 2). It seems that KRGE is more effective than VGE in the rota-rod test. The reason for this difference is unknown, but may be due to the fact that the tested KRGE drug is much more concentrated and is already a commercial product. Nevertheless, the results of the rota-rod test corroborate with the findings of the open-field test, showing that VGE treatment does not cause motor impairments.
Fig. 2.
Effects of Vietnamese ginseng extract on rota-rod test in mice (n = 8–10). Each bar represents the mean ± standard deviation of the latency time before the first fall (A, acute; C, repeated) and falling frequency (B, acute; D, repeated) of mice running on the rota-rod, for 10 min. * p < 0.05 significantly different from the control group (unpaired t test). Con, control; KRGE, Korean Red Ginseng extract; VGE, Vietnamese ginseng extract.
3.2. Sedative–hypnotic effects of VG on the pentobarbital-induced sleeping test
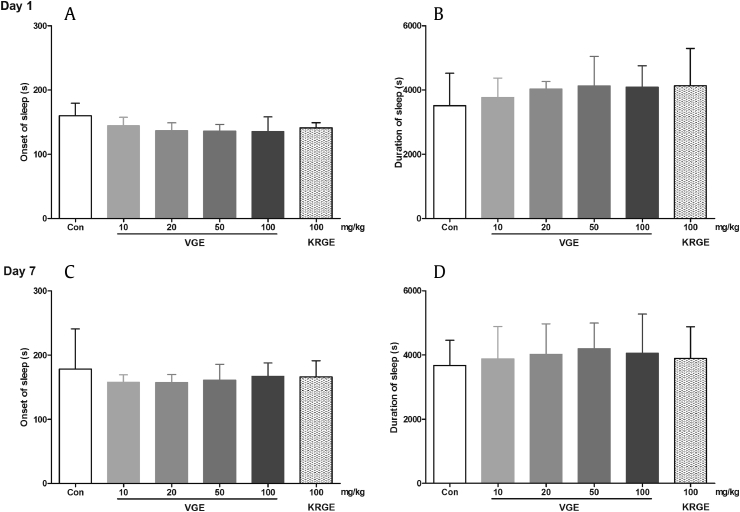
To assess the sedative–hypnotic properties of VGE, we subjected animals to the pentobarbital-induced sleeping test. Substances that potentiate the sleep induced by pentobarbital, a short-acting barbiturate, are known to have centrally acting sedative–hypnotic effects [29], [30]. As can be gleaned from Fig. 3, VGE treatment, both acute and repeated, failed to produce any significant changes on pentobarbital-induced sleeping behavior in mice, as compared to the control group. Similarly, KRGE treatment did not influence pentobarbital-induced sleep. The result with VGE corresponds with an earlier study reporting that Vietnamese ginseng does not have any effect on pentobarbital-induced sleep in mice [31]. These findings are also supported by the results of the open-field and rota-rod tests, wherein VGE treatment did not induce sedative-like effects. Together, these findings indicate that Vietnamese ginseng does not have sedative–hypnotic effect. In contrast to the present result, a study by Lee et al [32] showed that repeated KRGE administration improves the sleep condition of rats. This discrepancy may be related to the various methodological differences between our studies (e.g., difference in doses, animal species, treatment scheme, and others). Alternatively, the results could be interpreted that the sleep-promoting mechanism of KRGE is different from that of pentobarbital. This topic might be a worthwhile focus for future study.
Fig. 3.
Effects of Vietnamese ginseng extract on pentobarbital-induced sleeping test in mice (n = 8–10). Each bar represents the mean ± standard deviation of the onset of sleep (A, acute; C, repeated) and duration of sleep (B, acute; D, repeated) in mice, monitored for 120 min. Con, control; KRGE, Korean Red Ginseng extract; VGE, Vietnamese ginseng extract.
3.3. Antistress effects of VG on the cold swimming test
The cold swimming test measures the rodents' capability to withstand physiological (cold temperature, fatigue) and psychological (behavioral despair) stress [33]. The swimming latency time was used as an indicator of the animals' overall endurance to stressful situations. Treatment with VGE significantly increased the swimming latency time of mice at both acute [50 mg/kg and 100 mg/kg (p < 0.05)] and repeated [50 mg/kg (p < 0.05) and 100 mg/kg (p < 0.01)] treatments, as compared to the control group (Fig. 4). Likewise, KRGE-treated mice displayed a significant increase in swimming latency at acute [100 mg/kg (p < 0.01)] and repeated treatment [100 mg/kg (p < 0.01)]. These results demonstrate that VGE and KRGE possess antifatigue and antistress properties. This result bolsters the adaptogenic status of ginseng products. In support, previous studies have also shown that ginseng boosts endurance, promotes survival to cold and fatigue, and other stressful conditions [2], [18], [33], [34], [35], [36], [37]. Thus, the findings of the cold swimming test strongly suggest that VG, like other ginseng species, can improve physiological stamina and has antistress effects.
Fig. 4.
Effects of Vietnamese ginseng extract on cold swimming test in mice (n = 8–10). Each bar represents the mean ± standard deviation of the swimming latency time (A, acute and B, repeated) of mice in the temperature-controlled water, for 10 min. * p < 0.05 and ** p < 0 .01 significantly different from the control group (unpaired t test). Con, control; KRGE, Korean Red Ginseng extract; VGE, Vietnamese ginseng extract.
3.4. Anxiolytic effects of VG on the EPM test
To evaluate the anxiolytic property of VGE, the EPM test was performed. The EPM is a widely used and accepted tool to measure anxiety-like behaviors in rodents. An increase in the percentage of entries and time spent in the open arms of the EPM is indicative of a substance's anxiolytic effects [38]. In accordance with previous reports, the KRGE-treated group showed significant increase in the percentage of entries [acute (p < 0.01) and repeated treatment (p < 0.01)] and time spent [acute (p < 0.01) and repeated (p < 0.01)] in the open arms of the maze (Fig. 5) as compared to the control group [1], [9], [33]. In the same manner, the VGE-treated group (100 mg/kg) showed an increased percentage of entries [acute (p < 0.05) and repeated treatment (p < 0.05)] and time spent [acute (p < 0.05) and repeated (p < 0.05)] in the open arms (Fig. 5). These results indicate that VGE has an anxiolytic-like effect. Previous studies have also shown that ginseng and ginseng saponins (ginsenosides Rb1, Rg1) produce anxiolytic-like effects. Moreover, the anxiolytic profile of ginseng is believed to be advantageous because it is devoid of locomotor impairments [38], [39], [40], [41], [42]; a characteristic also observed in the present study.
Fig. 5.
Effects of Vietnamese ginseng extract on the elevated plus-maze test in mice (n = 8–10). Each bar represents the mean ± standard deviation of the percentage of entries (A, acute; C, repeated) or the percentage of time spent (B, acute; D, repeated] in the open arms, for 5 min. * p < 0.05 and ** p < 0.01 significantly different from the control group (unpaired t-test). Con, control; KRGE, Korean Red Ginseng extract; VGE, Vietnamese ginseng extract.
3.5. Cognitive effects of VG on the Y-maze and passive avoidance tests
To assess the effect of VGE on learning and memory, we conducted the Y-maze and the passive avoidance tests. The Y-maze test is used to evaluate attention and short-term working memory of rodents. It is based on the propensity of rodents to explore new environments. The indicator of short-term working memory in this test is the spontaneous alternation behavior [43]. Treatment with scopolamine, an acetylcholine receptor antagonist known to produce memory deficits, impairs the spontaneous alternation behavior of mice in this test [44]. As expected, mice treated with scopolamine displayed a significant decrease in spontaneous alternation (p < 0.01) and an increase in the total entry (p < 0.01), as compared with the control group (Fig. 6). The increase in the total entry may reflect disorientation of the subjects. However, pretreatment with VGE [acute (20 mg/kg, p < 0.05 mg/kg; 50, p < 0.01; 100 mg/kg, p < 0.001) and repeated treatment (20 mg/kg, p < 0.05; 50 mg/kg, p < 0.01; 100 mg/kg, p < 0.001)] and KRGE [acute (p < 0.001) and repeated (p < 0.001)] ameliorated this scopolamine-induced memory impairment (Fig. 6). These results suggest that VGE and KRGE improve short-term working memory of mice. This finding is further supported by the results of the passive-avoidance test.
Fig. 6.
Effects of Vietnamese ginseng extract on Y-maze test in mice (n = 8–10). Each bar represents the mean ± standard deviation of the spontaneous alternation (A, acute; C, repeated) or the total entry (B, acute; D, repeated] in the Y-maze arms, for 8 min. * p < 0.05, ** p < 0.01, and *** p < 0.001 significantly different from the scopolamine-treated group (unpaired t test). **** p < 0.01 significantly different from control group. # indicates the difference between scopolamine (sco) vs control group. Con, control; KRGE, Korean Red Ginseng extract; Sco, scopolamine; VGE, Vietnamese ginseng extract.
The passive-avoidance test has long been used to evaluate learning and memory in rodents. In this test, mice learned to avoid an environment where a foot-shock was previously delivered [45]. Similar to the Y-maze test, scopolamine induced learning and memory impairments in the passive-avoidance test. However, VGE [acute (10 mg/kg, p < 0.05; 20 mg/kg, p < 0.01; 50 mg/kg, p < 0.001; 100 mg/kg, p < 0.001) and repeated (10 mg/kg, p < 0.05; 20 mg/kg, p < 0.01); 50 mg/kg, p < 0.001; 100 mg/kg, p < 0.001)] treatment significantly ameliorated this scopolamine-induced memory impairment (Fig. 7). Likewise, treatment with KRGE [acute (p < 0.001) and repeated (p < 0.001)] also recovered the scopolamine-induced amnesia (Fig. 7). In line with our present findings, previous studies have also demonstrated that ginseng and its active component (e.g., Rg3 ginsenoside) have memory-enhancing effects [4], [5], [8], [46], [47].
Fig. 7.
Effects of Vietnamese ginseng extract on passive avoidance task in mice (n = 8–10). Each bar represents the mean ± standard deviation of the retention latency time (A, acute and B, repeated) of mice in the passive avoidance chamber, for 5 min. * p < 0.05, ** p < 0.01, and *** p < 0.001 significantly different from the scopolamine-treated group (unpaired t test). **** p < 0.01 significantly different from control group. Con, control; KRGE, Korean Red Ginseng extract; Sco, scopolamine; VGE, Vietnamese ginseng extract.
In summary, we have shown that orally administered VGE produces psychomotor stimulating, antistress, antifatigue, anxiolytic, and memory enhancing, but not sedative–hypnotic, effects in mice. Due to the limitations of the present study, the specific constituents involved in the psychopharmacological activities of VG are still unknown. This can be a worthwhile focus in future studies. Nevertheless, the present results demonstrate that VG, like other ginseng species, can potentially be used as a natural alternative remedy for various psychological disorders.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF, 2015M3C7A 1028926) and the Center for Women In Science, Engineering and Technology (WISET) Grant funded by the Ministry of Science, ICT and Future Planning of Korea (MSIP) under the Program for Returners into R&D (KW-2015-PPD-0038).
References
- 1.Kim H.J., Kim P., Shin C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J Ginseng Res. 2013;37:8–29. doi: 10.5142/jgr.2013.37.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 3.Zhao H., Li Q., Pei X., Zhang Z., Yang R., Wang J., Li Y. Long-term ginsenoside administration prevents memory impairment in aged C57BL/6J mice by up-regulating the synaptic plasticity-related proteins in hippocampus. Behav Brain Res. 2009;201:311–317. doi: 10.1016/j.bbr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Lee B., Sur B., Park J., Kim S.H., Kwon S., Yeom M., Shim I., Lee H., Hahm D.H. Ginsenoside Rg3 alleviates lipopolysaccharide-induced learning and memory impairments by anti-inflammatory activity in rats. Biomol Ther (Seoul) 2013;21:381–390. doi: 10.4062/biomolther.2013.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy D., Scholey A. Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Behav. 2003;75:687–700. doi: 10.1016/s0091-3057(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 6.Radad K., Gille G., Liu L., Rausch W.D. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 2006;100:175–186. doi: 10.1254/jphs.crj05010x. [DOI] [PubMed] [Google Scholar]
- 7.Le T.H.V., Lee S.Y., Kim T.R., Kim J.Y., Kwon S.W., Nguyen N.K., Park J.H., Nguyen M.D. Processed Vietnamese ginseng: preliminary results in chemistry and biological activity. J Ginseng Res. 2014;38:154–159. doi: 10.1016/j.jgr.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dela Peña I., Yoon S.Y., Kim H.J., Park S., Hong E.Y., Ryu J.H., Park I.H., Cheong J.H. Effects of ginseol k-g3, an Rg3-enriched fraction, on scopolamine-induced memory impairment and learning deficit in mice. J Ginseng Res. 2014;38:1–7. doi: 10.1016/j.jgr.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J.H., Cha H.Y., Seo J.J., Hong J.T., Han K., Oh K.W. Anxiolytic-like effects of ginseng in the elevated plus-maze model: comparison of red ginseng and sun ginseng. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:895–900. doi: 10.1016/j.pnpbp.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Van Le T.H., Lee S.Y., Lee G.J., Nguyen N.K., Park J.H., Nguyen M.D. Effects of steaming on saponin compositions and antiproliferative activity of Vietnamese ginseng. J Ginseng Res. 2015;39:274–278. doi: 10.1016/j.jgr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu G., Zhou Q., Sun S., Leung K.S., Zhang H., Zhao Z. Differentiation of Asian ginseng, American ginseng and Notoginseng by Fourier transform infrared spectroscopy combined with two-dimensional correlation infrared spectroscopy. J Mol Struct. 2008;883–884:91–98. [Google Scholar]
- 12.Yamasaki K. Bioactive saponins in Vietnamese ginseng, Panax vietnamensis. Pharm Biol. 2000;38:16–24. doi: 10.1076/phbi.38.6.16.5956. [DOI] [PubMed] [Google Scholar]
- 13.Huong N.T., Matsumoto K., Kasai R., Yamasaki K., Watanabe H. In vitro antioxidant activity of Vietnamese ginseng saponin and its components. Biol Pharm Bull. 1998;21:978–981. doi: 10.1248/bpb.21.978. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen M.D., Kasai R., Ohtani K., Ito A., Nguyen T.N., Yamasaki K., Tanaka O. Saponins from Vietnamese ginseng, Panax vietnamensis Ha et Grushv. collected in central Vietnam. III. Chem Pharm Bull. 1994;42:634–640. doi: 10.1248/cpb.42.634. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen M.D., Kasai R., Yamasaki K., Nguyen T.N., Tanaka O. New dammarane saponins from Vietnamese ginseng. In: Chong-Ren Y., Osamu T., editors. vol. 6. Elsevier; Philadelphia, PA: 1999. pp. 77–82. (Studies in plant science). [Google Scholar]
- 16.Jeong S.M., Nah S.Y. Ginseng and ion channels: are ginsenosides, active component of Panax ginseng, differential modulator of ion channels? J Ginseng Res. 2005;29:19–26. [Google Scholar]
- 17.Park J.D., Kim D.S., Kwon H.Y., Son S.K., Lee Y.H., Baek N.I., Kim S.I., Rhee D.K. Effects of ginseng saponin on modulation of multidrug resistance. Arch Pharm Res. 1996;19:213–218. [Google Scholar]
- 18.Tran L.Q., Adnyana I., Tezuka Y., Nagaoka T., Tran Q., Kadota S. Triterpene saponins from Vietnamese ginseng (Panax vietnamensis) and their hepatocytoprotective activity. J Nat Prod. 2001;64:456–461. doi: 10.1021/np000393f. [DOI] [PubMed] [Google Scholar]
- 19.Nhut D.T., Hai N.T., Huy N.P., Chien H.X., Nam N.B. New achievements in Panax vietnamensis research. In: Jain S.M., Dutta Gupta S., editors. Biotechnology of neglected and underutilized crops. Springer Science+Business Media; Dordrecht, the Netherlands: 2013. pp. 43–57. [Google Scholar]
- 20.Dong N.T., Luan T.C., Huong N.T.T. Vietnam: Science and Technology Publishing House; 2007. Ngoc Linh ginseng and some medicinal plants belong to ginseng family. [Google Scholar]
- 21.Noldus L.P., Spink A.J., Tegelenbosch R.A. Ethovision: a versatile video tracking system for automation of behavioral experiments. Behav Res Methods. 2001;33:398–414. doi: 10.3758/bf03195394. [DOI] [PubMed] [Google Scholar]
- 22.Dela Peña I.J., Hong E., Kim H.J., de la Peña J.B., Woo T.S., Lee Y.S., Cheong J.H. Artemisia capillaris Thunberg produces sedative-hypnotic effects in mice, which are probably mediated through potentiation of the GABAA receptor. Am J Chin Med. 2015;43:667–679. doi: 10.1142/S0192415X1550041X. [DOI] [PubMed] [Google Scholar]
- 23.Dela Peña I.J., Lee H.L., Yoon S.Y., de la Peña J.B., Kim H.K., Hong E.Y., Cheong J.H. The ethanol extract of Cirsium japonicum increased chloride ion influx through stimulating Gaba(a) receptor in human neuroblastoma cells and exhibited anxiolytic-like effects in mice. Drug Discov Ther. 2013;7:18–23. doi: 10.5582/ddt.2013.v7.1.18. [DOI] [PubMed] [Google Scholar]
- 24.Gould T.D., Dao D.T., Kovacsics C.E. The Open field test. Neuromethods. In: Gould Todd D., editor. Mood and anxiety-related phenotypes in mice. Humana Press; 2009. pp. 1–20. [Google Scholar]
- 25.Tan S.J., Li N., Zhou F., Dong Q.T., Zhang X.D., Chen B.C., Yu Z. Ginsenoside Rb1 improves energy metabolism in the skeletal muscle of an animal model of postoperative fatigue syndrome. J Surg Res. 2014;191:344–349. doi: 10.1016/j.jss.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z.J., Sun L., Peng W., Ma S., Zhu C., Fu F., Heinbockel T. Ginseng derivative ocotillol enhances neuronal activity through increased glutamate release: a possible mechanism underlying increased spontaneous locomotor activity in mice. Neuroscience. 2011;195:1–8. doi: 10.1016/j.neuroscience.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Euaruksakul P., Tansawat R., Rodsiri R. Ginseng extract G115 improves locomotor function in rotenone-induced parkinsonism rats via an antioxidant effect. Songklanakarin J Sci Technol. 2015;37:163–169. [Google Scholar]
- 28.Farkas S., Berzsenyi P., Karpati E., Kocsis P., Tarnawa I. Simple pharmacological test battery to assess efficacy and side effect profile of centrally acting muscle relaxant drugs. J Pharmacol Toxicol Methods. 2005;52:264–273. doi: 10.1016/j.vascn.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Hosseinzadeh H., Noraei N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin, and safranal, in mice. Phytother Res. 2009;23:768–774. doi: 10.1002/ptr.2597. [DOI] [PubMed] [Google Scholar]
- 30.Ma Y., Han H., Eun J.S., Kim H.C., Hong J.T., Oh K.W. Sanjoinine A isolated from Zizyphi spinosi semen augments pentobarbital-induced sleeping behaviors through the modification of gabaergic systems. Biol Pharm Bull. 2007;30:1748–1753. doi: 10.1248/bpb.30.1748. [DOI] [PubMed] [Google Scholar]
- 31.Huong N.T.T., Matsumoto K., Yamasaki K., Duc N.M., Nham N.T., Watanabe H. Effects of majonoside-R2 on pentobarbital sleep and gastric lesion in psychologically stressed mice. Pharmacol Biochem Behav. 1996;53:957–963. doi: 10.1016/0091-3057(95)02147-7. [DOI] [PubMed] [Google Scholar]
- 32.Lee C.I., Kim C.S., Han J.Y., Oh E.H., Oh K.W., Eun J.S. Repeated administration of Korea red ginseng extract increases non-rapid eye movement sleep via GABAAergic systems. J Ginseng Res. 2012;36:403–410. doi: 10.5142/jgr.2012.36.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi J.Y., Woo T.S., Yoon S.Y., dela Pena I.C., Choi Y.J., Ahn H.S., Lee Y.S., Yu G.Y., Cheong J.H. Red ginseng supplementation more effectively alleviates psychological than physical fatigue. J Ginseng Res. 2011;35:331–338. doi: 10.5142/jgr.2011.35.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen T.T., Matsumoto K., Yamasaki K., Nguyen M.D., Nguyen T.N., Watanabe H. Crude saponin extracted from Vietnamese ginseng and its major constituent majonoside-R2 attenuate the psychological stress- and foot-shock stress-induced antinociception in mice. Pharmacol Biochem Behav. 1995;52:427–432. doi: 10.1016/0091-3057(95)00133-h. [DOI] [PubMed] [Google Scholar]
- 35.Huong N.T., Matsumoto K., Yamasaki K., Duc N.M., Nahm N.T., Watanabe H. Effects of Vietnamese ginseng on opioid agonist- and conditioned fear stress-induced antinociception. Phytomedicine. 1996;3:33–39. doi: 10.1016/S0944-7113(96)80007-9. [DOI] [PubMed] [Google Scholar]
- 36.Yobimoto K., Matsumoto K., Huong N.T., Kasai R., Yamasaki K., Watanabe H. Suppressive effects of Vietnamese ginseng saponin and its major component majonoside-R2 on psychological stress-induced enhancement of lipid peroxidation in the mouse brain. Pharmacol Biochem Behav. 2000;66:661–665. doi: 10.1016/s0091-3057(00)00257-4. [DOI] [PubMed] [Google Scholar]
- 37.Oliynyk S., Oh S. Actoprotective effect of ginseng: improving mental and physical performance. J Ginseng Res. 2013;37:144–166. doi: 10.5142/jgr.2013.37.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pellow S., File S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 39.Rex A., Stephens D.N., Fink H. “Anxiolytic” action of diazepam and abecarnil in a modified open field test. Pharmacol Biochem Behav. 1996;53:1005–1011. doi: 10.1016/0091-3057(95)02121-3. [DOI] [PubMed] [Google Scholar]
- 40.Carr M.N., Bekku N., Yoshimura H. Identification of anxiolytic ingredients in ginseng root using the elevated plus-maze test in mice. Eur J Pharmacol. 2006;531:160–165. doi: 10.1016/j.ejphar.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Bhattacharya S.K., Mitra S.K. Anxiolytic activity of Panax ginseng roots: an experimental study. J Ethnopharmacol. 1991;34:87–92. doi: 10.1016/0378-8741(91)90193-h. [DOI] [PubMed] [Google Scholar]
- 42.Cha H.Y., Park J.H., Hong J.T., Yoo H.S., Song S., Hwang B.Y., Eun J.S., Oh K.W. Anxiolytic-like effects of ginsenosides on the elevated plus-maze model in mice. Biol Pharm Bull. 2005;28:1621–1625. doi: 10.1248/bpb.28.1621. [DOI] [PubMed] [Google Scholar]
- 43.Yang J.H., Han S.J., Ryu J.H., Jang I.S., Kim D.H. Ginsenoside Rh2 ameliorates scopolamine-induced learning deficit in mice. Biol Pharm Bull. 2009;32:1710–1715. doi: 10.1248/bpb.32.1710. [DOI] [PubMed] [Google Scholar]
- 44.Olton D.S., Papas B.C. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- 45.Klinkenberg I., Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev. 2010;34:1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Lorenzini C.A., Baldi E., Bucherelli C., Sacchetti B., Tassoni G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat’s passive avoidance response: a tetrodotoxin functional inactivation study. Brain Res. 1996;730:32–39. doi: 10.1016/0006-8993(96)00427-1. [DOI] [PubMed] [Google Scholar]
- 47.Jin S.H., Park J.K., Nam K.Y., Park S.N., Jung N.P. Korean Red Ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice. J Ethnopharmacol. 1999;66:123–129. doi: 10.1016/s0378-8741(98)00190-1. [DOI] [PubMed] [Google Scholar]