Abstract
Background
The present study investigated the effect of ginseng berry hot water extract (GBx) on blood flow via the regulation of lipid metabolites and blood coagulation in rats fed a high-fat diet (HFD).
Methods
Sixty rats were divided into five groups in descending order of body weight. Except for the control group, the other four groups were fed a HFD containing 45% kcal from fat for 11 wk without GBx. GBx groups were then additionally treated by gastric gavage with GBx dissolved in distilled water at 50 (GBx 50) mg/kg, 100 (GBx 100) mg/kg, or 150 (GBx 150) mg/kg body weight for 6 wk along with the HFD. To investigate the effects of GBx on rats fed a HFD, biochemical metabolite, blood coagulation assay, and histological analysis were performed.
Results
In the experiments to measure the serum levels of leptin and apolipoprotein B/A, GBx treatment attenuated the HFD-induced increases in these metabolites (p < 0.05). Adiponectin and apolipoprotein E levels in GBx-treated groups were significantly higher than the HFD group. Prothrombin time and activated partial thromboplastin time were increased in all GBx-treated groups. In the GBx-treated groups, the serum levels of thromboxane A2 and serotonin were decreased and concentrations of serum fibrinogen degradation products were increased (p < 0.05). Moreover, histomorphometric dyslipidemia-related atherosclerotic changes were significantly improved by treatment with GBx.
Conclusion
These results suggest the possibility that GBx can ameliorate blood flow by decreasing intima-media thickness via the regulation of blood coagulation factors related to lipid metabolites in rats fed a HFD.
Keywords: blood coagulation, blood flow, ginseng berry, high-fat diet, lipid metabolite
1. Introduction
Nowadays, foods that are palatable to most individuals are those high in fat, especially saturated fat. A high-fat diet (HFD) leads to the expansion of adipose tissue and abdominal obesity, which are associated with metabolic abnormalities including complex and chronic cardiovascular disease, liver disorders, and others [1], [2]. In particular, cardiovascular disease is considered a serious public health issue globally and is among the top five causes of death in most countries [1], [3]. Cardiovascular disease causes up to 17 million deaths every year and results from thrombosis, atherosclerosis, and myocardial infarction [4].
Hyperlipidemia is accompanied by platelet hyperactivity, hypercoagulability, and hypofibrinolysis, and plays a key role in the pathogenesis of cardiovascular diseases such as atherosclerosis [5], [6]. The major pathological pathway involved in these disorders is the development of an occlusive thrombus in the arteries. Collapses of atherosclerotic plaques by rupture or erosion leads to prothrombotic conditions involving circulating platelets and procoagulant factors. The main thrombotic elements related to atherosclerotic plaques are tissue factor and collagen [7]. These result in platelet aggregation and activation to induce cardiovascular disease [8]. Because studies on the regulation of platelet hyperactivity have focused on approaches to prevent thrombosis, many therapies aimed at suppressing platelet function have been developed [8], [9]. Moreover, for alternative treatments, numerous patients and healthcare specialists have sought oriental medicines or herbal remedies with potential effectiveness and without side effects such as gastrointestinal side effects, hemorrhage, and decreased platelets counts [1], [8].
Among the natural product alternative treatments, ginseng and its related components have been widely used for thousands of years, either in food or herbal medicines, to treat various diseases. Ginseng has antiaging, antidiabetic, anticarcinogenic, and antifatigue functions through the promotion of DNA, RNA, and protein synthesis [10]. In addition, ginseng has been classified into 11 different species and contains approximately 200 ingredients, including ginsenosides, polysaccharides, polyacetylenes, fatty acids, mineral oils, peptides, and amino acids [11].
In particular, the bioactivity of ginseng results from the presence of ginsenosides in its roots [8]. Thus, until now, many studies have focused on the effect of the ginseng root [12]. However, these active ingredients are also distributed in other parts of the ginseng plant, such as the berries and leaves. Current studies have reported that the ginseng berry (GB) has higher ginsenoside content than that of the root and may exert more pharmacological effects on various diseases via its antihyperglycemic and antiobesity activities [6], [13]. Moreover, the harvesting of GB is easier than that of the root because it can be harvested several times after the 3rd yr of growth, whereas the root is only harvested between the 4th and 6th yr of growth [14]. However, until now, GB has not received much attention [6].
Consumption of a HFD, which includes foods palatable to many individuals, induces cardiovascular disease through alterations in lipid metabolism and blood coagulation, for which treatments with potential effectiveness and without various side effects are needed. GB contains a high ginsenoside content, which might exert pharmacological effects on cardiovascular diseases. However, there have been no reports regarding GB. Therefore, the purpose of the present study was to investigate the possibility of GB as a putative agent to improve blood flow in rats fed a HFD.
2. Materials and methods
2.1. Animals and experimental diets
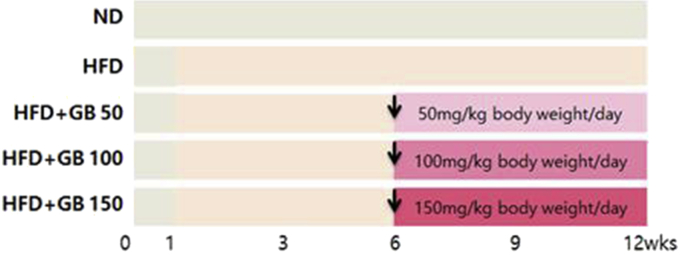
The animals used in this study were 5-wk-old male Sprague Dawley rats (140 ± 6.00 g) purchased from Central Lab. Animal Inc. (Seoul, Korea) and acclimatized for 1 wk. Each cage contained two rats. Rats were exposed to a 12-h light/dark cycle and maintained at a constant temperature of 22 ± 2°C and 55 ± 5% humidity. All animals were fed for 12 wk. The rats were selected randomly and assigned to five groups (12 rats/group). One group served as the control (CON) and was fed a normal diet (Zeigler Bros, Gardners, PA, USA). The other four groups were fed a HFD (D12451; Research Diets Inc., New Brunswick, NJ, USA) containing 45% kcal from fat for 11 wk without GB. Three of the HFD groups were additionally treated by gastric gavage with GB hot water extract (GBx) dissolved in distilled water at 50 (GBx 50) mg/kg, 100 (GBx 100) mg/kg, and 150 (GBx 150) mg/kg body weight for 6 wk (Fig. 1). This study was approved (EUIACUC 15-06) by the Eulji University Institutional Animal Care and Use Committee.
Fig. 1.
Experimental study design. The rats were selected randomly and assigned to five groups. One group served as the control and was fed a normal diet (ND). The other four groups were fed a high-fat diet (HFD) containing 45% kcal from fat for 11 wk without ginseng berry (GB). Three of the HFD groups were additionally treated by gastric gavage with ginseng berry hot water extract (GBx) dissolved in distilled water at 50 (GBx 50) mg/kg, 100 (GBx 100) mg/kg, and 150 (GBx 150) mg/kg body weight for 6 wk.
2.2. Preparation of GBx
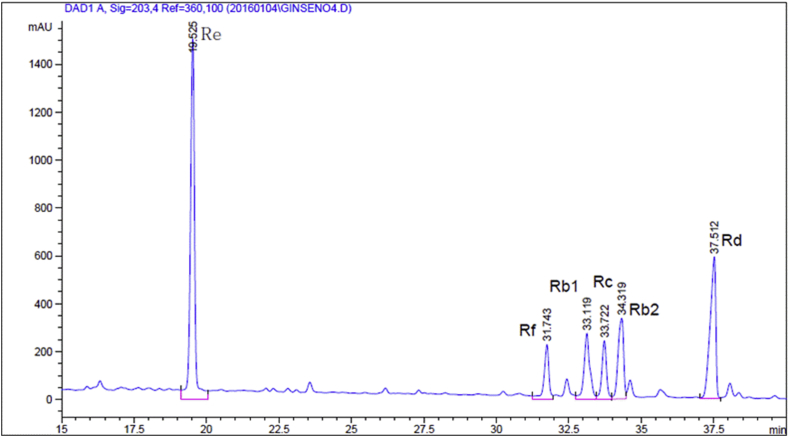
GB was extracted in three volumes of distilled water in a shaking incubator at 80°C for 10 h. The extract was filtered through a 60-mesh sieve filter and concentrated into a dry solid using a rotary vacuum evaporator (Daesung Machinery Co., Chuncheon, Korea) at 60°C, up to a 25% soluble solid content, depending on the GB ingredient. For freeze drying, extracts were frozen overnight at −40°C and lyophilized using a freeze dryer (PVTFA 10AT; Inshin Lab. Co. Ltd., Seoul, Korea) for 3 d. GB powder was obtained after drying for 12 h at 60°C, and the final dried extract was stored at −20°C until use. Constituents of the GBx were analyzed using HPLC. HPLC analysis of GB extract powder was done using an Agilent HPLC system (Agilent Technologies., Santa Clara, CA, USA) equipped with a binary pump and diode array detector. An aqueous solution of test materials (0.08 mg/mL) was injected at 10 μL. Separation was then done on a 5-μm Hector-M C18 column (4.6 mm × 250 mm; RS Tech Co., Daejeon, Korea). The mobile phase consisted of deionized water (A) and acetonitrile (B). The gradient was programmed as follows: 0–5 min: 20% B. 5–35 min: a linear gradient from 20% to 40% B; 35–45 min: 40% B. 45–45.1 min: a linear gradient from 40% to 20% B; 45.1–50 min: 20% B. The flow rate was 1.0 mL /min. The UV detector was set at 203 nm. Determination of major ginsenosides in the GB extract is shown in Fig. 2.
Fig. 2.
High-performance liquid chromatography analysis of the ginsenoside Re in the ginseng berry extract powder. The major ginsenosides of the ginseng berry hot water extract were analyzed using high-performance liquid chromatography.
2.3. Biochemical metabolite assay
Leptin (R&D Systems, Minneapolis, MN, USA) and adiponectin (Shibayagi Co. Ltd., Gunma, Japan) were measured using a commercial enzyme-linked immunosorbent assay kit. Apolipoproteins (apo) A, B100, and E, thromboxane A2, serotonin, and fibrinogen degradation products (FDP) were also measured using enzyme-linked immunosorbent assay kits (Elabscience Biotechnology Co. Ltd., Wuhan, China). After centrifugation, serum samples were mixed with substrate buffer and manganese solution and incubated for 2 h at 37°C, followed by further incubation with urea reagent at room temperature for another 30 min. Optical density was determined at 450 nm using a spectrophotometer.
2.4. Coagulation assays
Plasma for the coagulation assays was obtained by centrifugation (3,000 rpm, 10 min). Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were measured within 2 h of sample collection using automated coagulation analyzers (Diagnostica Stago, Asnières, France) according to the manufacturer's instructions.
2.5. Platelet function assays
The platelet function analyzer INNOVANCE PFA-200 in-vitro diagnostic system (Siemens Medical Solutions, Malvern, PA, USA) incorporates a high-shear flow system to simulate the in vivo hemodynamic conditions of platelet adhesion and aggregation as encountered at the site of a vascular lesion. The test has proved to be more sensitive than the template bleeding time [15]. Blood samples were collected in vacutainers containing 3.2% buffered sodium citrate. We measured the closure time using both collagen/epinephrine (Col/EPI) and collagen/adenosine-5-diphosphate (Col/ADP) cartridges for every blood sample. Col/EPI and Col/ADP analyses were run on a Siemens INNOVANCE PFA-200 analyzer.
2.6. Histological analysis
Regular parts of individual liver and aortic tissues were separated and fixed in 10% neutral buffered formalin. After embedding in paraffin, 3–4-μm serial sections were prepared. Representative sections were stained with Hematoxylin and Eosin (H&E) for examination using light microscopy. After the histological profiles of individual organs were examined, alternatively, portions of liver that had been dehydrated in 30% sucrose solutions were cryostat sectioned for Oil red (OR) staining. To observe more detailed histopathological changes, the areas of steatohepatitis and the mean hepatocyte diameters were calculated by automated image analysis using i-Solution FL version 9.1 (IMT i-Solution Inc., Vancouver, Quebec, Canada) on the restricted view fields according to previous methods [16], [17]. Steatohepatitis regions, the percentage of fatty deposited regions in hepatic parenchyma as lipid deposited regions in restricted histological view field of liver (%/mm2 hepatic parenchyma), and the number of ballooning/lipid droplet-deposited hepatocytes (per 1,000 hepatocytes) around centrilobular regions were calculated using a cryostat and OR staining. The mean diameters of hepatocytes from H&E-stained, paraffin-embedded tissues were also calculated (μm) in restricted fields of view on a computer monitor using automated image analysis; at least 10 hepatocytes in each view field per liver were considered. In addition, to show more detailed histomorphometric changes, the mean diameters of the total aortic wall, tunica intima, and tunica media (μm/vessel) were measured using H&E staining, and the percentages of areas showing lipid deposition (%/mm2 aorta parenchyma) and atherosclerotic plaques (%/mm aortic surface) were measured using OR staining. The histopathologist was blinded to the group distribution during the analyses. One histological field in each prepared liver and aortic tissue specimen was analyzed; consequently, 12 liver and aortic histological fields per group were considered for further histomorphometric analysis.
2.7. Statistical analysis
Results are expressed as the means ± standard deviation. Statistical analyses were conducted using SPSS for Windows (Release 18.0K; SPSS Inc., Chicago, IL, USA). Results were analyzed by one-way analysis of variance. When a significant difference was detected, Fisher's least significant difference test for multiple comparisons was performed to determine significant differences among the groups.
3. Results
3.1. Effect of GBx supplementation on lipid metabolites in rats fed a HFD
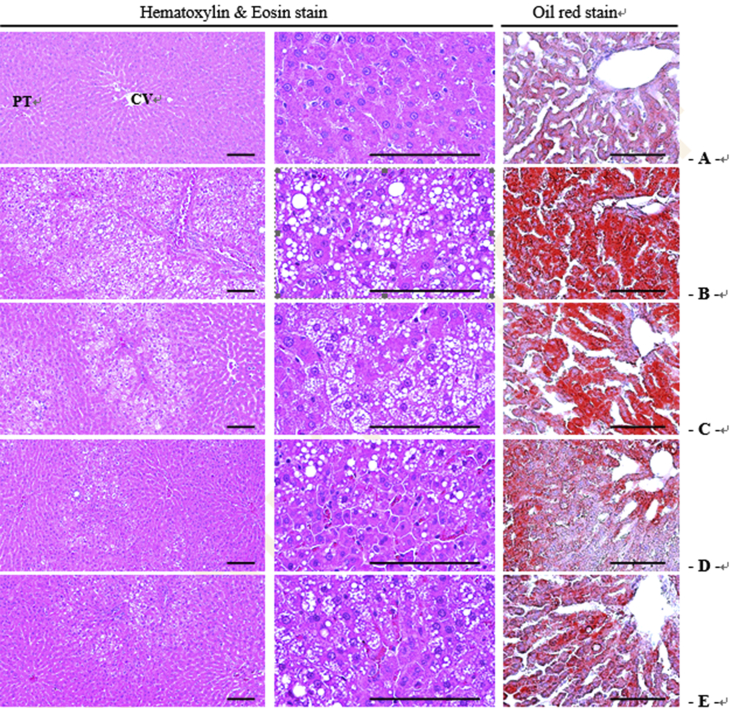
The effect of GBx on serum levels of lipid metabolites, including leptin, apoE, apoB/A, and adiponectin, were investigated in each group of rats. As shown in Table 1, while leptin levels in the HFD group were higher than those of the CON group, GBx (GBx 100 and GBx 150) significantly attenuated the HFD-induced increase in serum leptin levels (p < 0.05). GBx treatment significantly increased apoE levels in a dose-dependent manner (p < 0.05). ApoB/A levels were significantly lower in all three GBx groups compared with the CON and HFD groups (p < 0.05). Adiponectin levels were significantly higher in all GBx-treated groups compared with the HFD group (p < 0.05), and this effect was dose-dependent. Moreover, treatment with GBx 150 mg/kg induced almost a fourfold increase in adiponectin levels compared with rats fed a HFD only (p < 0.05). In addition, histological analysis of the liver was performed. As shown in Fig. 3, in the HFD group, noticeable steatohepatitis was induced due to the deposition of lipid droplets and related severe hypertrophic changes, including increases in hepatocyte size, steatohepatitis area, number of ballooning hepatocytes, and mean hepatocyte diameters. However, GBx treatment improved the HFD-induced steatohepatitis and related hypertrophic changes (Fig. 3).
Table 1.
Effect of ginseng berry extract (GBx) supplementation on serum biochemical values in rats fed a high-fat diet
| CON | HFD | HFD supplemented with |
|||
|---|---|---|---|---|---|
| GBx 50 | GBx 100 | GBx 150 | |||
| Leptin (ng/mL) |
55.20 ± 5.92 | 83.79 ± 3.16* | 75.10 ± 3.71* | 63.07 ± 10.31** | 66.27 ± 3.91** |
| Apo E (ng/mL) |
1111.89 ± 54.50 | 848.38 ± 51.52* | 1081.36 ± 106.24 | 1281.21 ± 125.05** | 1705.50 ± 170.62*,** |
| Apo B/A (ng/mL) |
0.77 ± 0.03 | 0.83 ± 0.06 | 0.50 ± 0.02*,** | 0.57 ± 0.03*,** | 0.51 ± 0.02*,** |
| Adiponectin (ng/mL) |
704.42 ± 50.94 | 138.59 ± 34.19* | 510.05 ± 31.89** | 530.70 ± 60.78** | 578.73 ± 71.38** |
Values are reported as the means ± standard deviation.
* p < 0.05 vs. CON group.
** p < 0.05 vs. HFD group.
Apo, apolipoprotein; CON, control group; GBx 50, 100, 150, groups treated with GB extract 50 mg/kg, 100 mg/kg, or 150 mg/kg body weight together with a HFD; HFD, high-fat diet group.
Fig. 3.
Liver histopathological images in the control group (A), high-fat diet (HFD) group (B), and groups treated with ginseng berry extract 50 mg/kg (C), 100 mg/kg (D), or 150 mg/kg (E) body weight together with a HFD. To examine hepatic histopathological changes in the control group, HFD, and groups treated with ginseng berry extract 50 mg/kg, 100 mg/kg, or 150 mg/kg body weight together with a HFD, Hematoxylin and Eosin and Oil red staining were performed as described in the Materials and methods section. CV, central vein; PT, potral triad.
3.2. Effect of GBx supplementation on blood coagulation and platelet function in rats fed a HFD
Blood coagulation factors related to platelet function, including PT, aPTT, Col/EPI, and Col/ADP, were measured. As shown in Table 2, PT values were increased significantly in all GBx groups, and the aPTT levels were increased compared with the HFD group (p < 0.05). However, there were no significant differences in Col/ADP or Col/EPI among the groups. The serum levels of thromboxane A2 (TXA2), serotonin, and FDP were also measured. In the GBx 100 and 150 groups, the serum levels of TXA2 were decreased significantly compared with the HFD group (p < 0.05). In addition, whereas the serum levels of serotonin were increased in the HFD group compared with the CON group, the GBx 150 group showed significantly lower serum serotonin levels compared with the CON and HFD groups (p < 0.05). The GBx 100 and 150 treatment groups also showed significantly higher serum FDP concentrations than those of the other groups (p < 0.05). These results indicate that GBx is involved in the regulation of blood coagulation, suggesting GBx as a possible agent for blood coagulation-related disorders.
Table 2.
Effect of ginseng berry extract (GBx) supplementation on blood coagulation in rats fed high fat diet
| CON | HFD | HFD supplemented with |
|||
|---|---|---|---|---|---|
| GBx 50 | GBx 100 | GBx 150 | |||
| PT (s) | 17.75 ± 0.74 | 15.66 ± 1.05* | 16.52 ± 0.60*,** | 16.75 ± 0.89*,** | 16.65 ± 0.52*,** |
| aPTT (s) | 17.62 ± 0.09 | 17.88 ± 0.47 | 17.99 ± 0.59 | 18.70 ± 0.87*,** | 18.43 ± 1.09* |
| Col/EPI (s) | 300.00 ± 0.00 | 289.70 ± 2.57 | 300.00 ± 0.00 | 300.00 ± 0.00 | 289.05 ± 4.15 |
| Col/ADP (s) | 260.33 ± 1.40 | 263.80 ± 9.03 | 300.00 ± 0.00 | 298.80 ± 3.79 | 293.33 ± 8.73 |
| TXA2 (pg/mL) | 295.87 ± 55.32 | 520.12 ± 25.12* | 459.61 ± 80.55* | 81.06 ± 10.48*,** | 73.60 ± 10.36*,** |
| Serotonin (pg/mL) | 108.43 ± 16.55 | 126.63 ± 10.93 | 116.12 ± 15.85 | 95.08 ± 5.38 | 64.85 ± 10.96*,** |
| FDP (pg/mL) | 574.29 ± 35.36 | 554.01 ± 52.55 | 667.23 ± 48.18 | 1182.36 ± 92.31*,** | 1053.94 ± 94.81*,** |
Values are reported as the means ± standard deviation.
* p < 0.05 versus CON group.
** p < 0.05 versus HFD group.
aPTT, activated partial thromboplastin time; Col/ADP, collagen/adenosine-5-diphosphate; Col/EPI, collagen/epinephrine; CON, control group; FDP, fibrinogen degradation products; GBx 50, 100, 150, groups treated with GB extract 50 mg/kg, 100 mg/kg, or 150 mg/kg body weight together with a HFD; HFD, high-fat diet group; PT, prothrombin time; TXA2, thromboxane A2.
3.3. Effect of GBx supplementation on aortic tissues of rats fed a HFD
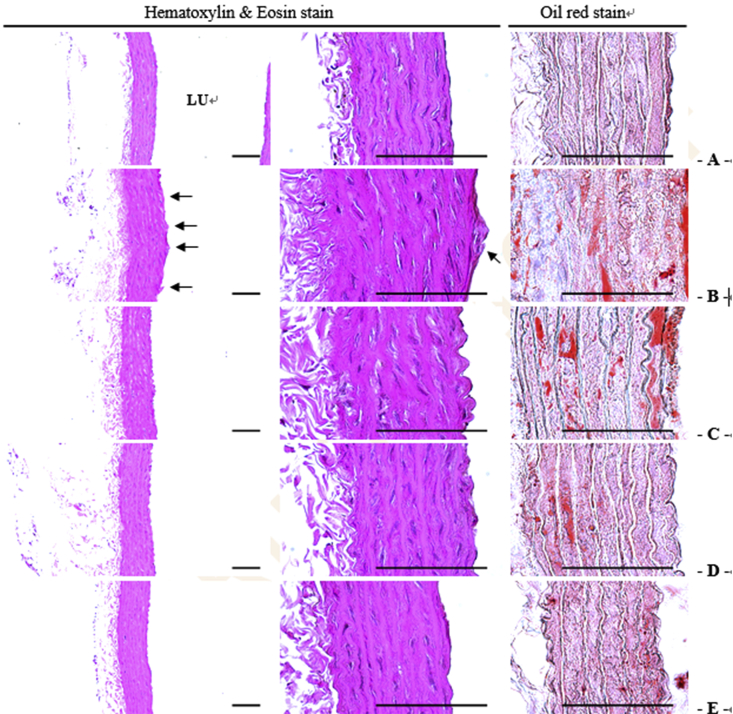
Histomorphometric analysis showed severe hypertrophic changes in the walls of the aorta and formation of atherosclerotic plaques induced by a HFD (Fig. 4; Table 3). In addition, significant increases in the mean thicknesses of the total aortic wall, tunica intima, and tunica media and in the areas of lipid deposition in the aortic wall and atherosclerotic plaques were induced compared with the CON group. However, these histomorphometric dyslipidemia-related atherosclerotic changes were significantly improved by treatment with GBx 50 mg/kg, 100 mg/kg, and 150 mg/kg in a dose-dependent manner (p < 0.05).
Fig. 4.
Aortic histopathological images in the control group (A), high-fat diet (HFD) group (B), and groups treated with ginseng berry extract 50 mg/kg (C), 100 mg/kg (D), or 150 mg/kg (E) body weight together with a HFD. To examine aortic histopathological changes in the control group, HFD, and groups treated with ginseng berry extract 50 mg/kg, 100 mg/kg, or 150 mg/kg body weight together with a HFD, Hematoxylin and Eosin and Oil red staining were performed as described in the Materials and methods section. LU, Lumen.
Table 3.
Histomorphometrical analysis of aorta tissues in rats fed high fat diet
| CON | HFD | HFD supplemented with |
|||
|---|---|---|---|---|---|
| GBx 50 | GBx 100 | GBx 150 | |||
| Mean thicknesses (μm/vessel) | |||||
| Total walls | 91.57 ± 12.43 | 147.64 ± 15.32* | 125.22 ± 15.21*,** | 107.95 ± 11.73*,** | 105.40 ± 13.44*,** |
| Tunica intima | 3.08 ± 0.63 | 19.68 ± 2.01* | 11.02 ± 1.48*,** | 7.95 ± 1.19*,** | 6.16 ± 1.25*,** |
| Tunica media | 80.06 ± 14.64 | 114.98 ± 15.22* | 97.89 ± 12.88*,** | 88.88 ± 12.50* | 86.50 ± 8.47** |
| Region percentages (%) | |||||
| Atherosclerotic plaque | 0.71 ± 0.32 | 30.35 ± 11.85* | 18.92 ± 7.75*,** | 8.40 ± 2.17*,** | 4.84 ± 2.00*,** |
| Lipids | 1.31 ± 0.95 | 16.82 ± 3.92* | 12.73 ± 3.51*,** | 8.47 ± 2.11*,** | 4.34 ± 1.54*,** |
Values are reported as the means ± standard deviation.
* p < 0.05 versus CON group.
** p < 0.05 versus HFD group.
CON, control group; GB, ginseng berry; GBx 50, 100, 150, groups treated with GB extract 50 mg/kg, 100 mg/kg, or 150 mg/kg body weight together with a HFD; HFD, high-fat diet group.
4. Discussion
A HFD induces cardiovascular disease through alterations in lipid metabolism and blood coagulation. GBx includes a high content of ginsenosides, which might exert pharmacological effects on cardiovascular disease. Therefore, the present study aimed to investigate the effect of GBx on blood flow in rats fed a HFD.
A HFD induces abnormalities in serum and hepatic lipid profiles [1]. To evaluate risk factors for obesity, insulin resistance, and diabetes, as well as liver disorders, apoA, apoB, leptin, adiponectin, free fatty acids, ghrelin, and tumor necrosis factor-alpha were proposed as biomarkers [18]. Therefore, to investigate the effect of GBx on lipid metabolites in this study, serum levels of leptin, apoE, apoB/A, and adiponectin were measured in each group of rats.
Leptin is a proteohormone that functions in the regulation of energy balance and body weight according to energy expenditure and is synthesized by differentiated adipocytes. Leptin levels are reportedly higher in obese individuals with nonalcoholic fatty liver disease [18], [19]. Similarly, in the present study, leptin levels in the HFD group were higher than those in the CON group, and GBx treatment significantly attenuated the HFD-induced increase in serum leptin levels. In addition, adiponectin, which is synthesized only by adipocytes, influences hepatocellular free fatty acid metabolism. Low levels of adiponectin have been linked to cardiovascular disease, considering that adiponectin potentially protects against cardiovascular disease by accumulating in injured vessel walls and acting as an antiatherogenic factor [5]. In our study, we found that adiponectin levels in all three GBx groups were significantly higher than those in the HFD group, and this effect was dose-dependent. Previous studies reported increased leptin levels and decreased adiponectin levels in patients with steatosis and steatohepatitis due to morbid obesity [18], [20]. These findings are similar to our histological results showing evident steatohepatitis, induced by lipid droplet deposition, and related severe hypertrophic changes, including increased hepatocyte size, areas of steatohepatitis, number of ballooning hepatocytes, and mean hepatocyte diameters, in the HFD group. However, GBx treatment improved these liver impairments in a dose-dependent manner. Therefore, these results indicate that GBx ameliorates lipid-metabolite regulation as well as steatosis and steatohepatitis, with antagonizing effects against HFD-induced serum lipid profile and hepatic lipid distribution abnormalities.
Meanwhile, our results showed that apoE levels increased significantly in a dose-dependent manner with GBx treatment, whereas they were decreased in the HFD group. The decrease in apoE levels may be related to the development of atherosclerotic plaques [21]. In addition, several studies have reported that adipocyte-derived hormones induced by HFD, including leptin, adiponectin, and apo, could be prominent regulators linking obesity and cardiovascular disease, since they participate as atherosclerotic factors affecting platelet hyperactivity, hypercoagulability, and hypofibrinolysis [5], [6], [20].
TXA2, a metabolite of arachidonic acid, is well known to regulate platelet aggregation and vasomotor response along with nitric oxide [22]. Endothelial cells secrete nitric oxide which inhibits clot formation through vasodilation, by inhibiting the production of TXA2 [23]. In addition, ADP and serotonin, in combination with thrombin, act as major mediators in the initiation of platelet recruitment and adhesion/activation, which are critical for blood coagulation [24]. Fibrinogen not only promotes arterial and venous thrombosis via increased fibrin formation, platelet aggregation, and plasma viscosity, but also increases progressively with the severity of atherosclerosis [5]. In our study, we found that the serum levels of TXA2 and serotonin in the GBx groups decreased significantly compared with the CON and HFD groups. In addition, the GBx 100 and 150 treatment groups showed significantly higher serum FDP concentrations than those of the other groups. These data suggest the possibility that GBx is involved in the regulation of blood flow.
PT is related to the extrinsic clotting pathway and aPTT is used to assess the intrinsic coagulation phase in plasma [22]. These factors can serve as important risk factors in cardiovascular disease and as independent indicators of the progression of atherosclerosis [25]. Therefore, PT and aPTT are the most widely used measures to investigate coagulation abnormalities [26]. To examine the possibility of GBx as an antithrombotic agent, we investigated the effects of GBx on the coagulation cascade, using the classical coagulation assays (PT and aPTT content). Such coagulation assays with GB extract in animal models have not previously been reported. In the present study, we found that the PT values increased significantly in all GBx-treated groups, and aPTT levels increased when compared with the HFD group. These findings suggest that GBx contributes to the amelioration of blood flow by regulating the extrinsic clotting pathway. Moreover, the ability of ginseng to inhibit platelet aggregation was recently demonstrated in a separate study [27]. Several other studies also reported that red ginseng inhibits platelet aggregation directly via nuclear factor-κB and mitogen-activated protein kinase signaling pathways [8], [11], [28]. Collectively with previously reported data, our findings suggest that GBx may exert an ameliorating effect on blood flow by modulating lipid metabolites.
Increased intima-media thickness is an important predictor of cardiovascular disease incidence [29]. The first signs of atherosclerosis include lipid deposits, resulting in fatty streaks in the intima of systemic arteries. Advanced atherosclerotic lesions arise from these fatty streaks [30]. Our results also demonstrated that a HFD induced severe hypertrophic changes in the aortic walls, formation of atherosclerotic plaques, as well as significant increases in the mean thicknesses of the total aortic wall, tunica intima, and tunica media, and in the areas of lipid deposits in the aortic walls and atherosclerotic plaques compared with the CON group. However, these dyslipidemia-related histomorphometric changes in the sclerotic aorta were significantly improved by treatment with 50 mg/kg, 100 mg/kg, and 150 mg/kg GBx in a dose-dependent manner. In addition, Jeong et al [11] recently reported that Korean Red Ginseng extract alleviates atherosclerotic lesions through monocyte chemoattractant protein-1 and tumor necrosis factor-α suppression in apoE knockout mice. Kim et al [6] also demonstrated in a hyperlipidemic mouse model that Korean GB extract suppresses atherosclerotic lesion development through the inhibition of atherogenic inflammatory gene expression. Taken together, these findings illustrate the effects of GBx on intima-media thickness and histomorphometric changes.
Therefore, these data imply that GBx contributes to improved blood flow by decreasing intima-media thickness via the regulation of blood coagulation factors related to lipid metabolites in rats fed a HFD. However, more systematic studies will be necessary to determine whether GBx is useful in preventing the development of atherosclerosis and improving blood flow. In particular, a hemodynamic study and the establishment of indexes of blood flow using serum biochemistry and its mechanisms are needed.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This work was supported by a grant from the Korea Food Research Institute, Republic of Korea.
References
- 1.Bassil M., Daher C.F., Mroueh M., Zeeni N. Salvia libanotica improves glycemia and serum lipid profile in rats fed a high fat diet. BMC Complement Altern Med. 2015;15:384. doi: 10.1186/s12906-015-0917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riccardi G., Giacco R., Rivellese A.A. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr. 2004;23:447–456. doi: 10.1016/j.clnu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Cordner Z.A., Tamashiro K.L. Effects of high-fat diet exposure on learning and memory. Physiol Behav. 2015;152:363–371. doi: 10.1016/j.physbeh.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahlof B. Cardiovascular disease risk factors: epidemiology and risk assessment. Am J Cardiol. 2010;105:3A–9A. doi: 10.1016/j.amjcard.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Darvall K.A., Sam R.C., Silverman S.H., Bradbury A.W., Adam D.J. Obesity and thrombosis. Eur J Vasc Endovasc Surg. 2007;33:223–233. doi: 10.1016/j.ejvs.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Kim C.K., Cho D.H., Lee K.S., Lee D.K., Park C.W., Kim W.G., Lee S.J., Ha K.S., Goo Taeg O., Kwon Y.G. Ginseng berry extract prevents atherogenesis via anti-inflammatory action by upregulating phase II gene expression. Evid Based Complement Alternat Med. 2012;2012:490301. doi: 10.1155/2012/490301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okafor O.N., Gorog D.A. Endogenous fibrinolysis: an important mediator of thrombus formation and cardiovascular risk. J Am Coll Cardiol. 2015;65:1683–1699. doi: 10.1016/j.jacc.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 8.Jeon B.R., Kim S.J., Hong S.B., Park H.J., Cho J.Y., Rhee M.H. The inhibitory mechanism of crude saponin fraction from Korean Red Ginseng in collagen-induced platelet aggregation. J Ginseng Res. 2015;39:279–285. doi: 10.1016/j.jgr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patrono C., Coller B., FitzGerald G.A., Hirsh J., Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:234S–264S. doi: 10.1378/chest.126.3_suppl.234S. [DOI] [PubMed] [Google Scholar]
- 10.Xie J.T., Wang C.Z., Zhang B., Mehendale S.R., Li X.L., Sun S., Han A.H., Du W., He T.C., Yuan C.S. In vitro and in vivo anticancer effects of American ginseng berry: exploring representative compounds. Biol Pharm Bull. 2009;32:1552–1558. doi: 10.1248/bpb.32.1552. [DOI] [PubMed] [Google Scholar]
- 11.Jeong E.S., Seo J.H., Yoo R., Lee Y.J., Kang J.H., Nah S.Y., Choi Y.K. Korean Red Ginseng extract alleviates atherosclerotic lesions in apolipoprotein E knockout mice. Food Sci Biotechnol. 2014;23:1267–1272. [Google Scholar]
- 12.Lu J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim Y.K., Yoo D.S., Xu H., Park N.I., Kim H.H., Choi J.E., Park S.U. Ginsenoside content of berries and roots of three typical Korean ginseng (Panax ginseng) cultivars. Nat Prod Commun. 2009;4:903–906. [PubMed] [Google Scholar]
- 14.Yang S.O., Park H.R., Sohn E.S., Lee S.W., Kim H.D., Kim Y.C., Kim K.H., Na S.W., Choi H.K., Arasu M.V. Classification of ginseng berry (Panax ginseng Meyer) extract using 1H NMR spectroscopy and its inhibition of lipid accumulation in 3 T3-L1 cells. BMC Complement Altern Med. 2014;14:455. doi: 10.1186/1472-6882-14-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cattaneo M., Federici A.B., Lecchi A., Agati B., Lombardi R., Stabile F., Bucciarelli P. Evaluation of the PFA-100 system in the diagnosis and therapeutic monitoring of patients with von Willebrand disease. Thromb Haemost. 1999;82:35–39. [PubMed] [Google Scholar]
- 16.Jung Y.M., Lee S.H., Lee D.S., You M.J., Chung I.K., Cheon W.H., Kwon Y.S., Lee Y.J., Ku S.K. Fermented garlic protects diabetic, obese mice when fed a high-fat diet by antioxidant effects. Nutr Res. 2011;31:387–396. doi: 10.1016/j.nutres.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Kang S.J., Lee J.E., Lee E.K., Jung D.H., Song C.H., Park S.J., Choi S.H., Han C.H., Ku S.K., Lee Y.J. Fermentation with Aquilariae Lignum enhances the antidiabetic activity of green tea in type II diabetic db/db mouse. Nutrients. 2014;6:3536–3571. doi: 10.3390/nu6093536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuman M.G., Cohen L.B., Nanau R.M. Biomarkers in nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol. 2014;28:607–618. doi: 10.1155/2014/757929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baranova A., Tran T.P., Afendy A., Wang L., Shamsaddini A., Mehta R., Chandhoke V., Birerdinc A., Younossi Z.M. Molecular signature of adipose tissue in patients with both non-alcoholic fatty liver disease (NAFLD) and polycystic ovarian syndrome (PCOS) J Transl Med. 2013;11:133. doi: 10.1186/1479-5876-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eriksson M., Johnson O., Boman K., Hallmans G., Hellsten G., Nilsson T.K., Soderberg S. Improved fibrinolytic activity during exercise may be an effect of the adipocyte-derived hormones leptin and adiponectin. Thromb Res. 2008;122:701–708. doi: 10.1016/j.thromres.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Wei S., Zhang Y., Su L., He K., Wang Q., Zhang Y., Yang D., Yang Y., Ma S. Apolipoprotein E-deficient rats develop atherosclerotic plaques in partially ligated carotid arteries. Atherosclerosis. 2015;243:589–592. doi: 10.1016/j.atherosclerosis.2015.10.093. [DOI] [PubMed] [Google Scholar]
- 22.Dang X., Miao J.J., Chen A.Q., Li P., Chen L., Liang J.R., Xie R.M., Zhao Y. The antithrombotic effect of RSNK in blood-stasis model rats. J Ethnopharmacol. 2015;173:266–272. doi: 10.1016/j.jep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Frendl C.M., Tucker S.M., Khan N.A., Esch M.B., Kanduru S., Cao T.M., Garcia A.J., King M.R., Butcher J.T. Endothelial retention and phenotype on carbonized cardiovascular implant surfaces. Biomaterials. 2014;35:7714–7723. doi: 10.1016/j.biomaterials.2014.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badimon L., Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014;276:618–632. doi: 10.1111/joim.12296. [DOI] [PubMed] [Google Scholar]
- 25.Okazaki M., Zhang H., Yoshida Y., Ichino K., Nakayama S., Oguchi K. Correlation between plasma fibrinogen and serum lipids in rats with hyperlipidemia induced by cholesterol free-high fructose or high cholesterol diet. J Nutr Sci Vitaminol (Tokyo) 1994;40:479–489. doi: 10.3177/jnsv.40.479. [DOI] [PubMed] [Google Scholar]
- 26.Liu S.Q., Guo J.Y., Du J., Deng Q., He Z.J., Lin H.Y., Lei S.H. Anticoagulant effect of Huisheng oral solution in a rat model of thrombosis. Indian J Pharmacol. 2013;45:359–364. doi: 10.4103/0253-7613.115018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee C.H., Kim J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Y.R., Yu J.Y., Lee J.J., You S.H., Chung J.H., Noh J.Y., Im J.H., Han X.H., Kim T.J., Shin K.S. Antithrombotic and antiplatelet activities of Korean Red Ginseng extract. Basic Clin Pharmacol Toxicol. 2007;100:170–175. doi: 10.1111/j.1742-7843.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 29.Sodhi K.S., Hondappanavar A., Saxena A.K., Dutta S., Khandelwal N. Intima-media complex thickness: preliminary workup of comparative evaluation of abdominal aorta and carotid artery of small-for-gestation-age term newborns and normal size term newborns. Acta Cardiol. 2015;70:351–357. doi: 10.1080/ac.70.3.3080640. [DOI] [PubMed] [Google Scholar]
- 30.Jarvisalo M.J., Jartti L., Nanto-Salonen K., Irjala K., Ronnemaa T., Hartiala J.J., Celermajer D.S., Raitakari O.T. Increased aortic intima-media thickness: a marker of preclinical atherosclerosis in high-risk children. Circulation. 2001;104:2943–2947. doi: 10.1161/hc4901.100522. [DOI] [PubMed] [Google Scholar]