Abstract
Background
The prevalence of allergic inflammatory diseases such as atopic dermatitis (AD), asthma, and allergic rhinitis worldwide has increased and complete recovery is difficult. Korean Red Ginseng, which is the heat-processed root of Panax ginseng Meyer, is widely and frequently used as a traditional medicine in East Asia. In this study, we investigated whether Korean Red Ginseng water extract (RGE) regulates the expression of proinflammatory cytokines and chemokines via the mitogen-activated protein kinases (MAPKs)/nuclear factor kappa B (NF-κB) pathway in allergic inflammation.
Methods
Compound 48/80-induced anaphylactic shock and 1-fluoro-2,4-dinitrobenzene (DNFB)-induced AD-like skin lesion mice models were used to investigate the antiallergic effects of RGE. Human keratinocytes (HaCaT cells) and human mast cells (HMC-1) were also used to clarify the effects of RGE on the expression of proinflammatory cytokines and chemokines.
Results
Anaphylactic shock and DNFB-induced AD-like skin lesions were attenuated by RGE administration through reduction of serum immunoglobulin E (IgE) and interleukin (IL)-6 levels in mouse models. RGE also reduced the production of proinflammatory cytokines including IL-1β, IL-6, and IL-8, and expression of chemokines such as IL-8, thymus and activation-regulated chemokine (TARC), and macrophage-derived chemokine (MDC) in HaCaT cells. Additionally, RGE decreased the release of tumor necrosis factor-α (TNF-α), IL-1β, IL-6, and IL-8 as well as expressions of chemokines including macrophage inflammatory protein (MIP)-1α, MIP-1β, regulated on activation, normal T cell expressed and secreted (RANTES), monocyte chemoattractant protein (MCP)-1, and IL-8 in HMC-1 cells. Furthermore, our data demonstrated that these inhibitory effects occurred through blockage of the MAPK and NF-κB pathway.
Conclusion
RGE may be a useful therapeutic agent for the treatment of allergic inflammatory diseases such as AD-like dermatitis.
Keywords: allergic inflammation, chemokine, keratinocytes, Korean Red Ginseng, mast cells
1. Introduction
Allergic inflammation is a hypersensitivity disorder of the immune system and occurs with several important medical conditions, such as atopic dermatitis (AD), allergic eczema, allergic rhinitis, and other allergic diseases. Most of the allergic diseases have increased in prevalence in the past few decades. They represent an important public health problem due to their impact on quality of life and medical expenditure burdens [1]. Among allergic diseases, AD correlates with the expression of proinflammatory cytokines and chemokines from keratinocytes, mast cells, and other immune cells [2], [3].
Keratinocytes are the major cell types in the epidermis, and they maintain the biochemical and physical condition of the skin. They are also involved in the progression of various inflammatory skin diseases [4]. Epidermal keratinocytes release inflammatory mediators such as proinflammatory cytokines and chemokines in response to immune triggers including UV light, allergens, and microbiological agents [5]. Activated keratinocytes are capable of producing biologically active interleukin (IL)-8, which mediates the influx of T cells and neutrophils into the epidermis [6]. Thymus and activation-regulated chemokine (TARC/CCL17) and macrophage-derived chemokine (MDC/CCL22), which are secreted from keratinocytes, play important roles in the infiltration of Th2 cells into inflammatory tissues [7].
Mast cells are also the major effector cells related to the allergic inflammatory reaction and are considered to be involved in the progression of AD because they show increases in the majority of AD patients [8], [9]. In response to several antigens, mast cell degranulation is initiated and these cells release a variety of bioactive substances such as histamine. Activated mast cells also produce various proinflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-1β, and IL-6 as well as chemokines [10]. These mediators contribute to inflammation through the recruitment and activation of immune cells [11].
Mitogen-activated protein kinases (MAPKs) including the extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK), and p38 MAPK regulate diverse cellular functions such as proliferation, activation, and degranulation [12]. The activation of MAPKs is associated with the allergic inflammatory response via the translocation of nuclear factor kappa B (NF-κB), which causes the production of proinflammatory cytokines and chemokines [13], [14], [15]. After phosphorylation of MAPKs, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα) proteins are degraded and NF-κB is translocated into the nucleus and initiates the transcription of target genes. Therefore, as the inhibition of MAPKs and NF-κB activation subsequently decrease secretion of allergic mediators, MAPKs are important in the prevention of allergic inflammation [16], [17], [18].
Ginseng, the root of Panax ginseng Meyer, has been used for the treatment of many diseases and for enhancing physical strength and immunity. Korean Red Ginseng, which is heat-processed ginseng, is a popular traditional medicine in East Asia and has been widely used to treat a number of diseases such as cancer, Alzheimer's disease, and vascular diseases [19], [20], [21]. Several studies have reported that Korean Red Ginseng has antiallergic effects in in vitro and in vivo models [22], [23]. Although the antiallergic effects of Korean Red Ginseng have already been reported, mechanistic understanding of its immunopharmacological roles is poorly elucidated.
In this study, we investigated the effects of Korean Red Ginseng water extract (RGE) on allergic inflammation and mechanisms. We evaluated the effects of RGE on systemic and local allergic reactions to assess its antiallergic effect in compound 48/80-induced anaphylactic shock and 1-fluoro-2,4-dinitrobenzene (DNFB)-induced dermatitis mice models. Moreover, the effects of RGE on the expression of proinflammatory cytokines and chemokines, as well as its mechanisms, were investigated in HaCaT cells and HMC-1 cells.
2. Materials and methods
2.1. Reagents
Compound 48/80, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), phorbol 12-myristate 13-acetate (PMA), and calcium ionophore A23187 (A23187) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) was purchased from Promega Corporation (Madison, WI, USA). Avidin peroxidase (AP), fetal bovine serum (FBS), RPMI 1640, and Iscove's Modified Dulbecco's Medium (IMDM) were purchased from Gibco BRL (Grand Island, NY, USA). Anti-human TNF-α/IL-6/IL-8, anti-mouse immunoglobulin E (IgE)/IL-6, recombinant TNF-α/IL-6/IL-8, and biotinylated TNF-α/IL-6/IL-8 were purchased from BD Pharmingen (San Diego, CA, USA). Anti-phospho-p38, -ERK, -JNK, -Akt, -IκBα, anti-GAPDH, IκBα, and NF-κB antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-p38, JNK, and histone antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-Akt and ERK antibodies were purchased from Bioworld Technology (Minneapolis, MN, USA).
2.2. Preparation of RGE
RGE was kindly provided by the Korea Ginseng Corporation (Daejeon, Korea). It was manufactured by Korea Ginseng Corporation, Seoul, Korea from roots of a 6-yr-old red ginseng, Panax ginseng Meyer, harvested in the Republic of Korea. Red ginseng was made by steaming fresh ginseng at 90–100°C for 3 h and then drying at 50–80°C. RGE was prepared from red ginseng water extract, which was extracted by circulating 85–90°C water for 8 h three times. The water content of the pooled extract was 36% of the total weight. RGE was analyzed by high-performance liquid chromatography. RGE contained major ginsenoside-Rb1: 7.44 mg/g, -Rb2: 2.59 mg/g, -Rc: 3.04 mg/g, -Rd: 0.91 mg/g, -Re: 1.86 mg/g, -Rf: 1.24 mg/g, -Rg1: 1.79 mg/g, -Rg2: 1.24 mg/g, -Rg3: 1.39 mg/g, and other minor ginsenosides.
2.3. Animals
BALB/c mice (5 wk, male, 19–20 g) were purchased from Da-Mool Science (Daejeon, Republic of Korea). The animals were housed in a laminar air-flow room maintained at a temperature of 22 ± 1°C and a humidity level of 55 ± 1% throughout the study. The research was conducted in accordance with the internationally accepted principles for laboratory animal use and care as stated in the Wonkwang University (Iksan, Republic of Korea) guidelines (WKU-14-35).
2.4. Cell culture
Human keratinocytes HaCaT cells were obtained from CLS Cell Lines Service (Eppelheim, Baden-Württemberg, Germany) and the human mast-cell line (HMC-1) was generously provided by Eichi Morri (Osaka University, Osaka, Japan). HaCaT cells were cultured in RPMI 1640 and HMC-1 cells were cultured in IMDM supplemented with 10% FBS, 100 units/mL of penicillin, and 100 μg/mL of streptomycin in a humidified atmosphere of 5% CO2 at 37°C.
2.5. DNFB-induced AD-like dermatitis mouse model
The dorsal skin of mice (n = 7) was shaved by depilatory equipment before the experiment. The mice were sensitized with a vehicle or 100 μL 0.15% solution of DNFB in acetone/olive oil (3:1) to the dorsal skin twice each week for 6 wk. The AD-like dermatitis-induced mice were divided into four groups: Blank, DNFB, RGE (5 mg/kg or 50 mg/kg), and Pre-RGE (50 mg/kg) group. RGE oral administration of the Pre-RGE group was initiated immediately after the first DNFB application to investigate the preventive effect. The RGE group was orally administered RGE after 4 wk of DNFB application.
2.6. Histological analysis
Mouse dorsal skin samples were excised from the mice 24 h after the final DNFB application. Samples were fixed with 10% formaldehyde, embedded in paraffin wax, routinely processed, and then sectioned into 4 μm thick slices. The skin sections were then stained with hematoxylin and eosin (H&E) and photographed by a light microscope for the presence of edema and for inflammatory cell accumulation.
2.7. Compound 48/80-induced anaphylactic shock
The mice (n = 5) were intraperitoneally injected with compound 48/80 (8 mg/kg). RGE (5 mg/kg and 50 mg/kg) was orally administered 1 h prior to the injection of compound 48/80. Mortality was monitored for 1 h after the induction of anaphylactic shock.
2.8. Cell viability
An MTT or MTS assay was performed to investigate the cell toxicity of RGE on HaCaT and HMC-1 cells, respectively. After treatment of HaCaT cells with RGE for 24 h, MTT solution (500 μg/mL) was added and followed by incubation at 37°C for 4 h. The crystallized formazan was dissolved in DMSO and the absorbance was measured at 540 nm by a microplate reader. Cell viability of RGE-treated HMC-1 cells was determined using a cell proliferation MTS reagent. MTS solution was added for 2 h and the absorbance was measured by a microplate reader at 490 nm.
2.9. RNA extraction and real-time reverse transcription polymerase chain reaction
Total RNA was extracted using an RNA-spin total RNA extraction kit (iNtRon Biotech, Seoul, Republic of Korea) according to the manufacturer's directions. First-strand cDNA was prepared from an RNA template (2 μg) using oligo dT primer and a power cDNA synthesis kit (iNtRon Biotech). Reverse transcription (RT) was performed at 42°C for 50 min and then at 70°C for 15 min. Real-time quantitative RT-polymerase chain reaction (PCR) was performed using a Power SYBR Green PCR Master Mix and Step-One Plus Real-Time PCR systems (Applied Biosystems, Foster City, CA, USA). All data were normalized to GAPDH mRNA. The primer sequences are summarized in Table 1.
Table 1.
Sequences of the Real-Time reverse Transcription Polymerase Chain Reaction (RT-PCR) primers
| Genes | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| IL-8 (m) | TGTAATGAAAGACGGCACACC | TCTTCTTTGGGTATTGCTTGG |
| MDC (m) | CCAAGGTGCCTTTGAAGACT | TCCTCCAGCTGGTGGTTACT |
| TARC (m) | CCAGGAAATCTCTGGTCTGC | CTGTGGAAGCAGTGGAGTCA |
| GAPDH (m) | GACATGCCGCCTGGAGAAAC | AGCCCAGGATGCCCTTTAGT |
| IL-8 (h) | AAGCTGGCCGTGGCTCTCTTG | AGCCCTCTTCAAAAACTTCTC |
| TNF-α (h) | CGCTCCCCAAGAAGACAG | AGAGGCTGAGGAACAAGCAC |
| MDC (h) | AGGACAGAGCATGGATCGCCTACAGA | TAATGGCAGGGAGGTAGGGCTCCTGA |
| TARC (h) | CTTCTCTGCAGCACATCC | AAGACCTCTCAAGGCTTTG |
| MIP-1α (h) | GTCTGTGCTGATCCCAGTGA | TTGTCACCAGACGCGGTGTG |
| MIP-1β (h) | GTCTGTGCTGATCCCAGTGA | GGACACTTATCCTTTGGCTA |
| RANTES (h) | CCGCGGCAGCCCTCGCTGTCATCC | CATCTCCAAAGAGTTGATGTACTCC |
| MCP-1 (h) | GTCTCTGCCGCCCTTCTGT | TTGCATCTGATGGCAGTAGCT |
| GAPDH (h) | TGCACCACCACCTGCTTAGC | GGCATGGACTGTGGTCATGAG |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; h, human; IL-8, interleukin-8; m, mouse; MCP-1, monocyte chemoattractant protein-1; MDC, macrophage-derived chemokine; RANTES, regulated on activation, normal T cell expressed and secreted; TARC, thymus and activation-regulated chemokine; TNF-α, tumor necrosis factor-α.
2.10. Enzyme-linked immunosorbent assay
Microplates (96-well) were coated with capture antibodies overnight at 4°C. For the blocking step, plates were washed with 0.05% phosphate buffer saline (PBS)-Tween20 (PBST) and 5% FBS in PBS was added. Between subsequent steps in this assay, plates were washed four times with 0.05% PBST. After 1 h, samples and standards were added to the plates and incubated for 3 h. Assay plates were exposed to biotin-conjugated antibodies for 1 h and incubated with AP for 30 min in a 37°C incubator. Finally, TBS substrate solution was added and color development was measured by a microplate reader at 405 nm.
2.11. Preparation of total cell lysates and nuclear extracts and Western blot analysis
Collected cells were rinsed with ice-cold PBS and lysed using lysis buffer (iNtRon Biotech, Seoul, Republic of Korea) for 1 h. Total cell lysates were centrifuged for 10 min at 4°C, and the supernatants were obtained. Nuclear extracts were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Thermo Scientific, Rockford, IL, USA). After bicinchoninic acid protein quantification, the supernatant was mixed with a 2 × sample buffer, boiled for 5 min, separated by 10% SDS-polyacrylamide gel electrophoresis, and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% skim milk for 1 h 30 min and incubated for 3 h with primary antibodies. After washing with 0.1% PBST for 45 min, membranes were then incubated with secondary antibodies for 45 min. After washing with 0.1% PBST for 1 h 30 min, protein bands were detected using an enhanced chemiluminescence (ECL) solution.
2.12. Statistical analysis
The results are shown as a summary of the data from three experiments and are presented as the mean ± standard deviation (SD). A statistical evaluation of the results was performed by the Student t test using SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). For all experiments, p < 0.05 was considered to be significant.
3. Results
3.1. Effect of RGE on compound 48/80-induced anaphylactic shock and DNFB-induced dermatitis
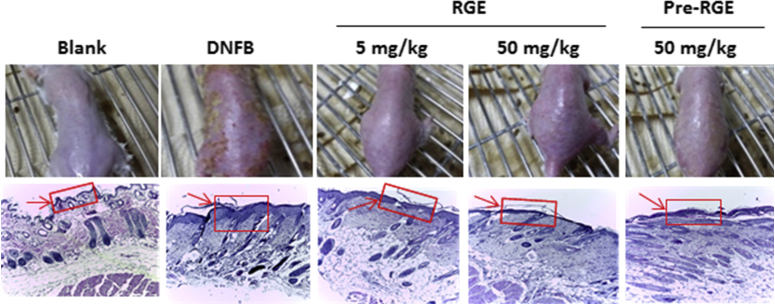
To evaluate the effect of RGE on systemic allergic reaction, we used the compound 48/80-induced anaphylactic shock model. The dose of 5–50 mg/kg of RGE was chosen because several studies had previously reported various pharmacological in vivo effects of RGE at such doses [24], [25], [26]. As shown in Table 2, RGE (5 mg/kg and 50 mg/kg) oral administration dose-dependently reduced the mortality rate induced by compound 48/80 (Table 2). The repeated cutaneous application of DNFB induces AD-like dermatitis in BALB/c mice [27]. To investigate the prevention and therapeutic effect of RGE on AD-like dermatitis, we used the DNFB-induced dermatitis mouse model and divided the administration group into pre-RGE (50 mg/kg) and RGE (5 mg/kg and 50 mg/kg). AD-like skin lesions were improved in the pre-RGE and RGE groups as compared to the DNFB group (Fig. 1, upper panels). The dorsal skins were stained using H&E reagents and DNFB-induced hyperplasia was inhibited by RGE administration. Accumulation of immune cells in AD-like skin lesions was decreased by RGE administration (Fig. 1, lower panels).
Table 2.
Effect of Korean Red Ginseng Extract (RGE) on the compound 48/80-induced anaphylactic reaction
| RGE (mg/kg) 1) | Compound 48/80 (8 mg/kg) 2) | Mortality (%) 3) |
|---|---|---|
| None (saline) | + | 100 |
| 5 | + | 40* |
| 50 | + | 26.7* |
| 50 | - | 0 |
*p < 0.05; significantly different from saline group.
The groups of mice (n = 5) were orally administrated with saline or Korean Red Ginseng extract (RGE) given at various doses 1 h before the compound 48/80 injection.
The compound 48/80 solution was intraperitoneally injected to the groups of mice
Mortality (%) within 1 h following compound 48/80 injection is presented as the “Number of dead mice × 100/total number of experimental mice”. This result was represented by average results from three independent experiments.
Fig. 1.
Effect of Korean Red Ginseng extract (RGE) on 1-fluoro-2,4-dinitrobenzene (DNFB)-induced atopic dermatitis (AD)-like dermatitis. Comparison of DNFB-induced dermatitis in BALB/c mice after oral administration of RGE (5 mg/kg and 50 mg/kg) or Pre-RGE (50 mg/kg; upper panels) and hematoxylin and eosin (H&E) stained tissue sections (lower panels). Skin tissues were fixed with 10% formaldehyde embedded in paraffin and H&E staining was conducted. The immune cells (arrows) are indicated. Photographs were taken under a regular light microscope (100 × magnification). Blank, DNFB-untreated normal mice; DNFB, DNFB-treated AD mice; RGE, DNFB-treated AD mice administered RGE after 4 wk of DNFB treatment; Pre-RGE, DNFB-treated AD mice administered RGE after the first DNFB treatment.
3.2. Effects of RGE on chemokines and inflammatory mediators in DNFB-induced dermatitis mice
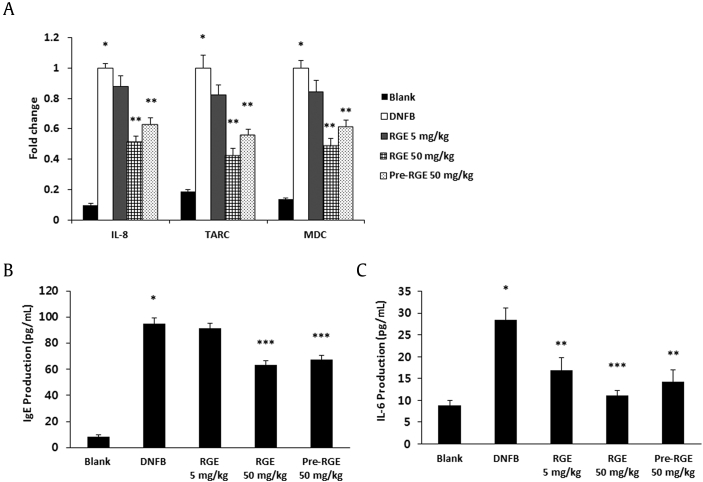
To investigate the effects of RGE on the inflammatory mediator production, dorsal skin lesions and blood samples were collected. These samples were analyzed using real-time RT-PCR and the enzyme-linked immunosorbent assay (ELISA) method. Expression levels of IL-8, TARC, and MDC were significantly decreased in the RGE 50 mg/kg and pre-RGE groups compared to the DNFB group (Fig. 2A). Moreover, serum IgE and IL-6 levels were reduced by RGE administration compared to the DNFB group (Figs. 2B and 2C).
Fig. 2.
Effects of Korean Red Ginseng extract (RGE) on chemokine expression in skin lesions and serum immunoglobulin E (IgE) and interleukin-6 (IL-6) levels. (A) mRNA levels of IL-8, thymus and activation-regulated chemokine (TARC), and macrophage-derived chemokine (MDC) in skin lesions. Total RNA was isolated from normal or 1-fluoro-2,4-dinitrobenzene (DNFB)-induced dermatitis dorsal skin. (B and C) Blood samples were collected from the mice, and the levels of serum IgE (B) and IL-6 (C) in the indicated groups were measured with the enzyme-linked immunosorbent assay (ELISA) method. Values represent the mean ± standard deviation (SD) of three independent experiments. *p < 0.05 versus blank and **p < 0.05, ***p < 0.01 versus DNFB alone. Blank, DNFB-untreated normal mice; DNFB, DNFB-treated atopic dermatitis (AD) mice; RGE, DNFB-treated AD mice administered RGE after 4 wk of DNFB treatment; Pre-RGE, DNFB-treated AD mice administered RGE after the first DNFB treatment.
3.3. Effect of RGE on the activation of MAPKs and NF-κB in skin lesions of DNFB-induced dermatitis mice
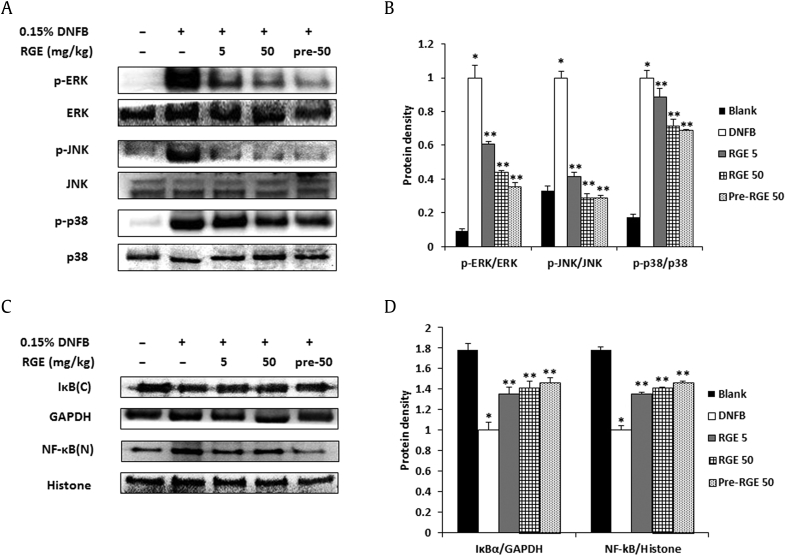
The activation of MAPKs and NF-κB induces the expression of chemokines and proinflammatory cytokines, which are pivotal factors in allergic reactions. Therefore, we evaluated the effect of RGE on the activation of MAPKs and NF-κB in AD-like dermatitis skin lesions. As shown in Fig. 3A, RGE administration suppressed the phosphorylation of ERK, JNK, and p38 in dorsal skin compared to the DNFB group. The relative expression levels of the MAPKs are represented in Fig. 3B. In addition, RGE inhibited the degradation of IκBα and NF-κB translocation into the nucleus (Fig. 3C). The relative expression levels of IκBα and NF-κB are represented in Fig. 3D.
Fig. 3.
Effects of Korean Red Ginseng extract (RGE) on the mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) signaling pathway in 1-fluoro-2,4-dinitrobenzene (DNFB)-induced skin lesions. Protein was isolated from normal or DNFB-induced dermatitis dorsal skin. (A) Phosphorylation levels of extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK), and p38 was assayed by a Western blot analysis. (B) Relative levels of p-ERK, p-JNK, and p-p38 were calculated using an image analyzer. Values represent the mean ± standard deviation (SD) of independent experiments. (C) The degradation of IκBα and the translocation of NF-κB were assayed by a Western blot analysis. (D) The relative levels of IκBα and NF-κB were calculated using an image analyzer. Values represent the mean ± SD of independent experiments. *p < 0.05 versus blank and **p < 0.05 versus DNFB alone. Blank, DNFB-untreated normal mice; DNFB, DNFB-treated atopic dermatitis (AD) mice; RGE, DNFB-treated AD mice administered RGE after 4 wk of DNFB treatment; Pre-RGE, DNFB-treated AD mice administered RGE after the first DNFB treatment.
3.4. Effects of RGE on the expression of proinflammatory cytokines and chemokines in HaCaT cells
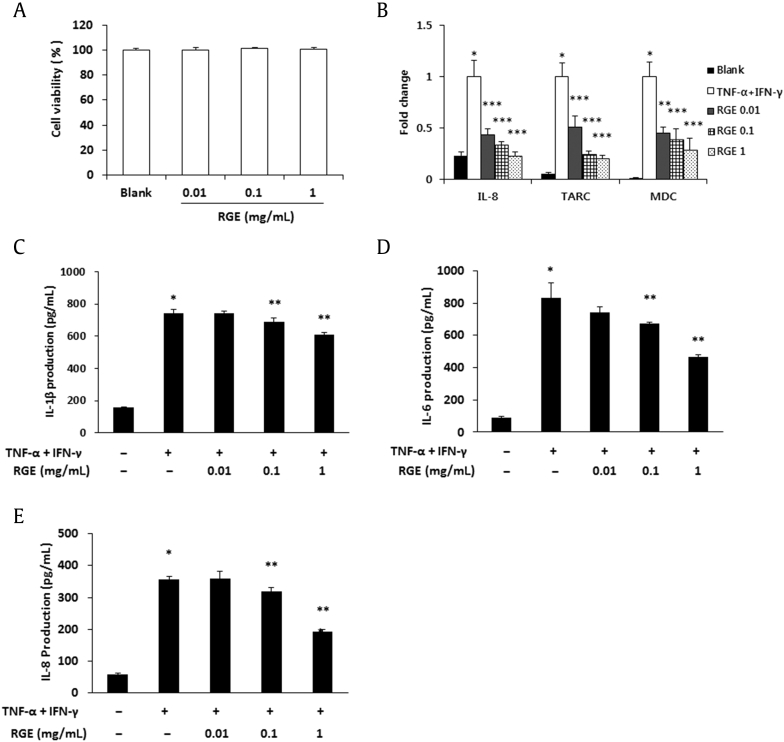
Epidermal keratinocytes play multiple roles in the immune reactions associated with allergic dermatitis and other skin diseases [28]. Therefore, the effect of RGE on the cell viability was first examined using an MTT assay. RGE treatment with 0.01–1 mg/mL did not show cytotoxicity in HaCaT cells (Fig. 4A). Due to this result, further assays were performed at concentrations of 0.01 mg/mL, 0.1 mg/mL, and 1 mg/mL. However, previous reports demonstrated that high concentrations of RGE (1–2.5 mg/mL) showed a neuroprotective effect without affecting cell viability [29], [30]. To determine whether RGE inhibits the expression of TNF-α/interferon (IFN)-γ-induced chemokines and proinflammatory cytokines, we carried out real-time RT-PCR and ELISA. The expression levels of chemokines (IL-8, TARC, and MDC) were suppressed by the RGE treatment in a dose-dependent manner (Fig. 4B). Moreover, RGE significantly suppressed the production of IL-1β, IL-6, and IL-8 in the TNF-α/IFN-γ-stimulated HaCaT cells (Figs. 4C–4E).
Fig. 4.
Effects of Korean Red Ginseng extract (RGE) on chemokine expressions and proinflammatory cytokine production in HaCaT cells. (A) Cell viability was analyzed by a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Cells were seeded in 24-well microplates at 5 × 104 cells/well and various concentrations of RGE were added to each well for 24 h. (B) The mRNA level of chemokines was assayed by a real-time reverse transcription polymerase chain reaction (RT-PCR) analysis. (C–E) The production levels of interleukin (IL)-1β (C), IL-6 (D), and IL-8 (E) were measured by the enzyme-linked immunosorbent assay (ELISA) method. HaCaT cells were pretreated with RGE for 30 min and were stimulated with tumor necrosis factor-α (TNF-α; 10 ng/mL) and interferon (IFN)-γ (10 ng/mL) for 18 h. Values represent the mean ± standard deviation (SD) of three independent experiments. *p < 0.05 versus blank and **p < 0.05 versus TNF-α + IFN-γ alone.
3.5. Effects of RGE on the activation of MAPKs and NF-κB in HaCaT cells
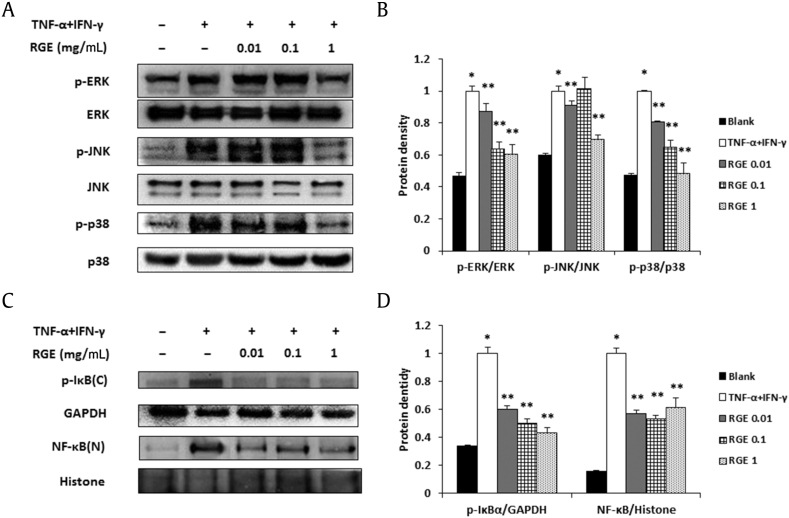
The intracellular mechanisms for the release of various inflammatory mediators are coordinated by MAPK and NF-κB in keratinocytes [31]. Thus, we investigated the regulatory effects of RGE on the activation of MAPKs and NF-κB in HaCaT cells by Western blot analysis. RGE decreased TNF-α/IFN-γ-induced phosphorylation of ERK, JNK, and p38 (Figs. 5A and 5B). RGE also inhibited the phosphorylation of IκBα and the translocation of NF-κB (Figs. 5C and 5D).
Fig. 5.
Effects of Korean Red Ginseng extract (RGE) on the mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) signaling pathway in HaCaT cells. Phosphorylation of MAPKs (A, B) and the phosphorylation of IκBα and translocation of NF-κB (C, D) were assayed by a Western blot analysis. HaCaT cells (1 × 106 cells/well) were incubated with various concentrations of RGE and stimulated with phorbol 12-myristate 13-acetate (PMA; 50 nM) + A23187 (1 μM) for 30 min (A, B) or 2 h (C, D). C, cytosol extracts; N, nuclear extracts. (B, D) Densitometry analyses presented as relative ratios of total protein (B) or housekeeping protein (D) were used as a loading control. The data shown are representative of three independent experiments. Values represent the mean ± standard deviation (SD) of three independent experiments. *p < 0.05 versus blank and **p < 0.05 versus tumor necrosis factor-α (TNF-α) + interferon (IFN)-γ alone.
3.6. Effects of RGE on the expression of proinflammatory cytokines and chemokines in HMC-1 cells
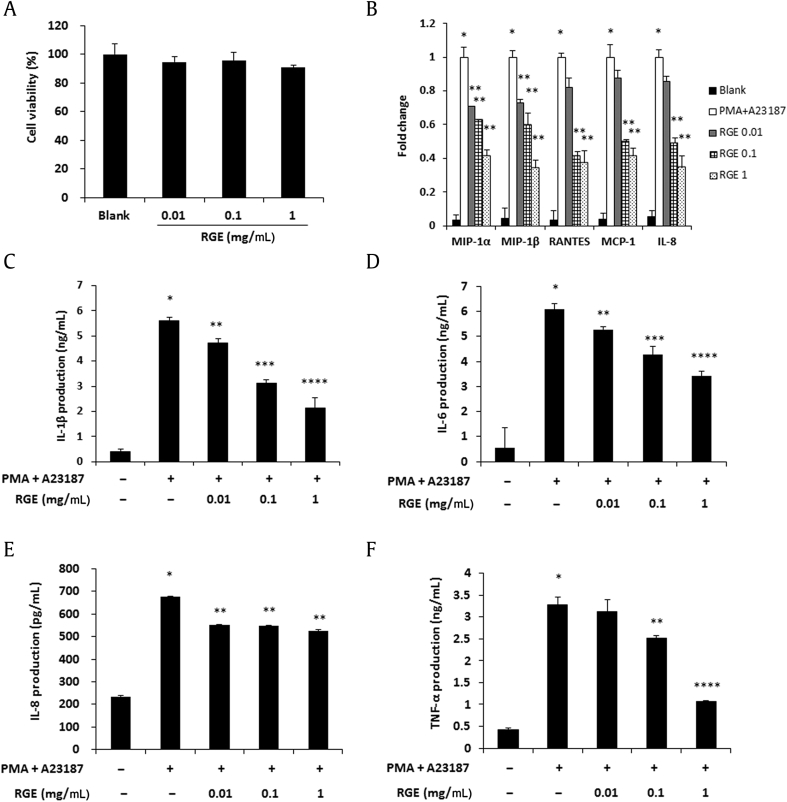
Mast cells also play a critical role in allergic reactions through the release of a number of mediators and cytokines [11]. Therefore, we determined whether RGE regulates the levels of chemokines and proinflammatory cytokines in HMC-1 cells. We first checked the effect of RGE on the cell viability of HMC-1 cells by an MTS assay (Fig. 6A) and treated HMC-1 cells with a nontoxic concentration of RGE (0.01–1 mg/mL). Expressions of macrophage inflammatory protein (MIP)-1α, MIP-1β, regulated upon activation normal T expressed and secreted (RANTES), monocyte chemoattractant protein (MCP)-1, and IL-8 were decreased by RGE treatment (Fig. 6B). In addition, RGE significantly suppressed the production of proinflammatory cytokines including IL-1β, IL-6, IL-8, and TNF-α (Figs. 6C–6F).
Fig. 6.
Effects of Korean Red Ginseng extract (RGE) on chemokine expression and proinflammatory cytokine production in HMC-1 cells. (A) HMC-1 cells (1 × 104 cells/well) were treated with RGE (0.01–1 mg/mL) for 24 h and the cell viability was analyzed with a MTS assay. (B) The mRNA level of chemokines was assayed by a real-time reverse transcription polymerase chain reaction (RT-PCR) analysis. (C–F) The production levels of interleukin (IL)-1β (C), IL-6 (D), IL-8 (E), and tumor necrosis factor-α (TNF-α) (F) were measured using the enzyme-linked immunosorbent assay (ELISA) method. HMC-1 cells (2 × 105 cells/well) were pretreated with RGE for 1 h and were stimulated with phorbol 12-myristate 13-acetate (PMA; 50 nM) + A23187 (1 μM) for 8 h. Values represent the mean ± standard deviation (SD) of three independent experiments. *p < 0.05 versus blank and **p < 0.05, ***p < 0.01, ****p < 0.001 versus PMA + A23187 alone.
3.7. Effects of RGE on the activation of MAPKs and NF-κB in HMC-1 cells
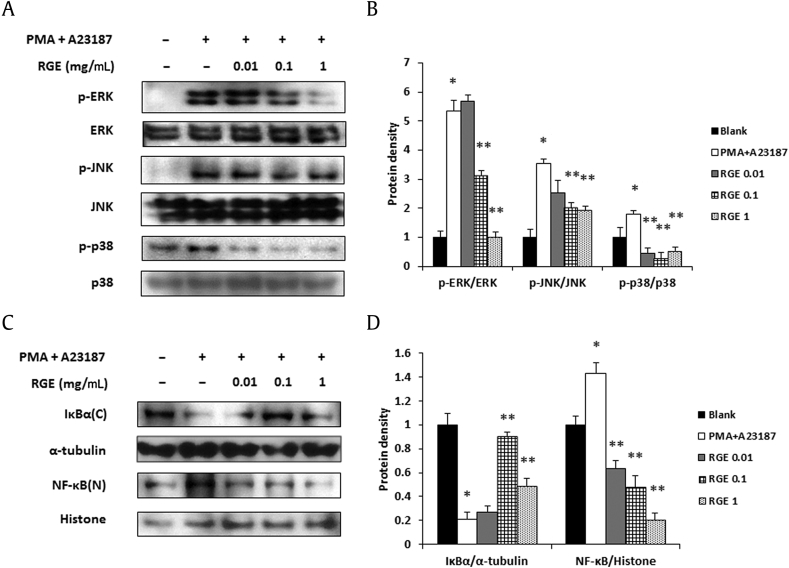
The expressions of proinflammatory cytokines and chemokines are induced by allergen stimulation via the MAPKs/NF-κB pathway in mast cells. For this reason, the MAPKs/NF-κB signaling pathway is an important component in the regulation of mast cell-mediated inflammatory responses [32]. To determine which molecules are regulated by RGE in an allergic reaction, we examined the effects of RGE on the phosphorylation of MAPKs. RGE significantly decreased PMA+A23187-induced phosphorylation of ERK, JNK, and p38 (Figs. 7A and 7B). Additionally, RGE inhibited the degradation of IκBα and the nuclear localization of NF-κB (Figs. 7C and7 D).
Fig. 7.
Effect of Korean Red Ginseng extract (RGE) on the mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) signaling pathway in HMC-1 cells. Phosphorylation of MAPKs (A, B) and the degradation of IκBα and translocation of NF-κB (C, D) were assayed by a Western blot analysis. HMC-1 cells (1 × 106 cells/well) were incubated with various concentrations of RGE and then stimulated with phorbol 12-myristate 13-acetate (PMA; 50 nM) + A23187 (1 μM) for 30 min (A, B) or 2 h (C, D). C, cytosol extracts; N, nuclear extracts. (B, D) Densitometry analyses presented as relative ratios of total protein (B) and housekeeping protein (D) were used as a loading control. The data shown are representative of three independent experiments. Values represent the mean ± standard deviation (SD) of three independent experiments. *p < 0.05 versus blank and **p < 0.05 versus PMA + A23187 alone.
4. Discussion
Korean Red Ginseng is known to afford protective effects against skin diseases including dermatitis. In a TNCB-induced AD mouse model, Korean Red Ginseng significantly reduced the total clinical severity score, ear thickness, and the level of thymic stromal lymphopoietin (TSLP) and TNF-α [33], [34]. Additionally, Korean Red Ginseng ethanol extract improved skin lesions and reduced the Th2-activated cytokines, such as IL-4, IL-10, and the serum IgE levels. These suppressive effects of Korean Red Ginseng ethanol extract appear to be mediated through the inhibition of MAPKs, such as ERK, JNK, and p38 signaling pathways in DNFB-induced AD-like mice [35]. However, the effects of RGE on AD-like dermatitis and related mechanisms have not been elucidated in the context of dermatitis.
Chemokines are small proteins secreted by the immune cells that mediate many functions including recruitment of their specific receptor-expressed cells [36]. Among these chemokines, IL-8 is a potent neutrophil chemoattractant and Th2 chemokines such as TARC and MDC also attract CCR4+ lymphocytes to sites of inflammation [6], [37]. These chemokines are produced by various cell types, including keratinocytes, and their levels were also elevated in the serum of AD patients and were closely correlated to disease severity [7], [38], [39], [40]. Moreover, mast cells are involved in the pathogenesis of AD through secretion of various chemokines such as MIP-1α, MCP-1, and IL-8 in AD patients [9]. Therefore, we focused on chemokines and proinflammatory cytokines released from AD-like skin lesions and their mechanisms in vivo and in vitro. RGE improves DNFB-induced AD-like skin lesions (Fig. 1) through inhibition of chemokine and proinflammatory cytokine expressions via MAPKs and the NF-κB pathway (Fig. 2, Fig. 3). These results suggest that RGE reduces the expression of cytokines and chemokines via inhibition of MAPKs and the NF-κB pathway in AD-like skin lesions.
HaCaT cells, a spontaneously immortalized human keratinocyte cell line, maintain normal keratinocyte morphology and play an important role in skin inflammation because they are constantly exposed to external stimuli [41]. These cells are thus commonly used in pharmacological in vitro studies of potential skin drugs [42]. Stimulation of keratinocytes with TNF-α and IFN-γ enhances the expression of chemokines and proinflammatory cytokines [28]. The HMC-1 cell line was established from leukemia patients; it expresses characteristic markers for mast cells [43]. Mast cells also play a critical role in both adaptive and innate immunity and show increases in immunological skin diseases, including dermatitis [44]. Therefore, HaCaT and HMC-1 cells are ideal candidates to confirm the biological activities of RGE in vitro. RGE decreases the secretion of proinflammatory cytokines such as TNF-α and IL-8 in LPS-stimulated HaCaT cells [45], but it has not been investigated whether this effect leads to improvement of dermatitis in vivo. In this study, RGE significantly inhibited the expressions of chemokines such as IL-8, TARC, and MDC in TNF-α/IFN-γ-stimulated HaCaT cells in a dose-dependent manner (Fig. 4). Moreover, we first demonstrated that the expression of chemokines including MIP-1α, MIP-1β, RANTES, MCP-1, and IL-8, was decreased by RGE treatment in PMA+A23187-stimulated HMC-1 cells (Fig. 6). Therefore, our data suggest that RGE may be useful to treat AD and other related allergic diseases, based on its regulatory effects on chemokine expression.
The MAPK signal transduction pathway has been shown to be important in the proliferation, degranulation, and activation of various immune cells. In particular, the expression of proinflammatory cytokines and chemokines is associated with the MAPK (ERK, JNK, and p38)/NF-κB pathway in keratinocytes and mast cells [10], [46], [47]. Therefore, we confirmed the regulatory effect of RGE on the MAPKs/NF-κB pathway to elucidate the mechanisms of RGE-induced inhibition of proinflammatory cytokine and chemokine expression in HaCaT and HMC-1 cells. RGE significantly prevented the phosphorylation of p38, ERK, and JNK in both HaCaT and HMC-1 cells (Fig. 5, Fig. 7). These results suggest that suppression of ERK, JNK, p38, and NF-κB activation by RGE treatment might lead to decreased production of proinflammatory cytokines and chemokines in keratinocytes or mast cells.
In conclusion, RGE showed a protective effect on the compound 48/80-induced anaphylactic shock and DNFB-induced AD-like mouse model through the inhibition of serum IgE and IL-6 release and chemokine expression. Additionally, RGE administration decreased activation of ERK, JNK, p38, and NF-κB in AD-like skin lesions. These effects of RGE on AD were due to inhibition of chemokines and proinflammatory cytokines, which resulted from blockage of the MAPK/NF-κB pathway in keratinocytes and mast cells. Taken together, the present results suggest that Korean Red Ginseng may be useful as a therapeutic agent for allergic inflammatory diseases such as dermatitis.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the 2013 grant from the Korean Society of Ginseng, and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP; NRF-2013R1A2A2A01067805, NRF-2015R1A4A1042399, and 2014R1A6A3A01056089).
Contributor Information
Jae-Young Um, Email: jyum@khu.ac.kr.
Seung-Heon Hong, Email: jooklim@wku.ac.kr.
References
- 1.Leung D.Y.M., Bieber T. Atopic dermatitis. Lancet. 2003;361:151–160. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- 2.Novak N. New insights into the mechanism and management of allergic diseases: atopic dermatitis. Allergy. 2009;64:265–275. doi: 10.1111/j.1398-9995.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 3.Sebastiani S., Albanesi C., De P.O., Puddu P., Cavani A., Girolomoni G. The role of chemokines in allergic contact dermatitis. Arch Dermatol Res. 2002;293:552–559. doi: 10.1007/s00403-001-0276-9. [DOI] [PubMed] [Google Scholar]
- 4.Gröne A. Keratinocytes and cytokines. Vet Immunol Immunopathol. 2002;88:1–12. doi: 10.1016/s0165-2427(02)00136-8. [DOI] [PubMed] [Google Scholar]
- 5.Uchi H., Terao H., Koga T., Furue M. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(Suppl. 1):S29–S38. doi: 10.1016/s0923-1811(00)00138-9. [DOI] [PubMed] [Google Scholar]
- 6.Barker J.N., Jones M.L., Mitra R.S., Crockett-Torabe E., Fantone J.C., Kunkel S.L., Warren J.S., Dixit V.M., Nickoloff B.J. Modulation of keratinocyte-derived interleukin-8 which is chemotactic for neutrophils and T lymphocytes. Am J Pathol. 1991;139:869–876. [PMC free article] [PubMed] [Google Scholar]
- 7.Horikawa T., Nakayama T., Hikita I., Yamada H., Fujisawa R., Bito T., Harada S., Fukunaga A., Chantry D., Gray P.W. IFN-gamma-inducible expression of thymus and activation-regulated chemokine/CCL17 and macrophage-derived chemokine/CCL22 in epidermal keratinocytes and their roles in atopic dermatitis. Int Immunol. 2002;14:767–773. doi: 10.1093/intimm/dxf044. [DOI] [PubMed] [Google Scholar]
- 8.Galli S.J., Kalesnikoff J., Grimbaldeston M.A., Piliponsky A.M., Williams C.M., Tsai M. Mast cells as ”tunable“ effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 9.Kawakami T., Ando T., Kimura M., Wilson B.S., Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009;21:666–678. doi: 10.1016/j.coi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theoharides T.C., Alysandratos K.D., Angelidou A., Delivanis D.A., Sismanopoulos N., Zhang B., Asadi S., Vasiadi M., Weng Z., Miniati A. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822:21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galli S.J., Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol. 2010;40:1843–1851. doi: 10.1002/eji.201040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson G.L., Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 13.Hommes D.W., Peppelenbosch M.P., van Deventer S.J. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut. 2003;52:144–151. doi: 10.1136/gut.52.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.May M.J., Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 15.Arthur J.S., Ley S.C. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 16.Finco T.S., Baldwin A.S. Mechanistic aspects of NF-kappa B regulation: the emerging role of phosphorylation and proteolysis. Immunity. 1995;3:263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 17.Barnes P.J., Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 18.Barnes P.J. Pathophysiology of allergic inflammation. Immunol Rev. 2011;242:31–50. doi: 10.1111/j.1600-065X.2011.01020.x. [DOI] [PubMed] [Google Scholar]
- 19.Choi J., Kim T.H., Choi T.Y., Lee M.S. Ginseng for health care: a systematic review of randomized controlled trials in Korean literature. PLoS ONE. 2013;8:e59978. doi: 10.1371/journal.pone.0059978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heo J.H., Lee S.T., Oh M.J., Park H.J., Shim J.Y., Chu K., Kim M. Improvement of cognitive deficit in Alzheimer's disease patients by long term treatment with Korean Red Ginseng. J Ginseng Res. 2011;35:457–461. doi: 10.5142/jgr.2011.35.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J.B., Kwon S.K., Nagar H., Jung S.B., Jeon B.H., Kim C.S., Oh J.H., Song H.J., Kim C.S. Rg3-enriched Korean Red Ginseng improves vascular function in spontaneously hypertensive rats. J Ginseng Res. 2014;38:244–250. doi: 10.1016/j.jgr.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung J.H., Kang I.G., Kim D.Y., Hwang Y.J., Kim S.T. The effect of Korean Red Ginseng on allergic inflammation in a murine model of allergic rhinitis. J Ginseng Res. 2013;37:167–175. doi: 10.5142/jgr.2013.37.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee E.J., Song M.J., Kwon H.S., Ji G.E., Sung M.K. Oral administration of fermented red ginseng suppressed ovalbumin-induced allergic responses in female BALB/c mice. Phytomedicine. 2012;19:896–903. doi: 10.1016/j.phymed.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Im E.J., Yayeh T., Park S.J., Kim S.H., Goo Y.K., Hong S.B., Son Y.M., Kim S.D., Rhee M.H. Antiatherosclerotic effect of Korean Red Ginseng extract involves regulator of g-protein signaling 5. Evid Based Complement Alternat Med. 2014;2014:985174. doi: 10.1155/2014/985174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han J.Y., Ahn S.Y., Oh E.H., Nam S.Y., Hong J.T., Oh K.W., Lee M.K. Red ginseng extract attenuates kainate-induced excitotoxicity by antioxidative effects. Evid Based Complement Alternat Med. 2012;2012:479016. doi: 10.1155/2012/479016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jhun J., Lee J., Byun J.K., Kim E.K., Woo J.W., Lee J.H., Kwok S.K., Ju J.H., Park K.S., Kim H.Y. Red ginseng extract ameliorates autoimmune arthritis via regulation of STAT3 pathway, Th17/Treg balance, and osteoclastogenesis in mice and human. Mediators Inflamm. 2014;2014:351856. doi: 10.1155/2014/351856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S.J., Kee J.Y., Choi I.Y., Kim M.C., Kim D.S., Jeon Y.D., Kim S.G., Kim B.S., Jung H.J., Kim H.M. Insamhodo-tang, a traditional Korean medicine, regulates mast cell-mediated allergic inflammation in vivo and in vitro. J Ethnopharmacol. 2011;134:339–347. doi: 10.1016/j.jep.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Albanesi C. Keratinocytes in allergic skin diseases. Curr Opin Allergy Clin Immunol. 2010;10:452–456. doi: 10.1097/ACI.0b013e32833e08ae. [DOI] [PubMed] [Google Scholar]
- 29.Ko H.M., Joo S.H., Kim P., Park J.H., Kim H.J., Bahn G.H., Kim H.Y., Lee J., Han S.H., Shin C.Y. Effects of Korean Red Ginseng extract on tissue plasminogen activator and plasminogen activator inhibitor-1 expression in cultured rat primary astrocytes. J Ginseng Res. 2013;37:401–412. doi: 10.5142/jgr.2013.37.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Im G.J., Chang J.W., Choi J., Chae S.W., Ko E.J., Jung H.H. Protective effect of Korean Red Ginseng extract on cisplatin ototoxicity in HEI-OC1 auditory cells. Phytother Res. 2010;24:614–621. doi: 10.1002/ptr.3082. [DOI] [PubMed] [Google Scholar]
- 31.Mitev V., Miteva L. Signal transduction in keratinocytes. Exp Dermatol. 1999;8:96–108. doi: 10.1111/j.1600-0625.1999.tb00355.x. [DOI] [PubMed] [Google Scholar]
- 32.Duan W., Wong W.S. Targeting mitogen-activated protein kinases for asthma. Curr Drug Targets. 2006;7:691–698. doi: 10.2174/138945006777435353. [DOI] [PubMed] [Google Scholar]
- 33.Lee J.H., Cho S.H. Korean Red Ginseng extract ameliorates skin lesions in NC/Nga mice: an atopic dermatitis model. J Ethnopharmacol. 2011;133:810–817. doi: 10.1016/j.jep.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Cho E., Cho S.H. Effects of Korean Red Ginseng extract on the prevention of atopic dermatitis and its mechanism on early lesions in a murine model. J Ethnopharmacol. 2013;145:294–302. doi: 10.1016/j.jep.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Sohn E.H., Jang S.A., Lee C.H., Jang K.H., Kang S.C., Park H.J., Pyo S. Effects of Korean Red Ginseng extract for the treatment of atopic dermatitis-like skin lesions in mice. J Ginseng Res. 2011;35:479–486. doi: 10.5142/jgr.2011.35.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pease J.E., Williams T.J. Chemokines and their receptors in allergic disease. J Allergy Clin Immunol. 2006;118:305–318. doi: 10.1016/j.jaci.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Saeki H., Tamaki K. Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J Dermatol Sci. 2006;43:75–84. doi: 10.1016/j.jdermsci.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Kimata H., Lindley I. Detection of plasma interleukin-8 in atopic dermatitis. Arch Dis Child. 1994;70:119–122. doi: 10.1136/adc.70.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jahnz-Rozyk K., Targowski T., Paluchowska E., Owczarek W., Kucharczyk A. Serum thymus and activation-regulated chemokine, macrophage-derived chemokine and eotaxin as markers of severity of atopic dermatitis. Allergy. 2005;60:685–688. doi: 10.1111/j.1398-9995.2005.00774.x. [DOI] [PubMed] [Google Scholar]
- 40.Shimada Y., Takehara K., Sato S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J Dermatol Sci. 2004;34:201–208. doi: 10.1016/j.jdermsci.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Boukamp P., Petrussevska R.T., Breitkreutz D., Hornung J., Markham A., Fusenig N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pastore S., Lulli D., Potapovich A.I., Fidanza P., Kostyuk V.A., Dellambra E., De Luca C., Maurelli R., Korkina L.G. Differential modulation of stress-inflammation responses by plant polyphenols in cultured Normal human keratinocytes and immortalized HaCaT cells. J Dermatol Sci. 2011;63:104–114. doi: 10.1016/j.jdermsci.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Hamann K., Grabbe J., Welker P., Haas N., Algermissen B., Czarnetzki B.M. Phenotypic evaluation of cultured human mast and basophilic cells and of Normal human skin mast cells. Arch Dermatol Res. 1994;286:380–385. doi: 10.1007/BF00371797. [DOI] [PubMed] [Google Scholar]
- 44.Navi D., Saegusa J., Liu F.T. Mast cells and immunological skin diseases. Clin Rev Allergy Immunol. 2007;33:144–155. doi: 10.1007/s12016-007-0029-4. [DOI] [PubMed] [Google Scholar]
- 45.Hong C.E., Lyu S.Y. Anti-inflammatory and anti-oxidative effects of Korean Red Ginseng extract in human keratinocytes. Immune Netw. 2011;11:42–49. doi: 10.4110/in.2011.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qi X.F., Kim D.H., Yoon Y.S., Li J.H., Song S.B., Jin D., Huang X.Z., Teng Y.C., Lee K.J. The adenylyl cyclase-cAMP system suppresses TARC/CCL17 and MDC/CCL22 production through p38 MAPK and NF-kappaB in HaCaT keratinocytes. Mol Immunol. 2009;46:1925–1934. doi: 10.1016/j.molimm.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 47.Yano C., Saeki H., Komine M., Kagami S., Tsunemi Y., Ohtsuki M., Nakagawa H. Mechanism of macrophage-derived chemokine/CCL22 production by HaCaT keratinocytes. Ann Dermatol. 2015;27:152–156. doi: 10.5021/ad.2015.27.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]