Abstract
Background
Fermented black ginseng (FBG) is processed ginseng by the repeated heat treatment and fermentation of raw ginseng. The protective effect and mechanism of FBG on cisplatin-induced nephrotoxicity was investigated to evaluate its therapeutic potential.
Methods
The free radical scavenging activity of FBG was measured using 1,1-diphenyl-2-picrylhydrazyl (DPPH). In addition, the protective effect against cisplatin-induced renal damage was tested in rats. FBG was orally administered every day at a dose of 150 mg/kg body weight for 10 d, and a single dose of cisplatin was administered intraperitoneally (7.5 mg/kg body weight) with 0.9% saline on the 4th d.
Results
The DPPH radical-scavenging activity of FBG (IC50 = 384 μg/mL) was stronger than that of raw ginseng. The improved DPPH radical-scavenging activity was mediated by the generation phenolic compounds. The decreased cell viability by cisplatin was recovered significantly after treatment with FBG in a dose-dependent manner. Then, the protective effect of FBG on cisplatin-induced oxidative renal damage was investigated in rats. The decreased creatinine clearance levels, which are a reliable marker for renal dysfunction in cisplatin-treated rats, were reduced to the normal level after the administration of FBG. Moreover, FBG showed protective effects against cisplatin-induced oxidative renal damage in rats through the inhibition of NF-κB/p65, COX-2, and caspase-3 activation.
Conclusion
These results collectively show that the therapeutic evidence for FBG ameliorates the nephrotoxicity via regulating oxidative stress, inflammation, and apoptosis.
Keywords: cisplatin, fermented black ginseng, oxidative stress, Panax ginseng, renal damage
1. Introduction
Herbal therapeutics of the nutrition field has become one of the most popular trends because herbal products not only contain an important group of multicomponent therapeutics, but are also known as being harmless [1], [2]. The appropriate and concomitant use of herbal medicines with modern medicines can prevent or ameliorate the development of complications of multiple chronic conditions. Several lines of evidence have underlined that many medicinal herbal supplements have the potential to become valuable complementary therapy for various renal disorders [2], [3].
Kidneys are vital organs that function to keep the blood clean and maintain the chemical balance within—that is, kidneys play important roles in excreting waste products and in maintaining electrolyte and water balance in the body. Therefore, kidney injury is considered to contribute to organ dysfunction of the lung, brain, liver, heart, and other organs [4]. Recent literature indicates that reactive oxygen species (ROS) play important roles in the progression of kidney damage [5], [6]. Oxidative stress caused by alterations in redox homeostasis can directly exert renal parenchymal damage and may intensify renal microvascular and functional dysregulation [7]. Then, increased oxidative stress in the kidney leads to deterioration of the renal function, inflammation, and apoptosis [8], [9]. Moreover, oxidative stress is closely associated with independent risk factors such as diabetes, hypertension, hyperlipidemia, and metabolic syndrome [10], [11], [12], [13].
Cisplatin is an important chemotherapeutic agent commonly used for the treatment of several tumors, but accumulates and causes severe damage in the kidneys. Although the exact mechanism of cisplatin nephrotoxicity is not fully understood, multiple studies have shown that it is associated with DNA fragmentation, ROS, and caspase activation [14], [15], [16]. It has also been recognized that apoptosis and inflammation are important factors in cisplatin-induced nephrotoxicity [17], [18].
Panax ginseng Meyer is one of the most widely used traditional herbal medicines, and there are various commercial ginseng products such as red and black ginsengs. The steaming process is known to induce deglycosylation of ginsenoside and to enhance the biological activities of ginseng [19], [20], [21]. Black ginseng (BG) is prepared by steaming at 85°C for 8 h and then drying until the water content decreases to less than 20%. This steaming and drying process is repeated nine times. This process turns white ginseng to BG [22]. Fermentation is known as a useful method to increase safety and efficacy [23]. In addition, based on a large number of scientific studies, ginseng is known to have a wide range of pharmacological and physiological properties such as anti-inflammation, immunoenhancement, antistress, and antitumor activities [24], [25], [26], [27]. For the preparation of fermented black ginseng (FBG), BG is ground and extracted with distilled water at 80°C for 72 h. Subsequently, this water extract is fermented with Saccharomyces cerevisiae at 35°C for 24 h [22]. Although the efficacy of FBG is poorly understood, augmentation of antioxidant activity and its beneficial effect on vascular dementia have recently been reported [28], [29]. The objective of this study is to evaluate how FBG shows renoprotective efficacy against cisplatin-induced renal oxidative stress.
2. Materials and methods
2.1. Chemicals and reagents
Cisplatin, 1,1-diphenyl-2-picrylhydrazyl (DPPH), and Folin–Ciocalteu's phenol reagent were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). NF-κBp/65, COX-2, cleaved caspase-3, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and horseradish peroxidase-conjugated antirabbit antibodies were purchased from Cell Signaling (Boston, MA, USA).
2.2. Preparation of herbal extract
FBG in the form of a dried powder extract was supplied by GINSENG BY PHARM Co., Ltd. (Wonju, Korea). FBG was prepared using a recently reported method [22]. In brief, BG was manufactured via nine cycles of repeated steaming of ginseng at 85°C for 8 h. Then, BG extract was fermented with S. cerevisiae (Lallemand, Birkerod, Denmark) at 34°C for 25 h.
2.3. Measurement of total phenolic contents
The total phenolic contents of samples were determined using the Folin–Ciocalteu method [30]. Contents were expressed as milligrams of gallic acid equivalent (GAE) per gram of ginseng extract, which was repeated three times.
2.4. DPPH radical scavenging activity test
The free radical scavenging effect of samples was evaluated according to the method described by Hatano et al [31]. Four concentrations were prepared for each sample. After mixing gently and leaving the samples to stand for 30 min at room temperature, the absorbance at 540 nm was determined using a microplate reader (PowerWave XS; Bio-Tek Instruments, Winooski, VT, USA), and a green tea extract was used as DPPH-scavenging positive control.
2.5. Renoprotective effect against cisplatin-induced damage in kidney cells
The renoprotective effect against oxidative renal cell damage was evaluated using LLC-PK1 cells [32], [33]. The LLC-PK1 (pig kidney epithelium, CL-101) cells were purchased from the American Type Culture Collection (Rockville, MD, USA), and cultured in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 4mM l-glutamine at 37°C with 5% CO2 in air. The cells were seeded in 96-well culture plates at 1 × 104 cells/well and allowed to adhere for 2 h. Thereafter, the test sample and/or 25μM cisplatin were added to the culture medium. Twenty-four hours later, the medium containing the test sample and/or cisplatin was removed. Next, the cells were incubated with serum-free medium (90 μL/well) and Ez-Cytox reagent (10 μL/well) at 37°C for 2 h. Cell viability was measured by absorbance at 450 nm using a microplate reader (PowerWave XS; Bio-Tek Instruments, Winooski, VT, USA).
2.6. Renoprotective effect against cisplatin-induced oxidative damage in rats
2.6.1. Treatment of animals
In this study, we followed the Guidelines for Animal Experimentation, which is approved by the Korea Institute of Science and Technology, Gangneung, Taiwan. Male Wistar rats weighing 140–160 g were used to evaluate the protective effect of the BG against cisplatin-induced nephrotoxicity. The rats were housed under fixed temperature (23 ± 2°C) and humidity (55 ± 5%) conditions with a standard light (12 h light/dark). The rats were given free access to water and normal diet (38057; Agribrands Purina Korea, Seongnam, Gyeonggi, Korea) containing 10 kcal% fat for a period of 1 wk after arrival. Then rats were divided into three groups based on their body weight (vehicle, cisplatin, cisplatin + FBG) and then treated: Group 1, vehicle (n = 4) received water (no sample treatment); Group 2, cisplatin (n = 4) received water (no sample treatment); and Group 3, cisplatin + FBG (n = 4) treated with FBG water extract (150 mg/kg) in aqueous solution orally for 10 d.
FBG was orally administered daily at a dose of 150 mg/kg body weight, whereas water was given orally to vehicle-treated rats. The dose used (150 mg/kg FBG) was chosen according to the literature [34]. After 4 d, rats in two groups (cisplatin and cisplatin + FBG) were intraperitoneally administered a single dose of cisplatin (7.5 mg/kg body weight) in 0.9% saline. Animals in the vehicle group were given an equivalent amount of normal saline for 10 d. The rats were sacrificed 6 d after cisplatin administration under light ether anesthesia. Next, 24-h urine samples were collected using a metabolic cage. Blood samples were collected from the abdominal aorta and kidneys were removed. Body weights of rats were measured daily during the experimental period.
2.7. Plasma and urine biomarker analysis
Blood samples were collected in test tubes containing 0.18M EDTA, which were then centrifuged at 3,000 g for 5 min at 4°C. The plasma creatinine levels were determined using a rate-blanked kinetic Jaffe method. Creatinine clearance was calculated on the basis of the urinary creatinine (Cr), serum Cr, urine volume, and body weight using the following equation:
| Creatinine clearance (mL/kg body weight/min) = [urinary Cr (mg/dL) × urine volume (mL)/serum Cr (mg/dL)] × [1,000/body weight (g)] × [1/1,440 (min)]. | (1) |
2.8. Histological analysis of kidney
Kidney samples were fixed in 10% buffered formalin phosphate (Fisher Scientific, Pittsburgh, PA, USA), and then dehydration and embedding in paraffin were performed sequentially. These samples were sectioned at 3-μm thickness and then stained with periodic acid-Schiff (PAS) reagents for histological examination. Tubular damage in PAS-stained sections was examined under the microscope.
2.9. Preparation of cytosolic and nuclear extracts from tissue
The frozen kidney tissues, weighing 30 mg, were powdered by grinding thoroughly with a pestle and mortar in liquid nitrogen. The tissue powders were resuspended in hypotonic buffer [10mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 7.9), 10mM KCl, 0.1mM EDTA, 1mM DTT, 1× protease inhibitor cocktail, 1mM phenylmethylsulfonyl (PMSF), and 1mM Na3VO4] for 15 min on ice. Subsequently, 10% Nonidet P-40 (USB, Cleveland, OH, USA) was added, and the mixture was vortexed and then centrifuged at 19,480 g for 30 s at 4°C. The supernatant containing cytosolic proteins was collected and stored at −80°C until further use. The nuclear pellets were rinsed twice with cold phosphate-buffered saline and resuspended in hypertonic buffer [20mM HEPES (pH 7.9), 0.4M NaCl, 0.1mM EDTA, 1mM DTT, 1× protease inhibitor cocktail, 1mM PMSF, and 1mM Na3VO4] by rocking at 4°C for 15 min. The resuspended nuclear fraction was then centrifuged at 13,200 rpm for 5 min at 4°C. The supernatant containing nuclear proteins was collected and stored at −80°C until further use.
2.10. Western blot analysis
Proteins (whole-cell extracts, 30 μg/lane; nuclear extracts, 10 μg/lane; cytosolic extracts, 20 μg/lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride (PVDF) membranes for 1 h at semidry, and then stopped with blocking buffer for 1 h at room temperature. The PVDF membranes were incubated with primary antibody against NF-κB/p65 (1:1,000 dilution), COX-2 (1:1,000 dilution), cleaved caspase-3 (1:1,000 dilution), GAPDH (1:1,000 dilution) for overnight at 4°C, and then washed three times for 5 min with wash buffer, incubated with horseradish peroxidase-conjugated secondary antibody (1:2,000 dilution, antirabbit) for 1 h at room temperature, washed three times, and then detected with ECL solution.
2.11. Statistical analysis
The quantitative data were expressed as means ± standard deviation. Statistical significance was determined using the analysis of variance followed by a multiple comparison test with a Bonferroni adjustment. A p value < 0.05 was considered statistically significant. The analysis was performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Total phenolic contents and DPPH radical scavenging activity of FBG extract
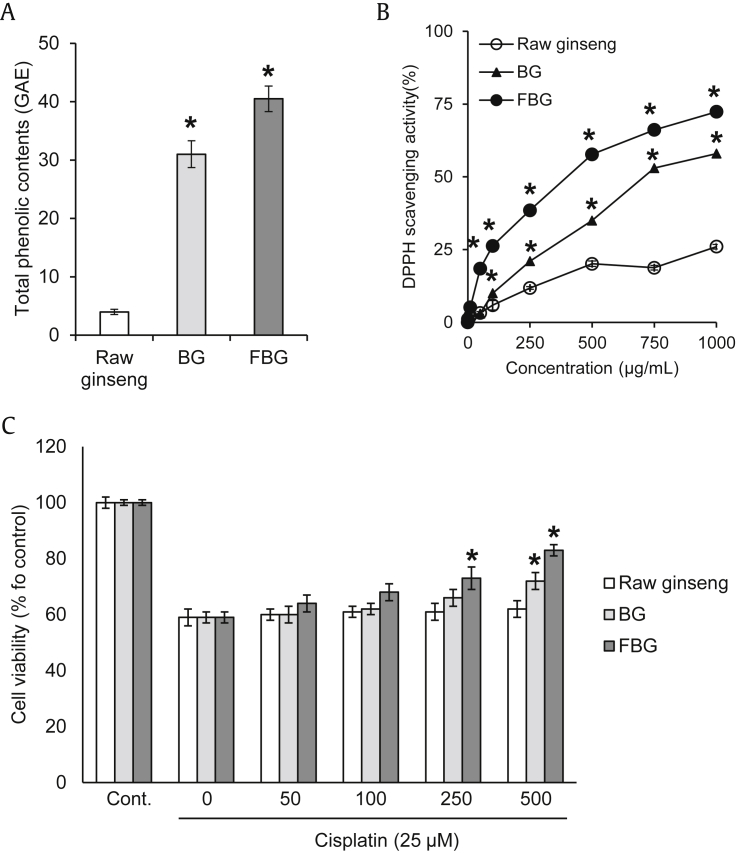
The contents of total phenolic compounds in raw ginseng, BG, and FBG were 3.9 ± 0.5, 31.3 ± 2.3, and 40.5 ± 2.2 GAE, respectively (Fig. 1A). The total phenolic content of FBG was 10 times more compared to that of raw ginseng (Fig. 1A). The DPPH radical scavenging activity of FBG (IC50 = 399.5 μg/mL) was increased in a dose-dependent manner, and its effect was significantly stronger than those of raw ginseng and BG (Fig. 1B). The free radical scavenging activities of raw ginseng and FBG extract were correlated with the total phenolic content.
Fig. 1.
Comparison of antioxidant effect and protective effects of raw ginseng, BG, and FBG against cisplatin-induced damage in LLC-PK1 cells. (A) Total phenolic contents. (B) DPPH radical scavenging activity. (C) Effects on cisplatin-induced damage in LLC-PK1 cells. The total phenolic contents and antioxidant effects of samples were determined using the Folin–Ciocalteu and DPPH radical scavenging assays. LLC-PK1 cells were pretreated with various concentrations (up to 500 μg/mL) of the ginsengs for 2 h, and then 25μM cisplatin was further treated for 24 h. Cell viability was assessed using the MTT assay. * p < 0.05 compared to raw ginseng value. BG, black ginseng; FBG, fermented black ginseng; DPPH, 1,1-diphenyl-2-picrylhydrazyl; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
3.2. Renoprotective effect of FBG extract against cisplatin-induced damage in kidney cells
The LLC-PK1 cell viability was decreased to 59% of the control value after cotreatment with 25μM cisplatin. The decreased cell viability by cisplatin was recovered significantly after treatment with FBG in a dose-dependent manner, whereas raw ginseng and BG showed no or moderate level of protective effect (Fig. 1C). FBG (250–500 μg/mL) ameliorated cisplatin-induced nephrotoxicity up to 80% of the control level, and further in vivo studies were carried out with FBG.
3.3. Protective effect of FBG on cisplatin-induced renal damage in rats
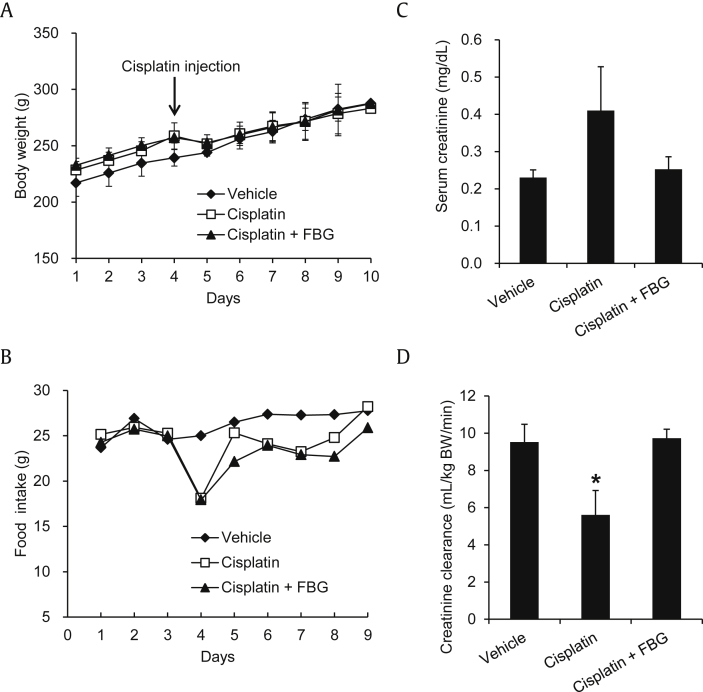
The body weight gain of rats in the cisplatin- and cisplatin + FBG-treated groups was markedly reduced compared to that in the vehicle-treated group (Fig. 2A). Similarly, food intake was slightly lowered after cisplatin treatment, but it gradually recovered in vehicle-treated groups (Fig. 2B). The food intake decreased sharply on the 4th d in both cisplatin- and cisplatin + FBG-treated groups. These results are in accordance with previous reports about the decrease in body weight gain after cisplatin injection [35].
Fig. 2.
Effect of fermented black ginseng (FBG) on body weight, food intake, and renal function parameters in the cisplatin-induced renal damage rats. (A) Changes in body weight. (B) Changes in food intake. (C) Serum creatinine levels. (D) Creatinine clearance. FBG was orally administered every day at a dose of 150 mg/kg body weight, whereas vehicle-treated rats were orally given water. The rats were sacrificed 6 d after cisplatin administration under light ether anesthesia. Twenty-four-hour urine samples were collected using metabolic cage. Blood samples were collected from the abdominal aorta, and kidneys were removed. Body weights of rats were measured daily during the experimental period. * p < 0.05 compared to the cisplatin-treated control value.
In the comparison of serum and urine biochemical parameters, cisplatin-injected rats showed increased serum creatinine level and decreased creatinine clearance levels than those of the vehicle-treated group (Figs. 2C and 2D). The elevated serum creatinine level of cisplatin-treated rats was slightly reduced by cotreatment with FBG. In particular, the decreased creatinine clearance level recovered nearly up to its normal levels after administration of FBG (Fig. 2D).
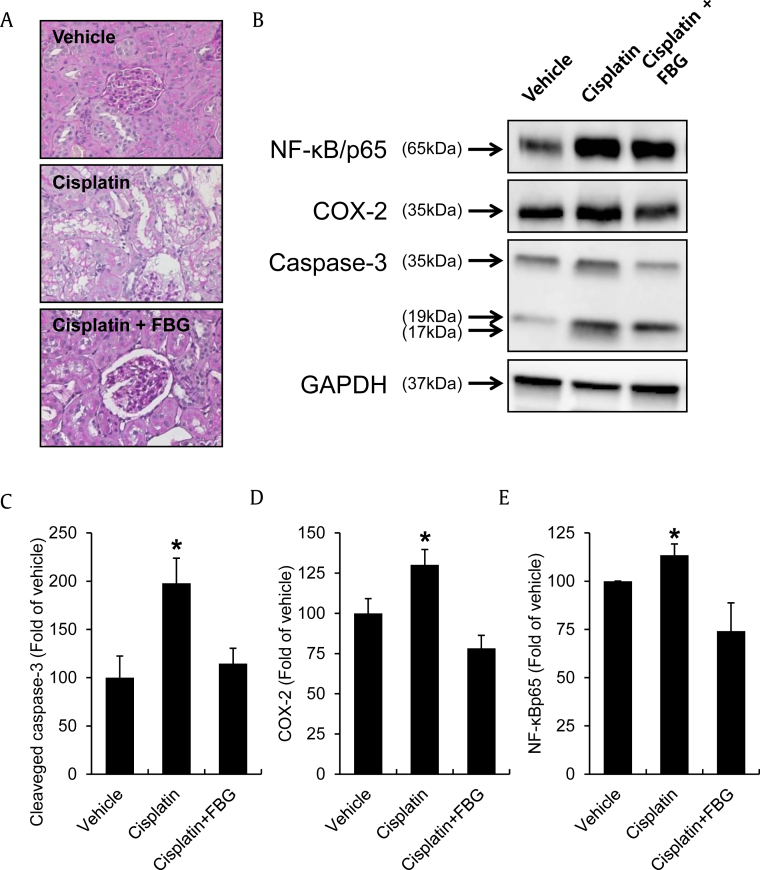
PAS staining was performed on renal tissue sections to measure tubular damage. As shown in the representative pictures of renal sections, we observed severe tubulointerstitial injuries including cystic dilatation of tubules, tubular epithelial cell detachments, and inflammatory cell infiltration in the cisplatin-exposed kidneys (Fig. 3A). However, the increased tubular damage in cisplatin-treated rats was significantly reduced by cotreatments with FBG (Fig. 3A). Fig. 3B shows the effect of FBG on NF-κBp65, COX-2, and cleaved caspase-3 protein expression in the cisplatin-treated rat kidneys. Proteins from nuclear extracts were used for the Western blot analysis of NF-κBp65, whereas whole-cell extracts were used for the analysis of COX-2 and cleaved caspase-3. NF-κBp65, COX-2, and cleaved caspase-3 protein expressions were significantly increased after cisplatin injection, and its cotreatment with FBG afforded almost complete kidney protection (Figs. 3C–3E).
Fig. 3.
Effect of FBG on renal histological changes and protein expressions of renal cortex tissues. (A) PAS staining of representative renal section and its quantitative data for the tubular damage. (B) NF-κB/p65, COX-2, and cleaved caspase-3 protein expressions. (C) Quantitative data for the COX-2 Western blot analysis of renal cortex tissues. (D) Quantitative data for the cleaved caspase-3 Western blot analysis of renal cortex tissues. (E) Quantitative data for the NF-κB/p65 Western blot analysis of renal cortex tissues. Kidney samples were fixed, dehydrated, embedded in paraffin, sectioned at 3-μm thickness, and stained with PAS reagents for histological examination. Proteins from kidneys were separated by SDS-PAGE, transferred to PVDF membranes, and it was analyzed for NF-κB/p65, COX-2 and cleaved caspase-3. * p < 0.05 compared to the cisplatin-treated control value. FBG, fermented black ginseng; PAS, periodic acid-Schiff; PVDF, polyvinylidene fluoride; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
4. Discussion
The recent literature indicates that ROS plays critical roles in the progression of renal damage [5], [7], [10]. Treatment with antioxidants can reduce oxidative damage, which might delay the development of kidney disease. Therefore, antioxidants as an inhibitor of ROS are considered to be an important therapeutic approach for kidney disorders. In the present study, we have investigated the protective potential and mechanism of FBG on the renal damage caused by cisplatin, oxidative stress, and inflammation to evaluate its possible use in treating kidney damage.
P. ginseng contains high concentrations of saponins such as ginsenosides Rb1, Rb2, Rc, Rd, and Re. Many studies have been conducted to develop novel methods for enhancing the biological effects of ginseng by conversion of the saponins via high-temperature or high-pressure thermal processing [22], [36], [37]. FBG is prepared by repeated steaming and drying processes with fresh ginseng followed by fermentation with S. cerevisiae. The contents of ginsenosides Re, Rg1, Rb1, Rc, and Rb2 were decreased, whereas the contents of less polar ginsenosides Rg2, Rg3, Rh1, Rh2, and Rf were newly detected in FBG [22]. In particular, ginsenoside Rg3, which is abundantly contained in FBG, was found to prevent the progression of renal damage and dysfunction in type 1 diabetic rats via inhibition of oxidative stress and inflammation [25], [38].
The DPPH radical scavenging activity test has been widely used to test the free radical scavenging ability of plant extracts or compounds [31], [39], and the phenolic contents of plants can be related to their antioxidant activities [40]. FBG extract showed a stronger DPPH radical scavenging activity than that of raw ginseng, which was thought to relate with its higher content of total phenolic compounds. We carried out in vitro kidney cell protection screening to compare the protective effect of raw ginseng, BG, and FBG on LLC-PK1 pig kidney epithelial cells. The kidney cell protection assay conditions were established using the LLC-PK1 cell line, which is commonly used to evaluate nephrotoxicity [32]. The potent protective effect by ameliorating reduced cell viability due to cisplatin was observed only after treatment with FBG. Then, we further examined the effect of FBG on cisplatin-induced nephrotoxicity in rats.
In line with in vitro results, FBG abrogated the cisplatin-induced renal dysfunction and tubulointerstitial injuries in rats. The lowered creatinine clearance as an indicator of kidney dysfunction in cisplatin-treated rats [14], [16] recovered to nearly normal levels after cotreatment with FBG. Renal tubules comprise 95% of the renal mass, so damage to the tubulointerstitium is an important predictor of renal dysfunction [41]. The severe tubulointerstitial injuries in cisplatin-treated kidneys, which were analyzed by PAS staining, were also reduced by cotreatments with FBG, reflecting its protective effect. ROS play an important role in mediating apoptosis by inducing the activation of caspases. Among all the caspase members, caspase-3 in particular is an essential apoptotic effector leading to cytoskeletal breakdown, nuclear demise, and other cell changes associated with apoptosis [42]. Therefore, caspase inhibitors have the potential to minimize uncontrolled apoptosis in cisplatin-induced nephropathy [43]. Our results also showed significant increases in cleaved caspase-3 expression levels of the cisplatin-treated rat kidney, but its elevated level was significantly reduced after FBG administration. Similarly, the elevated NF-κB/p65 and COX-2 protein expressions, which are reliable markers of inflammation in cisplatin-treated rat kidney [44], were lowered nearly back to its normal levels. These results imply that FBG may alleviate oxidative stress by preventing caspase-3 activation and related inflammation in the kidney.
In summary, the kidney cell damage induced by oxidative stress was significantly inhibited by the treatments with FBG. In addition, the renal dysfunction of cisplatin-treated mice was markedly ameliorated by FBG extract administration. The kidney protection effect of FBG was associated with the caspase-dependent anti-inflammatory pathway. Taken together, these results demonstrate that FBG exerted a renoprotective effect in cisplatin-treated rats.
Conflicts of interest
The authors have declared no conflicts of interest.
Acknowledgments
This research was funded by the Korea Institute of Science and Technology Institutional Program (2Z04371). This research was also funded by Gachon University Research Fund 2014 (GCU-2014-0116).
Contributor Information
Ki Sung Kang, Email: kkang@gachon.ac.kr.
Su-Nam Kim, Email: snkim@kist.re.kr.
References
- 1.Gesler W.M. Therapeutic landscapes: medical issues in light of the new cultural geography. Soc Sci Med. 1992;34:735–746. doi: 10.1016/0277-9536(92)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Wojcikowski K., Johnson D.W., Gobé G. Medicinal herbal extracts—renal friend or foe?: Part two. Herbal extracts with potential renal benefits. Nephrology. 2004;9:400–405. doi: 10.1111/j.1440-1797.2004.00355.x. [DOI] [PubMed] [Google Scholar]
- 3.Ceylan-Isik A.F., Fliethman R.M., Wold L.E., Ren J. Herbal and traditional Chinese medicine for the treatment of cardiovascular complications in diabetes mellitus. Curr Diabetes Rev. 2008;4:320–328. doi: 10.2174/157339908786241142. [DOI] [PubMed] [Google Scholar]
- 4.Li X., Hassoun H.T., Santora R., Rabb H. Organ crosstalk: the role of the kidney. Curr Opin Crit Care. 2009;15:481–487. doi: 10.1097/MCC.0b013e328332f69e. [DOI] [PubMed] [Google Scholar]
- 5.Forbes J.M., Coughlan M.T., Cooper M.E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 6.Nistala R., Whaley-Connell A., Sowers J.R. Redox control of renal function and hypertension. Antioxid Redox Signal. 2008;10:2047–2089. doi: 10.1089/ars.2008.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heyman S.N., Rosen S., Rosenberger C. A role for oxidative stress. Contrib Nephrol. 2011;174:138–148. doi: 10.1159/000329383. [DOI] [PubMed] [Google Scholar]
- 8.Manucha W., Vallés P.G. Apoptosis modulated by oxidative stress and inflammation during obstructive nephropathy. Inflamm Allergy Drug Targets. 2012;11:303–312. doi: 10.2174/187152812800958997. [DOI] [PubMed] [Google Scholar]
- 9.Ozbek E. Induction of oxidative stress in kidney. Int J Nephrol. 2012;2012:465897. doi: 10.1155/2012/465897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J.M., Shah A.M. ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S221–S226. doi: 10.1097/01.asn.0000077406.67663.e7. [DOI] [PubMed] [Google Scholar]
- 11.Gökhan S.H. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung H.W., Lim J.H., Kim M.Y., Shin S.J., Chung S., Choi B.S., Kim H.W., Kim Y.S., Park C.W., Chang Y.S. High-fat diet-induced renal cell apoptosis and oxidative stress in spontaneously hypertensive rat are ameliorated by fenofibrate through the PPARα–FoxO3a–PGC-1α pathway. Nephrol Dial Transplant. 2012;27:2213–2225. doi: 10.1093/ndt/gfr613. [DOI] [PubMed] [Google Scholar]
- 13.Lim J.H., Kim E.N., Kim M.Y., Chung S., Shin S.J., Kim H.W., Yang C.W., Kim Y.S., Chang Y.S., Park C.W. Age-associated molecular changes in the kidney in aged mice. Oxid Med Cell Longev. 2012;2012:171383. doi: 10.1155/2012/171383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathushima H., Yonemura K., Ohishi K., Hishida A. The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J Lab Clin Med. 1998;131:518–526. doi: 10.1016/s0022-2143(98)90060-9. [DOI] [PubMed] [Google Scholar]
- 15.Kaushal G.P., Kaushal V., Hong X., Shah S.V. Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int. 2001;60:1726–1736. doi: 10.1046/j.1523-1755.2001.00026.x. [DOI] [PubMed] [Google Scholar]
- 16.Basnakian A.G., Apostolov E.O., Yin X., Napirei M., Mannherz H.G., Shah S.V. Cisplatin nephrotoxicity is mediated by deoxyribonuclease I. J Am Soc Nephrol. 2005;16:697–702. doi: 10.1681/ASN.2004060494. [DOI] [PubMed] [Google Scholar]
- 17.Mitazaki S., Honma S., Suto M., Kato N., Hiraiwa K., Yoshida M., Abe S. Interleukin-6 plays a protective role in development of cisplatin-induced acute renal failure through upregulation of anti-oxidative stress factors. Life Sci. 2011;88:1142–1148. doi: 10.1016/j.lfs.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Yang F., Long W., Xuechuan H., Xueqin L., Hongyun M., Yonghui D. Upregulation of Fas in epithelial ovarian cancer reverses the development of resistance to cisplatin. BMB Rep. 2015;48:30–35. doi: 10.5483/BMBRep.2015.48.1.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitagawa I., Taniyama T., Shibuya H., Noda T., Yoshikawa M. Chemical studies on crude drug processing: V. On the constituents of ginseng radix rubra (2): comparison of the constituents of white ginseng and red ginseng prepared from the same Panax ginseng root. Yakugaku Zasshi. 1987;107:495–505. doi: 10.1248/yakushi1947.107.7_495. [DOI] [PubMed] [Google Scholar]
- 20.Wang C.Z., Zhang B., Song W.X., Wang A., Ni M., Luo X., Aung H.H., Xie J.T., Tong R., He T.C. Steamed American ginseng berry: ginsenoside analyses and anticancer activities. J Agric Food Chem. 2006;54:9936–9942. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- 21.Kang K.S., Kim H.Y., Baek S.H., Yoo H.H., Park J.H., Yokozawa T. Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 2007;30:724–728. doi: 10.1248/bpb.30.724. [DOI] [PubMed] [Google Scholar]
- 22.Bak M.J., Jeong W.S., Kim K.B. Detoxifying effect of fermented black ginseng on H2O2-induced oxidative stress in HepG2 cells. Int J Mol Med. 2014;34:1516–1522. doi: 10.3892/ijmm.2014.1972. [DOI] [PubMed] [Google Scholar]
- 23.Lee W., Park S.H., Lee S., Chung B.C., Song M.O., Song K.I., Ham J., Kim S.N., Kang K.S. Increase in antioxidant effect of ginsenoside Re–alanine mixture by Maillard reaction. Food Chem. 2012;135:2430–2435. doi: 10.1016/j.foodchem.2012.06.108. [DOI] [PubMed] [Google Scholar]
- 24.Kaneko H., Nakanishi K. Proof of the mysterious efficacy of ginseng; basic and clinical trials: clinical effects of medical ginseng, Korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004;95:158–162. doi: 10.1254/jphs.fmj04001x5. [DOI] [PubMed] [Google Scholar]
- 25.Kang K.S., Yamabe N., Kim H.Y., Park J.H., Yokozawa T. Therapeutic potential of 20(S)-ginsenoside Rg3 against streptozotocin-induced diabetic renal damage in rats. Eur J Pharmacol. 2008;591:266–272. doi: 10.1016/j.ejphar.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 26.Byeon S.E., Lee J., Kim J.H., Yang W.S., Kwak Y.S., Kim S.Y., Choung E.S., Rhee M.H., Cho J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramesh T., Kim S.W., Hwang S.Y., Sohn S.H., Yoo S.K., Kim S.K. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr Res. 2012;32:718–726. doi: 10.1016/j.nutres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Park H.J., Shim H.S., Kim K.S., Shim I. The protective effect of black ginseng against transient focal ischemia-induced neuronal damage in rats. Korean J Physiol Pharmacol. 2011;15:333–338. doi: 10.4196/kjpp.2011.15.6.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H.S., Kim M.R., Park Y., Park H.J., Chang U.J., Kim S.Y., Suh H.J. Fermenting red ginseng enhances its safety and efficacy as a novel skin care anti-aging ingredient: in vitro and animal study. J Med Food. 2012;15:1015–1023. doi: 10.1089/jmf.2012.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 31.Hatano T., Edamatsu R., Hiramatsu M., Mori A., Fujita Y., Yasuhara T., Yoshida T., Okuda T. Effects of the interaction of tannins with co-existing substances: VI. Effects of tannins and related polyphenols on superoxide anion radical, and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull. 1989;37:2016–2021. [Google Scholar]
- 32.Park J.Y., Choi P., Kim T., Ko H., Kim H.K., Kang K.S., Ham J. Protective effects of processed ginseng and its active ginsenosides on cisplatin-induced nephrotoxicity: in vitro and in vivo studies. J Agric Food Chem. 2015;63:5964–5969. doi: 10.1021/acs.jafc.5b00782. [DOI] [PubMed] [Google Scholar]
- 33.Han M.S., Han I.H., Lee D., An J.M., Kim S.N., Shin M.S., Yamabe N., Hwang G.S., Yoo H.H., Choi S.J. Beneficial effects of fermented black ginseng and its ginsenoside 20(S)-Rg3 against cisplatin-induced nephrotoxicity in LLC-PK1 cells. J Ginseng Res. 2017;42(2):135–140. doi: 10.1016/j.jgr.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh J.S., Lee S.R., Hwang K.T., Ji G.E. The anti-obesity effects of the dietary combination of fermented red ginseng with levan in high fat diet mouse model. Phytother Res. 2014;28(4):617–622. doi: 10.1002/ptr.5042. [DOI] [PubMed] [Google Scholar]
- 35.Takeda H., Sadakane C., Hattori T., Katsurada T., Ohkawara T., Nagai K., Asaka M. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology. 2008;134:2004–2013. doi: 10.1053/j.gastro.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 36.Kang K.S., Ham J., Kim Y.J., Park J.H., Cho E.J., Yamabe N. Heat-processed Panax ginseng and diabetic renal damage: active components and action mechanism. J Ginseng Res. 2013;37:379–388. doi: 10.5142/jgr.2013.37.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamabe N., Kim Y.J., Lee S., Cho E.J., Park S.H., Ham J., Kim H.Y., Kang K.S. Increase in antioxidant and anticancer effects of ginsenoside Re–lysine mixture by Maillard reaction. Food Chem. 2013;138:876–883. doi: 10.1016/j.foodchem.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Park J.Y., Choi P., Kim H.K., Kang K.S., Ham J. Increase in apoptotic effect of Panax ginseng by microwave processing in human prostate cancer cells: in vitro and in vivo studies. J Ginseng Res. 2016;40:62–67. doi: 10.1016/j.jgr.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Q.Y., Hackman R.M., Ensunsa J.L., Holt R.R., Keen C.L. Antioxidant activities of oolong tea. J Agric Food Chem. 2002;50:6929–6934. doi: 10.1021/jf0206163. [DOI] [PubMed] [Google Scholar]
- 40.Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tortorici M.A., Nolin T.D. Kidney function assessment and its role in drug development, review and utilization. Expert Rev Clin Pharmacol. 2014;7:523–532. doi: 10.1586/17512433.2014.922865. [DOI] [PubMed] [Google Scholar]
- 42.Bratton S.B., Cohen G.M. Apoptotic death sensor: an organelle's alter ego? Trends Pharmacol Sci. 2001;22:306–315. doi: 10.1016/s0165-6147(00)01718-1. [DOI] [PubMed] [Google Scholar]
- 43.Razzaque M.S. Cisplatin nephropathy: is cytotoxicity avoidable? Nephrol Dial Transplant. 2007;22:2112–2116. doi: 10.1093/ndt/gfm378. [DOI] [PubMed] [Google Scholar]
- 44.Domitrović R., Cvijanović O., Pugel E.P., Zagorac G.B., Mahmutefendić H., Škoda M. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology. 2013;310:115–123. doi: 10.1016/j.tox.2013.05.015. [DOI] [PubMed] [Google Scholar]