Abstract
The steaming process of Panax ginseng has been reported to increase its major known bioactive components, ginsenosides, and, therefore, its biological properties as compared to regular Panax ginseng. Biological functions of red Panax ginseng attenuating pro-oxidant environments associated with chronic diseases are of particular interest, since oxidative stress can be a key contributor to the pathogenesis of chronic diseases. Additionally, proper utilization of various biomarkers for evaluating antioxidant activities in natural products, such as ginseng, can also be important to providing validity to their activities. Thus, studies on the effects of red ginseng against various diseases as determined in cell lines, animal models, and humans were reviewed, along with applied biomarkers for verifying such effects. Limitations and future considerations of studying red ginseng were been discussed. Although further clinical studies are warranted, red ginseng appears to be beneficial for attenuating disease-associated symptoms via its antioxidant activities, as well as for preventing oxidative stress-associated chronic diseases.
Keywords: antioxidant, bioactives, Panax ginseng, red ginseng
1. Introduction
Panax ginseng Meyer root has been considered as a medicinal plant in Eastern countries for thousands of years, and has been widely used for its health promotion in various disease conditions [1]. Panax ginseng can be classified by its processing methods and include fresh ginseng, white ginseng (air-dried), red ginseng (steamed), and sun ginseng. Notably, red ginseng, which is harvested at 6 yr, steamed, and then further dried, is well known for its elevated content of ginsenosides, which are bioactive compounds [2], [3]. Red ginseng also exhibits various biological activities against chronic diseases, such as diabetes mellitus, cancer, and cardiovascular disease [4], [5].
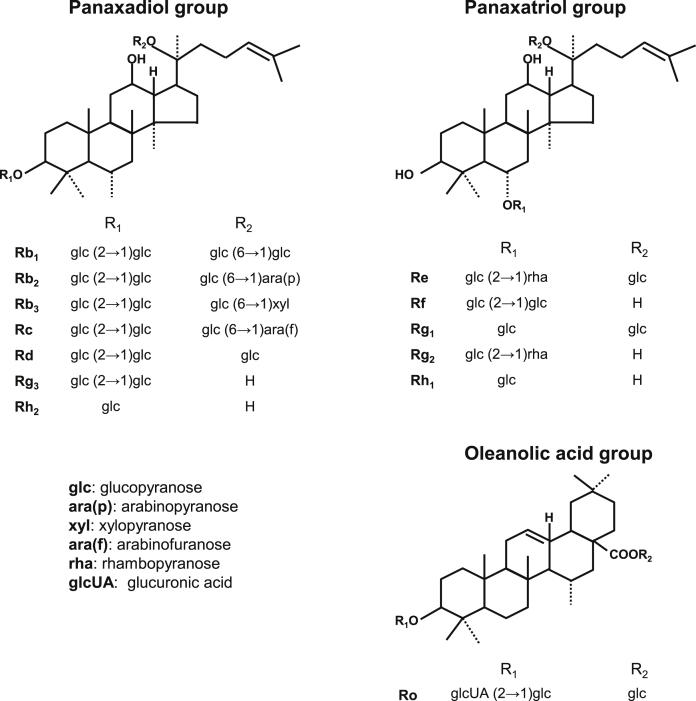
Red ginseng is composed of saponin (generally known as ginsenosides) and nonsaponin, including polysaccharides. The main active components in red ginseng are ginsenosides containing triterpene and sugar moieties [3]. Ginsenosides can be divided into three groups depending on their structures: (1) the panaxadiol group, including Rb1, Rb2, Rb3, Rc, Rd, Rg3, and Rh2; (2) the panaxatriol group, including Re, Rf, Rg1, Rg2, and Rh1; and (3) the oleanolic acid group, including Ro, as shown in Fig. 1 [1]. The amount of ginsenosides vary according to harvest time, storage condition, and processing methods [1]. Although there are other Panax species, such as Panax notoginseng, Panax japonicus, Panax quinquefolius, Panax pseudoginseng, and Panax vietnamensis as shown in Table 1, this review focuses on the biological activities of Panax ginseng.
Fig. 1.
Structures of ginsenosides. Ginsenosides of Panax ginseng are classified into three groups according to their structures: panaxadiol, panaxatriol, and oleanolic acid groups.
Table 1.
Origins of Panax species
| Panax species | Origin (habitat) |
|---|---|
| Panax ginseng | Korea, China, Japan |
| Panax notoginseng | China |
| Panax japonicus | Japan |
| Panax quinquefolius | Southern Canada, United States |
| Panax pseudoginseng | Nepal and eastern Himalayas |
| Panax vietnamensis | Vietnam |
2. Significance of proper determination of antioxidant activity
Certain levels of reactive oxygen species (ROS), such as superoxide, hydroxyl radical, and hydrogen peroxide, are necessary for crucial roles in differentiation, proliferation, and regulation of signal transduction in cells. ROS are constantly produced from mitochondria via the respiratory chain and the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system, and can be eliminated by enzymatic and non-enzymatic ROS scavenging factors, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), reduced glutathione (GSH), thioredoxin, and NF-E2-related factor 2 (Nrf 2)-mediated expression of heme oxygenase 1 (HO-1), resulting in redox homeostasis [6], [7]. However, impaired redox homeostasis results in excessive ROS, which is associated with the progression of several chronic diseases, such as diabetes, cancer, Alzheimer's disease, and cardiovascular diseases [8]. For these reasons, reducing oxidative stress by increased antioxidant intake is an attractive strategy for the prevention of such chronic diseases [9].
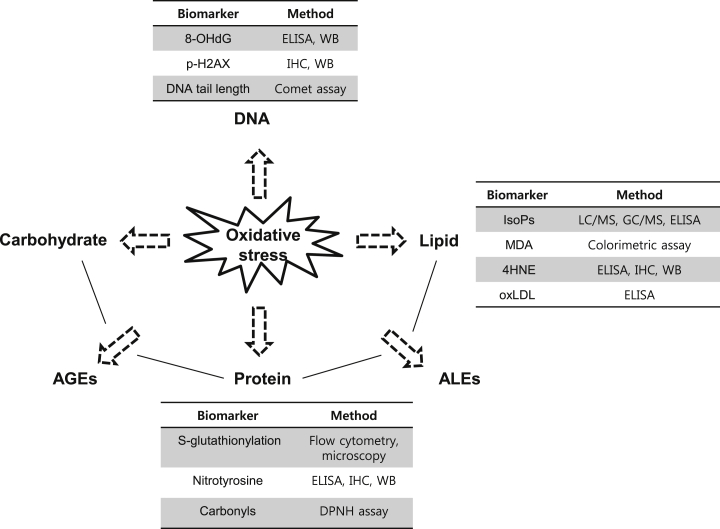
Proper biomarkers for oxidative stress validate the efficacy of red ginseng for evaluating the risk of chronic diseases. ROS determination in cells can be achieved by treatment with 2′,7′-dichlroflurescein diacetate (DCF-DA). Oxidized DCF-DA is changed to 2′,7′-dichlroflurescein, which appears under fluorescence examination and can be detected by a microscope, flow cytometry, or microplate reader [10]. Oxidative modification of macromolecules (DNA, lipids, proteins, and carbohydrates) are used to assess levels of oxidative stress, because of the short-lived nature of ROS. Oxidation of the eighth guanine of DNA can be detected by 8-hydroxydeoxyguanosine (8-OHdG) in serum, urine, and tissue [11]. Additionally, phosphorylation of a histone H2A variant (H2AX) at Ser19 [12], [13] can be determined by immunoblotting and immunohistochemistry in tissues as a novel biomarker for DNA damage by estimating the number of double-strand breaks. Comet assays (single-cell gel electrophoresis) also have been used for determination of DNA damage [14], which is closely related to cancer incidence [15].
Isoprostanes (IsoPs) and malondialdehyde (MDA) are by-products of lipid peroxidation from arachidonic acid and polyunsaturated fatty acid (PUFA), respectively [16]. Measurement of IsoPs in the plasma and urine can be performed by LC-MS, GC-MS, radioimmunoassay, and enzyme-linked immunosorbance assays (ELISA). MDA levels can be quantified through a reaction with thiobarbituric acid (TBA), resulting in an MDA-TBA adduct that appears as colored end products. This process is known as a TBA-reacting substances (TBARS) assay [17]. Additionally, oxidized low-density lipoprotein (oxLDL) is associated with oxidative stress [16], and 4-hydroxy-2-nonenal (HNE) can be produced by oxygen attack on ω-6-PUFA, which can make adducts with other macromolecules [18]. The HNE forms a Michael adduct with Cys, Lys, and His residues on proteins, and these adducts serve as advanced lipoxidation/glycation end products that are closely associated with various chronic diseases, such as cardiovascular diseases, chronic obstructive pulmonary diseases, and neurodegenerative diseases [16], [18], [19]. The advanced glycation end products can bind to their receptors, resulting in promotion of inflammatory factor production, which in turn produces oxidative stress and irreversible cellular dysfunction [20]. Additionally, protein modifications, such as nitrotyrosine and S-glutathionylation, can also be used as biomarkers for oxidative stress [16] (Fig. 2).
Fig. 2.
Biomarkers for oxidative stress and their analytical methods. 4HNE, 4-hydroxy-2-nonenal; 8-OHdG, 8-hydroxydeoxyguanosine; AGEs, advanced glycation end products; ALEs, advanced lipoxidation end products; DPNH, 2,4-dinitrophenyl-hydrazine; ELISA, enzyme-linked immunosorbent assay; IHC, immunohistochemistry; IsoPs, isoprostanes; MDA, malondialdehyde; oxLDL, oxidized low-density lipoprotein; WB, western blot.
The antioxidant activity of red ginseng has been studied for several decades. Red ginseng water extracts were shown to scavenge free radicals, such as 1,1-dipheny-2-picrylhydrazyl, hydroxyl, superoxide, and carbon-centered radical in vitro [21]. Additionally, red ginseng water extract decreased ROS-induced bacterial plasmid DNA-strand breaks in vitro [22]. The antioxidant function of red ginseng has been suggested for health benefits.
3. Assessment of antioxidant activities of red ginseng in cells
Due to the feasibility of diverse approaches, the antioxidant activities of red ginseng have been studied extensively in cells (Table 2). Red ginseng has a protective effect against cisplatin-induce ototoxicity [23], and pretreatment with red ginseng extract (2.5 mg/mL, major constituents: Rb1, Rg1) before inducing ototoxicity by cisplatin-attenuated apoptosis in HOI-OC1 auditory cells inhibited ROS production. Additionally, treatment with red ginseng extract (0.5 mg/mL) upregulated Nrf-induced HO-1 in polychlorinated biphenyl (PCB) 126-treated PC12 rat pheochromocytoma cells [24]. Red ginseng also rescues cells from oxidative stress by increasing the concentration of antioxidant enzymes, such as SOD, CAT, and GPx in hydrogen peroxide-treated HepG2 hepatoma cells, indicating cytoprotective effects in the liver [25]. Oxidative stress occurs following ethanol treatment in hepatocytes, leading to severe liver damage. Red ginseng (1–1,000 μg/mL) and its ginsenosides (Rg3 and Rh2 at 1–30 μg/mL) mitigated to the hepatocytic injury by increasing its antioxidant capacity [26]. The underlying mechanism of antioxidant activities associated with red ginseng is due to the downregulation of ROS-stimulated mitogen-activated protein kinase (MAPK) and Akt pathways [24], [25], [26]. When ROS was induced by hydrogen peroxide, the saponin fraction of red ginseng (50–100 μg/mL) significantly decreased ROS detected by DCF-DA assay and MDA levels, as well as improved antioxidant enzyme activities. It was suggested that saponin in red ginseng prevents oxidative stress-induced hepatic diseases, and that the effects were stronger as compared with those of white ginseng extract [27]. Additionally, red ginseng extract (1 mg/mL) eliminated arachiodonic acid + iron-induced oxidative stress in hepatocytes from diverse origins, including HepG2 (human), H4IIE (rat), and AML12 (mouse) cells. The inhibition of LKB1/AMP-activated protein kinases (AMPK) was reported to be a major mechanism associated with red ginseng antioxidant activity [28].
Table 2.
List of studies showing antioxidant activity of red ginseng in cells
| Disease model | Inducer | Cell line | Red ginseng type | Antioxidant biomarker | Ref |
|---|---|---|---|---|---|
| Ototoxicity | Cisplatin | HEI-OC1 (mouse auditory cell) |
Red ginseng water extract |
DCF ↓ | 23 |
| Pheochromocytoma | PCB126 | PC12 cell (rat adrenal gland) |
Red ginseng extract | DCF ↓ GCLC, SOD, catalase ↑ Nrf2, HO-1 ↑ |
24 |
| Hepatic disease | H2O2 | HepG2 cell (human hepatoma cell) |
Red ginseng essential oil | DCF ↓ SOD, GPx, CAT ↑ TBARS ↓ |
25 |
| Hepatic disease | Ethanol | TIB-73 cell (mouse hepatocyte) |
Red ginseng water extract Rg3, Rh2 |
DCF ↓ | 26 |
| Hepatic disease | H2O2 | HepG2 cell (human hepatoma cell) |
Red ginseng methanol extract |
DCF ↓ MDA ↓ GPx, GR, CAT, SOD↑ |
27 |
| Hepatic disease | AA+ iron | HepG2 cell (human hepatoma cell) H4IIE (rat hepatoma cell) AML12 (mouse hepatocyte) |
Red ginseng extract | DCF ↓ GSH ↑ |
28 |
| Neurodegenerative disease |
Glutamate NMDA β-amyloid |
Rat cortical cell | Red ginseng water extract |
DCF ↓ | 29 |
| Neurodegenerative disease |
Hippocampal Neuronal cell | Red ginseng extract | DCF ↓ | 30 | |
| Gastritis | Helicobacter pylori | AGS (human gastric epithelial cell) |
Red ginseng water extract |
DCF ↓ NADPH oxidase activity ↓ |
31 |
| Oral mucositis | Radiation | HaCaT (human keratinocytes) |
Red ginseng water extract |
DCF ↓ | 32 |
| Vascular disease | Acrolein | HUVECs (human umbilical vein endothelial cells) |
Red ginseng water extract |
DCF ↓ | 33 |
| Vascular disease | H2O2 | HUVECs (human umbilical vein endothelial cells) |
Red ginseng water extract |
TRX1 ↑ DCF ↓ |
34 |
| Diabetes | Cytokine | MIN6N8 (mouse pancreatic β-cells) |
Red ginseng extract | DCF ↓ | 35 |
AA, arachiodonic acid; CAT, catalase; DCF, 2′,7′-dichlroflurescein; GCLC, glutamate cysteine ligase; GPx, glutathione peroxidase; GR, glutathione reductase; GSH, glutathione; HO-1, heme oxygenase 1; MDA, malondialdehyde; NADPH, nicotinamide adenine dinucleotide phosphate; NMDA, N-methyl-D-aspartate; PCB126, polychlorinated biphenyls; SOD, superoxide dismutase; TBARS, thiobarbituric acid-reacting substances; TRX, thioredoxin reductase.
Studies of red ginseng in the area of neurodegenerative diseases have grown rapidly. Red ginseng water extract (0.3–3 mg/mL) blocked ROS generation and neuronal apoptosis, which was stimulated by glutamate, N-methyl-D-aspartate, or β-amyloid in rat cortical cells. These results implied the potential of red ginseng to aid in the prevention of neuronal diseases [29]. Additionally, red ginseng extract (0.01–1 mg/mL; major constituents: Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, and Rg3) was reported to have a neuroprotective effect on primary rat hippocampal neurons, where toxicity was induced by kainite, a glutamate analogue [30].
Helicobacter pylori has been studied as a causative bacteria that increases the risk of gastritis by triggering inflammation cascades. H. pylori-associated inflammation causes oxidative stress by recruiting neutrophils and macrophages to an infection site. Red ginseng extract (1–100 μg/mL; major constituents: Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, and Rh1) reduced expression of inflammatory factors by suppressing NADPH oxidase activity and Jak2/Stat3 in AGS human gastric epithelial cells [31]. Furthermore, treatment with red ginseng (10–50 μg/mL) was beneficial to enhancing cell survival under radiation exposure by inhibiting ROS generation and activation of the MAPK signaling pathways [32].
In an in vitro model of vascular diseases, red ginseng extract (0.5–2 mg/mL) exhibited a protective effect on oxidative stress-induced cell death in endothelial cells by upregulating thioredoxin reductase 1 and downregulating ROS generation, p38, and PKC-δ expression in endothelial cells damaged by α,β-unsaturated aldehyde acrolein and hydrogen peroxide [33], [34].
Furthermore, red ginseng extract (0–100 μg/mL) was effective at inhibiting cytokine-induced cell death by downregulating apoptosis cascades and ROS production in MIN6N8 cells and pancreatic β cells. In particular, ginsenosides at low concentrations of 0.1–1.0 μg/mL were responsible for such activity, introducing he possibility for red ginseng use in diabetic treatments [35].
4. Assessment of antioxidant activities of red ginseng in animal models
Aging is closely related to oxidative stress and is related to physiological status [36]. Therefore, various red ginseng studies evaluated biomarkers of oxidative stress in young versus aged animals, as well as sexual dysfunction and kidney dysfunction (Table 3). Aged rats fed red ginseng water extract (200 mg/kg/d; major constituents: Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, and Rh1) exhibited a significant reduction in oxidative stress as determined by MDA, as well as elevated concentration of antioxidant factors, such as SOD, CAT, GPx, glutathione reductase (GR), glutathione-S-transferase (GST), reduced glutathione, vitamin C, and vitamin E in various organs [37].
Table 3.
List of studies showing antioxidant activity of red ginseng in animals (rodents)
| Disease model | Inducer | Red ginseng type | Antioxidant biomarker | Ref |
|---|---|---|---|---|
| Aging | 12-mo old | Red ginseng water extract |
MDA ↓ SOD, CAT, GPx, GR, GST ↑ GSH, Vit C, Vit E ↑ |
37 |
| Age-related male sexual dysfunction | 12-mo old | Red ginseng water extract |
MDA ↓ SOD, CAT, GPx, GR, GST ↑ GSH, Vit C, α-tocopherol ↑ |
38 |
| Age-related renal injury | HFD, D-galactose | Red ginseng | 8-OHdG ↓ AGE ↓ |
39 |
| Hepatic disease | CCl4 | Red ginseng essential oil | TBARS ↓ SOD, GPx, CAT ↑ |
25 |
| Hepatic disease | Aflatoxin B1 | Red ginseng extract |
SOD, CAT, GPx ↑ MDA ↓ |
40 |
| Alcoholic liver disease | Ethanol | Red ginseng water extract |
4-HNE ↓ Nitrotyrosine ↓ |
41 |
| Diabetes | Streptozotocin | Fermented red ginseng extract | GSH ↑ MDA ↓ SOD, CAT, GPx, GR ↑ |
42 |
| Diabetes | Cyclosporine | Red ginseng water extract |
8-OHdG ↓ | 43 |
| Gastric ulcer | Hydrochloride/Ethanol indomethacin |
Red ginseng powered extract containing drug | TBARS ↓ | 44 |
| High intensive exercise | Treadmill for 3 wks | HRG | MDA ↓ SOD ↑ |
45 |
| Arthritis | Murine type II collagen | Red ginseng saponin extract | MDA ↓ Nitrotyrosine ↓ SOD, GSH, CAT ↑ |
46 |
| Skin cancer | 7,12-dimethylbenz(a)anthracene Croton oil |
Red ginseng hydroalcoholic extract | GSH, SOD, CAT, Vit C ↑ TBARS ↓ |
47 |
4HNE, 4-hydroxy-2-nonenal; 8-OHdG, 8-hydroxydeoxyguanosine; AA, arachiodonic acid; AGE, advanced glycation end product; CAT, catalase; DCF, 2′,7′-dichlroflurescein; GPx, glutathione peroxidase; GR, glutathione reductase; GSH, glutathione; HFD, high-fat diet; HRG, high pressure-treated red ginseng; HO-1, heme oxygenase 1; MDA, malondialdehyde; NADPH, nicotinamide adenine dinucleotide phosphate; NMDA, N-methyl-D-aspartate; PCB126, polychlorinated biphenyls; SOD, superoxide dismutase; TBARS, thiobarbituric acid-reacting substances; TRX, thioredoxin reductase; Vit, vitamin.
As male adults become older, one physical problem is sexual dysfunction. Red ginseng intake (200 mg/kg/d) in aged rats restored sexual function as estimated by enhancement of both sperm maturation and impaired testicular functions. The underlying mechanism for these alterations was revealed to be due to the antioxidant functions of red ginseng. Rats fed with red ginseng also displayed reduced MDA levels, while enzymatic and non-enzymatic antioxidants were elevated [38]. Other symptoms observed in aging rats was renal dysfunction; however one study showed that red ginseng treatment (200 mg/kg/d; major constituents: Rg1, Rb1, Rg3, Re, Rc, Rb2, and Rd) rescued oxidative-stress damaged renal tissue as detected by 8-OHdG and advanced glycation end-product levels [39]. As listed in Table 3, red ginseng oil (10–50 mg/kg/d)-fed mice exhibited improvements in carbon tetrachloride (CCl4)-damaged liver tissue following upregulation of antioxidant capacity, as well as downregulation of the MAPK pathway [25]. Additionally, rats administered 250mg/kg/d red ginseng extract prior to injection of aflatoxin B1 to induce hepatocyte damage showed decreased apoptosis levels in liver tissue and elevated antioxidant enzyme levels [40]. Red ginseng extract treatment (250–500 mg/kg/d) also relieved symptoms of fatty liver induced by chronic exposure to ethanol. In this study, lipid peroxidation as determined by 4-HNE and oxidative-protein modifications evaluated by nitrotyrosine were both decreased. Furthermore, AMPK/Sirt1 levels were reported to be involved in the observed decrease in oxidative stress [41].
Fermented red ginseng extract (100–200 mg/kg/d) treatment of diabetic rats exerted antioxidant effects, and red ginseng (0.2–0.4 mg/kg/d; major constituents: Rg1, Rb1, Rg3, Re, Rc, Rb2, Rd, Rf, Rh1, and Rg2) administration reduced cyclosporine-induced elevated 8-OHdG levels in mouse pancreas [42], [43], indicating anti-diabetic effects.
Gastric ulcers can be induced by oral administration of hydrochloride in ethanol or indomethacin in mice. Red ginseng treatment (30–300 mg/kg/d) with these drugs resulted in a decrease in ulcer area and TBARS [44]. Interestingly, high temperature- and high pressure-treated red ginseng (100 mg/kg/d)-fed mice ameliorated exercise-induced oxidative stress as compared with commercial red ginseng extracts (100 mg/kg/d) [45].
When saponin was fractionated from red-ginseng crude extract and administered to mice with arthritis, the red ginseng saponin extract (10 mg/kg/d; major constituents: Rg3, Rk1, and Rg5) reduced not only inflammatory factors, but also lipid peroxidation and nitrotyrosine levels. Additionally, the recovery of antioxidant enzymes in the liver and kidney that had been damaged by oxidative stress was also observed [46]. Furthermore, red ginseng (25 mg/kg/d; major constituents: Rb1 and Rg1) reduced incidence of skin cancer induced by 7,12-dimethylbenz(a)anthracene and croton oil through antioxidant mechanisms [47].
5. Assessment of antioxidant activities of red ginseng in humans
Biomarkers that were utilized to determine antioxidant activities of red ginseng in humans are listed in Table 4. Three grams of red ginseng per day and 6 g/d of red ginseng were randomly allocated to healthy participants for 8 wk along with a placebo. The participants that ingested red ginseng displayed elevated levels of antioxidant enzymes that were greater than the placebo group, along with decreased levels of DNA damage (detected by alkaline comet assay) and lipid peroxidation (detected as oxLDL levels) [48].
Table 4.
List of studies showing antioxidant activity of red ginseng in humans
| Participants | Red ginseng type | Antioxidant Biomarker | Ref |
|---|---|---|---|
| Healthy participants | Capsule containing red ginseng | SOD, CAT, GPx ↑ DNA tail length ↓ oxLDL ↓ |
48 |
| Postmenopausal women | Capsule containing red ginseng | SOD ↑ MDA ↓ |
49 |
CAT, catalase; GPx, glutathione peroxidase; MDA, malondialdehyde; oxLDL, oxidized low-density lipoprotein; SOD, superoxide dismutase.
Reduced estrogen levels in postmenopausal women are associated with high-risk of cardiovascular diseases. Postmenopausal women who ingested 3 g/d of red ginseng for 12 wk displayed higher concentrations of antioxidant enzymes relative to the placebo group. Furthermore, serum MDA levels were also reduced, although ingestion of red ginseng had no effect on serum 8-OHdG levels [49].
6. Limitations and perspectives
There have been limited studies on the antioxidant activity of red ginseng in humans. Although ginseng has been regarded as safe [50], [51], several studies warned about adverse effects of red ginseng, including allergies and toxicity to the heart, kidney, liver, and reproductive organs [52]. Additionally, depending on the processing method, the amount and type of ginsenosides vary. Therefore, application of red ginseng in humans requires development of a standard processing method and various other controlled conditions, such as proper daily dose duration and accurate determination of patient health status. As previously described, red ginseng is produced through a process of steaming and drying of white ginseng, resulting in improved stability and elevated ginsenoside content [53]. Despite its reported health benefits, studies on red ginseng are still limited in Asia, in contrast to worldwide studies on white ginseng. Therefore, further studies on the efficacy of red ginseng in Western countries and direct comparison with white ginseng regarding their reported functions are necessary.
In summary, red ginseng and ginsenosides, the major bioactive constituents in red ginseng, have biological functions ameliorating various disease symptoms via antioxidant mechanisms in cells and animals (especially in rodents). Although, there are limitations with respect to human studies on the effect of red ginseng, ingestion of red ginseng has also been shown to improve antioxidant activities in humans determined by various biomarkers of oxidative stress. Taken together, ingestion of red ginseng can be a preventive strategy against oxidative stress associated chronic diseases.
Conflicts of interest
None declared.
Acknowledgments
This study was supported in part by The Korean Society of Ginseng (2014).
References
- 1.Radad K., Gille G., Liu L., Rausch W.D. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 2006;100:175–186. doi: 10.1254/jphs.crj05010x. [DOI] [PubMed] [Google Scholar]
- 2.Bae J.S., Park H.S., Park J.W., Li S.H., Chun Y.S. Red ginseng and 20(S)-Rg3 control testosterone-induced prostate hyperplasia by deregulating androgen receptor signaling. J Nat Med. 2012;66:476–485. doi: 10.1007/s11418-011-0609-8. [DOI] [PubMed] [Google Scholar]
- 3.Choi Y.J., Choi H., Cho C.H., Park J.W. Red ginseng deregulates hypoxia-induced genes by dissociating the HIF-1 dimer. J Nat Med. 2011;65:344–352. doi: 10.1007/s11418-010-0504-8. [DOI] [PubMed] [Google Scholar]
- 4.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 5.Wong V.K., Cheung S., Li T., Jiang Z.H., Wang J.R., Dong H., Yi X.Q., Zhou H., Liu L. Asian ginseng extract inhibits in vitro and in vivo growth of mouse lewis lung carcinoma via modulation of ERK-p53 and NF-kappaB signaling. J Cell Biochem. 2010;111:899–910. doi: 10.1002/jcb.22778. [DOI] [PubMed] [Google Scholar]
- 6.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 7.Chen K., Gunter K., Maines M.D. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem. 2000;75:304–313. doi: 10.1046/j.1471-4159.2000.0750304.x. [DOI] [PubMed] [Google Scholar]
- 8.Khansari N., Shakiba Y., Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 9.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;1:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtin J.F., Donovan M., Cotter T.G. Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods. 2002;265:49–72. doi: 10.1016/s0022-1759(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 11.Wu L.L., Chiou C.C., Chang P.Y., Wu J.T. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clinica Chimica Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A., Singh K., Almasan A. Histone H2AX phosphorylation: a marker for DNA damage. Methods Mol Biol. 2012;920:613–626. doi: 10.1007/978-1-61779-998-3_40. [DOI] [PubMed] [Google Scholar]
- 13.Kuo L.J., Yang L.X. Gamma-H2AX – a novel biomarker for DNA double-strand breaks. Vivo. 2008;22:305–309. [PubMed] [Google Scholar]
- 14.Olive P.L., Banath J.P. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2006;1:23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- 15.Bartkova J., Horejsi Z., Koed K., Kramer A., Tort F., Zieger K., Guldberg P., Sehested M., Nesland J.M., Lukas C. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 16.Ho E., Karimi Galougahi K., Liu C.C., Bhindi R., Figtree G.A. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meagher E.A., FitzGerald G.A. Indices of lipid peroxidation in vivo: strengths and limitations. Free Radic Biol Med. 2000;28:1745–1770. doi: 10.1016/s0891-5849(00)00232-x. [DOI] [PubMed] [Google Scholar]
- 18.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53:S26–S33. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 19.Rahman I., van Schadewijk A.A., Crowther A.J., Hiemstra P.S., Stolk J., MacNee W., De Boer W.I. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- 20.Song B.C., Joo N.S., Aldini G., Yeum K.J. Biological functions of histidine-dipeptides and metabolic syndrome. Nutr Res Pract. 2014;8:3–10. doi: 10.4162/nrp.2014.8.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y.K., Guo Q., Packer L. Free radical scavenging activity of red ginseng aqueous extracts. Toxicology. 2002;172:149–156. doi: 10.1016/s0300-483x(01)00585-6. [DOI] [PubMed] [Google Scholar]
- 22.Park B.J., Lim Y.S., Lee H.J., Eum W.S., Park J., Han K.H., Choi S.Y., Lee K.S. Anti-oxidative effects of Phellinus linteus and red ginseng extracts on oxidative stress-induced DNA damage. BMB Rep. 2009;42:500–505. doi: 10.5483/bmbrep.2009.42.8.500. [DOI] [PubMed] [Google Scholar]
- 23.Im G.J., Chang J.W., Choi J., Chae S.W., Ko E.J., Jung H.H. Protective effect of Korean red ginseng extract on cisplatin ototoxicity in HEI-OC1 auditory cells. Phytother Res. 2010;24:614–621. doi: 10.1002/ptr.3082. [DOI] [PubMed] [Google Scholar]
- 24.Park S.H., Jang J.H., Chen C.Y., Na H.K., Surh Y.J. A formulated red ginseng extract rescues PC12 cells from PCB-induced oxidative cell death through Nrf2-mediated upregulation of heme oxygenase-1 and glutamate cysteine ligase. Toxicology. 2010;278:131–139. doi: 10.1016/j.tox.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Bak M.J., Jun M., Jeong W.S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H(2)O(2)-treated hepG2 cells and CCl(4)-treated mice. Int J Mol Sci. 2012;13:2314–2330. doi: 10.3390/ijms13022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park H.M., Kim S.J., Mun A.R., Go H.K., Kim G.B., Kim S.Z., Jang S.I., Lee S.J., Kim J.S., Kang H.S. Korean red ginseng and its primary ginsenosides inhibit ethanol-induced oxidative injury by suppression of the MAPK pathway in TIB-73 cell. J Ethnopharmacol. 2012;141:1071–1076. doi: 10.1016/j.jep.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 27.Sohn S.H., Kim S.K., Kim Y.O., Kim H.D., Shin Y.S., Yang S.O., Kim S.Y., Lee S.W. A comparison of antioxidant activity of Korean White and Red Ginsengs on H2O2-induced oxidative stress in HepG2 hepatoma cells. J Ginseng Res. 2013;37:442–450. doi: 10.5142/jgr.2013.37.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong G.Z., Jang E.J., Kang S.H., Cho I.J., Park S.D., Kim S.C., Kim Y.W. Red ginseng abrogates oxidative stress via mitochondria protection mediated by LKB1-AMPK pathway. BMC Complement Altern Med. 2013;13:64. doi: 10.1186/1472-6882-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S., Lee Y., Cho J. Korean red ginseng extract exhibits neuroprotective effects through inhibition of apoptotic cell death. Biol Pharm Bull. 2014;37:938–946. doi: 10.1248/bpb.b13-00880. [DOI] [PubMed] [Google Scholar]
- 30.Han J.Y., Ahn S.Y., Oh E.H., Nam S.Y., Hong J.T., Oh K.W., Lee M.K. Red ginseng extract attenuates kainate-induced excitotoxicity by antioxidative effects. Evid Based Complement Alternat Med. 2012;2012:479016. doi: 10.1155/2012/479016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho S.O., Lim J.W., Kim H. Red ginseng extract inhibits the expression of MCP-1 and iNOS in Helicobacter pylori-infected gastric epithelial cells by suppressing the activation of NADPH oxidase and Jak2/Stat3. J Ethnopharmacol. 2013;150:761–764. doi: 10.1016/j.jep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Chang J.W., Park K.H., Hwang H.S., Shin Y.S., Oh Y.T., Kim C.H. Protective effects of Korean red ginseng against radiation-induced apoptosis in human HaCaT keratinocytes. J Radiat Res. 2014;55:245–246. doi: 10.1093/jrr/rrt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park H.R., Lee S.E., Yang H., Son G.W., Jin Y.H., Park Y.S. Induction of thioredoxin reductase 1 by Korean red ginseng water extract regulates cytoprotective effects on human endothelial cells. Evid Based Complement Alternat Med. 2015;2015:972040. doi: 10.1155/2015/972040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S.E., Park Y.S. Korean Red Ginseng water extract inhibits COX-2 expression by suppressing p38 in acrolein-treated human endothelial cells. J Ginseng Res. 2014;38:34–39. doi: 10.1016/j.jgr.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H.Y., Kim K. Protective effect of ginseng on cytokine-induced apoptosis in pancreatic beta-cells. J Agric Food Chem. 2007;55:2816–2823. doi: 10.1021/jf062577r. [DOI] [PubMed] [Google Scholar]
- 36.Sahhugi Z., Hasenan S.M., Jubri Z. Protective effects of gelam honey against oxidative damage in young and aged rats. Oxid Med Cell Longev. 2014;2014:673628. doi: 10.1155/2014/673628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramesh T., Kim S.W., Hwang S.Y., Sohn S.H., Yoo S.K., Kim S.K. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr Res. 2012;32:718–726. doi: 10.1016/j.nutres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Kopalli S.R., Hwang S.Y., Won Y.J., Kim S.W., Cha K.M., Han C.K., Hong J.Y., Kim S.K. Korean red ginseng extract rejuvenates testicular ineffectiveness and sperm maturation process in aged rats by regulating redox proteins and oxidative defense mechanisms. Exp Gerontol. 2015;69:94–102. doi: 10.1016/j.exger.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Park S., Kim C.S., Min J., Lee S.H., Jung Y.S. A high-fat diet increases oxidative renal injury and protein glycation in D-galactose-induced aging rats and its prevention by Korea red ginseng. J Nutr Sci Vitaminol. 2014;60:159–166. doi: 10.3177/jnsv.60.159. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y.S., Kim Y.H., Noh J.R., Cho E.S., Park J.H., Son H.Y. Protective effect of Korean red ginseng against aflatoxin B1-Induced hepatotoxicity in rat. J Ginseng Res. 2011;35:243–249. doi: 10.5142/jgr.2011.35.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han J.Y., Lee S., Yang J.H., Kim S., Sim J., Kim M.G., Jeong T.C., Ku S.K., Cho I.J., Ki S.H. Korean Red Ginseng attenuates ethanol-induced steatosis and oxidative stress via AMPK/Sirt1 activation. J Ginseng Res. 2015;39:105–115. doi: 10.1016/j.jgr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim H.J., Lee S.G., Chae I.G., Kim M.J., Im N.K., Yu M.H., Lee E.J., Lee I.S. Antioxidant effects of fermented red ginseng extracts in streptozotocin- induced diabetic rats. J Ginseng Res. 2011;35:129–137. doi: 10.5142/jgr.2011.35.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim S.W., Doh K.C., Jin L., Piao S.G., Heo S.B., Zheng Y.F., Bae S.K., Chung B.H., Yang C.W. Oral administration of ginseng ameliorates cyclosporine-induced pancreatic injury in an experimental mouse model. PloS ONE. 2013;8:e72685. doi: 10.1371/journal.pone.0072685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oyagi A., Ogawa K., Kakino M., Hara H. Protective effects of a gastrointestinal agent containing Korean red ginseng on gastric ulcer models in mice. BMC Complement Altern Med. 2010;10:45. doi: 10.1186/1472-6882-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu S.Y., Yoon B.R., Lee Y.J., Lee J.S., Hong H.D., Lee Y.C., Kim Y.C., Cho C.W., Kim K.T., Lee O.H. Inhibitory effect of high temperature- and high pressure-treated red ginseng on exercise-induced oxidative stress in ICR mouse. Nutrients. 2014;6:1003–1015. doi: 10.3390/nu6031003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim K.R., Chung T.Y., Shin H., Son S.H., Park K.K., Choi J.H., Chung W.Y. Red ginseng saponin extract attenuates murine collagen-induced arthritis by reducing pro-inflammatory responses and matrix metalloproteinase-3 expression. Biol Pharm Bill. 2010;33:604–610. doi: 10.1248/bpb.33.604. [DOI] [PubMed] [Google Scholar]
- 47.Sharma J., Goyal P.K. Chemoprevention of chemical-induced skin cancer by Panax ginseng root extract. J Ginseng Res. 2015;39:265–273. doi: 10.1016/j.jgr.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J.Y., Park J.Y., Kang H.J., Kim O.Y., Lee J.H. Beneficial effects of Korean red ginseng on lymphocyte DNA damage, antioxidant enzyme activity, and LDL oxidation in healthy participants: a randomized, double-blind, placebo-controlled trial. Nutr J. 2012;11:47. doi: 10.1186/1475-2891-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seo S.K., Hong Y., Yun B.H., Chon S.J., Jung Y.S., Park J.H., Cho S., Choi Y.S., Lee B.S. Antioxidative effects of Korean red ginseng in postmenopausal women: a double-blind randomized controlled trial. J Ethnopharmacol. 2014;154:753–757. doi: 10.1016/j.jep.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 50.Park S.J., Lim K.H., Noh H., Jeong E.J., Kim Y.S., Han B.C., Lee S.H., Moon K.S. Subacute oral toxicity study of Korean red ginseng extract in Sprague-Dawley rats. Toxicol Res. 2013;29:285–292. doi: 10.5487/TR.2013.29.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bak M.J., Kim K.B., Jun J., Jeong W.S. Safety of red ginseng oil for single oral administration in Sprague-Dawley rats. J Ginseng Res. 2014;38:78–81. doi: 10.1016/j.jgr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paik D.J., Lee C.H. Review of cases of patient risk associated with ginseng abuse and misuse. J Ginseng Res. 2015;39:89–93. doi: 10.1016/j.jgr.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huu Tung N., Uto T., Morinaga O., Kim Y.H., Shoyama Y. Pharmacological effects of ginseng on liver functions and diseases: a mini review. Evid Based Complement Alternat Med. 2012;2012:173297. doi: 10.1155/2012/173297. [DOI] [PMC free article] [PubMed] [Google Scholar]