Abstract
Background
The incidence of halitosis has a prevalence of 22–50% throughout the world and is generally caused by anaerobic oral microorganisms, such as Fusobacterium nucleatum, Clostridium perfringens, and Porphyromonas gingivalis. Previous investigations on the structure-activity relationships of ginsenosides have led to contrasting results. Particularly, the antibacterial activity of less polar ginsenosides against halitosis-related bacteria has not been reported.
Methods
Crude saponins extracted from the Panax quinquefolius leaf-stem (AGS) were treated at 130°C for 3 h to obtain heat-transformed saponins (HTS). Five ginsenoside-enriched fractions (HTS-1, HTS-2, HTS-3, HTS-4, and HTS-5) and less polar ginsenosides were separated by HP-20 resin absorption and HPLC, and the antimicrobial activity and mechanism were investigated.
Results
HPLC with diode-array detection analysis revealed that heat treatment induced an extensive conversion of polar ginsenosides (-Rg1/Re, -Rc, -Rb2, and -Rd) to less polar compounds (-Rg2, -Rg3, -Rg6, -F4, -Rg5, and -Rk1). The antimicrobial assays showed that HTS, HTS-3, and HTS-4 were effective at inhibiting the growth of F. nucleatum, C. perfringens, and P. gingivalis. Ginsenosides-Rg5 showed the best antimicrobial activity against the three bacteria, with the lowest values of minimum inhibitory concentration and minimum bactericidal concentration. One major reason for this result is that less polar ginsenosides can more easily damage membrane integrity.
Conclusion
The results indicated that the less polar ginsenoside-enriched fraction from heat transformation can be used as an antibacterial agent to control halitosis.
Keywords: antimicrobial activity, halitosis, heat transformation, less polar ginsenosides, Panax quinquefolius
1. Introduction
Panax quinquefolius (American ginseng), Panax ginseng (Chinese ginseng), and Panax notoginseng (notoginseng) are ginseng botanicals that have been used for thousands of years as important health food resources throughout the world. The saponins in ginseng, also called ginsenosides, are considered to be its main biological constituents. Thus far, at least 289 saponins have been reported from different Panax species [1]. The variety of ginsenosides is coincident in different parts of ginseng botanicals, but the contents show diversity [2]. Accordingly, ginsenosides from the stem and leaf of ginseng have similar pharmacological activity as those from the roots [3]. However, as the ginseng leaf and stem can be harvested every year as compared to every 4 yr for the roots, it is more economical to produce ginsenosides from the leaf and stem.
Most harvested ginseng roots are air-dried to obtain white ginseng, while others are steamed at 100°C for a few hours before drying, resulting in what is known as red ginseng. Red ginseng has a unique, less polar ginsenoside profile that is different from that of white ginseng, including ginsenoside-F4, -Rg3, -Rg5, -Rg6, -Rk1, -Rk2, -Rk3, and -Rs5, as well as a different biological activity [4], [5]. Methods of bioconversion by enzymes and endophytes have been developed to transform saponins to less polar ginsenosides [6], [7]. It was confirmed that it is possible to transform polar ginsenosides to less polar ginsenosides by a microwave and vinegar process [8]. However, transformation by heating in a reaction kettle is more conducive for application in a factory as compared to the other methods.
In our previous research, it was shown that steaming American ginseng roots at a high temperature could favorably change the ginsenoside structures, resulting in superior antibacterial activity against Propionibacteria and Staphylococci species [9]. However, to the best of our knowledge, the antibacterial activity of less polar ginsenosides against halitosis-related bacteria, such as Fusobacterium nucleatum, Clostridium perfringens, and Porphyromonas gingivalis, has not been reported.
The incidence of halitosis is 22–50% throughout the world [10]. Halitosis represents a global healthcare problem that greatly affects daily activity, hinders interpersonal communication, and can even cause psychological barriers in people of all ages [11]. Its cause is the oral production of volatile sulfide compounds, including hydrogen sulfide (H2S), methyl mercaptan (CH3SH), and dimethylsulfide (CH3SCH3), through proteolytic degradation by predominantly anaerobic Gram-negative oral microorganisms [12]. These bacterial species, such as P. gingivalis, Porphyromonas endodontalis, Prevotella intermedia, and F. nucleatum, are the most likely to cause oral malodor [13]. Chlorhexidine, essential oils, metal ions, oxidizing agents, triclosan, and cetylpyridinium chloride are often used as active ingredients in clinical treatments [13], [14]. However, there are some limitations regarding the use of these drugs, such as widespread periodontal anaerobe resistance and the risk of urticaria [15], [16]. Therefore, in the present study, the profile of heat-transformed leaf-stem ginsenosides was characterized, and their potential as antibacterials for curing halitosis was investigated.
2. Materials and methods
2.1. Chemicals
American ginseng leaf-stem saponins (AGS) were purchased from Jilin Hongju Biotechnology Co., Ltd. (Jilin, China). Ginsenoside standards (-Rg1, -Re, -Rb1, -Rc, -Rb2, -Rd, -20(R)-Rg2, -20(S)-Rg2, -20(R)-Rg3, and -20(S)-Rg3) with > 98% purity were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Ginsenoside-Rg6, -F4, -Rh4, -Rh2, -Rg5, and -Rk1 (> 98% purity) were isolated and identified in our laboratory by a previously reported method with some modifications [17]. Erythromycin, tetracycline, chlorhexidine, rhodamine 123, Coomassie Blue G250, and dimethyl sulfoxide (DMSO) were all purchased from Sigma-Aldrich (Shanghai, China). Agar, Gifu Anaerobic Broth (GAM broth), and CDC anaerobic blood agar base medium were purchased from Beijing Suolaibao Biotech Co., Ltd. (Beijing, China). The other chemicals are analytical or chromatographic grade.
2.2. Material and sample preparation
The AGS (500 g) was dissolved in distilled water (2,500 mL) and then subjected to an autoclave (MLS-3750; SANYO, Osaka, Japan) at 130°C for 3 h. The heat-transformed saponins (HTS, 400 g) were loaded onto a HP-20 column and sequentially eluted with an ethanol gradient from 0% to 30%, 60%, 80%, and 95%. The fractions were collected, evaporated using a rotary evaporator (Buchi, Flawil, St. Gallen, Switzerland) at 45°C to remove the ethanol, and were lyophilized to obtain a dry powder. The fractions were named HTS-1, HTS-2, HTS-3, HTS-4, and HTS-5.
2.3. HPLC analyses
Chromatographic analysis was performed using a SHIMADZU Prominence LC-20A HPLC instrument (Shimadzu Corporation, Kyoto, Japan) equipped with a YMC-Pack ODS-AM column (4.6 mm × 250 mm; YMC Co., Ltd., Kyoto, Japan). The detection wavelength was set at 202 nm and the column oven at 25°C. The mobile phase consisted of water (A) and acetonitrile (B). A gradient elution was used as follows: 25% B at 0–5 min, 30–32% B at 5–14 min, 32–38% B at 14–28 min, 38–46% B at 28–30 min, 46–74% B at 30–50 min, 74–80% B at 50–51 min, 80–90% B at 51–60 min, 90–100% B at 60–65 min, and 100–25% B at 65–70 min. The flow rate was kept at 1 mL/min, and the injected volume was 10 μL.
2.4. HPLC-ESI-MS conditions
The column effluent of the HPLC was introduced into an Agilent-LC-1100 (Agilent, Santa Clara, CA, USA) mass spectrometer equipped with an ESI source 6,460 (Agilent). The parameters of the ESI were set according to a previous report with slight modifications [18]. Briefly, the collision-gas (N2) rate was maintained at 10 mL/min and the column oven at 25°C. ESI-MS data were acquired in negative mode to generate [M-H]− ginsenoside ions by fully scanning m/z over 50–2,000. The spray voltage was 4.5 kV, the capillary voltage was 10 V, and the capillary temperature was 250°C.
2.5. Preparation of ginsenoside standard curves
Stock solutions were prepared by dissolving ginsenoside-Rg1 (1.53 mg), -Re (1.52 mg), -Rb2 (0.52 mg), -20(S)-Rg2 (1.55 mg), -Rd (0.83 mg), -20(S)-Rg3 (0.43 mg), -20(R)-Rg3 (0.62 mg), -Rg6 (2.32 mg), -F4 (1.08 mg), -Rh4 (2.51 mg), -Rk1 (0.98 mg), -Rg5 (1.01 mg), and -Rh2 (1.52 mg) in 70% (v/v) ethanol (1 mL). The injected volumes were 1 μL, 2 μL, 4 μL, 8 μL, 10 μL, and 12 μL. The working standard solutions were analyzed by the established method in triplicate. Calibration curves were plotted as the peak area (y) versus the amount of each ginsenoside standard (x). The content of ginsenoside in each sample was evaluated by the standard curve of each analyte. The recovery test was performed by a previously reported method [19]. Three different amounts of ten ginsenosides were added to known concentrations of the reanalyzed HTS-3 sample solutions. The spiked samples were analyzed in triplicate by the HPLC method described.
2.6. Antimicrobial assay
The antimicrobial activity was analyzed by the method of Wang et al [20]. All standard strains were purchased from Guangdong Microbiology Culture Center (Guangzhou, China). F. nucleatum (ATCC 10953), C. perfringens (ATCC 13124), and P. gingivalis (ATCC 33277) were cultured in CDC anaerobic blood agar base medium for 48 h at 37°C in a YQX-II anaerobic incubator (Shanghai, China) for further use. The anaerobic incubation atmosphere contained 5% (v/v) CO2, 10% (v/v) H2, and 85% (v/v) N2. Cell suspensions were diluted in sterile GAM to provide initial cell counts of ∼108 colony-forming units per mL (CFU/mL). Erythromycin, tetracycline, and chlorhexidine were dissolved in DMSO to a concentration of 3 mg/mL for the subsequent antibacterial experiments as positive controls. All samples were dissolved in DMSO at a concentration of 20 mg/mL, except for ginsenoside-Rg6, -F4, -20(R)-Rg3, -20(S)-Rg3, -Rh4, -Rk1, -Rg5, and -Rh2, which were added at concentrations of 1 mg/mL.
2.7. Determination of bacterial growth
The inhibitory effect on bacterial growth was determined by the disk-diffusion method [20], [21]. Briefly, the bacteria were adjusted to the Mcfarland 0.5 standard and used to inoculate CDC agar or poly-beta-hydroxybutyric acid plates. The disk (6 mm in diameter) was impregnated with 10 μL of 10 mg/mL (100 μg/disc) samples and placed on the seeded agar. Erythromycin, tetracycline, and chlorhexidine (30 μg/disc) were used as positive controls. Discs containing only DMSO were used as negative controls.
2.8. Determination of minimum inhibitory concentration and minimum bactericidal concentration
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values were determined by microbroth-dilution methods [21]. Diluted suspensions (100 μL) of F. nucleatum, C. perfringens, and P. gingivalis were inoculated into each well of 96-well microplates, followed by addition of 100 μL of samples with different concentrations. Agent-free broths were incubated as growth controls. The final range of test-sample dilutions was 1.25–1.225 μg/mL in the GAM broth, and the final bacteria concentration in each dilution was 1 × 105 CFU/mL. The plates were incubated in an anaerobic incubator at 37°C for 48 h. The MIC was defined as the lowest concentration of antibacterial agent that inhibited bacterial growth as indicated by the absence of turbidity. MBC was determined by inoculating 10 μL of medium from each of the wells of the MIC test that showed no turbidity onto CDC agar plates and incubating them for 48 h. The MBC values were defined as the lowest concentration of antibacterial agents for which there was no bacterial growth on the plates. All determinations were performed in triplicate.
2.9. Cell membrane integrity
The integrity of the cell membrane was evaluated by measuring the release of cell constituents, including nucleic acids and proteins, into a cell suspension [22]. Bacterial cells from suspensions (5 mL) of P. gingivalis, C. perfringens, and F. nucleatum were collected by centrifugation for 3 min at 6,000g. The cells were washed three times and resuspended in phosphate-buffered saline [PBS; 0.1M (pH 7.4)]. Five milliliters of the washed suspension was incubated at 37°C for 12 h in the presence of variable concentrations of HTS-4 (control, MIC, and MBC) and AGS (0.5 mg/mL). Then, the suspensions were centrifuged at 6,500g for 3 min, and the supernatants were diluted with PBS. One-hundred microliters of each supernatant was transferred into the wells of 96-well microplates, and the absorbance at 260 nm was measured using a Spectramax Plus384 UV-vis spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). Correction was carried out for the absorbance of the suspension with 0.1M PBS (pH 7.4) containing the same concentration of HTS-4 after a 2-min reaction with the tested strains. The untreated cells (control) were corrected with 0.1M PBS (pH 7.4). The suspension was also collected to determine the concentrations of proteins according to the Bradford method with some modifications [23].
2.10. Membrane potential
To analyze the effects of HTS-4 on the metabolic activity of bacterial cells, the membrane potential (MP) of the bacteria was measured according to the rhodamine 123 fluorescence method, as described by Comas and Vives-Rego [24], with modifications. Bacterial cells were incubated in GAM medium at 37°C for 24 h. The cell solutions (∼1 × 107 CFU/mL) were added with different concentrations of HTS-4 (control, MIC, and MBC levels) and AGS (0.5 mg/mL) and incubated for 8 h. The suspensions were washed with PBS, and rhodamine 123 was then added to a final concentration of 2 μg/mL. After standing in the dark for 30 min, the samples were completely washed and resuspended in PBS. One-hundred microliters of the cell suspension was transferred into the wells of a 96-well microplate and placed in a SpectraMax M2e spectrofluorometer (Molecular Devices). The rhodamine 123 fluorescence was excited at 480 nm, and the emission wavelength was 530 nm. The data were expressed as the mean fluorescence intensity (MFI).
2.11. Statistical analysis
Data were presented as the mean of three replicates ± standard deviation. The statistical analysis of the results was performed in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) and Sigma Plot 10.0 (SPSS Inc., Chicago, IL, USA). The Student t test was used to compare the differences in mean values at the 5% level.
3. Results and discussion
3.1. Characterization of ginsenosides
The linearity of the calibration curves of the ginsenosides is demonstrated in Table 1. It was found that the reference compounds showed good linearity (R2 ≥ 0.992), except for ginsenoside-Rd (R2 = 0.983) and -Rg3 (R) (R2 = 0.989). The average recovery of the ginsenosides ranged from 98.1% to 102.8%, with a relative standard deviation of < 3.0%.
Table 1.
Analytical characteristics of ginsenosides (mg/mg)
| Peak | Ginsenoside | Retention time | Calibration curve | R2 | AGS | HTS1) | HTS-1 | HTS-2 | HTS-3 | HTS-4 | HTS-5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Re(Rg1) | 9.387 | y = 481,999, x + 93,235 | 0.9985 | 0.41 ± 0.01 | n.d | n.d | n.d | 0.04 ± 0.01 | n.d | n.d |
| 2 | Rg2(S) | 28.011 | y = 465,264, x + 200,215 | 0.9956 | 0.07 ± 0.01 | 0.05 ± 0.01 | n.d | n.d | 0.07 ± 0.01 | n.d | n.d |
| 3 | Rb2 | 28.842 | y = 108,262, x + 11,323 | 0.9933 | 0.16 ± 0.01 | 0.02 ± 0.01 | n.d | n.d | 0.11 ± 0.01 | n.d | n.d |
| 4 | Rd | 32.543 | y = 271,969, x + 427,367 | 0.9837 | 0.16 ± 0.01 | 0.01 ± 0.00 | n.d | n.d | 0.03 ± 0.01 | n.d | n.d |
| 5 | Rg6 | 38.751 | y = 719,696, x + 188,528 | 0.9982 | 0.07 ± 0.01 | 0.10 ± 0.03 | n.d | n.d | 0.07 ± 0.01 | 0.06 ± 0.01 | n.d |
| 6 | F4 | 39.378 | y = 1E+06, x − 660,748 | 0.9863 | 0.01 ± 0.01 | 0.02 ± 0.01 | n.d | n.d | 0.04 ± 0.01 | 0.04 ± 0.01 | n.d |
| 7 | Rh4 | 41.168 | y = 774,675, x + 146,497 | 0.9995 | n.d | 0.08 ± 0.01 | n.d | n.d | 0.02 ± 0.01 | 0.07 ± 0.01 | n.d |
| 8 | Rg3(S) | 41.631 | y = 177,999, x + 62,300 | 0.9926 | 0.01 ± 0.01 | 0.07 ± 0.00 | n.d | n.d | 0.02 ± 0.01 | 0.11 ± 0.01 | n.d |
| 9 | Rg3(R) | 42.105 | y = 218,319, x − 56,590 | 0.9893 | 0.01 ± 0.01 | 0.04 ± 0.01 | n.d | n.d | 0.01 ± 0.01 | 0.07 ± 0.01 | n.d |
| 10 | Rk1 | 48.011 | y = 198,283, x − 56,590 | 0.9904 | 0.01 ± 0.01 | 0.20 ± 0.02 | n.d | n.d | 0.02 ± 0.01 | 0.27 ± 0.01 | n.d |
| 11 | Rg5 | 48.653 | y = 506,274, x + 31,631 | 0.9991 | 0.01 ± 0.01 | 0.25 ± 0.01 | n.d | n.d | 0.02 ± 0.01 | 0.27 ± 0.01 | n.d |
| 12 | Rh2 | 51.384 | y = 485,961, x + 194,713 | 0.9942 | 0.01 ± 0.01 | 0.01 ± 0.001 | n.d | n.d | 0.02 ± 0.01 | 0.02 ± 0.01 | n.d |
| Total content of | 0.91 ± 0.03 | 0.83 ± 0.02 | n.d | n.d | 0.45 ± 0.04 | 0.91 ± 0.03 | n.d | ||||
| less polar ginsenosides | 0.10 ± 0.01 | 0.76 ± 0.04 | n.d | n.d | 0.20 ± 0.03 | 0.91 ± 0.03 | n.d |
Data are expressed as mean ± standard deviation of triplicate samples
AGS, Panax quinquefolius leaf-stem; HTS, heat-transformed saponins; n.d., not detected
HTS-1, HTS-2, HTS-3, HTS-4, and HTS-5 were from the 0, 30%, 60%, 80%, and 95% methanol-eluted fractions from an HP-20 column, respectively
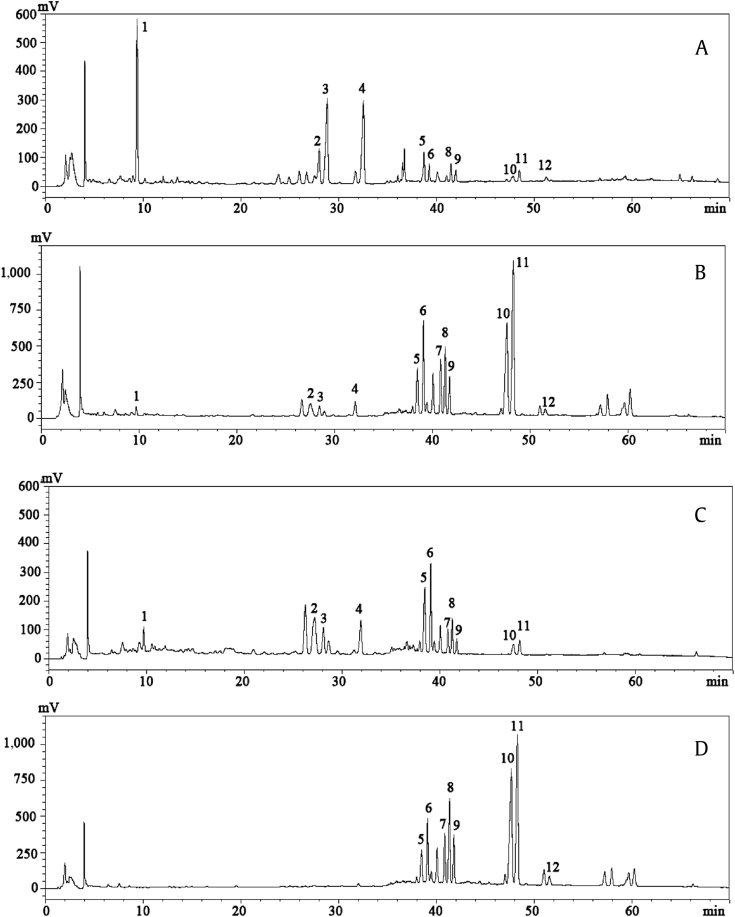
Thirteen ginsenosides were simultaneously identified according to the standard retention times and spectra, HPLC-ESI-MS ion fragments, and nuclear magnetic resonance (results not shown). The fragment ion results of the ginsenosides were consistent with those previously reported [18]. Typical HPLC UV chromatograms of the ginsenoside-enriched fractions are shown in Fig. 1. The content of each ginsenoside in every sample is shown in Table 1. As shown in Fig. 1, 12 peaks (peak 1 contains -Rg1 and -Re) were successfully separated under a gradient elution of water and acetonitrile. AGS contained a high concentration of polar ginsenosides (0.81 ± 0.03 mg/mg), such as ginsenoside-Re/Rg1 (0.41 ± 0.01 mg/mg), -Rb2 (0.16 ± 0.01 mg/mg), and -Rd (0.16 ± 0.01 mg/mg). The contents of ginsenoside-Rb2, -Rg2(S), and –Rd, which were abundantly present in AGS, were significantly decreased in HTS (Fig. 1B), while the contents of less polar ginsenosides in HTS (0.76 ± 0.02 mg/mg) were significantly increased (p < 0.05) as compared to those in AGS (0.10 ± 0.01 mg/mg). The amount of crude saponins in HTS (0.83 ± 0.02 mg/mg) was slightly lower than that observed in AGS (0.91 ± 0.03 mg/mg). The polar ginsenosides, such as ginsenoside-Re/Rg1, -Rb2, and –Rd, hydrolyzed to form low-polarity ginsenosides, such as ginsenoside-Rg6, -F4, -Rh4, -20S-Rg3, -20R-Rg3, -Rk1, and -Rg5. After purification by HP-20, the contents of ginsenoside-Rk1 and -Rg5 were significantly increased in HTS-4 as compared to those in HTS-3 (Fig. 1C, 1D). The content of the less polar ginsenosides in the HTS fraction was 0.76 ± 0.04 mg/mg, but it increased to 0.91 ± 0.03 mg/mg in HTS-4. Additionally, the less polar ginsenosides in HTS-4 (0.91 ± 0.03 mg/mg) were approximately nine-fold higher than that observed in the unpurified AGS sample. The HTS-1, HTS-2, and HTS-5 fractions did not show any ginsenosides, because 0% and 30% methanol could not elute saponins from the HP-20 column, while 80% methanol eluted all of the remaining saponins from the resin.
Fig. 1.
Chromatograms. (A) Chromatogram of America ginseng saponins, (B) steamed ginseng at 130°C for 4 h, (C) 60% fraction, and (D) 80% fraction. 1, Rg1/Re; 2, Rg2(R); 3, Rc; 4, Rd; 5, Rg6; 6, F4; 7, Rh4; 8, Rg3(S); 9, Rg3(R); 10, Rk1; 11, Rg5; 12, Rh2.
3.2. Structural changes in the heat-transformation process
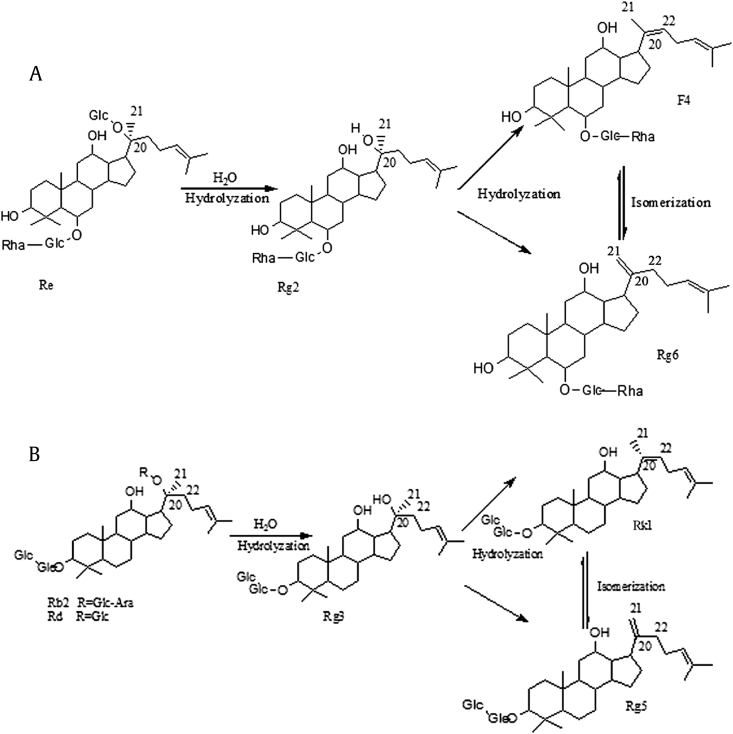
The structural transformation processes of the ginsenosides are summarized in Fig. 2. The results showed that natural ginsenosides could convert to low-polarity ginsenosides by deglycosylation at high temperature. Previous research provided ample evidence to establish the possible mechanisms involved in the heat-induced chemical conversion of ginsenosides [4]. Ginsenoside-Re could transform to ginsenosides -Rg2, -Rg6, and -F4, while ginsenoside-Rg1 could convert to ginsenosides -Rk3 and -Rh4. Protopanaxdiol ginsenosides, such as -Rb2 and -Rd, all transformed to ginsenoside-Rg3. Simultaneously, ginsenoside-Rg3 loses one molecular H2O at the position of C-20/22(20S) or C-20/21 (20R) and is converted to ginsenosides -Rk1 or -Rg5, respectively [5]. It is particularly noteworthy that the amount of less polar ginsenosides increased with the steaming temperature from 100°C to 120°C [5]. The total amount of less polar ginsenosides in ginseng that was steamed for 3 h was higher than that in ginseng that was steamed for 1 h [25]. Thus, we can conclude that the content of less polar ginsenosides was positively related to the steaming temperature and time.
Fig. 2.
Hydrolysis processes from polar ginsenosides to less polar ginsenosides. (A) Hydrolysis process of ginsenoside Re to ginsenosides Rg2, F4, and Rg6. (B) Hydrolysis process of ginsenosides Rb2 and Rd to ginsenosides Rg3, Rk1, and Rg5.
3.3. Antimicrobial activity
The antimicrobial activity results for the different ginsenoside fractions and standard antibiotics of erythrocin, tetracycline, and chlorhexidine are presented in Table 2. HTS, HTS-3, and HTS-4 had inhibition zones of 100 μg/disk against the three tested anaerobic bacteria. The results of the antibacterial test demonstrated a dose-dependent increase in the inhibition-zone diameter with higher amounts of less polar ginsenosides in the medium. AGS, HTS-1, HTS-2, and HTS-5 showed no inhibitory effect on the bacteria at the tested concentration. The MICs and MBCs of seven ginsenoside fractions (AGS, HTS, HTS-1, HTS-2, HTS-3, HTS-4, and HTS-5) against the three bacterial strains, along with erythrocin and chlorhexidine, are shown in Table 3. Similar to the inhibition-zone results, the MICs and MBCs of HTS-1, HTS-2, and HTS-5 (results not shown) were not detected, even at a concentration of 2.5 mg/mL. C. perfringens and P. gingivalis were mostly susceptible to HTS-4, with a MIC of 19.5 μg/mL and a MBC of 312.5 μg/mL. In our test, the less polar ginsenoside HTS-4 fraction demonstrated the highest activity against standard strains of anaerobic bacteria.
Table 2.
Growth inhibition of ginsenoside-enriched fractions and the positive control diameter of inhibition zone (mm) at 100 μg/disk
| Erythrocin | Chlorhexidine | AGS | HTS1) | HTS-1 | HTS-2 | HTS-3 | HTS-4 | HTS-5 | |
|---|---|---|---|---|---|---|---|---|---|
| Clostridium perfringens | 28.2 | 15.2 | 6.2 | 12.6 | 6.2 | 6.2 | 11.2 | 16.6 | 6.2 |
| Fusobacterium nucleatum | 28.4 | 14.4 | 6.1 | 11.7 | 6.1 | 6.1 | 10.5 | 17.4 | 6.1 |
| Porphyromonas gingivalis | 30.1 | 11.0 | 6.1 | 9.6 | 6.1 | 6.1 | 8.1 | 11.2 | 6.1 |
AGS, Panax quinquefolius leaf-stem; HTS, heat-transformed saponins
HTS-1, HTS-2, HTS-3, HTS-4, and HTS-5 were from the 0, 30%, 60%, 80%, and 95% methanol-eluted fractions from an HP-20 column, respectively
Table 3.
MIC (μg/mL) and MBC (μg/mL) of erythrocin, chlorhexidine, AGS, HTS, HTS-3, and HTS-41)
| Erythrocin |
Chlorhexidine |
AGS |
HTS2) |
HTS-3 |
HTS-4 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Clostridium perfringens | <2.45 | 19.5 | 19.5 | 312.5 | >2,500 | >2,500 | 39.06 | 1,250 | 312.5 | 1,250 | 19.5 | 312.5 |
| Fusobacterium nucleatum | <2.45 | 19.5 | 39.0 | 312.5 | >2,500 | >2,500 | 156.25 | 1,250 | 156.25 | 1,250 | 39.0 | 625.0 |
| Porphyromonas gingivalis | <2.45 | 19.5 | 39.0 | 1,250 | >2,500 | >2,500 | 39.06 | 1,250 | 156.25 | 1,250 | 19.5 | 312.5 |
AGS, Panax quinquefolius leaf-stem; HTS, heat-transformed saponins; MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration
The MIC and MBC of HTS-1, HTS-2, and HTS-5 were all not detected, even when the concentration was at 2.5 mg/mL (results not shown)
HTS-1, HTS-2, HTS-3, and HTS-4 were from the 0, 30%, 60%, and 80% methanol-eluted fractions from an HP-20 column, respectively
We also investigated which ginsenosides were responsible for the HTS-4 antibacterial effects. The MICs and MBCs of the eight less polar ginsenosides in HTS-4 were tested. As shown in Table 4, ginsenoside-Rg5 exhibited the lowest MIC and MBC among all of the ginsenosides in HTS-4 (MIC: 16.0 μg/mL; MBC: 31.3 μg/mL). Ginsenoside-Rg6 and -F4 played the same role for the antifungal activity of HTS-4, with the same highest MIC and MBC values (MIC: 125 μg/mL; MBC: 250 μg/mL). The other four less polar ginsenosides showed medium antimicrobial effects.
Table 4.
MIC and MBC of different ginsenoside monomers against the three strains of bacteria (μg/mL)
| Samples |
Clostridium perfringens |
Fusobacterium nucleatum |
Porphyromonas gingivalis |
|||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| Rg6 | 125.0 | 250.0 | 125.0 | 250.0 | 125.0 | 250.0 |
| F4 | 125.0 | 250.0 | 125.0 | 250.0 | 125.0 | 250.0 |
| Rh4 | 16.0 | 62.5 | 16.0 | 62.5 | 31.3 | 62.5 |
| Rg3(S) | 31.3 | 125.0 | 31.3 | 125.0 | 31.3 | 250.0 |
| Rg3(R) | 31.3 | 125.0 | 31.3 | 250.0 | 31.3 | 125.0 |
| Rk1 | 31.3 | 125.0 | 16.0 | 125.0 | 62.5 | 125.0 |
| Rg5 | 16.0 | 62.5 | 16.0 | 62.5 | 16.0 | 62.5 |
| Rh2 | 31.3 | 125.0 | 16.0 | 125.0 | 16.0 | 62.5 |
| Erythrocin | 8.0 | 16.0 | 8.0 | 31.3 | 16.0 | 62.5 |
MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration
Previous investigations of the structure-activity relationships of saponins reported contrasting results [26], [27], [28]. According to Wu et al [29], ginseng extracts containing ginsenoside-Rb1 and -Rg1 did not affect the growth rate of P. aeruginosa. Moreover, ginsenosides in Asian ginseng did not show any in vitro antibacterial effect against P. aeruginosa growth [30], [31]. Additionally, an in vitro study showed that ginsenoside-Rb1 did not inhibit the growth of H. pylori; however, protopanaxadiol significantly inhibited H. pylori growth, with a MIC of 50–100 μg/mL [32]. Interestingly, a refined ginsenoside mixture prepared from red Asian ginseng was useful in the treatment of S. aureus [33]. In summary, the antibacterial activities of ginseng can be affected by both extraction and processing methods [34], [35].
3.4. Antibacterial mechanism
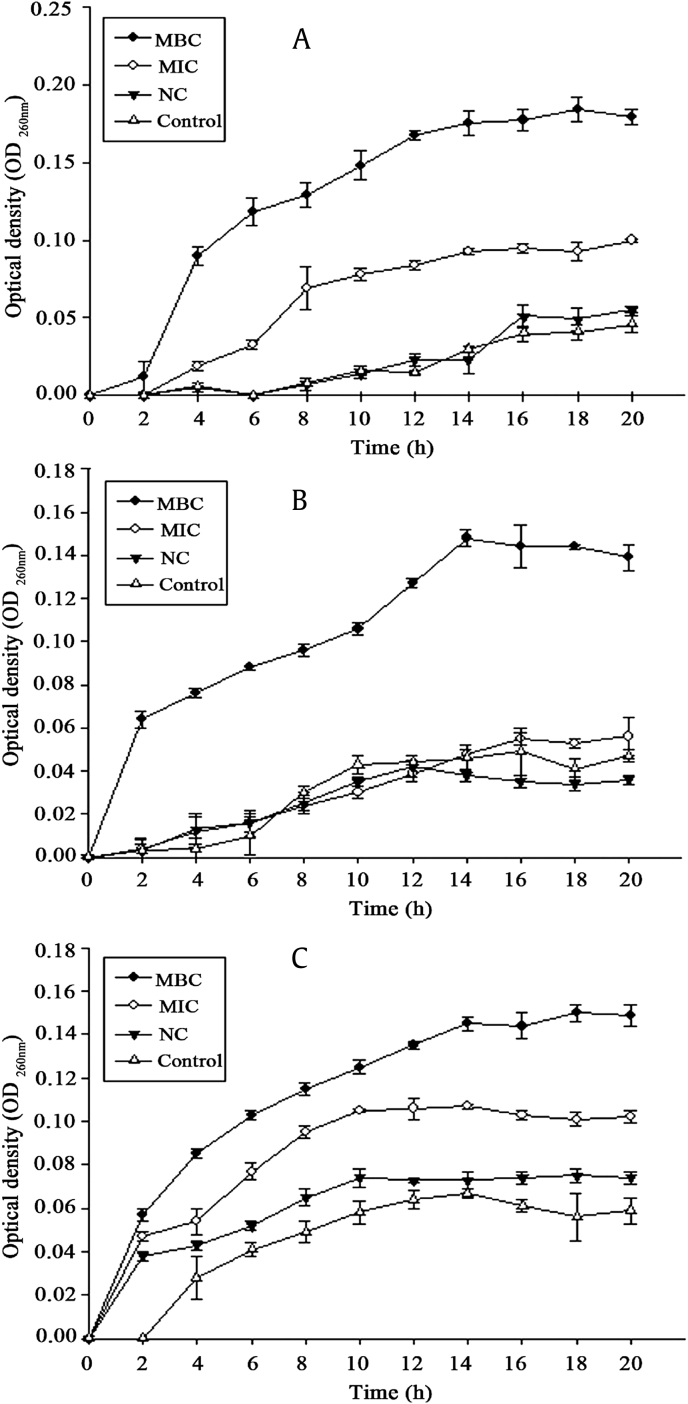
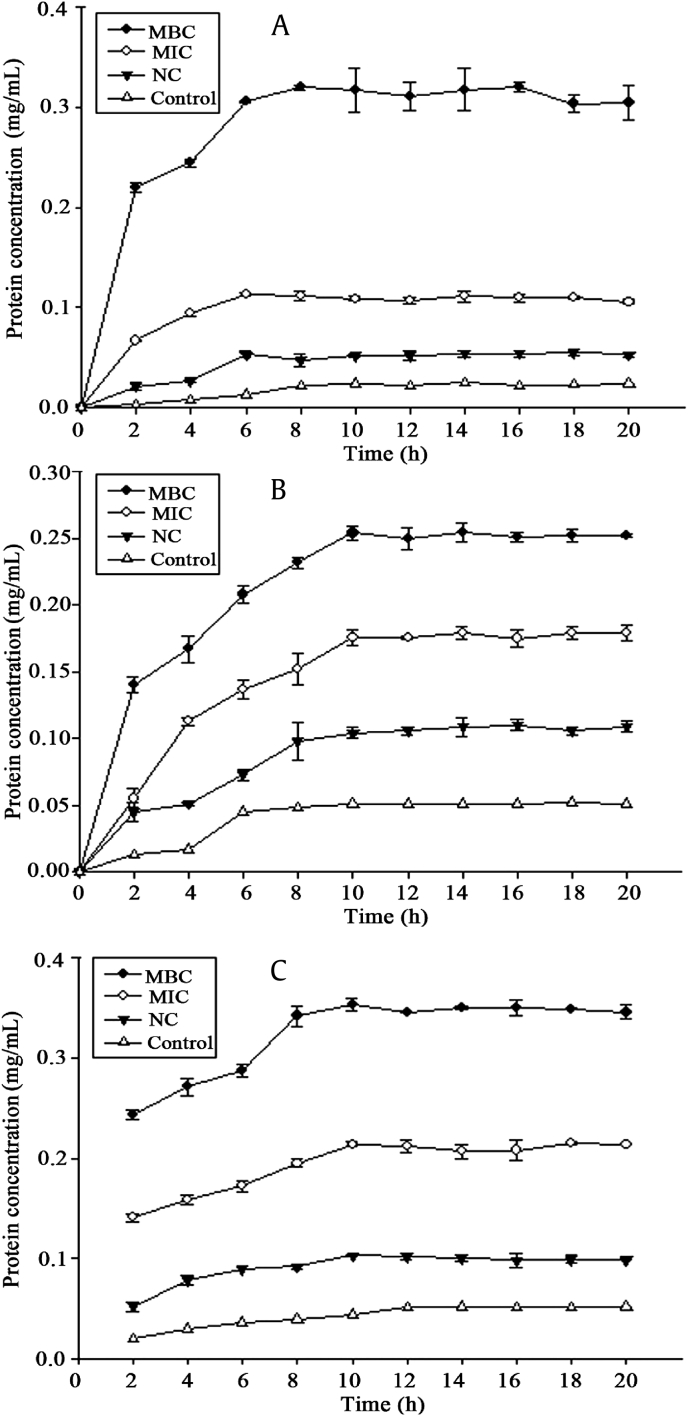
Information on the release of cell constituents revealed the integrity of the cell membrane. It was evident in our study that nucleic acids (Fig. 3) and proteins (Fig. 4) were released into the cell suspension, and that their levels increased multi-fold after contact with increasing HTS-4 concentrations. The absorbance value for the nucleic acids (OD260nm) of P. gingivalis increased significantly (p < 0.05), from 0.047 for the control to 0.100 and 0.184 in the presence of HTS-4 at the levels of the MIC and MBC at 22 h, respectively. After 18 h, the OD260nm value of P. gingivalis remained almost stable. Under similar conditions, there was a progressive release of nucleic acids from C. perfringens and F. nucleatum for up to almost 18 h, followed by a steady state. In the present study, the differences in the OD260nm between samples were narrower than those for Escherichia coli and Staphylococcus [36]. This phenomenon was likely due to the bacterial biofilm formed by P. gingivalis, C. perfringens, and F. nucleatum, and structural differences in the outer membranes of the bacteria. P .gingivalis, C. perfringens, and F. nucleatum are critical species in biofilm development [37], [38], [39]. The fragments of DNA or RNA combined with the matrix of biofilm may be removed by centrifugation. The protein values (mg/mL) also showed a significant increase (p < 0.05) for all bacteria, and the values of proteins at the MBC level increased faster than those at the MIC level. Interestingly, the concentrations of proteins and nucleic acids in bacteria treated with AGS showed no difference from those of untreated bacteria. The integrity of the cytoplasmic membrane is a critical factor to bacterial growth; therefore, analyzing the leakage of cell constituents can provide further insight into the mechanism of antibacterial action. Previous studies reported that the evaluation of cell-leakage markers, including absorbance at 260 nm for nucleic acids, and the determination of proteins are indicators of membrane integrity [36]. Our results clearly indicated that bacterial cell membrane integrity was compromised after exposure to less polar ginsenosides, which could consequently lead to cell death.
Fig. 3.
Nucleic acid absorbance. Release of 260-nm absorbing material from (A) Porphyromonas gingivalis, (B) Clostridium perfringens, and (C) Fusobacterium nucleatum treated with HTS-4 and AGS. AGS, Panax quinquefolius leaf-stem; HTS, heat-transformed saponins; MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration; NC, negative control.
Fig. 4.
Protein release. Release of protein from (A) Porphyromonas gingivalis, (B) Clostridium perfringens, and (C) Fusobacterium nucleatum treated with HTS-4 and AGS. AGS, Panax quinquefolius leaf-stem; HTS, heat-transformed saponins; MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration; NC, negative control.
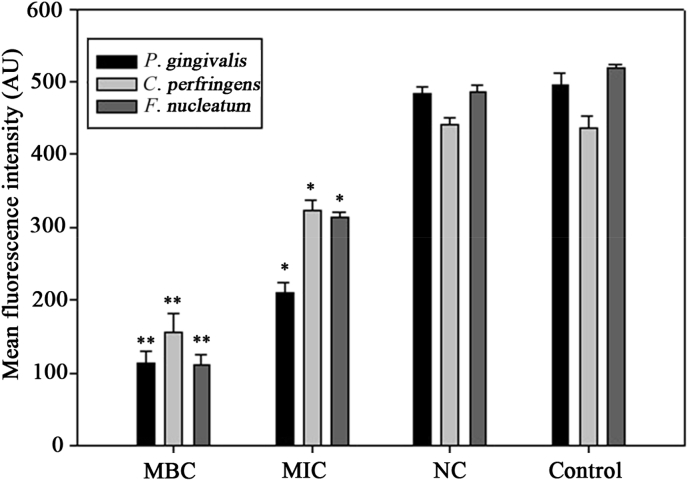
The result of the bacterial MP is shown in Fig. 5. The magnitude of the MP was illuminated by the MFI of rhodamine 123. After the addition of HTS-4 at the MBC level, a rapid decrease occurred in all bacteria, with MFI values decreasing by 77.21%, 64.53%, and 78.7% as compared with those of the controls. The MFIs of the three bacteria treated with HTS-4 at the MBC level decreased by 57.66%, 25.85%, and 40.54%. The bacteria treated with AGS were almost similar to the untreated bacteria. The MP was measured as the difference in electrical potential between the interior and exterior of a biological cell, and MP alterations in bacteria can affect their metabolic activity. An MP of normal bacteria is generated by differences in the concentrations of ions on opposite sides of the cell membrane. For this study, the fluorescence intensity was directly correlated with the bacterial MP. Our results showed that the mean fluorescence intensity of rhodamine 123 was significantly reduced after the addition of less polar ginsenosides. The loss of fluorescence indicated cell membrane depolarization, leading to irregular cell metabolic activity and bacterial death.
Fig. 5.
Membrane potential of Porphyromonas gingivalis, Clostridium perfringens, and Fusobacterium nucleatum treated with HTS-4 and AGS. AGS, Panax quinquefolius leaf-stem; AU, absorbance units; HTS, heat-transformed saponins; MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration; NC, negative control.
4. Conclusions
Our study showed that less polar ginsenosides exhibited higher antimicrobial efficacy relative to polar ginsenosides. One reason for this result was that less polar ginsenosides can more easily interact with and damage bacterial cell-membrane integrity. The antibacterial activities of less polar ginsenosides obtained by heat treatment from American ginseng leaf-stem saponins against F. nucleatum, C. perfringens, and P. gingivalis were reported here for the first time. The spectrum of action of less polar ginsenosides against medically important anaerobic bacteria and their considerable minimal inhibitory concentrations can be taken as good evidence for possible application of those compounds as antibacterial agents to control halitosis.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Yang W.Z., Hu Y., Wu W.Y., Ye M., Guo D.A. Saponins in the genus Panax L. (Araliaceae): a systematic review of their chemical diversity. Phytochemistry. 2014;106:7–24. doi: 10.1016/j.phytochem.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Xue Y., Wen L. RP-HPLC determination of twelve ginsenosides in extract of root and stem leaf from Panax quinquefolius L. Chin J Pharmaceut Ana. 2009;29:79–81. [Google Scholar]
- 3.Li F.L. Research Progress of Pharmacology effect about ginsenosides from stems and leaves of Panax ginseng. Guizhou Agric Sci. 2013;41:54–57. [Google Scholar]
- 4.Sun B.S., Pan F.Y., Sung C.K. Repetitious steaming-induced chemical transformations and global quality of black ginseng derived from Panax ginseng by HPLC-ESI-MS/MSn based chemical profiling approach. Biotechnol Bioproc E. 2011;16:956–965. [Google Scholar]
- 5.Kim W.Y., Kim J.M., Han S.B., Lee S.K., Kim N.D., Park M.K., Kim C.K., Park J.H. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 6.Luo S.L., Dang L.Z., Li J.F., Zou C.G., Zhang K.Q., Li G.H. Biotransformation of saponins by endophytes isolated from Panax notoginseng. Chem Biodivers. 2013;11:2021–2031. doi: 10.1002/cbdv.201300005. [DOI] [PubMed] [Google Scholar]
- 7.Du J., Cui C.H., Park S.C., Kim J.K., Yu H.S., Jin F.X., Sun C.K., Kim S.C., Im W.T. Identification and characterization of a ginsenoside-transforming β-glucosidase from Pseudonocardia sp. Gsoil 1536 and its application for enhanced production of minor ginsenoside Rg2(S) PLoS ONE. 2014;9:e96914. doi: 10.1371/journal.pone.0096914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S.J., Kim J.D., Ko S.K. Changes in ginsenoside composition of ginseng berry extracts after a microwave and vinegar process. J Ginseng Res. 2013;37:269–272. doi: 10.5142/jgr.2013.37.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L.J., Yang X.S., Yu X., Yao Y., Ren G.X. Evaluation of antibacterial and antiinflammatory activities of less polar ginsenosides produced from polar ginsenosides by heat-transformation. J Agric Food Chem. 2013;61:12274–12282. doi: 10.1021/jf404461q. [DOI] [PubMed] [Google Scholar]
- 10.Akaji E.A., Folaranmi N., Ashiwaju O. Halitosis: a review of the literature on its prevalence, impact and control. Oral Health Prev Dent. 2014;12:297–304. doi: 10.3290/j.ohpd.a33135. [DOI] [PubMed] [Google Scholar]
- 11.Lee S.S., Zhang W., Li Y. Halitosis update: a review of causes, diagnoses, and treatments. J Calif Dent Assoc. 2007;4 258–60,262,264–8. [PubMed] [Google Scholar]
- 12.Cortelli J.R., Barbosa M.D., Westphal M.A. Halitosis: a review of associated factors and therapeutic approach. Braz Oral Res. 2008;22:44–54. doi: 10.1590/s1806-83242008000500007. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho M.D., Tabchoury C.M., Cury J.A., Toledo S., Nogueira G.R. Impact of mouth rinses on morning bad breath in healthy subjects. J Clin Periodontol. 2004;31:85–90. doi: 10.1111/j.0303-6979.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 14.Fine D.H., Furgang D., Sinatra K., Charles C., McGuire A., Ku L.D. In vivo antimicrobial effectiveness of an essential oil-containing mouth rinse 12 h after a single use and 14 days' use. J Clin Periodontol. 2005;32:335–340. doi: 10.1111/j.1600-051x.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 15.Muller J.F., Ghosh S., Ikuma K., Stevens A.M., Love N.G. Chlorinated phenol-induced physiological antibiotic resistance in Pseudomonas aeruginosa. FEMS Microbiol Lett. 2015;362:1–7. doi: 10.1093/femsle/fnv172. [DOI] [PubMed] [Google Scholar]
- 16.Sharma A., Chopra H. Chlorhexidine urticaria: a rare occurrence with a common mouthwash. Indian J Dent Res. 2009;3:377–379. doi: 10.4103/0970-9290.57368. [DOI] [PubMed] [Google Scholar]
- 17.Park I.H., Kim N.Y., Han S.B., Kim J.M., Kwon S.W., Kim H.J., Park M.K., Park J.H. Three new dammarane glycosides from heat processed ginseng. Arch Pharm Res. 2002;25:428–432. doi: 10.1007/BF02976595. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y.C., Pi Z.F., Liu C.M., Song F.R., Liu Z.Q., Liu S.Y. Analysis of low-polar ginsenosides in steamed panax ginseng at high-temperature by HPLC-ESI-MS/MS. Chem Res Chinese U. 2012;28:31–36. [Google Scholar]
- 19.In G., Ahn N.G., Bae B.S., Han S.T., Noh K.B., Kim C.S. New method for simultaneous quantification of 12 ginsenosides in red ginseng powder and extract: In-house method validation. J Ginseng Res. 2012;36:205–210. doi: 10.5142/jgr.2012.36.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L.J., Yang X.S., Qin P., Shan F., Ren G.X. Flavonoid composition, antibacterial and antioxidant properties of tartary buckwheat bran extract. Ind Crop Prod. 2013;49:312–317. [Google Scholar]
- 21.Živkovic J., Barreira J.C.M., Stojkovic D., Cebovic T., Santos C., Maksimovic Z., Ferreira I.C. Phenolic profile, antibacterial, antimutagenic and antitumor evaluation of Veronica urticifolia Jacq. J Funct Foods. 2014;9:192–201. [Google Scholar]
- 22.Lv F., Liang H., Yuan Q., Li C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res Int. 2011;44:3057–3064. [Google Scholar]
- 23.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1975;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Comas J., Vives-Rego J. Assessment of the effects of gramicidin, form aldehyde, and surfactants on Escherichia coli by flow cytometry using nucleic acid and membrane potential dyes. Cytometry. 1997;29:58–64. doi: 10.1002/(sici)1097-0320(19970901)29:1<58::aid-cyto6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Xie Y.Y., Luo D., Chen Y.J., Ma J.F., Wang Y.M., Liang Q.L., Luo G.A. Steaming-induced chemical transformations and holistic quality assessment of red ginseng derived from by means of HPLC-ESI-MS/MSn-based multicomponent quantification fingerprint. J Agric Food Chem. 2012;60:8213–8224. doi: 10.1021/jf301116x. [DOI] [PubMed] [Google Scholar]
- 26.Oleszek W. Alfalfa saponins: structure, biological activity, and chemotaxonomy. In: Waller G.R., Yamasaki K., editors. In saponins used in food and agriculture. Plenum press; New York: 1996. pp. 155–170. [DOI] [PubMed] [Google Scholar]
- 27.Oleszek W., Naidu A.S. CRC Press; London: 2000. Saponins in natural food antimicrobial systems; pp. 1–30. [Google Scholar]
- 28.Sun H., Fang W.S., Wang W.Z., Hu C. Structure-activity relationships of oleanane- and ursane-type triterpenoids. Bot Stud. 2006;47:339–368. [Google Scholar]
- 29.Wu H., Lee B.L., Yang L., Wang H.G., Givskov M., Molin S., Høiby N., Song Z.J. Effects of ginseng on Pseudomonas aeruginosa motility and biofilm formation. FEMS Immunol Med Microbiol. 2011;62:49–56. doi: 10.1111/j.1574-695X.2011.00787.x. [DOI] [PubMed] [Google Scholar]
- 30.Song Z.J., Johansen H.K., Faber V., Høiby N. Ginseng treatment enhances bacterial clearance and decreases lung pathology in athymic rats with chronic P. aeruginosa pneumonia. APMIS. 1997;105:438–444. [PubMed] [Google Scholar]
- 31.Song Z.J., Johansen H.K., Faber V., Moser C., Kharazmi A., Rygaard J., Høiby N. Ginseng treatment reduces bacterial load and lung pathology in chronic Pseudomonas aeruginosa pneumonia in rats. Antimicrob Agents Chemother. 1997;41:961–964. doi: 10.1128/aac.41.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae E.A., Han M.J., Choo M.K., Park S.Y., Kim D.H. Metabolism of 20 (S)- and 20 (R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25:58–63. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- 33.Sung W.S., Lee D.G. The combination effect of Korean red ginseng saponins with kanamycin and cefotaxime against methicillin-resistant Staphylococcus aureus. Biol Pharm Bull. 2008;31:1614–1617. doi: 10.1248/bpb.31.1614. [DOI] [PubMed] [Google Scholar]
- 34.Lee K.A., Kim W.J., Kim H.J., Kim K.T., Paik H.D. Antibacterial activity of ginseng (Panax ginseng C. A. Meyer) stems-leaves extract produced by subcritical water extraction. Int J Food Sci Tech. 2013;48:947–953. [Google Scholar]
- 35.Norajit K., Ryu G.H. Preparation and properties of antibacterial alginate films incorporating extruded white ginseng extract. J Food Process Pres. 2011;35:387–393. [Google Scholar]
- 36.Bajpai V.K., Sharma A., Baek K.H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control. 2013;32:582–590. [Google Scholar]
- 37.Charlebois A., Jacques M., Archambault M. Biofilm formation of Clostridium perfringens and its exposure to low-dose antimicrobials. Front Microbiol. 2016 doi: 10.3389/fmicb.2014.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachrach G., Haake S.K., Glick A., Hazan R., Naor R., Andersen R.N., Kolenbrander P.E. Characterization of the novel Fusobacterium nucleatum plasmid pKH9 and evidence of an addiction system. Appl Environ Microbiol. 2004;70:6957–6962. doi: 10.1128/AEM.70.12.6957-6962.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lasserre J.F., Leprince J.G., Toma S., Brecx M.C. Electrical enhancement of chlorhexidine efficacy against the periodontal pathogen Porphyromonas gingivalis within a biofilm. New Microbiol. 2015;38:511–519. [PubMed] [Google Scholar]