Abstract
Peanut allergy is an IgE-mediated severe hypersensitivity disorder. The lack of a treatment of this potentially fatal allergy has led to intensive research on vaccine development. Here, we describe the design and initial characterization of a carrier-bound peptide derived from the most potent peanut allergen, Ara h 2, as a candidate vaccine. Based on the adjuvant capability of bacterial surface (S-) layers, a fusion protein of the S-layer protein SlpB from Lactobacillus buchneri CD034 and the Ara h 2-derived peptide AH3a42 was produced. This peptide comprised immunodominant B-cell epitopes as well as one T cell epitope. The fusion protein SlpB-AH3a42 was expressed in E. coli, purified, and tested for its IgE binding capacity as well as for its ability to activate sensitized rat basophil leukemia (RBL) cells. The capacity of Ara h 2-specific IgG rabbit-antibodies raised against SlpB-AH3a42 or Ara h 2 to inhibit IgE-binding was determined by ELISA inhibition assays using sera of peanut allergic patients sensitized to Ara h 2. IgE specific to the SlpB-AH3a42 fusion protein was detected in 69% (25 of 36) of the sera. Despite the recognition by IgE, the SlpB-AH3a42 fusion protein was unable to induce β-hexosaminidase release from sensitized RBL cells at concentrations up to 100 ng per ml. The inhibition of IgE-binding to the natural allergen observed after pre-incubation of the 20 sera with rabbit anti-SlpB-AH3a42 IgG was more than 30% for four sera, more than 20% for eight sera, and below 10% for eight sera. In comparison, anti-Ara h 2 rabbit IgG antibodies inhibited binding to Ara h 2 by 48% ±13.5%. Our data provide evidence for the feasibility of this novel approach towards the development of a peanut allergen peptide-based carrier-bound vaccine. Our experiments further indicate that more than one allergen-peptide will be needed to induce a broader protection of patients allergic to Ara h 2.
Keywords: Ara h 2, Food allergy, Lactobacillus buchneri, Peanut allergy vaccine, S-layer protein
1. Introduction
Peanut seeds are widely utilized in the food industry due to their high protein and oil content but, at the same time, they are one of the most common causes for severe allergic reactions (Bock et al., 2001; Bock et al., 2007). Allergen sensitization develops early in life, but in contrast to other food allergies in childhood, such as cow’s milk allergy, peanut allergy tends to persist into adulthood and thereby affects 0.6–0.8% of the adult population (Osborne et al., 2011; Skolnick et al., 2001; Soller et al., 2012). An efficient therapy is not available and therapeutic approaches that modify the immune response to peanut allergens and induce oral tolerance are being intensively investigated (reviewed in (Bublin and Breiteneder, 2014a; Commins et al., 2016; Kingwell, 2016)).
Several attempts have been made to design hypoallergenic variants of the major peanut allergens to avoid serious side effects, but still to generate tolerance or desensitization when used as vaccine components. Use of genetically modified peanut allergens with a highly reduced capacity to bind IgE but without compromising the ability of T cell stimulation is widely investigated (Burks et al., 1999; King et al., 2005; Rabjohn et al., 2002; Rolland et al., 2009; van Hoffen et al., 2014). Several immunotherapy trials were conducted with hypoallergenic peanut proteins and heat-killed bacteria as adjuvants (Li et al., 2003a,b; Wood et al., 2013). Another attractive approach is the use of short, linear peptides representing T cell epitopes of the major allergens that are able to modulate allergen-specific T cell responses without the ability to cross-link IgE and to activate effector cells (Sicherer and Sampson, 2007).
Bacterial surface (S-) layers, which are two-dimensional crystalline arrays of (glyco)protein subunits that make up the outermost layer of many bacteria, are excellent candidates to be used as antigen carriers in vaccine development (Sleytr et al., 2007; Sleytr et al., 2014). Proof-of-principle was accomplished by the translational fusion of the major birch pollen allergen Bet v 1 to the S-layer protein SbsC from Geobacillus stearothermophilus resulting in an Escherichia coli-produced fusion protein which showed reduced allergenicity and immunomodulatory capacity (Bohle et al., 2004; Gerstmayr et al., 2007; Gerstmayr et al., 2009). S-layers from lactic acid bacteria are particularly interesting candidates for carrier molecules in vaccine research because of their “generally regarded as safe” (GRAS) status. Currently, lactic acid bacteria are being intensively investigated as live vaccine delivery systems (Berlec et al., 2012; Trombert, 2015). Furthermore, their ability to withstand the passage through the gastrointestinal tract makes them an ideal oral antigen delivery system (Hynönen and Palva, 2013). In this context, the development of Lactobacillus strains carrying S-layers composed of tailored fusion proteins and, thus, displaying thousands of regularly arranged copies of antigenic peptides on the bacterial cell surface, is of great interest. Model peptides have already been fused to and displayed on each monomer of the S-layer of Lactobacillus brevis (Avall-Jääskeläinen et al., 2002) and Lactobacillus acidophilus (Smit et al., 2002) by chromosomal integration based on homologous recombination.
An important feature of B-cell epitope–based vaccines is that the use of a strongly immunogenic carrier allows induction of allergen-specific IgG antibodies also against allergens that intrinsically are poorly immunogenic and, thus, would induce a poor blocking IgG response when used as natural allergen. However, regarding food allergy, little is known until now about such vaccines based on an immunogenic carrier protein.
In this study, we investigated the S-layer protein SlpB from Lactobacillus buchneri CD034 as a carrier of a peptide derived from a major peanut allergen for peanut allergen-specific immunotherapy. Out of sixteen peanut allergens reported to date (www.allergen.org; reviewed in (Bublin and Breiteneder, 2014b)), Ara h 2 was described as the most dangerous one and was identified as a predictor of clinical reactivity to peanut (Nicolaou et al., 2010; Nicolaou et al., 2011; van Erp et al., 2016). Of ten identified Ara h 2 IgE-binding epitopes, three are immunodominant and located of the exposed and structurally flexible regions in the native protein (King et al., 2005; Stanley et al., 1997). Multiple T cell epitopes of Ara h 2, with three amino acid regions containing the majority of peptides that reacted with T cells from most patients, have been reported and the results hinted at the potential use of these peptides to treat peanut allergy (Glaspole et al., 2005; Prickett et al., 2011, 2013).
Here, we produced and characterized a carrier-bound Ara h 2-derived peptide. This fusion protein consisted of the recombinant S-layer protein SlpB from Lactobacillus buchneri CD034 and the Ara h 2-derived immunodominant peptide AH3a42. The recombinant fusion protein (His6-SlpB-AH3a42) was expressed in E. coli, purified, and tested for its IgE-binding capacity as well as for the ability to release mediators from sensitized rat basophilic leukemia (RBL) cells. The recombinant construct without the Ara h 2-derived peptide served as control. Additionally, the generation of peanut allergen blocking IgG antibodies in rabbits after immunization with the SlpB-AH3a42 construct was investigated.
2. Materials and methods
2.1. Bacterial strains and culture conditions
Escherichia coli DH5α (Life Technologies, Vienna, Austria) and E. coli BL21 (DE3) (Thermo Fisher Scientific, Life Technologies, Waltham, MA USA) were cultivated at 37 °C and 200 rpm in lysogeny broth (LB) medium supplemented with 50 μg kanamycin (Kan) per ml.
2.2. Cloning of the recombinant SlpB fusion constructs
The expression vector pET28a(+) (Novagen, Madison, WI, USA) was used for the production of N-terminally hexahistidyl-tagged SlpB and the Ara h 2-derived peptide fusion construct. The gene encoding SlpB devoid of its N-terminal signal sequence was PCR-amplified from genomic DNA of L. buchneri CD034. The construct His-SlpB was PCR amplified with the primer pairs (Thermo Fisher Scientific, Life Technologies, Waltham, MA USA) His_SlpB_NcoI_for/SlpB_XhoI_rev (Table 1). The construct His-SlpB-AH3a42 was in a first step PCR-amplified with primer pair SlpB_NheI_for/SlpB_AH3a42_1_rev and in a second step, with the primer pair SlpB_NheI_for/SlpB_AH3a42_2_XhoI_rev using the PCR product from step 1 as a template (Table 1). The final products His-SlpB and His-SlpB-AH3a42 were digested with NcoI/XhoI and NheI/XhoI and ligated into the NcoI/XhoI and NheI/XhoI linearized expression vector.
Table 1.
Oligonucleotide primers used for PCR amplification reactions.
| Oligonucleotide | Sequence (5′ → 3′)a |
|---|---|
| His_SlpB_NcoI_for | ctgctgCCATGGGCCACCACCACCACCACCATAAATCATATGCCAAAGTTACATC |
| SlpB_NheI_for | aatcaGCTAGCAAATCATATGCCAAAGTTACATC |
| SlpB_XhoI_rev | ctgctgCTCGAGTTAATTAAACGGTGTAACAGTAACAG |
| SlpB_His_XhoI_rev | aatcaCTCGAGTTAATGATGATGATGATGATGGCTGCTGCCGCTGCCGCGCGGCACCAGCGCATTAAACGGTGTAACAGTAAC |
| SlpB_AH3a42_1_rev | CTGCATTAAATGCTGTTCGCACGGGCGTAAGTTCGCGCGTTCTAACTGGCTCTGGCAGCGGCGATTAAACGGTGTAACAGTAAC |
| SlpB_AH3a42_2_XhoI_rev | aatcaCTCGAGTTA ATACGGATCCTGGCTCGGGCTATACGGATCGCGGCCATAGCTATCTTCATCGCGCTGAATTTTCTGCATTAAATGCTGTTCG |
Artificial restriction sites are underlined. Lowercase letters indicate artificially introduced nucleotides to improve restriction enzyme cutting. The AH3a-42 gene is indicated in bold italic.
2.3. Expression and purification of the recombinant SlpB and SlpB-AH3a42 fusion protein
The resulting recombinant plasmids (pET28a-His-SlpB and pET28a-His-SlpB-AH3a42) were propagated into E. coli BL21 Star (DE3) cells for production of hexahistidyl-tagged proteins. The transformed strains were grown in 800 ml of LB medium, each, supplemented with kanamycin (50 μg/ml) at 37 °C and 200 rpm. At the mid-exponential growth phase (OD600 ~ 0.6), protein expression was induced with 1 mM isopropyl β-d-1-thiogalactopyranoside and cultivation was continued for 4.5 h at 37 °C. Cells were pelleted by centrifugation, washed with 0.9% NaCl, resuspended in lysis buffer (50 mM sodium citrate buffer, pH = 6.2, 0.1% Triton X-100) and, after addition of lysozyme (800 μg/ml; Sigma-Aldrich, St. Louis, MO, USA) and benzonase (50 U/ml; Sigma-Aldrich), incubated for 30 min at 37 °C. Bacteria were further lysed by ultrasonication (Branson Sonifier, duty cycle 50%; output 6) applying ten cycles of 10 pulses with 30 s breaks, each, and insoluble inclusion bodies containing the recombinant proteins were pelleted. The proteins were extracted from the pellets with binding buffer (50 mM sodium citrate buffer, pH = 5.5, 5 M GdHCl, 20 mM imidazol, 0.5 M NaCl) for 1 h at 4 °C. The extracts were centrifuged and membrane-filtered (0.45-μm pore size). The resulting protein samples were applied to a 1-ml HisTrap HP column (Thermo Fisher Scientific, Waltham, MA USA) and recombinant proteins were recovered with elution buffer (50 mM sodium citrate buffer, pH = 5.5, 5 M GdHCl, 1 M imidazol, 0.5 M NaCl). Recombinant SlpB and the SlpB-based fusion protein were dialyzed against PBS at 4 °C for 24 h and concentrated using Amicon ultra-15 centrifugal filter units (Merck Millipore, Darmstadt, Germany) with 30 kDa cut off. Precipitates were removed from soluble proteins by centrifugation. The absorption of the protein solutions at 280 nm was measured by a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA USA) and protein concentrations were calculated. The aggregation profile of the recombinant proteins His-SlpB and His-SlpB-AH3a42 were measured on a Dynamic Light Scattering DynaPro NanoStar (Wyatt Technology Corp., Santa Barbara, CA, USA).
2.4. SDS-PAGE
SDS-PAGE was carried out on an 8% slab gel in a Mini Protean electrophoresis apparatus (Bio-Rad, Vienna, Austria) according to Laemmli (Laemmli, 1970). Protein bands were visualized with colloidal Coomassie Brilliant Blue R-250 (CBB) staining reagent, and the gel was visualized at 700 nm using the Odyssey imaging system (LICOR, Lincoln, NB, USA).
2.5. Immunoblotting
Polyclonal rabbit antiserum was raised against purified recombinant His6-tagged SlpB (EF-BIO s.r.o., Bratislava, Slovakia). Polyclonal rabbit anti-Ara h 2 antibodies were obtained from the Leibniz-Zentrum Borstel (Borstel, Germany).
Proteins were transferred from the SDS-PAGE gel to a polyvinylidene difluoride membrane (Bio-Rad) using a Mini Trans-Blot Cell (Bio-Rad). S-layer-specific antiserum and Ara h 2-specific antiserum were used in combination with IR Dye 800CW goat anti-mouse antibody and IR Dye 680CW goat anti-rabbit antibody (LI-COR), respectively, and detection was performed at 800 nm and 700 nm, respectively, using the Odyssey Infrared Imaging System.
2.6. Sera of peanut allergic patients
Serum samples from 36 peanut allergic patients were included in the study and tested for IgE reactivity to His-SlpB-AH3a42. Sera were selected according to clinical allergen reactivity on peanut exposure and sensitization to Ara h 2 as determined by IgE Immuno-CAP (Ackerbauer et al., 2015). The characteristics of the 20 sera (10 adolescents and adults older than 14 years and 10 children) used for the IgE inhibition assays are shown in Table 2. A negative control consisted of three sera from atopic but not peanut-sensitized subjects and three sera from non-atopic subjects. Ethical approval was obtained by the respective institutional review boards of Lower Austria (GS4-EK-4/143-2011) and the Medical University of Graz (24-006 ex11/12) and signed informed consent was obtained.
Table 2.
Clinical characteristics of peanut allergic patients reacting to S-layer fusion protein SlpB-AH3a42.
| Patient | Age | Gender | symptoms to peanut | Other food allergies | IgE(IU/ml) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Peanut | Ara h 1 | Ara h 2 | Ara h 3 | |||||
| 1 | 17 | m | anaphylaxis | soy | 2236 | >100 | >100 | >100 | 93.3 |
| 2 | 25 | f | anaphylaxis | egg, milk, hazelnut | 212 | 12.8 | 0.84 | 8.48 | 0.67 |
| 3 | 10 | m | angioedema | none | 345 | 15.9 | 8.57 | 7.36 | 0.32 |
| 4 | 15 | f | anaphylaxis | none | 157 | 5.57 | 23.1 | 84.5 | 7.16 |
| 5 | 5 | f | anaphylaxis | none | 157 | 5.57 | 23.1 | 84.5 | 7.16 |
| 6 | 20 | m | anaphylaxis | milk | 208 | 66.4 | 20.05 | 98.4 | 4.08 |
| 8 | 7 | m | anaphylaxis | soy and buckwheat | 732 | >100 | >100 | 87.7 | 13.6 |
| 9 | 7 | f | urticaria, erythema | egg | 95.1 | 40.9 | 1.85 | 17 | 0.08 |
| 10 | 6 | m | anaphylaxis | none | ND | 10.1 | 0.41 | 6.21 | 0.07 |
| 12 | 6 | f | deterioration of eczema | hazelnut, sesame | 135 | 22.8 | 0.11 | 10.7 | 0.03 |
| 14 | 24 | f | anaphylaxis | soy, coconut | 708 | >100 | 60.4 | 47.3 | 10.3 |
| 15 | 5 | m | anaphylaxis | egg | 1268 | 56.1 | 10.6 | 38.9 | 2.75 |
| 16 | 10 | m | anaphylaxis | none | 421 | >100 | 60.4 | >100 | 9.07 |
| 17 | 10 | f | deterioration of eczema | soy, hazelnut, walnut | 1010 | >100 | 32.5 | 90.3 | 1.19 |
| 19 | 9 | m | anaphylaxis | other nuts | 3000 | >100 | >100 | >100 | <100 |
| 24 | 23 | m | anaphylaxis | hazelnut | 677 | >100 | >100 | >100 | 26.4 |
| 28 | 32 | f | angioedema | hazelnut | 229 | 4.13 | 0.21 | 1.71 | 0.14 |
| 30 | 22 | f | urticaria | hazelnut, walnut | 297 | 80.1 | 66.7 | 40.5 | 8.6 |
| 32 | 24 | m | anaphylaxis | soy, hazelnut, apple | 717 | >100 | >100 | >100 | 41.7 |
| 33 | 25 | f | anaphylaxis | hazelnut, walnut | 4357 | 7.72 | 0.18 | 8.35 | 0.2 |
2.7. IgE ELISA
Peanut allergic patients’ IgE reactivity to recombinant His-SlpB and His-SlpB-AH3a42 was determined as described previously (Bublin et al., 2013) with some modifications. Briefly, microtiter plates (Nunc Maxisorp, Roskilde, Denmark) were coated with 0.5 μg/ml of fusion protein. After blocking, diluted sera from peanut allergic patients containing Ara h 2-specific IgE were applied in duplicates onto the plates. Bound IgE was detected with alkaline phosphatase-conjugated mouse anti-human IgE antibody (BD Pharmingen, San Diego, CA, USA). Sera of individuals without peanut allergy were included as negative controls. Optical densisty (OD) values at 450 nm were counted as positive if they exceeded the mean OD of the negative controls by more than 3 SDs.
2.8. Mediator release assay
The biological activity of recombinant His-SlpB-AH3a42 and His-SlpB was compared to that of natural Ara h 2 by mediator release assays. Rat basophilic leukemia cells RS-ATL8 expressing α-, β- and γ-chains of the human high-affinity IgE receptor (FcεRI) (Nakamura et al., 2010; Nakamura et al., 2012) were plated into 96-well polystyrene cell culture plates (Corning Inc., Corning, NY, USA) and incubated with diluted peanut allergic patients’ sera in minimum essential medium containing 10% fetal calf serum (Gibco, Life Technologies, Carlsbad, CA, USA) at 37 °C overnight. Ten-fold serial dilutions of allergens (0.01–100 ng/ml) were added for 1 h. Degranulation was measured in the supernatants as β-hexosaminidase release using the fluorescent substrate 4-methylumbelliferyl-N-acetyl-β-d-galactosaminide (Sigma-Aldrich, St. Louis, MO, USA) at 355 nm excitation and 460 nm detection wavelength. Percent activation was calculated relative to the β-hexosaminidase content of cells lysed with Triton X 100 (100% release) and the supernatant of untreated cells (0% release).
2.9. Analysis of the peptide-specific rabbit antibody response
His-SlpB-AH3a42-specific rabbit antibodies were obtained by immunization of New Zealand white rabbits three times in monthly intervals with 330 μg of the antigen adsorbed to complete (immunization I) or incomplete Freund’s adjuvant (immunizations II–III). For control purposes, rabbits were immunized with purified natural Ara h 2 or His-SlpB. Rabbits were maintained in the animal care unit of the Department of Toxicology (Slovak Medical University, Bratislava, Slovakia) according to the local guidelines for animal welfare.
Induction of allergen- and peptide-specific rabbit IgG responses were measured by ELISA. Microtiter plates (Nunc Maxisorp) were coated with 0.5 μg/ml of His-SlpB, His-SlpB-AH3a42 and Ara h 2, and incubated with 1:500-, 1:1000-, and 1:2000-diluted rabbit sera. Antigen-specific IgG antibodies were detected by 1:1000-diluted alkaline phosphatase-conjugated swine anti-rabbit IgG antibodies (DAKO, Hamburg, Germany). For negative controls, the ELISA was performed with pre-immune rabbit sera, without any sera or without the primary antibody.
2.10. IgE ELISA inhibition experiments
Inhibition of binding of IgE from peanut allergic patients to Ara h 2 was determined by IgE-inhibition ELISA. ELISA plates were coated with 0.5 μg/ml of Ara h 2 and incubated at 37 °C overnight. After blocking, plates were pre-incubated overnight with pooled and 1:50-diluted rabbit sera obtained by immunization with His-SlpB-AH3a42, and for control proposes, with rabbit sera obtained by immunization with His-SlpB or Ara h 2. After washing, diluted sera from patients allergic to peanut were added for 4 h, and bound human IgE was detected with a 1:6000-diluted HRP-conjugated goat anti-human IgE antibody (KPL, Gaithersburg, MD, USA). The percentage of inhibition of IgE-binding to Ara h 2 after pre-incubation with anti-His-SlpB-AH3a42 rabbit IgG and anti-Ara h 2 rabbit IgG are given as percent reduction of bound IgE compared to the controls where anti-His-SlpB rabbit IgG has been added.
3. Results
3.1. Expression, purification and characterization of recombinant fusion proteins consisting of the L. buchneri S-layer protein SlpB and an Ara h 2-derived immunodominant peptide
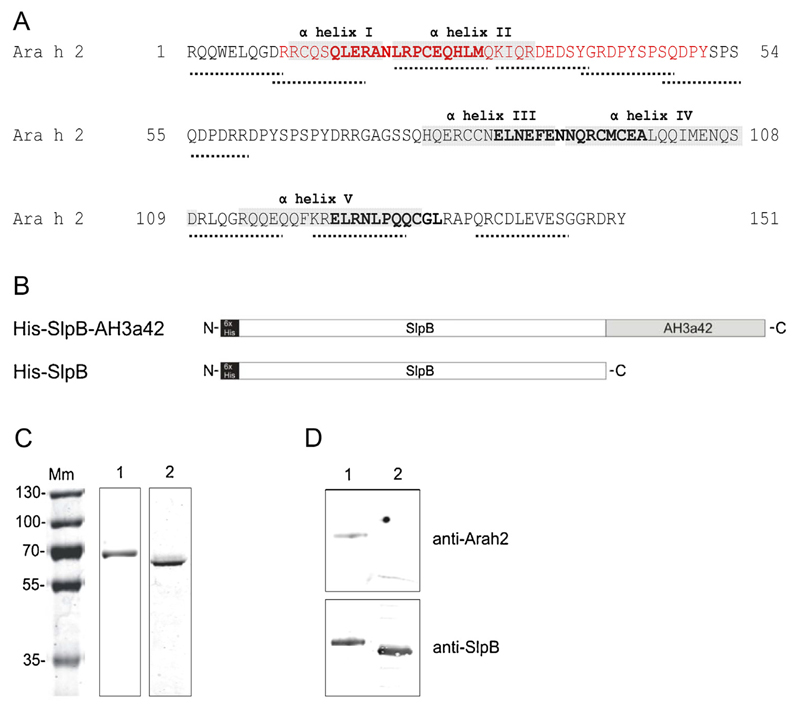
The peptide AH3a42 (Fig. 1A) derived from a region of Ara h 2 consisting of immunodominant B-cell epitopes and one T cell epitope (Glaspole et al., 2005; King et al., 2005; Prickett et al., 2011; Stanley et al., 1997) was fused to the C-terminal end of the His6-tagged S-layer protein SlpB of L. buchneri CD034 (Fig. 1B) and produced as a recombinant fusion protein in E. coli BL21 Star (DE3). His6-tagged SlpB devoid of the allergen peptide was produced as a negative control. The hexahistidyl tag was added to both protein constructs for the purification by nickel affinity chromatography. Proteins were purified to homogeneity as revealed by a CBB-stained SDS-PAGE and concentrated to 0.5 mg/ml. His-SlpB and His-SlpB-AH3a42 migrated close to their theoretical molecular weight of 56 kDa and 63 kDa, respectively (Fig. 1C). Recognition by SlpB- and Ara h 2-specific antisera verified the expression of the S-layer protein and the AH3a42 peptide, respectively (Fig. 1D). The result of dynamic light scattering analysis showed that only monomeric forms of both recombinant proteins were present in solution (data not shown).
Fig. 1.
Construction and characterization of the SlpB-based proteins. (A) Amino acid sequence of Ara h 2 indicating IgE epitopes (dotted lines), T cell epitopes in bold, α-helices in grey and the fragment which was fused to the L. buchneri S-layer protein in red. (B) Schematic presentation of the recombinant S-layer-based proteins, (C) CBB-stained SDS-PAGE (12%) and (D) immunoblot analysis of recombinant proteins probed with Ara h 2- and SlpB-specific antisera. Lane 1, His-SlpB-AH3a42; lane 2, His-SlpB; Mm, PageRuler Plus pre-stained protein ladder (Thermo Scientific).
3.2. IgE reactivity and biological activity of recombinant His-SlpB-AH3a42 fusion protein
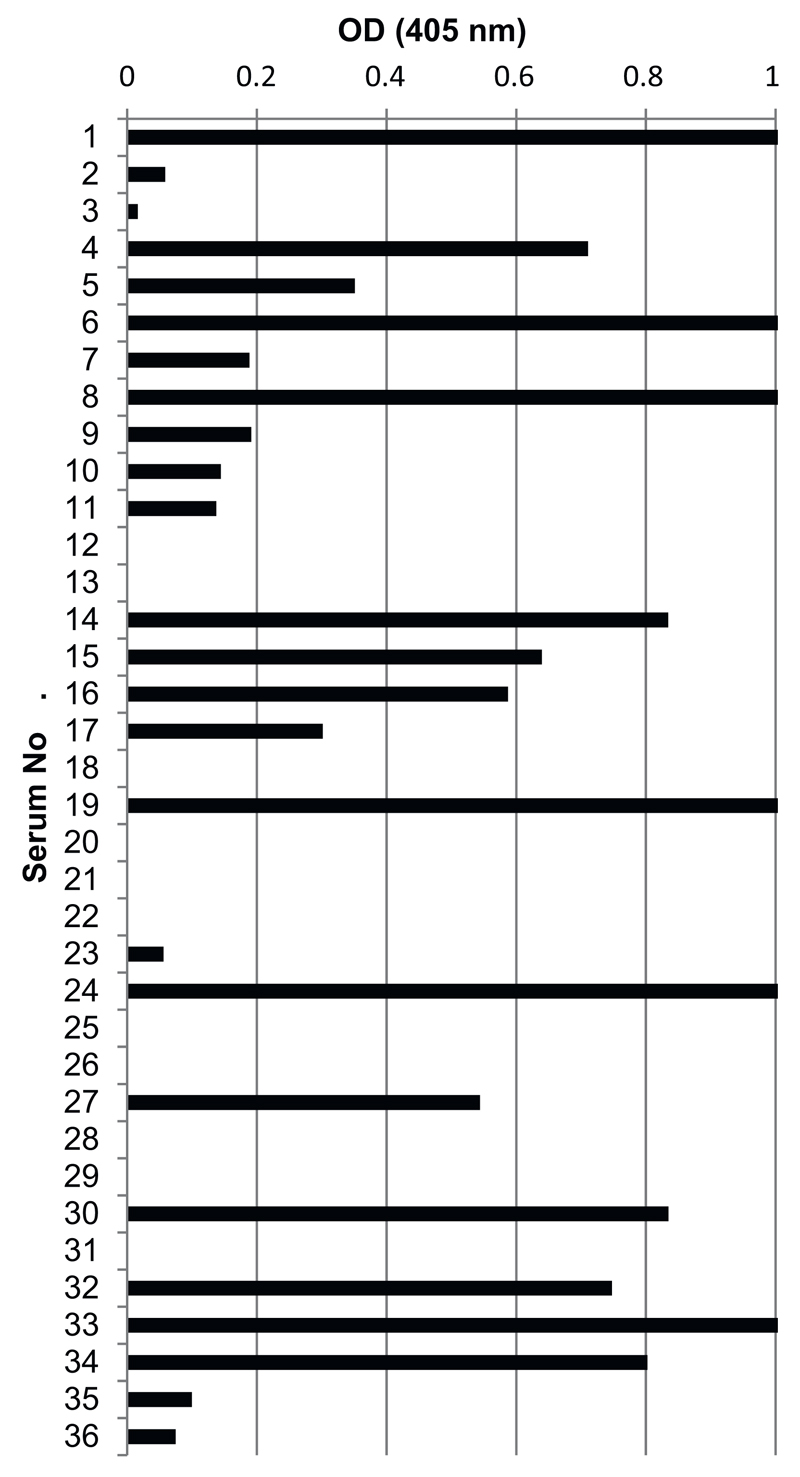
In preliminary experiments to select sera containing IgE specific for the AH3a42 peptide, IgE reactivity to the purified His-SlpB and the His-SlpB-AH3a42 fusion protein was tested using 36 peanut allergic patients’ sera containing Ara h 2-specific IgE. IgE specific for the AH3a42 peptide was detected in 69% (25 of 36) of the sera from patients sensitized to Ara h 2 (Fig. 2). AH3a42-positive sera showed IgE reactivity of varying intensity but with no significant association to Ara h 2-specific IgE levels. None of the 36 Ara h 2-positive sera showed IgE reactivity to His-SlpB. Sera from peanut non-allergic patients and non-atopic controls reacted neither with His-SlpB-AH3a42 nor with His –SlpB (data not shown).
Fig. 2.
IgE reactivity of Ara h 2-positive sera to His-SlpB-AH3a42 tested by ELISA.
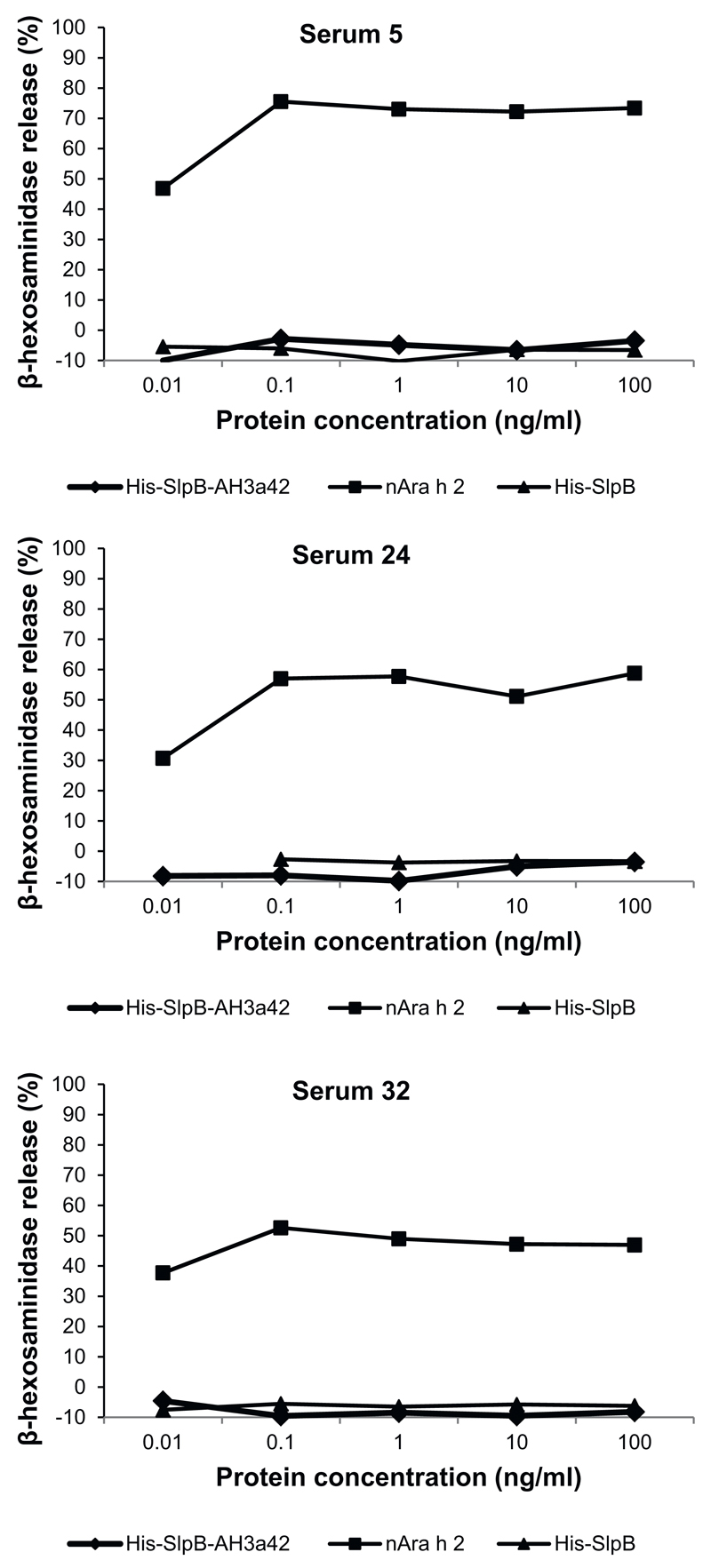
Next, we compared the biological activity of His-SlpB-AH3a42 and His-SlpB with natural Ara h 2 in RBL cell mediator release assays. RBL cells expressing the high-affinity receptor for human IgE were incubated with sera from three peanut allergic patients (serum number 5, 24 and 32) positive for His-SlpB-AH3a42 and containing high levels of Ara h 2-specific IgE (>84 IU/ml) (Table 2). Beta-hexosaminidase release was measured in a concentration range between 0.01 and 100 ng/ml. Already the lowest concentration of natural Ara h 2 (0.01 ng/ml) induced a high β-hexosaminidase (30–46%) release when incubated with sera of all three allergic patients (Fig. 3). The maximal β-hexosaminidase release of 75, 56 and 52% was induced for all three sera at a 0.1 ng/ml concentration of natural Ara h 2. The carrier protein SlpB alone did not induce any release of β-hexosaminidase up to the maximum concentration of 100 ng/ml (Fig. 3).
Fig. 3.
RBL assay showing the ability of His-SlpB-AH3a42, His-SlpB, and natural Ara h 2 to induce mediator release Comparison of the ability of His-SlpB-AH3a42, His-SlpB, and natural Ara h 2 to induce mediator release in an RBL assay. RBL RS-ATL8 cells were sensitized with sera from three peanut allergic patients positive to Ara h 2 and His-SlpB-AH3a42 followed by stimulation with increasing concentrations of the antigens (0.01–100 ng/ml).
3.3. His-SlpB-AH3a42 fusion protein induces Ara h 2-specific IgG antibodies in rabbits and inhibits the binding of Ara h 2-specific IgE
The ability of the His-SlpB-AH3a42 fusion protein to induce an IgG antibody response to the natural allergen was investigated in rabbits. Immunization of rabbits with His-SlpB-AH3a42 or natural Ara h 2 induced a strong Ara h 2–specific IgG response, however, the level of the Ara h 2-specific IgG response induced with His-SlpB-AH3a42 was approximately 60% lower than the response induced with natural Ara h 2 (data not shown).
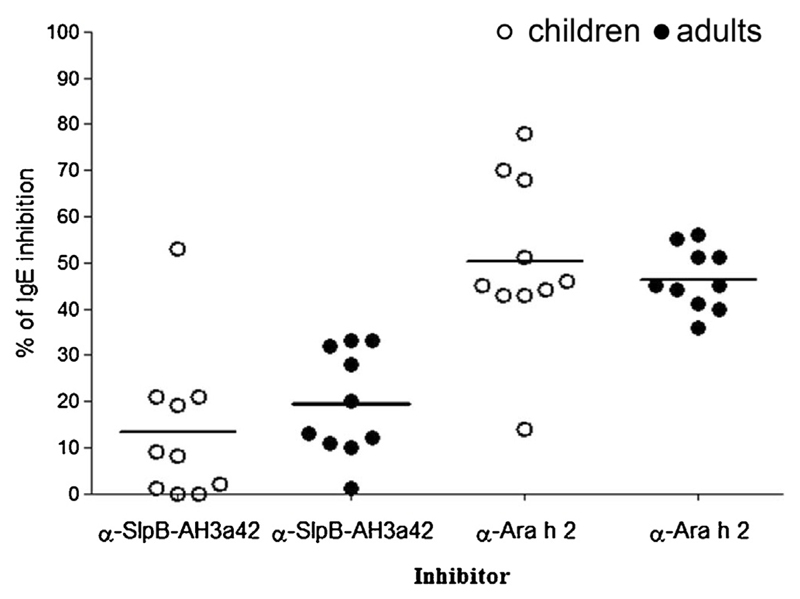
The capacity of the Ara h 2-specific IgG rabbit-antibodies raised against His-SlpB-AH3a42 or Ara h 2 to inhibit IgE-binding was determined by ELISA inhibition assays using 20 sera of peanut allergic patients sensitized to Ara h 2 and containing IgE specific for AH3a42 (Table 2 and Fig. 2). The mean inhibition of IgE-binding to the natural allergen observed after pre-incubation of the 20 sera with anti-His-SlpB-AH3a42 was 16.4% ± 14.1% (range 0–53%) (Fig. 4). Seven of 20 sera (35%) showed an inhibition of IgE binding lower than 10% and IgE binding of eight sera (40%) was inhibited by anti-His-SlpB-AH3a42 more than 20%. The mean inhibition of IgE observed after pre-incubation of the 10 sera from adult patients (19.3% ± 11.53%) was higher than the mean inhibition observed after preincubation of the 10 sera from peanut allergic children (13.4% ± 16.35%) (Fig. 4).
Fig. 4.
Percentages of inhibition of IgE binding of sera from peanut allergic patients to Ara h 2 by anti-His-SlpB-AH3a42 and anti-Ara h 2 antibodies. Open circles, sera from peanut allergic children (n = 10); black circles, sera from peanut allergic adults (n = 10). Mean values are indicated.
IgE binding to Ara h 2 of four sera was inhibited by more than 30%. Anti-Ara h 2 rabbit IgG antibodies inhibited binding to Ara h 2 by 48.3% ± 13.5% (range 14–78%) (Fig. 4). The inhibition of IgE-binding to Ara h 2 by anti-Ara h 2 rabbit IgG antibodies was comparable in both children (50.2% ± 18.2%) and adults (46.4% ± 6.6%). No correlation between inhibitions of anti-Ara h 2 rabbit IgG antibodies and anti-His-SlpB-AH3a42 antibodies was observed (P > 0.5; r = 0.28).
4. Discussion
In this study, we constructed a fusion protein consisting of the L. buchneri CD034 S-layer protein and a peptide derived from a major peanut allergen as a potential vaccine-component for peanut allergy. The DNA coding for the S-layer protein SlpB from L. buchneri CD034 was fused to the DNA encoding the peptide AH3a42 derived from the peanut allergen Ara h 2 and expressed in E. coli cells (Fig. 1). The peptide AH3a42 was previously determined as containing immunodominant IgE epitopes of Ara h 2 and also defined as an IgE-reactive biomarker for the identification of patients with symptomatic peanut allergy (King et al., 2005; Lin et al., 2012; Stanley et al., 1997). In addition to the IgE-binding epitopes, the peptide AH3a42 also contains an immunodominant T cell epitope (Glaspole et al., 2005; Prickett et al., 2011). Pricket et al. demonstrated that this Ara h 2 peptide contains epitopes which can be presented by all three HLA types (Prickett et al., 2011). HLA-DQ and HLA-DP alleles are much less diverse than HLA-DR alleles and, therefore, inclusion of HLA-DQ and HLA-DP-restricted epitopes in a vaccine component will more likely result in a positive immune response when treating a population of HLA-DR diverse individuals (Larché, 2008).
Vaccines based on allergen-derived peptides bound to allergen-unrelated carrier molecules are currently being tested as candidates for allergy treatment by inducing allergen-specific IgG with T cell help from carrier-derived epitopes (Focke et al., 2010; Valenta et al., 2016). Such B cell epitope–based vaccines appear to be safer than vaccines comprising allergen-specific T cell epitopes (Valenta et al., 2016). Furthermore, the use of peptides which contain IgE epitopes allows to better induce an allergen-specific IgG response that is directed against these IgE-binding sites of the allergens resulting in better therapeutic outcomes (Marth et al., 2013). The first proof-of-principle studies used allergen-derived peptides from the major timothy grass pollen allergen Phl p 1 and from the major birch pollen allergen Bet v 1 chemically coupled to keyhole limpet hemocyanin (Focke et al., 2001, 2004). As further carrier proteins, the viral proteins VP1 from human rhinovirus74 and PreS from hepatitis B75 were used (Edlmayr et al., 2011). This study found that these fusion proteins induced allergen-specific IgG responses similar to those induced by an allergen specific immunotherapy trial.
Bacterial S-layers have been shown to possess strong adjuvant properties (Sleytr et al., 2014). S-layers from Lysinibacillus sphaericus or Thermoanaerobacter thermohydrosulfuricus chemically conjugated to the major birch pollen allergen Bet v 1 modulated the cytokine production from a Th2 phenotype towards a Th0/Th1 one (Jahn-Schmid et al., 1996, 1997). Subsequently, S-layer-allergen fusion proteins consisting of SbsC from G. stearothermophilus or SpbA from L. sphaericus fused to Bet v 1 promoted the induction of allergen-specific Th0/1 cells and regulatory T -cells (Bohle et al., 2004; Breitwieser et al., 2002; Gerstmayr et al., 2007, 2009; Ilk et al., 2002).
We selected the L. buchneri CD034 S-layer protein as a strong adjuvant and as a source of T cell epitopes to induce an IgG antibody response that would also be directed against the peptide. In fact, the fusion protein of the carrier and the peptide derived from the major peanut allergen Ara h 2 resulted in a highly immunogenic vaccine component (Fig. 1).The peptide AH3a42 was fused to the C-terminus of SlpB because in this orientation it is better accessible to antibodies. We were able to show that 70% of the tested sera containing Ara h 2-specific IgE showed reactivity to AH3a42 fused to SlpB, confirming the previously described importance of this Ara h 2-derived peptide for binding patients’ IgE (King et al., 2005; Lin et al., 2012; Stanley et al., 1997) (Fig. 2). Despite the IgE recognition of this peptide by more than two thirds of the patients, SlpB-AH3a42 was unable to induce β-hexosaminidase release from RBL cells at concentrations up to 100 ng/ml (Fig. 3). This is in stark contrast to the allergen Ara h 2, which provoked a release already at 10 pg/ml (Fig. 3). Although show only for three sera from peanut allergic patients, these results suggest that the fusion protein has a strongly reduced ability to cross-link IgE and activate effectors cells. Whether this might indicate reduced side effects of such a vaccine in a clinical setting would need to be proven by additional in vitro and in vivo experiments
The induction of blocking IgG antibodies is a good indication for the efficacy of an allergy vaccine (Valenta et al., 2016). We tested the ability of IgG antibodies induced in rabbits by our vaccine to inhibit the binding of IgE present in peanut allergic patients’ sera specific for the allergen Ara h 2. We observed inhibition values ranging from 0 to 53% in contrast to Ara h 2-induced antibodies with inhibition values from 14 to 78% (Fig. 4). In detail, IgE-binding from eight of 20 sera were inhibited by less than 10%, eight sera were inhibited by more than 20%, and four sera were inhibited by more than 30% (Fig. 4). This reflects the fact that Ara h 2-specific blocking IgG antibodies were induced by our peptide vaccine. The range of inhibition values illustrates that individual patients possess distinct IgE recognition profiles for a given allergen. This was previously shown for the birch pollen allergen Bet v 1 (Gepp et al., 2014), the soybean allergen Gly m 4 (Husslik et al., 2016), and for the major peanut allergens Ara h 1 and Ara h 2 (Hoh et al., 2016). We demonstrated that an allergen-derived peptide that contains IgE epitopes when fused to an L. buchneri surface protein can induce blocking antibodies that interfere with the binding of IgE to the allergen. As each patient recognizes different epitopes, additional allergen-derived peptides used as vaccine components would induce a broader spectrum of protective IgG as was previously shown for Bet v 1 and Fel d 1 (Marth et al., 2013; Niespodziana et al., 2011).
5. Conclusions
In conclusion, this study describes the initial immunological characterization of a fusion protein consisting of an L. buchneri S-layer protein and an Ara h 2-derived peptide. Our data provide evidence for a novel approach for the development of a peanut allergen peptide-based carrier-bound vaccine. Our experiments clearly indicate that more than one allergen-peptide will be needed to induce a broader protection of patients allergic to Ara h 2. So far, several attempts to produce peanut allergy vaccines have not produced the desired results (Bublin and Breiteneder, 2014a). The expression of the AH3a42 fusion protein on the cell surface of L. buchneri CD034 could provide the basis for a future form of oral vaccination against peanut allergy.
Acknowledgements
We thank Ryosuke Nakamura (National Institute of Health Sciences, Tokyo, Japan) for kindly providing RS-ATL8 cells for basophil activation assays and Arnd Petersen (Leibniz-Zentrum Borstel, Borstel, Germany) for providing polyclonal rabbit anti-Ara h 2 antibodies.
Funding
This work was supported by the Austrian Science Fund projects P21954-B22 (to C.S.), P24305-B20 (to P. Messner), W1224 (PhD Program “Biomolecular Technology of Proteins”) and W1248-B30 (PhD Program “Molecular, Cellular and Clinical Allergology”).
Abbreviations
- CBB
colloidal Coomassie Brilliant Blue R, 250
- GdHCl
guanidinuium hydrochloride
- GRAS
generally regarded as safe
- OD
optical density
- RBL cells
rat basophilic leukemia cells
- S-layer
cell surface layer
Footnotes
Conflict of interest
None.
References
- Ackerbauer D, Bublin M, Radauer C, Varga EM, Hafner C, Ebner C, Szepfalusi Z, Froschl R, Hoffmann-Sommergruber K, Eiwegger T, Breiteneder H. Component-resolved IgE profiles in Austrian patients with a convincing history of peanut allergy. Int Arch Allergy Immunol. 2015;166:13–24. doi: 10.1159/000371422. [DOI] [PubMed] [Google Scholar]
- Avall-Jääskeläinen S, Kyla-Nikkila K, Kahala M, Miikkulainen-Lahti T, Palva A. Surface display of foreign epitopes on the Lactobacillus brevis S-layer. Appl Environ Microbiol. 2002;68:5943–5951. doi: 10.1128/AEM.68.12.5943-5951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlec A, Ravnikar M, Strukelj B. Lactic acid bacteria as oral delivery systems for biomolecules. Pharmazie. 2012;67:891–898. [PubMed] [Google Scholar]
- Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119:1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- Bohle B, Breitwieser A, Zwölfer B, Jahn-Schmid B, Sára M, Sleytr UB, Ebner C. A novel approach to specific allergy treatment: the recombinant fusion protein of a bacterial cell surface (S-layer) protein and the major birch pollen allergen Bet v 1 (rSbsC-Bet v 1) combines reduced allergenicity with immunomodulating capacity. J Immunol. 2004;172:6642–6648. doi: 10.4049/jimmunol.172.11.6642. [DOI] [PubMed] [Google Scholar]
- Breitwieser A, Egelseer EM, Moll D, Ilk N, Hotzy C, Bohle B, Ebner C, Sleytr UB, Sára M. A recombinant bacterial cell surface (S-layer)-major birch pollen allergen-fusion protein (rSbsC/Bet v1) maintains the ability to self-assemble into regularly structured monomolecular lattices and the functionality of the allergen. Protein Eng. 2002;15:243–249. doi: 10.1093/protein/15.3.243. [DOI] [PubMed] [Google Scholar]
- Bublin M, Breiteneder H. Developing therapies for peanut allergy. Int Arch Allergy Immunol. 2014a;165:179–194. doi: 10.1159/000369340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublin M, Breiteneder H. Cross-reactivity of peanut allergens. Curr Allergy Asthma Rep. 2014b;14:426. doi: 10.1007/s11882-014-0426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublin M, Kostadinova M, Radauer C, Hafner C, Szepfalusi Z, Varga EM, Maleki SJ, Hoffmann-Sommergruber K, Breiteneder H. IgE cross-reactivity between the major peanut allergen Ara h 2 and the nonhomologous allergens Ara h 1 and Ara h 3. J Allergy Clin Immunol. 2013;132:118–124. doi: 10.1016/j.jaci.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Burks AW, King N, Bannon GA. Modification of a major peanut allergen leads to loss of IgE binding. Int Arch Allergy Immunol. 1999;118:313–314. doi: 10.1159/000024114. [DOI] [PubMed] [Google Scholar]
- Commins SP, Kim EH, Orgel K, Kulis M. Peanut allergy: new developments and clinical implications. Curr Allergy Asthma Rep. 2016;16:35. doi: 10.1007/s11882-016-0613-x. [DOI] [PubMed] [Google Scholar]
- Edlmayr J, Niespodziana K, Focke-Tejkl M, Linhart B, Valenta R. Allergen-specific immunotherapy: towards combination vaccines for allergic and infectious diseases. Curr Top Microbiol Immunol. 2011;352:121–140. doi: 10.1007/82_2011_130. [DOI] [PubMed] [Google Scholar]
- Focke M, Mahler V, Ball T, Sperr WR, Majlesi Y, Valent P, Kraft D, Valenta R. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p1, for allergy vaccination. FASEB J. 2001;15:2042–2044. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- Focke M, Linhart B, Hartl A, Wiedermann U, Sperr WR, Valent P, Thalhamer J, Kraft D, Valenta R. Non-anaphylactic surface-exposed peptides of the major birch pollen allergen, Bet v1, for preventive vaccination. Clin Exp Allergy. 2004;34:1525–1533. doi: 10.1111/j.1365-2222.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010;40:385–397. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- Gepp B, Lengger N, Bublin M, Hemmer W, Breiteneder H, Radauer C. Chimeras of Bet v 1 and Api g 1 reveal heterogeneous IgE responses in patients with birch pollen allergy. J Allergy Clin Immunol. 2014;134:188–194. doi: 10.1016/j.jaci.2013.12.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstmayr M, Ilk N, Schabussova I, Jahn-Schmid B, Egelseer EM, Sleytr UB, Ebner C, Bohle B. A novel approach to specific allergy treatment: the recombinant allergen-S-layer fusion protein rSbsC-Bet v 1 matures dendritic cells that prime Th0/Th1 and IL-10-producing regulatory T cells. J Immunol. 2007;179:7270–7275. doi: 10.4049/jimmunol.179.11.7270. [DOI] [PubMed] [Google Scholar]
- Gerstmayr M, Ilk N, Jahn-Schmid B, Sleytr UB, Bohle B. Natural self-assembly of allergen-S-layer fusion proteins is no prerequisite for reduced allergenicity and T cell stimulatory capacity. Int Arch Allergy Immunol. 2009;149:231–238. doi: 10.1159/000199718. [DOI] [PubMed] [Google Scholar]
- Glaspole IN, de Leon MP, Rolland JM, O’Hehir RE. Characterization of the T-cell epitopes of a major peanut allergen, Ara h 2. Allergy. 2005;60:35–40. doi: 10.1111/j.1398-9995.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- Hoh RA, Joshi SA, Liu Y, Wang C, Roskin KM, Lee JY, Pham T, Looney TJ, Jackson KJ, Dixit VP, King J, et al. Single B-cell deconvolution of peanut-specific antibody responses in allergic patients. J Allergy Clin Immunol. 2016;137:157–167. doi: 10.1016/j.jaci.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husslik F, Nürnberg J, Seutter von Loetzen C, Mews T, Ballmer-Weber BK, Kleine-Tebbe J, Treudler R, Simon JC, Randow S, Volker E, Reuter A, et al. The conformational IgE epitope profile of soya bean allergen Gly m 4. Clin Exp Allergy. 2016;46:1484–1497. doi: 10.1111/cea.12796. [DOI] [PubMed] [Google Scholar]
- Hynönen U, Palva A. Lactobacillus surface layer proteins: structure, function and applications. Appl Microbiol Biotechnol. 2013;97:5225–5243. doi: 10.1007/s00253-013-4962-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilk N, Völlenkle C, Egelseer EM, Breitwieser A, Sleytr UB, Sára M. Molecular characterization of the S-layer gene, sbpA, of Bacillus sphaericus CCM 2177 and production of a functional S-layer fusion protein with the ability to recrystallize in a defined orientation while presenting the fused allergen. Appl Environ Microbiol. 2002;68:3251–3260. doi: 10.1128/AEM.68.7.3251-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn-Schmid B, Graninger M, Glozik M, Küpcü S, Ebner C, Unger FM, Sleytr UB, Messner P. Immunoreactivity of allergen (Bet v 1) conjugated to crystalline bacterial cell surface layers (S-layers) Immunotechnology. 1996;2:103–113. doi: 10.1016/1380-2933(96)00041-3. [DOI] [PubMed] [Google Scholar]
- Jahn-Schmid B, Siemann U, Zenker A, Bohle B, Messner P, Unger FM, Sleytr UB, Scheiner O, Kraft D, Ebner C. Bet v 1, the major birch pollen allergen, conjugated to crystalline bacterial cell surface proteins, expands allergen-specific T cells of the Th1/Th0 phenotype in vitro by induction of IL-12. Int Immunol. 1997;9:1867–1874. doi: 10.1093/intimm/9.12.1867. [DOI] [PubMed] [Google Scholar]
- King N, Helm R, Stanley JS, Vieths S, Luttkopf D, Hatahet L, Sampson H, Pons L, Burks W, Bannon GA. Allergenic characteristics of a modified peanut allergen. Mol Nutr Food Res. 2005;49:963–971. doi: 10.1002/mnfr.200500073. [DOI] [PubMed] [Google Scholar]
- Kingwell K. Food allergy drugs step up to Phase III. Nat Rev Drug Discov. 2016;15:149–150. doi: 10.1038/nrd.2016.24. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larché M. Of cats and men: immunodominance and the role of HLA-DP/DQ. Clin Exp Allergy. 2008;38:1709–1711. doi: 10.1111/j.1365-2222.2008.03112.x. [DOI] [PubMed] [Google Scholar]
- Li XM, Srivastava K, Grishin A, Huang CK, Schofield B, Burks W, Sampson HA. Persistent protective effect of heat-killed Escherichia coli producing engineered-recombinant peanut proteins in a murine model of peanut allergy. J Allergy Clin Immunol. 2003a;112:159–167. doi: 10.1067/mai.2003.1622. [DOI] [PubMed] [Google Scholar]
- Li XM, Srivastava K, Huleatt JW, Bottomly K, Burks AW, Sampson HA. Engineered recombinant peanut protein and heat-killed Listeria monocytogenes coadministration protects against peanut-induced anaphylaxis in a murine model. J Immunol. 2003b;170:3289–3295. doi: 10.4049/jimmunol.170.6.3289. [DOI] [PubMed] [Google Scholar]
- Lin J, Bruni FM, Fu Z, Maloney J, Bardina L, Boner AL, Gimenez G, Sampson HA. A bioinformatics approach to identify patients with symptomatic peanut allergy using peptide microarray immunoassay. J Allergy Clin Immunol. 2012;129:1321–1328, e1325. doi: 10.1016/j.jaci.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth K, Breyer I, Focke-Tejkl M, Blatt K, Shamji MH, Layhadi J, Gieras A, Swoboda I, Zafred D, Keller W, Valent P, et al. A nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype. J Immunol. 2013;190:3068–3078. doi: 10.4049/jimmunol.1202441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R, Uchida Y, Higuchi M, Nakamura R, Tsuge I, Urisu A, Teshima R. A convenient and sensitive allergy test: IgE crosslinking-induced luciferase expression in cultured mast cells. Allergy. 2010;65:1266–1273. doi: 10.1111/j.1398-9995.2010.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R, Ishiwatari A, Higuchi M, Uchida Y, Nakamura R, Kawakami H, Urisu A, Teshima R. Evaluation of the luciferase assay-based in vitro elicitation test for serum IgE. Allergol Int. 2012;61:431–437. doi: 10.2332/allergolint.11-OA-0407. [DOI] [PubMed] [Google Scholar]
- Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, Harlin A, Woodcock A, Ahlstedt S, Custovic A. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–197, e191–113. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Nicolaou N, Murray C, Belgrave D, Poorafshar M, Simpson A, Custovic A. Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. J Allergy Clin Immunol. 2011;127:684–685. doi: 10.1016/j.jaci.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Niespodziana K, Focke-Tejkl M, Linhart B, Civaj V, Blatt K, Valent P, van Hage M, Gronlund H, Valenta R. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol. 2011;127:1562–1570, e1566. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, Ponsonby AL, Wake M, Tang ML, Dharmage SC, Allen KJ, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127:668–676, e661–662. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- Prickett SR, Voskamp AL, Dacumos-Hill A, Symons K, Rolland JM, O’Hehir RE. Ara h 2 peptides containing dominant CD4+ T-cell epitopes: candidates for a peanut allergy therapeutic. J Allergy Clin Immunol. 2011;127:608–615, e601–605. doi: 10.1016/j.jaci.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Prickett SR, Voskamp AL, Phan T, Dacumos-Hill A, Mannering SI, Rolland JM, O’Hehir RE. Ara h 1 CD4+ T cell epitope-based peptides: candidates for a peanut allergy therapeutic. Clin Exp Allergy. 2013;43:684–697. doi: 10.1111/cea.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabjohn P, West CM, Connaughton C, Sampson HA, Helm RM, Burks AW, Bannon GA. Modification of peanut allergen Ara h 3: effects on IgE binding and T cell stimulation. Int Arch Allergy Immunol. 2002;128:15–23. doi: 10.1159/000057999. [DOI] [PubMed] [Google Scholar]
- Rolland JM, Gardner LM, O’Hehir RE. Allergen-related approaches to immunotherapy. Pharmacol Ther. 2009;121:273–284. doi: 10.1016/j.pharmthera.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007;120:491–503. doi: 10.1016/j.jaci.2007.07.015. quiz 504–495. [DOI] [PubMed] [Google Scholar]
- Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W, Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol. 2001;107:367–374. doi: 10.1067/mai.2001.112129. [DOI] [PubMed] [Google Scholar]
- Sleytr UB, Huber C, Ilk N, Pum D, Schuster B, Egelseer EM. S-layers as a tool kit for nanobiotechnological applications. FEMS Microbiol Lett. 2007;267:131–144. doi: 10.1111/j.1574-6968.2006.00573.x. [DOI] [PubMed] [Google Scholar]
- Sleytr UB, Schuster B, Egelseer EM, Pum D. S-layers: principles and applications. FEMS Microbiol Rev. 2014;38:823–864. doi: 10.1111/1574-6976.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit E, Jäger D, Martinez B, Tielen FJ, Pouwels PH. Structural and functional analysis of the S-layer protein crystallisation domain of Lactobacillus acidophilus ATCC 4356: evidence for protein-protein interaction of two subdomains. J Mol Biol. 2002;324:953–964. doi: 10.1016/s0022-2836(02)01135-x. [DOI] [PubMed] [Google Scholar]
- Soller L, Ben-Shoshan M, Harrington DW, Fragapane J, Joseph L, St Pierre Y, Godefroy SB, La Vieille S, Elliott SJ, Clarke AE. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130:986–988. doi: 10.1016/j.jaci.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, Helm RM, West CM, Bannon GA. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch Biochem Biophys. 1997;342:244–253. doi: 10.1006/abbi.1997.9998. [DOI] [PubMed] [Google Scholar]
- Trombert A. Recombinant lactic acid bacteria as delivery vectors of heterologous antigens: the future of vaccination? Benef Microbes. 2015;6:313–324. doi: 10.3920/BM2014.0068. [DOI] [PubMed] [Google Scholar]
- van Erp FC, Knol EF, Pontoppidan B, Meijer Y, van der Ent CK, Knulst AC. The IgE and basophil response to Ara h 2 and Ara h 6 are good predictors of peanut allergy in children. J Allergy Clin Immunol. 2016;139:358–360, e8. doi: 10.1016/j.jaci.2016.06.041. [DOI] [PubMed] [Google Scholar]
- van Hoffen E, van der Kleij HP, den Hartog Jager CF, van Doorn WA, Knol EF, Opstelten DJ, Koppelman SJ, Knulst AC. Chemical modification of peanut conglutin reduces IgE reactivity but not T cell reactivity in peanut-allergic patients. Clin Exp Allergy. 2014;44:1558–1566. doi: 10.1111/cea.12319. [DOI] [PubMed] [Google Scholar]
- Valenta R, Campana R, Focke-Tejkl M, Niederberger V. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol. 2016;137:351–357. doi: 10.1016/j.jaci.2015.12.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RA, Sicherer SH, Burks AW, Grishin A, Henning AK, Lindblad R, Stablein D, Sampson HA. A phase 1 study of heat/phenol-killed, E. coli-encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 (EMP-123) for the treatment of peanut allergy. Allergy. 2013;68:803–808. doi: 10.1111/all.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]