Abstract
The patient was a 13-year-old boy who complained of pain in both buttocks. Plain and reconstructive computed tomography (CT) images showed an ossified lesion within the dura mater at the L5–S2 levels, and arachnoiditis ossificans in the lumbosacral area was suspected. In the operative findings obtained after cutting the dura, a bone fragment 4.5 × 0.5 × 0.5 cm in size was observed in the center of the strongly adhesive nerve bundle of the cauda equina, which was removed en bloc. The postoperative clinical course of the patient was excellent. The case, along with a review of literature is presented.
Keywords: arachnoiditis ossificans , lumbosacral spine , surgery , cauda equina
Introduction
Arachnoiditis ossificans is a rare disease.1–3) We treated a boy presenting with a very rare case of lumbosacral adhesive arachnoiditis ossificans without any distinct antecedent triggers. He was treated by subtotal resection of a highly thickened arachnoid and total removal of the ossified lesion from within the dura mater. We present the case findings and a literature review.
Case Report
The patient was a 13-year-old boy. The chief complaint was pain in both buttocks that started around May 2012. Difficulty in lumbar anteflexion and a “stretched” feeling in both lower extremities occurred gradually. In February 2013, the left buttock pain aggravated, and he experienced severe electric-shock-like pain radiating from the buttocks to the lower extremities while walking; the patient visited us subsequently.
Regarding physical findings, sensation, muscles, and tendon reflex of the lower extremities were normal. The result of the straight leg raising (SLR) test was 30 degrees in both right and left legs. Sitting continuously in a position with the knees extended was not possible. The average urination frequency was 12 times/day, and stool frequency was as high as 4 times/day from childhood. Nothing remarkable was found in the patient’s past history, family history, and trauma history. As for sports history, he had played tennis for 1 year as a club activity in junior high school.
Hematological examination showed no abnormal findings, and X-ray examination showed spondylosis of L5. Magnetic resonance imaging (MRI) showed no distinct abnormalities on a T1-weighted image, but showed an irregular image with a mix of low and high signals at L5 vertebra or lower on a T2-weighted image (Fig. 1A, B). Plain and reconstructive computed tomography (CT) images showed an ossified lesion within the dura mater at the L5–S2 levels (Fig. 2A, B). Thus, arachnoiditis ossificans in the lumbosacral area was suspected, and surgery was conducted under general anesthesia.
Fig. 1.
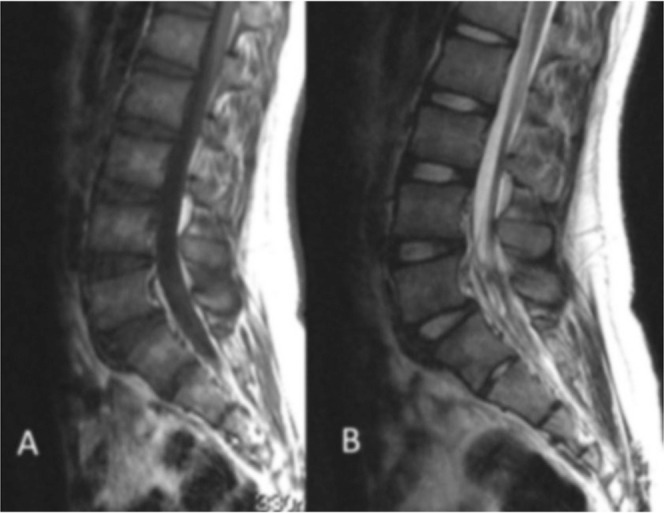
Magnetic resonance imaging (MRI) showed no distinct abnormalities on a T1-weighted image (A), but showed an irregular image with a mix of low and high signals at L5 vertebra or lower on a T2-weighted image (B).
Fig. 2.
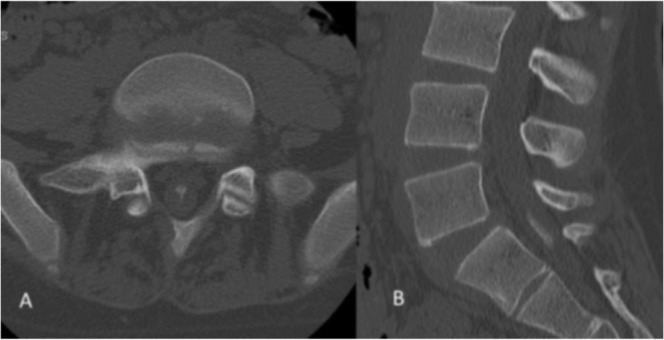
Plain computed tomography (CT) at L5/S1 level (A) and reconstructive CT images at the L5–S2 level (B) showed an ossified lesion within the dura mater.
The surgical procedure was as follows: laminectomy on L5 was conducted in accordance with the Gill method and the S1 vertebral arch was resected using an air drill. When the dura mater was dissected, a highly thickened arachnoid was observed, and upon cutting it open, the nerves of the cauda equina, which were in a bundled state due to adhesion, were found inside. When the nerves of the cauda equina were individually separated with care, a bone chip 4.5 × 0.5 × 0.5 cm in size was found in its center, which was removed en bloc (Fig. 3). These operations remarkably improved the flow of cerebral spinal fluid within the dura mater. L5 spondylosis was treated by fixing a pedicle screw system between L5/S. Additionally, posterolateral fusion was performed using the local bone, since the possibility of the onset of postoperative instability between L5/S1 was of great concern. Histological observation of the harvested bone chips showed a normal osseous structure (Fig. 4).
Fig. 3.
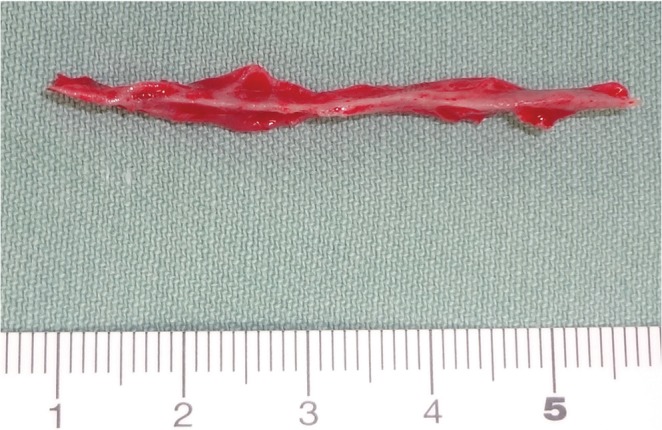
A bone chip 4.5 × 0.5 × 0.5 cm in size was found in a bundled state of the nerves of the cauda equina, which was removed en bloc.
Fig. 4.
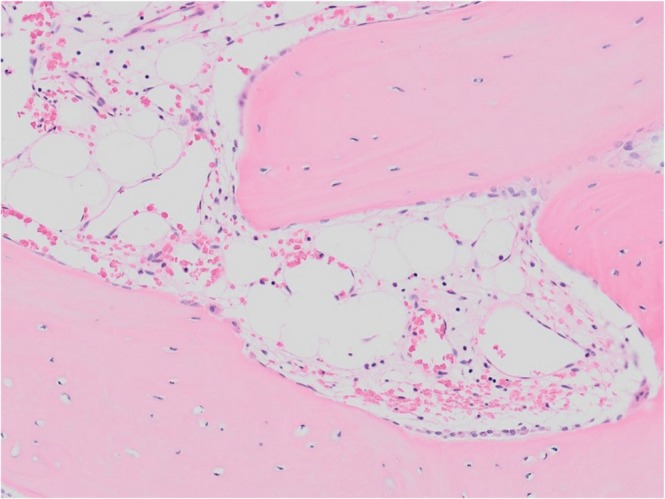
Histological observation of the harvested bone chips showed a normal osseous structure.
Immediately after surgery, the electric-shock-like pain that radiated to the lower extremities while walking disappeared. The result of SLR test improved to 70 degrees. Urination frequency decreased to 7 times/day, and stool frequency was now once daily. One year after the surgery, the patient was making favorable progress without any complaints. CT reconstruction images obtained 2 years after surgery showed no ossified lesions (Fig. 5).
Fig. 5.
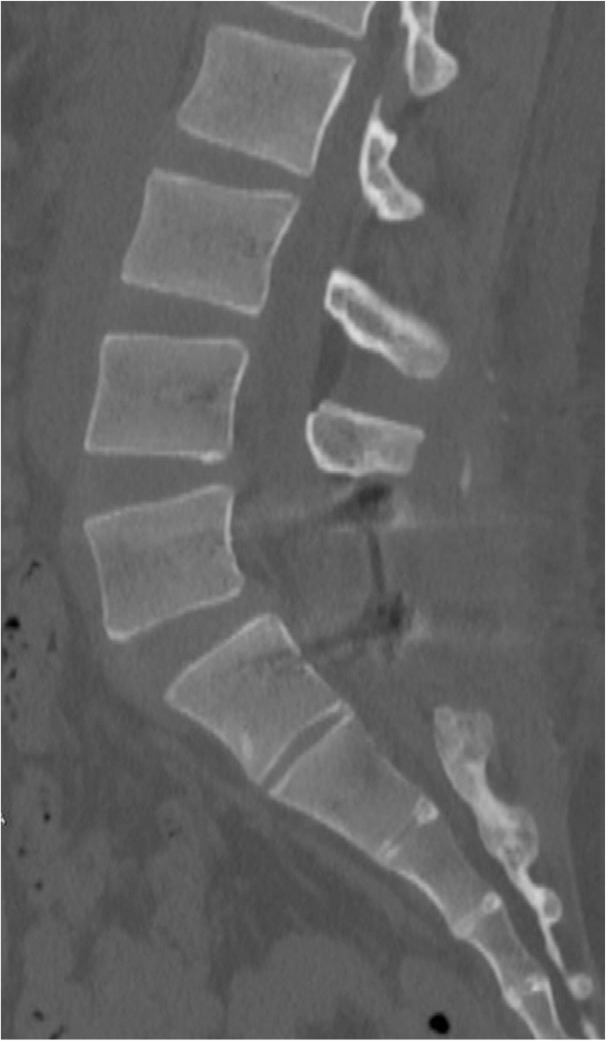
Computed tomography reconstruction images obtained 2 years after surgery showed no ossified lesions.
Discussion
The earliest clinicopathologic association of arachnoid calcification with syringomyelia was described by Schwarz4) in 1897, and the term “arachnoiditis ossificans” was originally used by Gatzke et al.5) in 1957. In 2014, when Maulucci et al.3) surveyed research papers on this disease, 72 cases in 50 papers were found to be reported; thus, it is an extremely rare disease.
Domenicucci et al.1) reviewed 47 cases of this disease and reported the onset age to be 22–71 years (average, 53.4 years); they did not find any sex differences in the age of onset. Junewick et al.6) reported a case of cerebral palsy in a 14-year-old patient with arachnoiditis ossificans. Our patient was 13 years of age at the time of disease occurrence; therefore, this report presents the youngest patient with this disease. As for the site of occurrence, Kitagawa et al.7) described the site of occurrence as the thoracic vertebra area (66%), lumbosacral area (24%), and cervical vertebra area (only a few patients). Various clinical symptoms were reported, such as low back pain, headache, hypesthesia of lower extremities, pain in lower extremities, paralysis of lower extremities, and vesicorectal disorder. In general, a patient with thoracic vertebra lesion tends to develop paralytic symptoms, and a patient with lumbosacral lesion develops comparatively milder symptoms.3,8)
Domenicucci et al.1) described that antecedent triggers such as a direct invasion into the dura mater or arachnoid membrane (caused by vertebra/spinal cord surgery and myelography, spinal cord tumor, trauma, spinal vascular malformation, subarachnoid hemorrhage, and cerebral meningitis) are observed in a number of patients with arachnoiditis ossificans. They also described rare cases (2 of 47 cases) without distinct antecedent triggers similar to the present case. In the current case, spondylosis of L5 was observed; however, no previous reports have considered spondylosis to be the antecedent trigger. We are also of the opinion that there is no direct relationship between spondylosis and occurrence of arachnoiditis ossificans. Although the cause of arachnoiditis ossificans remains unclear, it is presumed that chronic inflammation in the dura mater or an increase in proliferative activity of arachnoidal and osteoblastic cells due to multiple factors causes bony metaplasia of the arachnoid membrane.9) Furthermore, multiple factors such as systemic metabolism or hormones may be involved.10) We presume the following onset mechanism of symptoms in our patient; since there were no distinct antecedent triggers such as spinal puncture, and the patient had a high urination and stool frequency from childhood, he had an acquired predisposition for ossification within the dura mater since childhood. The ossified lesion grew as he grew, and it stimulated the nerves of the cauda equina, leading to pain in the buttocks and lower extremities.
For diagnosis of this disease, CT is considered to be extremely effective, and in this patient’s case, the CT findings were a decisive factor in the diagnosis.11) In general, MRI shows low signal on a T1-weighted image, and an irregular image with a mix of high and low signals on a T2-weighted image.12) In addition, complications of arachnoid cysts and syringomyelia are often observed.13)
Arachnoiditis ossificans is treated using conservative therapy for mild symptoms, and surgical treatment such as ebonation or laminectomy is performed for severe pain or paralysis; however, the use of the surgical method is controversial.1,3) Bagley et al.8) reported that surgery improved the symptoms in 60% of the patients, caused no change in symptoms in 30%, and worsened the symptoms in 10%. Braz et al.14) described that the key objective of surgery is to thoroughly treat the arachnoid cyst, intramedullary cyst, and syringomyelia without insisting on the removal of bone from within the dura mater. In our patient, subtotal removal of the highly thickened arachnoid resulted in excellent perfusion of the cerebrospinal fluid, which led to an improvement of the disturbance in urinary/stool frequency. Furthermore, successful removal of the bone from within the dura mater led to total amelioration of the electric-shock-like pain that presumably occurred when the bone chips came in contact with the nerve tissue while walking.
Footnotes
Conflicts of Interest Disclosure
No funds were received in support of this work. No benefits in any form have been or will be received from any commercial party related directly to the subject of this manuscript. Informed consent of the patient for the use of this information was obtained, and this publication was performed with the approval (No. 2930) of the ethics committee of our university.
References
- 1). Domenicucci M, Ramieri A, Passacantilli E, Russo N, Trasimeni G, Delfini R: Spinal arachnoiditis ossificans: report of three cases. Neurosurgery 55: 985, 2004. [DOI] [PubMed] [Google Scholar]
- 2). Ibrahim GM, Kamali-Nejad T, Fehlings MG: Arachnoiditis ossificans associated with syringomyelia: An unusual cause of myelopathy. Evid Based Spine Care J 1: 46– 51, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Maulucci CM, Ghobrial GM, Oppenlander ME, Flanders AE, Vaccaro AR, Harrop JS: Arachnoiditis ossificans: clinical series and review of the literature. Clin Neurol Neurosurg 124: 16– 20, 2014. [DOI] [PubMed] [Google Scholar]
- 4). Schwarz E: Präparate von einem Falle syphilitischer Meningomyelitis mit Höhlenbildung im Rükenmarke und besonderen degenerativen Veränderungen der Neuroglia. Wien klin Wchnschr 110: 17, 1897. [Google Scholar]
- 5). Gatzke LD, Dodge HW, Dockerty MB: Arachnoiditis ossificans; report of two cases. Proc Staff Meet Mayo Clin 32: 698– 704, 1957. [PubMed] [Google Scholar]
- 6). Junewick J, Culver SK: Clinical image. Arachnoiditis ossificans in a pediatric patient. Pediatr Radiol 40: 228, 2010. [DOI] [PubMed] [Google Scholar]
- 7). Kitagawa H, Kanamori M, Tatezaki S, Itoh T, Tsuji H: Multiple spinal ossified arachnoiditis. A case report. Spine 15: 1236– 1238, 1990. [DOI] [PubMed] [Google Scholar]
- 8). Bagley JH, Owens TR, Grunch BH, Moreno JR, Bagley CA: Arachnoiditis ossificans of the thoracic spine. J Clin Neurosci 21: 386– 389, 2014. [DOI] [PubMed] [Google Scholar]
- 9). Wijdicks CA, Williams JM: Spinal arachnoid calcifications. Clin Anat 20: 521– 523, 2007. [DOI] [PubMed] [Google Scholar]
- 10). Wagner JA, Slager UT, Tucker L: Hypoparathyroidism with cerebral calcification. I. Report of a case. Bull Sch Med Univ Md 39: 102– 109, 1954. [PubMed] [Google Scholar]
- 11). Faure A, Khalfallah M, Perrouin-Verbe B, Caillon F, Deschamps C, Bord E, Mathe JF, Robert R: Arachnoiditis ossificans of the cauda equina. Case report and review of the literature. J Neurosurg 97 (2 Suppl): 239– 243, 2002. [DOI] [PubMed] [Google Scholar]
- 12). Frizzell B, Kaplan P, Dussault R, Sevick R: Arachnoiditis ossificans: MR imaging features in five patients. AJR Am J Roentgenol 177: 461– 464, 2001. [DOI] [PubMed] [Google Scholar]
- 13). Papavlasopoulos F, Stranjalis G, Kouyialis AT, Korfias S, Sakas D: Arachnoiditis ossificans with progressive syringomyelia and spinal arachnoid cyst. J Clin Neurosci 14: 572– 576, 2007. [DOI] [PubMed] [Google Scholar]
- 14). Braz A, Gonçalves C, Diogo C, Reis FC: [Arachnoiditis ossificans]. Acta Med Port 16: 183– 184, 2003. [PubMed] [Google Scholar]


