Abstract
Objective
To explore the impact of primary debulking surgery (PDS) to minimal but gross residual disease (RD) in women with bulky stage IIIC ovarian, fallopian tube, or primary peritoneal cancer.
Methods
We retrospectively reviewed all patients with the aforementioned diagnosis who underwent PDS at our institution from 01/2001–12/2010. Those with disease of non-epithelial histology or borderline tumors were excluded. Clinicopathologic data were abstracted, and appropriate statistical tests were used.
Results
We identified 496 eligible patients. Median age was 62 years; 91% had disease of serous histology. Patients were grouped by RD status: no gross RD, 184 (37%); RD of 1–5 mm, 127 (26%); RD of 6–10 mm, 54 (11%); and RD >10 mm, 131 (26%). With a median follow-up of 53 months, the median progression-free survivals (PFS) were: 26.7, 20.7, 16.2, and 13.6 months, respectively (p<0.001). The median overall survivals (OS) were 83.4, 54.5, 43.8, and 38.9 months, respectively (p<0.001). Among patients with RD following PDS, those with RD of 1–10 mm had improved PFS (p<0.001) and OS (p=0.001) compared with those with RD >10 mm. Patients with RD 1–10 mm who received intravenous/intraperitoneal (IV/IP) chemotherapy were younger and had prolonged OS compared with those solely exposed to IV chemotherapy (p<0.001 and p=0.002, respectively).
Conclusions
PDS to no gross RD was associated with the longest PFS and OS. However, cytoreduction to 1–10 mm of RD was also associated with better survival outcomes compared with cytoreduction to >10 mm of RD. We conclude that PDS remains an appropriate option for patients with a high likelihood of achieving RD ≤10 mm, especially for younger patients who can receive IV/IP chemotherapy after PDS.
Keywords: ovarian cancer, primary debulking surgery, neoadjuvant chemotherapy, optimal cytoreduction, residual disease
Introduction
Ovarian cancer accounts for 5% of cancer mortality among American women and is the leading cause of death from gynecologic cancer in the United States. In 2016, an estimated 22,280 women will be diagnosed with ovarian cancer and 14,240 will die of this disease in the United States [1]. The vast majority of women present with advanced disease; in this setting, primary debulking surgery (PDS) followed by platinum- and taxane-based chemotherapy is the traditional treatment of choice [2]. Surgical cytoreduction has been considered optimal if the residual tumor is ≤1cm in maximum diameter or thickness [2, 3]. However, over the past few years, converging evidence has highlighted the incremental survival benefit with cytoreduction to no gross residual disease (NGR) [3–7]. On this basis, it is now well established that the ultimate goal of PDS should be to remove all macroscopic disease, and maximal effort should be made toward this [2, 8].
The role of neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS) in patients with advanced ovarian cancer is somewhat controversial [2]. Although two randomized controlled trials (RCTs) showed that NACT is not inferior to PDS in terms of survival outcomes [9, 10], factors related to patient selection and surgical comprehensiveness might have undermined the prognosis of participants treated with PDS [2, 11]. Indeed, a recent literature review of publications on women with stage III or IV disease cytoreduced to NGR at primary or interval surgery revealed that IDS compromised the median overall survival (OS) by almost 2 years [12]. However, it is generally accepted that NACT should be offered to patients deemed to be poor surgical candidates or those with unresectable disease [2, 13]. Similarly to PDS, complete resection of all macroscopic disease at IDS is the strongest independent predictor of OS in NACT cases [10].
Despite its limited reproducibility, especially when measured in millimeters in cases with disseminated disease, the critical role of the maximum residual disease (RD) size in the prognosis of patients with advanced ovarian cancer is universally acknowledged. In contrast, the sequence of therapeutic interventions when RD of 1–10 mm is expected at PDS is under debate. On this basis, we sought to explore the impact on progression free-survival (PFS) and OS of PDS to minimal but gross RD in women with bulky, stage IIIC ovarian, fallopian tube, or primary peritoneal carcinoma.
Methods
After obtaining Institutional Review Board (IRB) approval, we retrospectively reviewed the records of patients with ovarian, fallopian tube, or primary peritoneal carcinoma treated at our institution. Our analysis was restricted to those with stage IIIC disease who underwent PDS from 01/2001–12/2010. Patients with borderline ovarian tumors or disease of non-epithelial histology, as well as those primarily cytoreduced at other hospitals, were excluded from the study. Based on the current International Federation of Gynecology and Obstetrics (FIGO) staging system, women with positive retroperitoneal lymph nodes as the only site of extrapelvic disease did not qualify as having stage IIIC disease.
All clinical variables, including age at diagnosis, body mass index (BMI), serum albumin and CA-125, as well as histologic grade, were obtained from the medical records. Details on the operative time, procedures performed, volume of RD, ascites, and estimated blood loss (EBL) were retrieved from the operative notes. Based on the reported RD, patients were classified into 4 groups: Group 1 included patients left with NGR; Group 2 included those left with RD of 1–5 mm; Group 3 included those left with RD of 6–10 mm; and Group 4 included those left with RD >10 mm. We used our prospectively collected, institutional morbidity and mortality data to identify surgical secondary events. Their classification followed the modified Clavien-Dindo system, as published elsewhere [14].
Any laparotomy undertaken with the intent of optimal cytoreduction was considered a PDS. Intraoperatively, initial disease burden was measured with the use of our previously described “OR tumor index.” Patients with a score of 0 had neither carcinomatosis nor bulky upper abdominal disease. Women with a score of 1 had either carcinomatosis or bulky upper abdominal disease, and those with a score of 2 had both carcinomatosis and bulky upper abdominal disease [15]. Debulking procedures included: standard hysterectomy, salpingo-oophorectomy, omentectomy with or without gastrointestinal surgery, lymphadenectomy and radical upper abdominal procedures, such as diaphragm peritonectomy/resection, splenectomy, distal pancreatectomy, liver resection, and cholecystectomy. As described by Aletti et al., a surgical complexity score (CS), which incorporates the number and complexity of surgical procedures performed, was assigned to all patients. A CS ≤3 was defined as low, CS 4–7 as intermediate, and CS ≥8 as high [16].
PFS and OS were the endpoints of our study. The date of progression was determined by computed tomography (CT) scan and/or CA-125 levels. When using CT imaging, the progression date was taken as the first appearance of one or more new lesions or increased size of existing lesions. When using CA-125 levels, the date of progression was defined as the first date of the initial CA-125 of greater than or equal to two times the nadir value or upper limit of normal, as applicable [17, 18]. When a subsequent CT scan confirmed that the rise in CA-125 indicated progression, the date of progression was defined as the date of CA-125 rise. PFS was defined as the time interval from the date of PDS to the date of disease progression, death, or last follow-up. OS was calculated from the date of PDS to the date of death or last follow-up. Patients who were lost to follow-up were censored from the analysis. Median PFS and OS were obtained through the Kaplan-Meier method, and p-values for categorical variables were obtained through the log-rank test. Differences in age, grade, histology, American Society of Anesthesiologists (ASA) score, and OR tumor index between the intravenous/intraperitoneal (IV/IP) chemotherapy and IV chemotherapy groups of patients with RD 1–10 mm were evaluated with the chi square test for trend, Fisher’s exact test, and the student’s t test, as appropriate. All statistical analyses were performed on IBM SPSS Statistics v. 22.0 (IBM SPSS Statistics for Windows 2013, Armonk, NY).
Results
A total of 496 patients met inclusion criteria. Patient characteristics are presented in Table 1. The median age was 62 years, median BMI was 25.3 kg/m2, median preoperative CA125 was 519 U/mL, and median preoperative albumin was 4.1 g/dL. Greater than 90% of patients had an ASA score of 2 or 3. Surgicopathologic findings are shown in Table 2. The median amount of ascites was 1000 mL; 55% had an OR tumor index of 2; 45% had an intermediate CS; median EBL was 700 mL, and median surgical time was 265 minutes. Greater than 90% of patients had grade 3 tumors; similarly, serous histology was diagnosed in 91%. Based on RD reported at the end of PDS, patients were grouped as follows: Group 1, 184 patients (37%); Group 2, 127 (26%); Group 3, 54 (11%); Group 4, 131 (26%).
Table 1.
Patient Characteristics (N=496)
| Factor | Median (range) |
|---|---|
| Age at PDS, years | 62 (23–96) |
| BMI, kg/m2 | 25.3 (16.7–54.6) |
| Preoperative CA-125, U/mL (range) | 519 (2.9–38,100) |
| Preoperative albumin, g/dL (range) | 4.1 (2.7–4.8) |
|
| |
| ASA score | Number of Patients (%) |
| 1 | 29 (6) |
| 2 | 291 (59) |
| 3 | 175 (35) |
| 4 | 1(0.2) |
PDS, primary debulking surgery; BMI, body mass index; ASA, American Society of Anesthesiologists
Table 2.
Surgicopathologic Findings (N=496)
| Factor | Number of patients (%) |
|---|---|
| Presence of ascites | |
| Yes | 354 (71) |
| No | 123 (25) |
| Unknown | 19 (4) |
| OR tumor index | |
| 0 | 91 (18) |
| 1 | 131 (27) |
| 2 | 274 (55) |
| Surgical complexity score | |
| Low | 135 (27) |
| Intermediate | 224 (45) |
| High | 137 (28) |
| Tumor grade | |
| 1 | 15 (3) |
| 2 | 26 (5) |
| 3 | 455 (92) |
| Histology | |
| Serous | 451 (91) |
| Endometrioid | 4 (0.8) |
| Clear cell | 1 (0.2) |
| Mixed | 40 (8) |
| Median (range) | |
| Ascites, mL | 1000 (0–13,000) |
| EBL, mL | 700 (5–8,000) |
| Surgical time, min | 265 (34–893) |
EBL, estimated blood loss
Median follow-up time was 53 months (range, 0.3–171 months). Median PFS for the entire cohort was 18.6 months (95% CI: 16.7–20.4 months). Median PFS was 26.7 months (95% CI: 23.8–29.7) for Group 1; 20.7 months (95% CI: 17.2–24.2) for Group 2; 16.2 months (95% CI: 12.3–20.1) for Group 3; and 13.6 months (95% CI: 12.3–14.9) for Group 4 (p<0.001). Median OS for the entire study population was 54.7 months (95% CI: 50.8–58.7 months). Median OS was 83.4 (95% CI: 67.6–99.2), 54.5 (95% CI: 48.9–60.1), 43.8 (95% CI: 24.9–62.7), and 38.9 months (95% CI: 32.9–44.8), respectively (p<0.001; Figure 1A).
Figure 1.
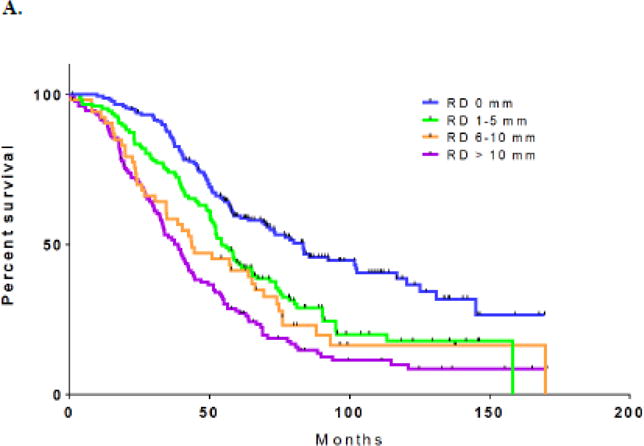
A. Overall survival by residual disease: 0 mm vs. 1–5 mm vs. 6–10 mm vs. >10 mm
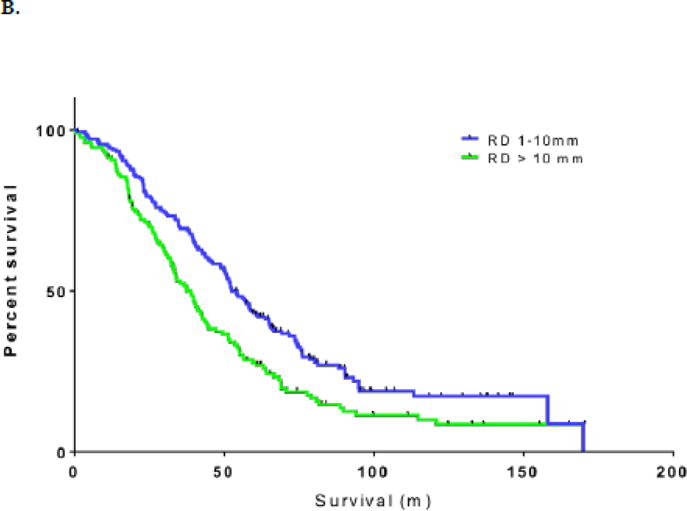
B. Overall survival by residual disease: 1–10 mm vs. >10 mm
Given the relatively small number of patients with RD of 6–10 mm (n=54), we were unable to draw any clinically meaningful conclusions from comparisons with other subsets. In order to be consistent with the cut-off of 10 mm, which is used in the vast majority of studies in the existing literature to define optimal RD, we combined groups 2 (RD 1–5 mm) and 3 (RD 6–10 mm) into one group for the remaining analyses. The median PFS and OS for the combined group were 18 (95% CI: 15.2–20.8) and 52.6 months (95% CI: 46.9–58.3), respectively. Compared with patients with RD >10 mm, those with RD 1–10 mm had significantly better PFS (p<0.001) and OS (p=0.001; Figure 1B).
Of 365 patients who achieved NGR or RD 1–10 mm, 153 (42%) were given at least 1 cycle of primary IV/IP chemotherapy. Details regarding the IV/IP chemotherapy regimen used at our institution have been published [19]. The median PFS and OS for those treated with IV/IP chemotherapy were 25.8 (95% CI: 21.8–29.8) and 77.1 months (95% CI: 67.6–86.6), respectively. In focusing on the survival outcomes of patients with RD 1–10 mm after PDS, PFS did not significantly differ between patients with RD 1–10 mm who received IV/IP chemotherapy compared with those solely treated with IV chemotherapy (median PFS: 21.2 vs. 14.9 months; p=0.097). OS was significantly prolonged in those treated with at least 1 cycle of IV/IP chemotherapy (median OS: 65.1 vs. 40.6 months; p=0.002; Figure 2). However, patients with RD 1–10 mm exposed to IV/IP chemotherapy were younger in comparison with those who received IV chemotherapy (mean age±SD: 57.9±9.8 vs. 64.9±10.5 years; p<0.001; Table 3).
Figure 2.
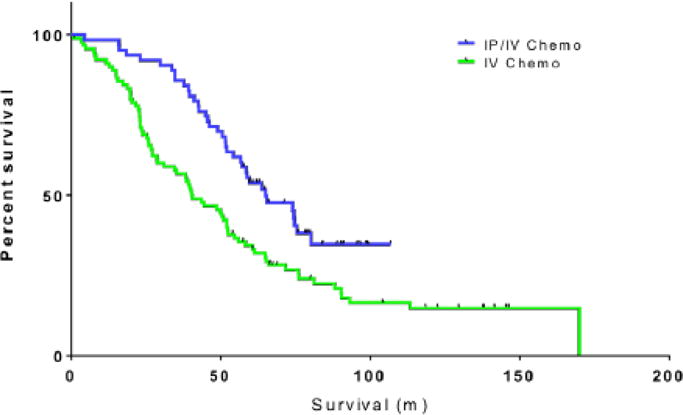
Overall survival in patients with residual disease 1–10 mm by type of chemotherapy: intravenous/intraperitoneal (IV/IP) chemotherapy vs. IV chemotherapy
Table 3.
Differences in age, tumor grade, histology, ASA score and OR tumor index among patients in the IV/IP chemotherapy and IV chemotherapy-alone groups with residual disease 1–10 mm
| Factor | No. of patients (%) | p | |
|---|---|---|---|
|
| |||
| IV chemotherapy (n=120) |
IV/IP chemotherapy (n=61) |
||
|
| |||
| Tumor grade | 0.725CT | ||
| 1 | 3 (2.5) | 3 (5) | |
| 2 | 7 (6) | 2 (3) | |
| 3 | 110 (92) | 56 (92) | |
|
| |||
| Histology | 0.667F | ||
| Serous | 111 (92.5) | 59 (97) | |
| Endometrioid | 1 (1) | 0 (0) | |
| Mixed | 8 (7) | 2 (3) | |
|
| |||
| ASA score | 0.535CT | ||
| 1 | 8 (7) | 1 (2) | |
| 2 | 66 (55) | 43 (70) | |
| 3 | 46 (38) | 17 (28) | |
|
| |||
| OR tumor index | 0.399CT | ||
| 0 | 14 (12) | 7 (11) | |
| 1 | 44 (37) | 17 (28) | |
| 2 | 62 (52) | 37 (61) | |
|
| |||
| Factor | mean±SD | mean±SD | p |
|
| |||
| Age (years) | 64.9±10.5 | 57.9±9.8 | <0.001T |
ASA: American Society of Anesthesiologists
CT: p value derived from chi square test for trend; F: p value derived from Fisher’s exact test; T: p value derived from student’s t test
In total, 318 patients (64%) experienced at least one surgical complication, while grade 3–5 complications were recorded in 85 patients (17%). Two patients died within 30 days of surgery, for a perioperative mortality rate of 0.4%. The distribution of OR tumor index, surgical CS, total complications, and grade 3–5 complications per RD group is listed in Table 4.
Table 4.
OR tumor index, surgical complexity score, total complications, and grade 3–5 complications per residual disease group
| Factor | No. of patients (%) | |||
|---|---|---|---|---|
|
| ||||
| NGR (n= 184) |
RD 1–5 mm (n= 127) |
RD 6–10 mm (n= 54) |
RD >10 mm (n=131) |
|
|
| ||||
| OR tumor index | ||||
| 0 | 64 (35) | 15 (12) | 6 (11) | 6 (5) |
| 1 | 50 (27) | 42 (33) | 19 (35) | 20 (15) |
| 2 | 70 (38) | 70 (55) | 29 (54) | 105 (80) |
|
| ||||
| Surgical complexity score | ||||
| Low | 25 (14) | 25 (20) | 5 (9) | 80 (61) |
| Intermediate | 104 (56) | 53 (42) | 25 (46) | 42 (32) |
| High | 55 (30) | 49 (39) | 24 (44) | 9 (7) |
|
| ||||
| Complications | ||||
| Yes | 111 (60) | 88 (69) | 39 (72) | 80 (61) |
| No | 73 (40) | 39 (31) | 15 (28) | 51 (39) |
|
| ||||
| Grade 3–5 complications | ||||
| Yes | 28 (15) | 28 (22) | 18 (33) | 11 (8) |
| No | 156 (85) | 99 (78) | 36 (67) | 120 (92) |
Discussion
Our study presents the survival outcomes of patients with bulky, stage IIIC ovarian, fallopian tube, or primary peritoneal carcinoma who underwent PDS between 01/2001 and 12/2010. When compared to our previous report, which reflected our experience in a similar cohort treated from 01/1989 to 12/2003, the rate of NGR after PDS increased from 15% to 37% [3]. Accordingly, there was a 23% decrease in the rate of suboptimally debulked patients in our current study [3]. The above differences can be attributed to the change in our surgical paradigm from 2001 onwards. At that time, studies from our institution and others showed that resection of upper abdominal disease was both feasible and safe, while also enabling for higher rates of optimal debulking [20].
Interestingly, the increased rates of optimal cytoreduction during our study period were not accompanied by better survival outcomes per RD. When compared to our results published in 2006, the median OS of patients cytoreduced to NGR decreased from 106 to 83.4 months. A similar trend was noted in patients with RD 1–5 mm or 6–10 mm; their median OS decreased 11.5 and 4.2 months, respectively [3]. We attribute this to the increased incorporation of extensive upper abdominal surgical procedures, which has led to improved survival outcomes in patients who would have been otherwise left with RD >10 mm [20, 21]. However, as a recent analysis of Gynecologic Oncology Group (GOG) study 182 pointed out, the negative prognostic role of initial disease burden remains despite the achievement of complete gross and optimal cytoreduction, indicating that comprehensive surgery cannot fully overcome tumor biology [22]. Consequently, the inclusion of patients who would have been suboptimally debulked before the expansion of our surgical armamentarium in our current NGR or optimal RD groups may account for the observed decrease in median OS within each RD group. However, the overall benefits on survival outcomes of complete gross and optimal cytoreduction still remain.
In our study, women with NGR exhibited the best survival outcomes. Our findings are consistent with the contemporary literature and underscore that resection of all visible disease should be the goal of primary cytoreduction [2–8]. When this cannot be accomplished, many experts recommend NACT as the preferable treatment regimen so that NGR at IDS may be achieved [22, 23]. In this context, multiple algorithms encompassing serologic markers, imaging modalities, and laparoscopy have been proposed as a means to preoperatively estimate the likelihood of NGR at PDS and, subsequently, determine the best therapeutic strategy. However, even when they are implemented, the achievement of RD 1–10 mm is still a possible scenario [23, 24]. More importantly, do we have adequate evidence to strongly support that NACT/IDS is the best option for all patients with acceptable perioperative risk profile if NGR at PDS is not deemed feasible?
Our study clearly demonstrates that patients with RD 1–10 mm have better PFS and OS than patients left with RD >10 mm. In the ovarian cancer literature, a limited number of authors have aimed to shed light on the survival outcomes of patients with minimal but gross RD at PDS as a separate group prognostically. Recently, a retrospective review of 13 publications on patients with stage III to IV ovarian cancer demonstrated that the median disease-free survival (DFS) and OS for patients with RD 1–10 mm at PDS was 16 (range, 15–22) and 40 months (range, 27–55), respectively. As expected, their prognosis was significantly worse than that of patients with complete removal of all macroscopic disease at PDS. Nonetheless, in line with our conclusions, women with RD 1–10 mm obtained a significant benefit of 4 months in median DFS and 10 months in median OS over suboptimally debulked patients [25]. In the same report, primary cytoreduction to RD 1–5 mm led to significantly better median OS than RD 6–10 mm (53 vs. 44 months). On this basis, its authors proposed a cut-off of RD >5 mm to define suboptimally debulked patients [25]. Although we did not address this issue in the current study, our survival results in patients with RD 1–5 and 6–10 mm are strikingly similar to the aforementioned study. Nonetheless, differentiating a residual of 5 mm vs. 6 mm to define optimal vs. suboptimal debulking may be undermined by the intra- and inter-observer variability.
The role of primary IV/IP chemotherapy in patients with gross but minimal RD seems to be crucial. Numerous RCTs and meta-analyses have highlighted that stage III ovarian cancer patients who are primarily debulked to NGR or RD 1–10 mm benefit from IP delivery of chemotherapy [26–28]. In 2015, a retrospective analysis of GOG 114 and 172 showed that, despite the 1.89-fold increase in risk of death compared with those with NGR, patients with RD 1–10 mm had prolonged survival with IV/IP chemotherapy [29]. Consistent with those findings, the median OS of our patients with RD 1–10 mm treated with at least 1 cycle of primary IV/IP chemotherapy increased by 24.5 months. Although the confounding role of patient age in our results could not be excluded, these data suggest that primary IV/IP chemotherapy should be strongly considered in patients with RD 1–10 mm at PDS to maximize survival benefit [25].
Survival outcomes following the NACT/IDS approach, which emerges as the indicated treatment regimen when primary cytoreduction to NGR seems unlikely, were best illustrated in two RCTs, which reported median PFS and OS of 12 and 24–30 months, respectively [9, 10]. In addition, a review of 24 articles published from 2008 to 2014 showed that patients with advanced-stage ovarian cancer treated with NACT/IDS had a median DFS of 14 months and median OS of 33 months [12]. Similarly, a median OS of 33 months was recently recorded even for patients with stage IIIC disease treated with NACT/IDS at high standards, National Cancer Institute-designated cancer centers [30]. These results are comparable to our survival outcomes for those patients who underwent suboptimal (>10 mm RD) primary cytoreduction (median PFS: 13.6 months; median OS: 38.9 months).
Given that achieving NGR at IDS is associated with the best prognosis in NACT patients [10], the obvious question is whether or not those patients have better survival outcomes compared with those who undergo PDS and have RD 1–10 mm. To the best of our knowledge, well-designed studies that directly compare survival outcomes in patients with RD 1–10 mm at PDS with those who achieve NGR at IDS do not exist. Importantly, with a complete pathologic response of 6.5% and microscopic pathologic response of 32.3% after NACT [31], there is no evidence that patients with RD 1–10 mm at PDS will obtain NGR at IDS if the NACT approach is utilized instead. Indeed, it was reported that even in those select stage IIIC to IV tubo-ovarian high-grade serous carcinoma patients who showed the best response to NACT, complete resection at IDS was achieved in only 78% [32]. Therefore, it is impossible to conclude that disease unamenable to complete gross resection in the primary setting due to its size/location or surgeons’ lack of expertise will definitely respond to NACT to a degree that could render it completely removable at IDS.
The inclusion of a large number of patients with comparable extent of disease is among the strengths of this study. Furthermore, our results incorporate a time period marked by cardinal changes in the management of patients with advanced ovarian, fallopian tube, and primary peritoneal carcinoma, such as the implementation of extensive upper abdominal resections and the administration of IV/IP chemotherapy. It is noteworthy that all patients underwent PDS in a high-volume cancer center, with surgeons capable of performing the full spectrum of procedures that may be required in this setting; the homogeneity in the surgical management of our study population certainly adds value to our study. On the other hand, our report has the limitations inherent to retrospective analyses. The interobserver variability in estimating the maximum diameter of residual tumor, as well as the use of a variety of IV chemotherapy regimens after PDS or at the time of disease recurrence, should be also cited as our study’s weaknesses. In addition, the study period did not permit the role of the dose-dense weekly paclitaxel and carboplatin regimen or bevacizumab to be adequately investigated; the small number of patients who received them would preclude analysis and, subsequently, meaningful conclusions [33–37]. Lastly, clinically sound comparison between groups with RD 1–5 mm and 6–10 mm, so as to possibly redefine the cut-off for optimal RD, was not attainable given the relatively small number of patients in the latter group.
In conclusion, as the very recent clinical practice guideline by the Society of Gynecologic Oncology (SGO) and the American Society of Clinical Oncology (ASCO) states, complete removal of all macroscopic disease should be the objective of PDS [38]. It should be noted that the associated morbidity may occasionally have detrimental effects on the disease course and patient survival. Therefore, the required surgical procedures to meet the above goal, along with the individual risk factors for perioperative morbidity or mortality (e.g., age, co-morbidities, nutritional status or albumin levels, the presence of ascites or newly diagnosed deep vein thrombosis, BMI, stage and performance status [38]), should always be taken into serious consideration. If obtaining NGR at PDS is deemed feasible with acceptable (according to each patient’s specific characteristics) morbidity, primary cytoreduction should be offered to patients with bulky stage IIIC ovarian, fallopian tube, or primary peritoneal cancer. When the extent of disease precludes the achievement of RD ≤10 mm or in case of patients with high perioperative risk profiles, NACT/IDS should be the preferred treatment regimen [38]. In line with SGO/ASCO recommendations, our results showed cytoreduction to minimal but gross residual disease remains an effective treatment strategy, especially in younger patients who receive IV/IP chemotherapy after PDS [38]. Future research is needed to prove whether or not patients who can only be primarily cytoreduced to RD 1–10 mm would have had NGR at IDS; and whether survival outcomes of those left with NGR at IDS are better than those with RD 1–10 mm at PDS. Until then, the acquisition of the necessary surgical skills through training and intraoperative consultations, as well as referral to surgeons able to perform extensive surgical cytoreduction prior to commencement of primary therapy, is still of paramount importance [39].
Research Highlights.
PDS to no gross RD confers the best survival outcomes
PDS to RD of 1–10 mm is associated with survival benefit compared to RD of >10 mm
IV/IP chemotherapy use is associated with better OS in patients with RD of 1–10 mm
Acknowledgments
Financial Support
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 47th Annual Meeting of the Society of Gynecologic Oncology, San Diego, California
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.NCCN Guidelines. Morgan RJ, Armstrong DK, Alvarez RD, et al. NCCN Clinical Practice Guidelines in Oncology. Ovarian Cancer, Including Fallopian Tube Cancer and Primary Peritoneal Cancer Version 1.2016. Available at nccn.org. Accessed Accessed September 26, 2016.
- 3.Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 4.Aletti GD, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006;107:77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 5.Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 6.Du Bois A, Reuss A, Pujade-Lauraine E, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 7.Winter WE, 3rd, Maxwell GL, Tian C, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;26:83–89. doi: 10.1200/JCO.2007.13.1953. [DOI] [PubMed] [Google Scholar]
- 8.Stuart GC, Kitchener H, Bacon M, et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer. 2011;21:750–755. doi: 10.1097/IGC.0b013e31821b2568. [DOI] [PubMed] [Google Scholar]
- 9.Kehoe S, Nankivell M. Primary chemotherapy versus primary surgery for ovarian cancer – Authors’ reply. Lancet. 2015;386:2143. doi: 10.1016/S0140-6736(15)01052-1. [DOI] [PubMed] [Google Scholar]
- 10.Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 11.Chi DS, Musa F, Dao F, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT) Gynecol Oncol. 2012;124:10–14. doi: 10.1016/j.ygyno.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Chiva L, Lapuente F, Castellanos T, et al. What Should We Expect After a Complete Cytoreduction at the Time of Interval or Primary Debulking Surgery in Advanced Ovarian Cancer? Ann Surg Oncol. 2016;23:1666–1673. doi: 10.1245/s10434-015-5051-9. [DOI] [PubMed] [Google Scholar]
- 13.Narasimhulu DM, Khoury-Collado F, Chi DS. Radical surgery in ovarian cancer. Curr Oncol Rep. 2015;17:16. doi: 10.1007/s11912-015-0439-z. [DOI] [PubMed] [Google Scholar]
- 14.Strong VE, Selby LV, Sovel M, et al. Development and assessment of Memorial Sloan Kettering Cancer Center’s surgical secondary events grading system. Ann Surg Oncol. 2015;22:1061–1067. doi: 10.1245/s10434-014-4141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanner EJ, Long KC, Zhou Q, et al. Impact of operative start time on surgical outcomes in patients undergoing primary cytoreduction for advanced ovarian cancer. Gynecol Oncol. 2012;126:58–63. doi: 10.1016/j.ygyno.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2007;197:e1–7. doi: 10.1016/j.ajog.2007.10.495. [DOI] [PubMed] [Google Scholar]
- 17.Rustin GJ, Vergote I, Eisenhauer E, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer InterGroup (GCIG) Int J Gynecol Cancer. 2011;21:419–423. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Barlin JN, Dao F, Bou Zgheib N, et al. Progression-free and overall survival of a modified outpatient regimen of primary intravenous/intraperitoneal paclitaxel and intraperitoneal cisplatin in ovarian, fallopian tube, and primary peritoneal cancer. Gynecol Oncol. 2012;125:621–624. doi: 10.1016/j.ygyno.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EL, Abu-Rustum NR, Sonoda Y, et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol Oncol. 2006;103:1083–1090. doi: 10.1016/j.ygyno.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz NS, Miller A, Rungruang B, et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. J Clin Oncol. 2015;33:937–943. doi: 10.1200/JCO.2014.56.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nick AM, Coleman RL, Ramirez PT, Sood AK. A framework for a personalized surgical approach to ovarian cancer. Nat Rev Clin Oncol. 2015;12:239–245. doi: 10.1038/nrclinonc.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez-Hidalgo NR, Martinez-Cannon BA, Nick AM, et al. Predictors of optimal cytoreduction in patients with newly diagnosed advanced-stage epithelial ovarian cancer: Time to incorporate laparoscopic assessment into the standard of care. Gynecol Oncol. 2015:137553–558. doi: 10.1016/j.ygyno.2015.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiva LM, Castellanos T, Alonso S, Gonzalez-Martin A. Minimal Macroscopic Residual Disease (0.1–1 cm). Is It Still a Surgical Goal in Advanced Ovarian Cancer? Int J Gynecol Cancer. 2016;26:906–911. doi: 10.1097/IGC.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 27.Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2016;12:CD005340. doi: 10.1002/14651858.CD005340.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 29.Tewari D, Java JJ, Salani R, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2015;33:1460–1466. doi: 10.1200/JCO.2014.55.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer LA, Cronin AM, Sun CC, et al. Use and effectiveness of neoadjuvant chemotherapy for treatment of ovarian cancer. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.68.1239. pii: JCO681239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrillo M, Zannoni GF, Tortorella L, et al. Prognostic role and predictors of complete pathologic response to neoadjuvant chemotherapy in primary unresectable ovarian cancer. Am J Obstet Gynecol. 2014;211:632.e1–8. doi: 10.1016/j.ajog.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 32.Böhm S, Faruqi A, Said I, et al. Chemotherapy response score: Development and validation of a system to quantify histopathologic response to neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J Clin Oncol. 2015;33:2457–2463. doi: 10.1200/JCO.2014.60.5212. [DOI] [PubMed] [Google Scholar]
- 33.Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 34.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 35.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 36.Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 38.Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34:3460–73. doi: 10.1200/JCO.2016.68.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang SJ, Bristow RE, Chi DS, Cliby WA. Role of aggressive surgical cytoreduction in advanced ovarian cancer. J Gynecol Oncol. 2015;26:336–342. doi: 10.3802/jgo.2015.26.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]


