Abstract
Background
In the era of biologic agents, risk factors for complications following resection for Crohn’s disease have not been fully identified. In particular, the association of preoperative use of immunosuppressive and biologic agents with the incidence of complications after resection remains to be elucidated.
Aim
This retrospective multicentre study aimed to identify risk factors for complications after ileocolonic resection for Crohn’s disease, with a major focus on the impact of preoperative immunosuppressive and biologic therapy.
Methods
A total of 231 consecutive patients who underwent ileocolonic resections for active Crohn’s disease in seven inflammatory bowel disease referral centres from three countries (Japan, Brazil and Italy) were included. The following variables were investigated as potential risk factors: age at surgery, gender, behaviour of Crohn’s disease (perforating vs. non-perforating disease), smoking, preoperative use (within eight weeks before surgery) of steroids, immunosuppressants and biologic agents, previous resection, blood transfusion, surgical procedure (open vs. laparoscopic approach), and type of anastomosis (side-to-side vs. end-to-end). Postoperative complications occurring within 30 days after surgery were recorded.
Results
The rates of overall complications, intra-abdominal sepsis, and anastomotic leak were 24%, 12% and 8%, respectively. Neither immunosuppressive nor biologic therapy prior to surgery was significantly associated with the incidence of overall complications, intra-abdominal sepsis or anastomotic leak. In multivariate analysis, blood transfusion, perforating disease and previous resection were significant risk factors for overall complications (odds ratio [OR] 3.02, 95% confidence interval [CI] 1.21–7.52; P = 0.02), intra-abdominal sepsis (OR 2.67, 95% CI 1.04–6.86; P = 0.04) and anastomotic leak (OR 2.87, 95% CI 1.01–8.18; P = 0.048), respectively.
Conclusions
Blood transfusion, perforating disease and previous resection were significant risk factors for overall complications, intra-abdominal sepsis and anastomotic leak after ileocolonic resection for Crohn’s disease, respectively. Preoperative immunosuppressive or biologic therapy did not increase the risk of postoperative complications.
Keywords: Anastomotic leak, biologics, Crohn’s disease, ileocolonic resection, immunosuppressants, intra-abdominal sepsis, postoperative complications
Introduction
The introduction of biologic agents has led to a dramatic improvement in the care of patients with inflammatory bowel disease (IBD).1,2 Nevertheless, the need for surgical intervention remains high in patients with Crohn’s disease (CD).3 The goal of surgery similarly to medical treatment is to provide long-lasting symptomatic relief while avoiding excessive morbidity.4 Since CD frequently affects the terminal ileum with or without the right side of the colon, ileocolonic resection is the most frequently performed surgical procedure in patients with CD.4
Serious postoperative complications such as anastomotic leak and intra-abdominal sepsis develop more frequently in CD than in other intestinal diseases.5 These complications can markedly affect patients’ quality of life and may negatively impact their mental and physical health. Various risk factors specific for the patients with conditions related to CD can influence the outcomes of surgical treatment especially in the early postoperative period, such as the first 30 days after surgery.6,7 Those risk factors may include preoperative conditions like poor nutritional status, duration of symptoms before surgery and site of the disease, and operative factors such as indication for surgery, intra-operative findings and type of operation.
In the era of biologic agents, risk factors for complications following resection for CD have not been fully identified. In particular, the association of preoperative use of immunosuppressive and biologic agents with the incidence of complications after resection remains to be elucidated. The present study was designed to identify risk factors for complications after ileocolonic resection for CD, with a major focus on the impact of preoperative immunosuppressive and biologic therapy.
Patients and methods
Study design
This was a retrospective international multicentre study. In this study, seven IBD referral centres from three countries (Japan, Brazil and Italy) were involved. Our study protocol was approved by the ethics committee of each institution involved.
Patient inclusion and exclusion criteria
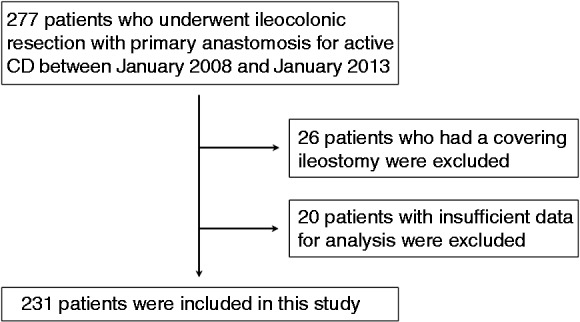
Patients who underwent ileocolonic resection (by open or laparoscopic approach) with primary anastomosis for active CD between January 2008 and January 2013 were included. Patients who underwent concomitant intestinal resection or strictureplasty for small bowel or colorectal CD were included. Patients who had a covering ileostomy were excluded. Patients with insufficient data for analysis were also excluded.
Surgical technique and strategy
Before starting this study, we had confirmed that indications for surgery, surgical technique and strategy, and perioperative management were similar between the centres. In severely compromised patients, the patient’s medical status was optimised by correcting anaemia, fluid depletion, electrolyte and acid-base disorders, and malnutrition prior to operation. Regarding surgical techniques, laparoscopic resection was performed by experienced surgeons, but it was avoided in patients with a large inflammatory mass, dense adhesions, markedly thickened mesentery and bowel, acute enteric fistulae, and severe abscesses. The selection of anastomotic technique mainly depended on individual surgeon experience and personal preference. Two-stage operation (avoid a primary anastomosis or construct a covering stoma to protect an anastomosis at the first operation) was selected for patients with some of the following factors such as poor nutritional status, use of steroids or immunosuppressive drugs, and the presence of abscess or fistula at the time of laparotomy.
Postoperative complications
Postoperative complications occurring within 30 days after surgery were analysed. The incidence of overall complications, intra-abdominal sepsis and anastomotic leak was evaluated. Intra-abdominal sepsis was defined as anastomotic leak, intra-abdominal abscess or entero-cutaneous fistula. Although no routine examinations were performed after surgery to detect anastomotic leak or intra-abdominal abscess, in cases of unexplained fever or abdominal tenderness, imaging studies including ultrasound, computed tomographic scan, or contrast x-ray were performed.
Potential risk factors for postoperative complications
The following variables were investigated as potential risk factors for postoperative complications: age at surgery, gender, behaviour of CD (perforating vs. non-perforating disease), smoking, preoperative use (within eight weeks before surgery) of steroids (prednisolone), immunosuppressants (azathioprine or 6-mercaptopurine) and biologic agents (infliximab or adalimumab), previous resection, blood transfusion, surgical procedure (open vs. laparoscopic approach), and type of anastomosis (side-to-side vs. end-to-end). Perforating disease was defined as perforation, abscess, or internal or external fistula from findings at laparotomy.8
Statistical analysis
Univariate analysis (the chi-square test with Yates’ correction) was conducted to investigate the association of each clinical variable with the incidence of postoperative complications. To determine risk factors for postoperative complications, a multivariate analysis using multiple regression model was conducted. P < 0.05 was considered to be statistically significant.
Results
Baseline characteristics
A flow diagram for patient inclusion and exclusion is shown in Figure 1. A total of 231 consecutive patients were included in this study. Baseline characteristics of the 231 patients are presented in Table 1. The indication for surgery was perforating disease in 99 patients (43%) and non-perforating disease in 132 patients (57%). At the time of ileocolonic resection, 63 patients (27%) underwent synchronous procedures: small bowel strictureplasty in 12 patients, small bowel resection in 14 patients, colonic resection in 29 patients, and others in eight patients. Of the patients, 24 (10%) required blood transfusion in the perioperative period. Open and laparoscopic approaches were used in 156 patients (68%) and 75 patients (32%), respectively. A hand-sewn side-to-side anastomosis was performed in 18 patients (8%), a stapled side-to-side anastomosis (functional end-to-end anastomosis) in 152 patients (66%), and a hand-sewn end-to-end anastomosis in 61 patients (26%).
Figure 1.
A flow diagram for patient inclusion and exclusion.
Table 1.
Baseline characteristics of the 231 patients
| Age at surgery (mean ± SE) | 33 ± 0.8 years |
| Male : female | 144 (62%) : 87 (38%) |
| Nationality (Brazil : Japan : Italy) | 85 (37%) : 96 (41%) : 50 (22%) |
| Behaviour of CD (perforating : non-perforating disease) | 99 (43%) : 132 (57%) |
| Smokers | 37 (16%) |
| Preoperative medications | |
| Steroids | 68 (29%) |
| Immunosuppressants (azathioprine or 6-mercaptopurine) | 65 (28%) |
| Biologics (infliximab or adalimumab) | 79 (34%)a |
| Previous resection | 71 (31%) |
| Synchronous procedures | 63 (27%) |
| Blood transfusion | 24 (10%) |
| Surgical procedure (open : laparoscopic approach) | 156 (68%) : 75 (32%) |
| Type of anastomosis (side-to-sideb : end-to-end) | 170 (74%) : 61 (26%) |
Infliximab for 55 patients, adalimumab for 23 patients, both for one patient.
Hand-sewn side-to-side anastomosis in 18 patients (8%), stapled side-to-side anastomosis (functional end-to-end anastomosis) in 152 patients (66%).
Preoperative immunosuppressive and biologic therapy
Within eight weeks before surgery, 65 patients (28%) were receiving immunosuppressive drugs (azathioprine or 6-mercaptopurine). Of the patients, 79 (34%) received biologic agents (infliximab for 55 patients, adalimumab for 23 patients, both for one patient) within eight weeks before surgery. Of the patients, 31 (13%) received both immunosuppressive and biologic agents. In contrast, 118 patients (51%) received neither immunosuppressants nor biologics. The relationship between the preoperative use of immunosuppressants and biologics, and clinical parameters are shown in Tables 2 and 3, respectively. Patients treated with immunosuppressive drugs significantly more often received biologics. Similarly, patients on biologic agents significantly more frequently received immunosuppressive drugs. The following parameters were not significantly different between patients treated with and without these agents: gender, behaviour of CD, smoking, preoperative use of steroids, previous resection, blood transfusion, and type of anastomosis.
Table 2.
Preoperative use of immunosuppressants and clinical parameters
| Immunosuppressants n = 65 | No immunosuppressants n = 166 | P | |
|---|---|---|---|
| Age at surgery | |||
| <17 years (n = 27) | 11 (17%) | 16 (10%) | |
| 17–40 years (n = 161) | 45 (69%) | 116 (70%) | |
| >40 years (n = 43) | 9 (14%) | 34 (20%) | 0.20 |
| Gender | |||
| Male (n = 144) | 39 (60%) | 105 (63%) | |
| Female (n = 87) | 26 (40%) | 61 (37%) | 0.76 |
| Behaviour of CD | |||
| Perforating disease (n = 99) | 28 (43%) | 71 (43%) | |
| Non-perforating disease (n = 132) | 37 (57%) | 95 (57%) | >0.99 |
| Smoking | |||
| Yes (n = 37) | 9 (14%) | 28 (17%) | |
| No (n = 194) | 56 (86%) | 138 (83%) | 0.72 |
| Preoperative steroids | |||
| Yes (n = 68) | 18 (28%) | 50 (30%) | |
| No (n = 163) | 47 (72%) | 116 (70%) | 0.84 |
| Preoperative biologics | |||
| Yes (n = 79) | 31 (48%) | 48 (29%) | |
| No (n = 152) | 34 (52%) | 118 (71%) | 0.01 |
| Previous resection | |||
| Yes (n = 71) | 26 (40%) | 45 (27%) | |
| No (n = 160) | 39 (60%) | 121 (73%) | 0.08 |
| Blood transfusion | |||
| Yes (n = 24) | 9 (14%) | 15 (9%) | |
| No (n = 207) | 56 (86%) | 151 (91%) | 0.40 |
| Surgical procedure | |||
| Open approach (n = 156) | 51 (78%) | 105 (63%) | |
| Laparoscopic approach (n = 75) | 14 (22%) | 61 (37%) | 0.04 |
| Type of anastomosis | |||
| Side-to-side (n = 170) | 54 (83%) | 116 (70%) | |
| End-to-end (n = 61) | 11 (17%) | 50 (30%) | 0.06 |
Table 3.
Preoperative use of biologics and clinical parameters
| Biologics n = 79 | No biologics n = 152 | P | |
|---|---|---|---|
| Age at surgery | |||
| <17 years (n = 27) | 14 (18%) | 13 (9%) | |
| 17–40 years (n = 161) | 56 (71%) | 105 (69%) | |
| >40 years (n = 43) | 9 (11%) | 34 (22%) | 0.03 |
| Gender | |||
| Male (n = 144) | 47 (59%) | 97 (64%) | |
| Female (n = 87) | 32 (41%) | 55 (36%) | 0.62 |
| Behaviour of CD | |||
| Perforating disease (n = 99) | 38 (48%) | 61 (40%) | |
| Non-perforating disease (n = 132) | 41 (52%) | 91 (60%) | 0.31 |
| Smoking | |||
| Yes (n = 37) | 11 (14%) | 26 (17%) | |
| No (n = 194) | 68 (86%) | 126 (83%) | 0.66 |
| Preoperative steroids | |||
| Yes (n = 68) | 26 (33%) | 42 (28%) | |
| No (n = 163) | 53 (67%) | 110 (72%) | 0.49 |
| Preoperative immunosuppressants | |||
| Yes (n = 65) | 31 (39%) | 34 (22%) | |
| No (n = 166) | 48 (61%) | 118 (78%) | 0.01 |
| Previous resection | |||
| Yes (n = 71) | 29 (37%) | 42 (28%) | |
| No (n = 160) | 50 (63%) | 110 (72%) | 0.20 |
| Blood transfusion | |||
| Yes (n = 24) | 10 (13%) | 14 (9%) | |
| No (n = 207) | 69 (87%) | 138 (91%) | 0.56 |
| Surgical procedure | |||
| Open approach (n = 156) | 52 (66%) | 104 (68%) | |
| Laparoscopic approach (n = 75) | 27 (34%) | 48 (32%) | 0.80 |
| Type of anastomosis | |||
| Side-to-side (n = 170) | 64 (81%) | 106 (70%) | |
| End-to-end (n = 61) | 15 (19%) | 46 (30%) | 0.09 |
The incidence of postoperative complications
Postoperative complications observed within 30 days after surgery are listed in Table 4. Several patients developed multiple complications. The rates of overall complications, intra-abdominal sepsis, and anastomotic leak were 24% (55 patients), 12% (27 patients) and 8% (19 patients), respectively.
Table 4.
Postoperative complications
| Anastomotic leak | 19 (8%) |
| Intra-abdominal abscess | 10 (4%) |
| Entero-cutaneous fistula | 7 (3%) |
| Wound dehiscence | 8 (3%) |
| Haemorrhage | 7 (3%) |
| Pneumonia | 5 (2%) |
| Pancreatitis | 2 (1%) |
| Urinary tract infection | 3 (1%) |
| Others | 7 (3%) |
| Overall | 55 (24%) |
Risk factors for postoperative complications
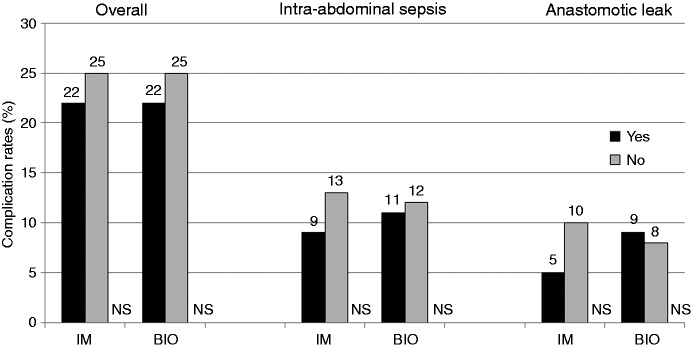
In univariate analysis (Table 5), the association of each clinical variable with the incidence of postoperative complications is investigated. The incidence of overall complications and intra-abdominal sepsis was lower in patients who received immunosuppressive or biologic agents as compared with those who did not; however, the difference did not reach statistical significance (Table 5 and Figure 2). Thus, neither immunosuppressive nor biologic therapy prior to surgery was significantly associated with the incidence of overall complications, intra-abdominal sepsis or anastomotic leak. Blood transfusion significantly increased the risk of overall complications (46% vs. 21%, P = 0.02). Further, patients with perforating disease were at a significantly higher risk of intra-abdominal sepsis as compared with those with non-perforating disease (19% vs. 6%, P = 0.004). An end-to-end anastomosis was significantly associated with a higher risk of intra-abdominal sepsis as compared with a side-to-side anastomosis (20% vs. 9%, P = 0.04). No parameters were associated with the incidence of anastomotic leak.
Table 5.
The association between clinical parameters and the incidence of postoperative complications: Univariate analysis
| Overall complications n (%) | Intra-abdominal sepsis n (%) | Anastomotic leak n (%) | |
|---|---|---|---|
| Age at surgery | P = 0.87 | P = 0.87 | P = 0.86 |
| < 17 years (n = 27) | 7 (26%) | 4 (15%) | 3 (11%) |
| 17–40 years (n = 161) | 39 (24%) | 18 (11%) | 13 (8%) |
| > 40 years (n = 43) | 9 (21%) | 5 (12%) | 3 (7%) |
| Gender | P = 0.70 | P = 0.78 | P = 0.75 |
| Male (n = 144) | 36 (25%) | 18 (13%) | 13 (9%) |
| Female (n = 87) | 19 (22%) | 9 (10%) | 6 (7%) |
| Behaviour of CD | P = 0.22 | P = 0.004 | P = 0.86 |
| Perforating disease (n = 99) | 28 (28%) | 19 (19%) | 9 (9%) |
| Non-perforating disease (n = 132) | 27 (20%) | 8 (6%) | 10 (8%) |
| Smoking | P = 0.48 | P = 0.22 | P = 0.34 |
| Yes (n = 37) | 11 (30%) | 7 (19%) | 5 (14%) |
| No (n = 194) | 44 (23%) | 20 (10%) | 14 (7%) |
| Preoperative steroids | P = 0.66 | P = 0.49 | P = 0.13 |
| Yes (n = 68) | 18 (26%) | 10 (15%) | 9 (13%) |
| No (n = 163) | 37 (23%) | 17 (10%) | 10 (6%) |
| Preoperative immunosuppressants | P = 0.74 | P = 0.62 | P = 0.32 |
| Yes (n = 65) | 14 (22%) | 6 (9%) | 3 (5%) |
| No (n = 166) | 41 (25%) | 21 (13%) | 16 (10%) |
| Preoperative biologics | P = 0.67 | P > 0.99 | P > 0.99 |
| Yes (n = 79) | 17 (22%) | 9 (11%) | 7 (9%) |
| No (n = 152) | 38 (25%) | 18 (12%) | 12 (8%) |
| Previous resection | P = 0.12 | P = 0.59 | P = 0.06 |
| Yes (n = 71) | 22 (31%) | 10 (14%) | 10 (14%) |
| No (n = 160) | 33 (21%) | 17 (11%) | 9 (6%) |
| Blood transfusion | P = 0.02 | P = 0.25 | P = 0.68 |
| Yes (n = 24) | 11 (46%) | 5 (21%) | 3 (13%) |
| No (n = 207) | 44 (21%) | 22 (11%) | 16 (8%) |
| Surgical procedure | P = 0.08 | P = 0.06 | P = 0.17 |
| Open approach (n = 156) | 43 (28%) | 23 (15%) | 16 (10%) |
| Laparoscopic approach (n = 75) | 12 (16%) | 4 (5%) | 3 (4%) |
| Type of anastomosis | P = 0.30 | P = 0.04 | P = 0.18 |
| Side-to-side (n = 170) | 37 (22%) | 15 (9%) | 11 (6%) |
| End-to-end (n = 61) | 18 (30%) | 12 (20%) | 8 (13%) |
Figure 2.
Neither immunosuppressive nor biologic therapy prior to surgery was significantly associated with the incidence of overall complications, intra-abdominal sepsis or anastomotic leak.
IM: immunosuppressants; BIO: biologics; NS: not significant.
In multivariate analysis (Table 6), only blood transfusion was a significant independent risk factor for overall complications (odds ratio [OR] 3.02, 95% confidence interval [CI] 1.21–7.52; P = 0.02). Perforating disease was the only significant risk factor for intra-abdominal sepsis (OR 2.67, 95% CI 1.04–6.86; P = 0.04). Further, previous resection was the only significant risk factor for anastomotic leak (OR 2.87, 95% CI 1.01–8.18; P = 0.048).
Table 6.
Risk for postoperative complications: multivariate analysis
| Overall complications OR (95% CI) | Intra-abdominal sepsis OR (95% CI) | Anastomotic leak OR (95% CI) | |
|---|---|---|---|
| Age at surgery: < 17 years | aP = 0.69, bP = 0.55 | cP = 0.72, dP = 0.96 | eP = 0.24, fP = 0.29 |
| vs. 17–40 years | 1.23 (0.44–3.45)a | 1.28 (0.34–4.75)c | 2.56 (0.54–12.11)e |
| vs. > 40 years | 1.46 (0.42–5.04)b | 1.04 (0.21–5.08)d | 2.79 (0.42–18.04)f |
| Gender: male | P = 0.79 | P = 0.91 | P = 0.66 |
| vs. female | 1.10 (0.55–2.17) | 0.95 (0.37–2.42) | 1.27 (0.43–3.79) |
| Behaviour of CD: perforating disease | P = 0.63 | P = 0.04 | P = 0.47 |
| vs. non-perforating disease | 1.18 (0.60–2.30) | 2.67 (1.04–6.86) | 0.67 (0.23–1.99) |
| Smoking: yes | P = 0.38 | P = 0.19 | P = 0.11 |
| vs. no | 1.45 (0.63–3.35) | 2.02 (0.71–5.78) | 2.76 (0.80–9.47) |
| Preoperative steroids: yes | P = 0.75 | P = 0.52 | P = 0.10 |
| vs. no | 1.12 (0.56–2.23) | 1.34 (0.55–3.29) | 2.33 (0.84–6.43) |
| Preoperative immunosuppressants: yes | P = 0.41 | P = 0.47 | P = 0.13 |
| vs. no | 0.73 (0.34–1.54) | 0.68 (0.24–1.93) | 0.35 (0.09–1.37) |
| Preoperative biologics: yes | P = 0.50 | P = 0.84 | P = 0.70 |
| vs. no | 0.79 (0.39–1.59) | 1.10 (0.43–2.81) | 1.23 (0.42–3.59) |
| Previous resection: yes | P = 0.16 | P = 0.68 | P = 0.048 |
| vs. no | 1.64 (0.82–3.30) | 1.21 (0.48–3.03) | 2.87 (1.01–8.18) |
| Blood transfusion: yes | P = 0.02 | P = 0.36 | P = 0.52 |
| vs. no | 3.02 (1.21–7.52) | 1.72 (0.54–5.48) | 1.61 (0.38–6.75) |
| Surgical procedure: open approach | P = 0.31 | P = 0.18 | P = 0.28 |
| vs. laparoscopic approach | 1.50 (0.68–3.32) | 2.25 (0.68–7.40) | 2.18 (0.54–8.82) |
| Type of anastomosis: side-to-side | P = 0.53 | P = 0.12 | P = 0.13 |
| vs. end-to-end | 0.80 (0.39–1.64) | 0.48 (0.19–1.20) | 0.43 (0.14–1.28) |
OR: odds ratio; CI: confidence interval.
Discussion
The major focus of this study was to investigate the impact of preoperative immunosuppressive or biologic therapy on complications after ileocolonic resection for CD. There may be two opposite possibilities for the impact of these drugs. Diminished inflammatory activity of CD with preoperative immunomodulator therapy may contribute to the reduction of postoperative complications. In contrast, deleterious immunomodulatory effects of these agents may increase the risk of postoperative complications. In this study, 28% and 34% of the patients received immunosuppressive and biologic agents within eight weeks before surgery, respectively. Of the patients 13% received both immunosuppressive and biologic agents. In contrast, 51% received neither immunosuppressants nor biologics. We found that the incidence of overall complications and intra-abdominal sepsis was slightly lower in patients with exposure to immunosuppressive or biologic therapy as compared with those without exposure; however, the difference did not reach statistical significance. In multivariate analysis, neither immunosuppressive nor biologic therapy prior to surgery was significantly associated with the incidence of overall complications, intra-abdominal sepsis or anastomotic leak.
Several studies9–19 investigated whether immunosuppressive or biologic therapy increases the risk of postoperative complications, particularly septic complications in patients with CD. Conflicting results exist regarding the impact of preoperative biologic therapy. A number of meta-analyses20–24 were conducted on this issue. In a meta-analysis,20 preoperative biologic therapy was associated with a modestly increased risk of infectious complications (OR 1.50, 95% CI 1.08–2.08), mostly remote from the surgical site (OR 2.07 95% CI 1.30–3.30) and with a trend towards a higher rate of non-infectious complications (OR 2.00, 95% CI 0.89–4.46). A more recent study22 found that there was significant association between infliximab therapy prior to surgery and total (OR 1.45, 95% CI 1.04–2.02), infectious (OR 1.47, 95% CI 1.08–1.99) and non-infectious (OR 2.29, 95% CI 1.14–4.61) postoperative complications, respectively. In contrast, two meta-analyses23,24 reported that there was no significant associations between preoperative biologic therapy and the incidence of postoperative complications. Clinicians may be confused because of these conflicting results. Most of the studies included in these meta-analyses are retrospective or case-control studies and thus do not allow the control of important confounding factors such as preoperative conditions (the presence of abscess or fistula) or concomitant steroid therapy. A multicentre prospective study (the PUCCINI trial)25 aiming to evaluate the impact of previous immunosuppressive and biologic therapy in patients submitted to abdominal resections in both CD and ulcerative colitis is currently being performed, and its results will clarify in a near future the real impact of these medications in postoperative scenario, with a robust scientific level.
The timing of biological infusion prior to surgery varies through studies, and the impact of time between the last biologic infusion and operation has not been fully elucidated. In one study,18 operations performed within 14 days from last biologic dose had similar rates of infections and other complications when compared with those performed within 15–30 days or 31–180 days. Further, patients with detectable preoperative infliximab levels had similar rates of wound infection compared with those with undetectable levels. These results indicate that a shorter time interval from last biological dose is not associated with increased postoperative complications. A recent study26 investigated whether preoperative serum levels of biologic agents correlate with postoperative morbidity. In patients with CD, there was a higher but statistically insignificant rate of adverse outcomes in the detectable vs. undetectable groups. Using a cut off level of 3 µg/mL, postoperative morbidity (OR 2.5) and infectious complications (OR 3.0) were significantly higher in the ≥3 µg/mL group. There were significantly higher rates of postoperative morbidity and hospital readmissions in the ≥8 µg/mL compared with <3 µg/mL group. These results indicate that increasing preoperative serum levels of biologic agents are associated with adverse postoperative outcomes.
We found that blood transfusion significantly increased the risk of postoperative complications (OR 3.02). To our knowledge, few studies have identified blood transfusion as a risk factor for complications after surgery for CD.27 Blood transfusion has been reported to be associated with declines in lymphocyte numbers and inhibition of lymphocyte function.28 In patients undergoing surgical procedures, the receipt of blood may increase the risk of postoperative infectious complications. Interestingly, decreased recurrence of active IBD has been reported in transfused patients with CD.29 Conversely, deleterious immunomodulatory effects of transfusion may explain the association between transfusion and increased susceptibility to cancer recurrence and bacterial and viral infection.30 Blood transfusion may cause profound and prolonged alterations in immune function which result in clinical phenomena which can be either beneficial or detrimental to the recipient. Further well-designed research is warranted to evaluate the effects of blood transfusion on morbidity after bowel resection for CD.
In this study, perforating disease (perforation, abscess or fistula) was a significant risk factor for intra-abdominal sepsis (OR 2.67). The deleterious effect of perforating disease has been confirmed in the previous studies.6,7 In univariate analysis, side-to-side anastomosis was significantly associated with a lower incidence of intra-abdominal abscess as compared with end-to-end anastomosis (9% vs. 20%). Stapled side-to-side anastomosis (functional end-to-end anastomosis) has become a popular procedure in colorectal surgery. Its potential benefits include a wide anastomotic lumen, minimal contamination and a quick method. In our study, this anastomotic technique was used in 66% of the patients. In a meta-analysis comparing outcomes between end-to-end anastomosis and other anastomotic configurations after bowel resection for CD,31 end-to-end anastomosis was associated with increased anastomotic leak rates. Side-to-side anastomosis reduced the risk of anastomotic leaks and overall postoperative complications. We also found that a history of previous resection significantly increased the risk of anastomotic leak (OR 2.87). To our knowledge, this finding has not been reported in the previous studies, so further studies are necessary to confirm it.
Our study has certain limitations that need to be considered in the interpretation of its results. The methodological features of a retrospective multicentre and observational analysis, without a fixed perioperative management protocol, may have led to some bias, even if the database in each institution was prospectively maintained. Secondly, the sample size calculation was not done based on a primary statistical endpoint. Further, the nutritional status of patients was not included as a potential risk factor for postoperative complications. However, in our institutions, two-stage operation was performed in most patients with poor nutritional status. Those patients were not included in the present study.
In conclusion, blood transfusion, perforating disease and previous resection were significant risk factors for overall complications, intra-abdominal sepsis and anastomotic leak after ileocolonic resection for CD, respectively. These significant factors may be surrogate markers for aggressive disease, extensive resection, and dense adhesions. Preoperative immunosuppressive or biologic therapy did not increase the risk of postoperative complications. Our results may suggest that surgery does not need to be delayed, and appropriate immunosuppressive or biologic therapy can be continued preoperatively in patients with CD. In clinical practice, the decision to choose between one-stage or two-stage operation is based not only on the use of immunosuppressive or biologic agents, but also multiple confounding factors such as malnutrition, use of steroids, and preoperative sepsis should be taken into consideration. A large-scale prospective study is necessary to rigorously evaluate the effects of preoperative immunosuppressive or biologic therapy on the incidence of postoperative complications. Furthermore, data able to control confounding factors and various pharmacokinetic conditions are also needed.
Footnotes
Author note: TY, AS, YS, FVT and PGK designed the study. All authors did data collection and gave scientific contribution to the study design and discussion. TY, AS and PGK drafted the article. All authors read and approved the final version of the manuscript.
Conflict of interest
TY, YS, RNS, IFB, KT, AY, TS, LMSK, MS: None. AS: Consultancy: Abbvie. RSH: Financial support for research: FAPESP. FVT: Lecture fees: Abbvie, Ferring, Janssen, Consultancy: Abbvie, Ferring, Janssen. ICA: Lecture fees: Abbvie, Janssen, Consultancy: Abbvie, Janssen. SD: Lecture fees: Schering-Plough, Abbott Laboratories, Abbvie, Merck & Co, UCB Pharma, Ferring, Cellerix, Celtrion, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, Alphawasserman, Genentech, Grunenthal, Pfizer, Astra Zeneca, Novo Nordisk, Cosmo Pharmaceuticals, Pharmacosmos, Tigenix, Vifor, Johnson and Johnson, Consultancy: Schering-Plough, Abbott Laboratories, Abbvie, Merck & Co, UCB Pharma, Ferring, Cellerix, Celtrion, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, Alphawasserman, Genentech, Grunenthal, Pfizer, Astra Zeneca, Novo Nordisk, Cosmo Pharmaceuticals, Pharmacosmos, Tigenix, Vifor, Johnson and Johnson. PGK: Lecture fees: Abbvie, Astrazeneca, Janssen, Takeda, Ferring, Consultancy: Abbvie, Astrazeneca, Janssen, Takeda, Ferring.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Hanauer SB, Feagan BG, Lichtenstein GR, et al. ACCENT I study group. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet 2002; 359: 1541–1549. [DOI] [PubMed] [Google Scholar]
- 2.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: The CHARM trial. Gastroenterology 2007; 132: 52–65. [DOI] [PubMed] [Google Scholar]
- 3.Cosnes J, Nion-Larmurier I, Beaugerie L, et al. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut 2005; 54: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto T, Watanabe T. Surgery for luminal Crohn’s disease. World J Gastroenterol 2014; 20: 78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farmer RG, Hawk WA, Turnbull RB Jr. Indications for surgery in Crohn’s disease: Analysis of 500 cases. Gastroenterology 1976; 71: 245–250. [PubMed] [Google Scholar]
- 6.Yamamoto T, Allan RN, Keighley MR. Risk factors for intra-abdominal sepsis after surgery in Crohn’s disease. Dis Colon Rectum 2000; 43: 1141–1145. [DOI] [PubMed] [Google Scholar]
- 7.Alves A, Panis Y, Bouhnik Y, et al. Risk factors for intra-abdominal septic complications after a first ileocecal resection for Crohn’s disease: A multivariate analysis in 161 consecutive patients. Dis Colon Rectum 2007; 50: 331–336. [DOI] [PubMed] [Google Scholar]
- 8.Greenstein AJ, Lachman P, Sachar DB, et al. Perforating and non-perforating indications for repeated operations in Crohn’s disease: Evidence for two clinical forms. Gut 1988; 29: 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tay GS, Binion DG, Eastwood D, et al. Multivariate analysis suggests improved perioperative outcome in Crohn’s disease patients receiving immunomodulator therapy after segmental resection and/or strictureplasty. Surgery 2003; 134: 565–572. [DOI] [PubMed] [Google Scholar]
- 10.Appau KA, Fazio VW, Shen B, et al. Use of infliximab within 3 months of ileocolonic resection is associated with adverse postoperative outcomes in Crohn’s patients. J Gastrointest Surg 2008; 12: 1738–1744. [DOI] [PubMed] [Google Scholar]
- 11.Syed A, Cross RK, Flasar MH. Anti-tumor necrosis factor therapy is associated with infections after abdominal surgery in Crohn’s disease patients. Am J Gastroenterol 2013; 108: 583–593. [DOI] [PubMed] [Google Scholar]
- 12.Colombel JF, Loftus EV Jr, Tremaine WJ, et al. Early postoperative complications are not increased in patients with Crohn’s disease treated perioperatively with infliximab or immunosuppressive therapy. Am J Gastroenterol 2004; 99: 878–883. [DOI] [PubMed] [Google Scholar]
- 13.Marchal L, D’Haens G, Van Assche G, et al. The risk of post-operative complications associated with infliximab therapy for Crohn’s disease: A controlled cohort study. Aliment Pharmacol Ther 2004; 19: 749–754. [DOI] [PubMed] [Google Scholar]
- 14.El-Hussuna A, Andersen J, Bisgaard T, et al. Biologic treatment or immunomodulation is not associated with postoperative anastomotic complications in abdominal surgery for Crohn’s disease. Scand J Gastroenterol 2012; 47: 662–668. [DOI] [PubMed] [Google Scholar]
- 15.Bafford AC, Powers S, Ha C, et al. Immunosuppressive therapy does not increase operative morbidity in patients with Crohn’s disease. J Clin Gastroenterol 2013; 47: 491–495. [DOI] [PubMed] [Google Scholar]
- 16.Nørgård BM, Nielsen J, Qvist N, et al. Pre-operative use of anti-TNF-α agents and the risk of post-operative complications in patients with Crohn’s disease: A nationwide cohort study. Aliment Pharmacol Ther 2013; 37: 214–224. [DOI] [PubMed] [Google Scholar]
- 17.Krane MK, Allaix ME, Zoccali M, et al. Preoperative infliximab therapy does not increase morbidity and mortality after laparoscopic resection for inflammatory bowel disease. Dis Colon Rectum 2013; 56: 449–457. [DOI] [PubMed] [Google Scholar]
- 18.Waterman M, Xu W, Dinani A, et al. Preoperative biological therapy and short-term outcomes of abdominal surgery in patients with inflammatory bowel disease. Gut 2013; 62: 387–394. [DOI] [PubMed] [Google Scholar]
- 19.Myrelid P, Marti-Gallostra M, Ashraf S, et al. Complications in surgery for Crohn’s disease after preoperative antitumour necrosis factor therapy. Br J Surg 2014; 101: 539–545. [DOI] [PubMed] [Google Scholar]
- 20.Kopylov U, Ben-Horin S, Zmora O, et al. Anti-tumor necrosis factor and postoperative complications in Crohn’s disease: Systematic review and meta-analysis. Inflamm Bowel Dis 2012; 18: 2404–2413. [DOI] [PubMed] [Google Scholar]
- 21.Billioud V, Ford AC, Tedesco ED, et al. Preoperative use of anti-TNF therapy and postoperative complications in inflammatory bowel diseases: a meta-analysis. J Crohns Colitis 2013; 7: 853–867. [DOI] [PubMed] [Google Scholar]
- 22.Yang ZP, Hong L, Wu Q, et al. Preoperative infliximab use and postoperative complications in Crohn’s disease: A systematic review and meta-analysis. Int J Surg 2014; 12: 224–230. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld G, Qian H, Bressler B. The risks of post-operative complications following pre-operative infliximab therapy for Crohn’s disease in patients undergoing abdominal surgery: A systematic review and meta-analysis. J Crohns Colitis 2013; 7: 868–877. [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Tang Y, Nong L, et al. Risk factors for postoperative intra-abdominal septic complications after surgery in Crohn’s disease: A meta-analysis of observational studies. J Crohns Colitis 2015; 9: 293–301. [DOI] [PubMed] [Google Scholar]
- 25.http://ccfacra.org/?page_id=85 Last accessed 30 July 2015.
- 26.Lau C, Dubinsky M, Melmed G, et al. The impact of preoperative serum anti-TNFα therapy levels on early postoperative outcomes in inflammatory bowel disease surgery. Ann Surg 2015; 261: 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tartter PI, Driefuss RM, Malon AM, et al. Relationship of postoperative septic complications and blood transfusions in patients with Crohn’s disease. Am J Surg 1988; 155: 43–48. [DOI] [PubMed] [Google Scholar]
- 28.Tartter PI. Immunologic effects of blood transfusion. Immunol Invest 1995; 24: 277–288. [DOI] [PubMed] [Google Scholar]
- 29.Gooszen HG, Silvis R. Protective effect of blood transfusions on postoperative recurrence of Crohn’s disease in parous women. Neth J Med 1994; 45: 65–71. [PubMed] [Google Scholar]
- 30.Triulzi DJ, Blumberg N, Heal JM. Association of transfusion with postoperative bacterial infection. Crit Rev Clin Lab Sci 1990; 28: 95–107. [DOI] [PubMed] [Google Scholar]
- 31.Simillis C, Purkayastha S, Yamamoto T, et al. A meta-analysis comparing conventional end-to-end anastomosis vs. other anastomotic configurations after resection in Crohn’s disease. Dis Colon Rectum 2007; 50: 1674–1687. [DOI] [PubMed] [Google Scholar]