Abstract
Background and objective
Endoscopic mucosal dissection (ESD) is a treatment option for oesophagus tumours localized to the mucosa enabling en bloc removal of large lesions. The resulting larger mucosal defects have resulted in an increase in the occurrence of post-treatment strictures. Transplantation of autologous cell sheets, cultured from oral mucosa, has been shown to prevent post-ESD strictures. The aim of the study was to assess the efficacy and safety of cell sheet transplantation after oesophageal ESD in a Western patient population where reflux-associated pre-malignant and malignant conditions predominate.
Methods
Patients with Barrett’s oesophagus associated high-grade dysplasia or early adenocarcinoma where ESD entailed a resection >3 cm in length and ≥75% of the circumference were eligible for treatment under hospital exemption. Cell sheets were cultured from buccal mucosa according to Good Manufacturing Practice and were endoscopically applied to the post-ESD defect directly after resection. Patients were followed with weekly endoscopy examinations, including confocal laser microscopy, for a total of four weeks.
Results
Five patients were treated. ESD was extensive with resections being circumferential in three patients and 9–10 cm in length in two. The number of transplanted cell sheets ranged from two to six. Three patients developed strictures requiring two to five dilatation sessions.
Conclusions
Cell sheet transplantation shows to be safe and feasible in a Western population. Results suggest that transplantation has a protective effect on the mucosal defect after ESD, decreasing both the risk for and extent of stricture formation.
Keywords: Barrett’s oesophagus, early oesophageal cancer, endoscopic submucosal dissection, cell sheets, oesophagus stricture prevention
Introduction
The incidence of oesophageal cancer, specifically adenocarcinoma, is increasing in the Western world and has been linked to overweight and gastro-oesophageal reflux disease (GERD).1 Despite development in surgical technique, post-operative care and neo-adjuvant therapy, oesophageal cancer has an overall five-year survival rate of about 20%.2–4 However, when diagnosed at a precancerous or early tumour stage survival rates approach 95–100%.5 Improved staging, better understanding of metastatic patterns and advances in endoscopic techniques and technology have opened the prospect of endoscopic treatment of pre-neoplastic lesions and early oesophageal cancer.6 Endoscopic resection is an option for tumours localized to the mucosa (T1a) where the risk of lymph node metastases is around 1–3%. In contrast, tumours infiltrating the submucosa (T1b) have a risk of lymph node metastases around 20%, precluding endoscopic resection.7 As opposed to endoscopic mucosal resection (EMR), where piecemeal resections are required to remove larger lesions, endoscopic mucosal dissection (ESD) enables removal of lesions en bloc, irrespective of size. More extensive resections have resulted in an increase in the occurrence of post-endoscopic resection strictures, representing a major source of post-treatment morbidity.8–14 Transplantation of autologous cell sheets cultured from oral mucosa epithelial cells has been shown to prevent strictures after endoscopic resection.15 In this paper we report data on efficacy and safety of cell sheet transplantation after ESD in a Western patient population with reflux-associated pre-malignant and malignant conditions.
Materials and methods
Patients
In Stockholm county, Sweden, all patients with malignant or pre-malignant oesophageal lesions are discussed at an inter-hospital multidisciplinary team (MDT) conference for assessment and treatment planning. Patients with Barrett’s oesophagus associated high-grade dysplasia (HGD) or early oesophageal cancer where ESD entailed a resection >3 cm in length and ≥75% of the circumference of the oesophagus were eligible for inclusion in the study. Inclusion and exclusion criteria are shown in Table 1. Patients were treated under hospital exemption. The efficacy and safety of the treatment are reported. Written informed consent was obtained from all participating patients and the treatment was approved by the local ethics committee. The treatment was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Manufacturing of the cell sheets was done according to Good Manufacturing Practice (GMP) and was certified by the Swedish Medical Product Agency.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| Age > 18 years |
| Barrett’s oesophagus associated high-grade dysplasia or early adenocarcinoma |
| Planned ESD expecting a mucosal defect >3 cm in length and ≥75% of the oesophageal circumference |
| Exclusion criteria |
| Previous oesophageal surgery |
| Women who are pregnant, lactating or who are of childbearing age without a negative urine pregnancy test |
| Portal hypertension |
| Hepatitis B or C, or HIV |
| Patients with impaired renal function (e.g. acute renal failure) or patients on dialysis |
| INR > 1.6; thrombocytes < 50 000 |
| Patients with any physical or mental status precluding signing of informed consent |
ESD: endoscopic mucosal dissection; INR: international normalized ratio
Cell harvesting
At inclusion a clinical history was taken, physical examination was performed, blood for analysis was collected and a quality of life (QOL) assessment (EORTC QLQ – OES18) was completed. The oral cavity was sterilized by rinsing with a 0.5% iodine solution followed by swabbing the area of the incision with a 1% solution. Using an ellipse-shaped template covering a surface of 1.75 cm2 the incision line was marked on the buccal mucosa. A full-thickness mucosal specimen was harvested under local anaesthesia (Xylocain® 10 mg/ml + adrenalin 5 µg/ml), and the wound closed with interrupted 4.0 Vicryl® sutures.
Preparation of oral mucosal epithelial cells
The biopsy was placed in transport medium (Dulbecco’s modified Eagle’s medium high glucose (DMEM; Sigma-Aldrich, St Louis, USA) supplemented with 4.4 mM L-glutamine (Sigma-Aldrich, St Louis, USA), 0.14 µg/ml Fungizone (Bristol-Myers Squibb, Solna, Sweden), 1 µg/ml clindamycin (Stragen, Hilleröd, Denmark) and 40 µg/ml Gensumycin (Sanofi, Stockholm, Sweden) for transfer to the GMP-facility. The biopsy was disinfected twice using a 1% povidone iodine solution (Pharmaxim, Markaryd, Sweden) and rinsed with transport medium. The biopsy was cut into approximately 3 mm3 pieces and rinsed three times with transport medium before incubation in 1000 PU/ml dispase (Invitrogen, Carlsbad, CA, USA) for 15–24 h at +4℃. The mucosal epithelium was separated from the submucosa using a surgical forceps. The epithelial layers were treated with 0.25% trypsin-EDTA solution (Invitrogen, Carlsbad, CA, USA) for 20 min at 37℃. During the incubation period, the cells were pipetted up and down several times to disperse cells into a single cell suspension. The trypsin was inactivated using keratinocyte culture medium (KSM) containing 75% (v/v) Dulbecco’s Modified Eagle’s Medium-high glucose (Sigma-Aldrich, St Louis, USA), 25% (v/v) Nutrient Mixture F-12 Ham (Sigma-Aldrich, St Louis, MO, USA), 0.475 g/L L-glutamin (Sigma-Aldrich, St Louis, MO, USA), 5 μg/mL Humulin Regular (insulin) (Eli Lilly, Solna, Sweden), 0.4 μg/mL Solu-Cortef (hydrocortisone) (Pfizer, Stockholm, Sweden), 1 nM Cholera toxin (List Biological Laboratories, Campbell, CA, USA) 2 nM T3 (3,3′,5-Triiodo-L-thyronine Sodium Salt) (MP Biomedicals, Santa Ana, CA, USA), 10 ng/mL EGF (recombinant Human Epidermal Growth Factor) (Invitrogen, Carlsbad, CA, USA), 40 μg/mL Gensumycin (Sanofi, Stockholm, Sweden), 0.14 μg/mL Fungizone (Bristol-Myers Squibb, Solna, Sweden), supplemented with 5% human autologous serum. The cell suspension was filtered through a cell strainer (40 µm, BD Falcon, Franklin Lakes, NJ, USA) to remove aggregates.
Manufacturing of oral mucosal epithelial cell sheets
The human oral epithelial cells in KCM supplemented with 5% autologous serum were seeded on temperature-sensitive culture inserts (CellSeed, Tokyo, Japan) at a density of 2–5 × 105 cells/insert. Cells were cultured for two weeks at 37℃ and 5% CO2. The culture medium was changed at days 5, 8, 10, 12, 13, 14, 15 and 16. Harvesting of the cell sheets was performed at day 16 (for two patients the culturing continued until day 20). Harvesting was performed in theatre by bringing the cell sheets to room temperature for 30 min using a surgical forceps. A support membrane of polyvinylidene difluoride was placed on the detached cell sheet before lifting it for transplantation.
Quality control of cell sheets
The product specifications for the cell sheets are summarized in Table 2. Cells were inspected visually under a phase-contrast microscope at each medium change. The discarded culture medium was analysed for endotoxin at days 8 and 13. At day 13 a microbiological control was performed. At day 15 the discarded medium was sent for mycoplasma testing using a qPCR-method according to Ph.Eur 2.6.7. One cell sheet was harvested in the clean room the day before harvest to evaluate detachment from the culture insert. This cell sheet was then treated with 0.25% trypsin-EDTA solution (Invitrogen, St. Louis, MO, USA) for 20 min at 37℃. Cells were pipetted up and down several times to disperse them into a single cell suspension. The cell suspension was filtered through a cell strainer (40 µm, BD Falcon, Franklin Lakes, NJ, USA) to remove aggregates and cells counted before flow cytometry analysis. Cells were fixed and permeabilized with a BD Cytofix/Cytoperm kit (BD Biosciences, Franklin Lakes, NJ, USA). Cells were stained with a FITC-conjugated anti-pan-cytokeratin antibody (Progen Biotechnik GmbH, Heidelberg, Germany) for 1 h at room temperature. A FITC-conjugated mouse-IgG 2a isotype (Santa Cruz, Heidelberg, Germany) was used as control. Before analysis, treated cells were washed with BD Perm/Wash buffer (BD Biosciences, Franklin Lakes, NJ, USA). Flow cytometry was performed with CyFlow ML (Partec, Görlitz, Germany). Before harvesting the cell sheets on day 16, quality control testing was performed on the discarded medium. Endotoxin and sterility were analysed according to Ph.Eur 2.6.14 and 2.6.1 respectively. These results were available post-release.
Table 2.
Product specifications for the cell sheets. Samples for microbiological testing are collected three days before release and transplantation. The result is a preliminary result after two days’ incubation. The total incubation period is seven days
| Analysis | Limit |
|---|---|
| Detachment test | Detached with no defect in the oral epithelial sheet |
| Cell number | ≥1 × 105 cells/sheet |
| Cell viability | ≥70% |
| Cell purity | ≥70% pan-CK positive |
| Endotoxin test (of culture medium) | <5 EU/ml |
| Microbiological testing (of culture medium) | No growth in 4 ml cell culture medium |
In vitro analysis
A series of in vitro analyses were performed on excess cell sheets in order to understand the composition and status of the transplanted sheets. Histology and transmission electron microscopy (TEM) revealed the histological architecture and cell organization. Immunohistochemistry was performed to find key proteins within the sheets including extracellular matrix proteins, markers for cell-to-cell contact, pluripotency and cell proliferation. We further evaluated the health of the sheets by functional analyses. In contrast to immunohistochemistry, these evaluations (live and dead staining and MTT assay) are dependent on metabolic activity and give a better indicator of the cells’ health. Gene expression analyses were performed by real time polymerase chain reaction (PCR) for genes that are related to pluripotency and epithelial phenotype.
Histology and immunohistochemistry
Excess cell sheets from three patients were used for histology and immunohistochemistry. Detached cell sheets were fixed in 4% formalin (HistoLab, Göteborg, Sweden), frozen in optimum cutting temperature (OCT) compound (HistoLab, Göteborg, Sweden) and cut into 8 µm sections using a cryostat (CM1860, Leica, Nussloch, Germany). Histology was performed by haematoxylin and eosin (H&E) staining. For immunohistochemistry, the samples were blocked in 5% goat serum (Life Technologies, Carlsbad, CA, USA) in phosphate-buffered saline (PBS) with 0.3% Triton X (Sigma-Aldrich, St Louis, MO, USA) incubated with the primary antibodies on a rocking platform in 4℃ overnight, using the following concentrations: connexin 43 (1:200; 3512; Cell Signaling Technology, Danvers, MA, USA), collagen I (1:100; 34710; Abcam, Cambridge, UK), collagen IV (1:100; 6586; Abcam, Cambridge, UK), elastin (1:100; 21610; Abcam, Cambridge, UK), laminin (1:200; 11575; Abcam, Cambridge, UK), Ki-67 (1:400; SP6; Abcam, Cambridge, UK), C-kit (1:400; 3074; Cell Signaling Technology, Danvers, MA, USA), Oct-4 (1:200; C30A3; Cell Signaling Technology, Danvers, MA, USA), Sox-2 (1:200; D69D; Cell Signaling Technology, Danvers, MA, USA), NANOG (1:200; #9656; Cell Signaling Technology, Danvers, MA, USA), SSEA4 (1:200; MC813; Cell Signaling Technology, Danvers, MA, USA). As controls, primary antibody was omitted. The following day, sections were washed and secondary antibodies were added and incubated for 1 h. The following secondary antibodies were used: goat anti-rabbit (1:500; a11037; Life Technologies, Carlsbad, CA, USA) for Oct-4, Sox-2, NANOG, C-kit, connexin 43, collagen I, collagen IV, Ki67, laminin, elastin; for SSEA4, goat anti-mouse (1:500; a11032; Life Technologies, Carlsbad, CA, USA). Cover slips were then mounted using Fluoroshield DAPI (Sigma-Aldrich, St Louis, MO, USA). Images were acquired by inverted fluorescent microscope (IX71, Olympus Medical Systems, Tokyo, Japan). Cells positive for Ki-67 were counted at eight different randomly chosen locations in six different sections, to quantify the number of proliferating cells.
Gene expression analyses
Total RNA was purified according to the manufacturer’s instructions from human foreskin fibroblasts (HFFs) (CRL-2429; ATCC, USA) and patients’ cell sheets using a commercially available RNeasy Mini Kit (Qiagen, Hilden, Germany). Complementary DNA (cDNA) samples were prepared from 100 ng of total RNA with Superscript III (Invitrogen, Carlsbad, CA, USA). Real time PCR was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA) with Taqman Universal Master Mix and Taqman primer/probe for OCT4 (HS03005111_g1), NANOG (HS04260366_g1), SOX2 (HS01053049_s1), KRT18 (HS01941416_g1). The housekeeping gene GAPDH (HS02758991_g1) was used as an endogenous control and water was used as negative control. The expression levels for each sample were normalized to GAPDH and relative quantification of expression was estimated using the ΔΔCT method and was presented as relative fold change.
Transmission electron microscopy
The cell sheet was fixed at room temperature for 30 min in 2% glutaraldehyde (Merck, Darmstadt, Germany) and 1% paraformaldehyde (HistoLab, Göteborg, Sweden) in 0.1 M phosphate buffer, pH 7.4. Samples were rinsed in 0.1 M phosphate buffer pH 7.4 and post-fixed in 2% osmium tetroxide, 0.1 M phosphate buffer pH 7.4 at 4℃ for 2 h, dehydrated in ethanol followed by acetone and embedded in LX-112 (Ladd). Semi-thin sections were cut and stained with toluidine blue O (Sigma-Aldrich, St Louis, MO, USA) and used for light microscopy analysis. Ultra-thin sections (approximately 40 to 50 nm) were cut with a Leica EM UC 6 and contrasted with uranyl acetate followed by lead citrate and were examined in a Tecnai 12 Spirit Bio TWIN transmission electron microscope (FEI company) at 100 kV. Digital images were taken with a Veleta camera (Olympus Soft Imaging Solutions, Tokyo, Japan).
Viability assessment and cell activity assay
To assess the viability of cell sheets pre-transplantation, two sheets were handled in the same manner as transplanted sheets. Sheets were taken to the operating room, left on a heat plate for 2 h and then detached by temperature reduction. After an additional 2 h at room temperature, they were taken back to the laboratory for viability evaluation. Cell sheets were cut in half and the viability of two halves was assessed by determining plasma membrane integrity and esterase activity using a commercially available kit (Live/Dead L-3224, Life Technologies, Carlsbad, CA, USA). Briefly, reagents were mixed, diluted in PBS (Gibco, Carlsbad, CA, USA) and incubated for 30 min in room temperature. Images were then acquired by inverted fluorescent microscope (IX71, Olympus, Japan). Two cell sheet halves were put in wells of 96-well plates and evaluated for cell activity using Cell Proliferation Kit I (MTT, Roche, Basel, Switzerland). 100 µl media and 10 µL MTT substrate was added to each well, including wells for negative control. After 4 h incubation 100 µl solubilization agent (10% sodium dodecyl sulphate) was added and incubated overnight. Samples were read on a spectrophotometer (SpectraMax 250, Molecular Devices, Sunny Vale, CA, USA) at 560 nm.
Endoscopic assessment and treatment
Treatment was performed under general anaesthesia using an EVIS EXERA III endoscopy system with compatible gastroscope (GIF HQ190) fitted with a distal cap (MH-594), irrigation pump (OFP-2) and CO2-insufflator (UCR) (Olympus Medical Systems, Tokyo, Japan). A complete oesophagogastroduodenoscopy (EGD) was performed, including narrow-band imaging (NBI) and chromoscopy (Lugol’s iodine 2% and/or indigocarmine 1%) before and after spraying with 1% acetic acid administered with a spray catheter (PW-205 V, Olympus Medical Systems, Tokyo, Japan). The lesion was marked using a DualKnife (KD-650U, Olympus Medical Systems, Tokyo, Japan) followed by submucosal expansion with a mixture of 0.4% hyaluronate sodium solution (Sigmavisc, Hyaltech Ltd, Livingston, UK), 1% indigocarmine (1 ml/100 ml hyaluronate sodium) and 0.1% adrenalin (0.5 ml/100 ml hyaluronate sodium) injection into the submucosal space using a 21-gauge injection needle (NM-400 L-0423, Olympus Medical Systems, Tokyo, Japan). An Erbe VIO 300D electrosurgical generator (ERBE Co., Tübingen, Germany) was used for dissection applying the EndoQ (tissue effect 2; cutting width 1; cutting interval 6) and Swift coag (tissue effect 3 at 30 W) modes. Circumferential cutting was performed using a DualKnife and/or ITknife 2 (KD-611 L, Olympus Medical Systems, Tokyo, Japan) and submucosal dissection using mainly the DualKnife. Haemostasis was achieved by coagulation with the dissecting knife and in the case of larger vessels a Coagrasper haemostatic forceps (FD-411 QR, Olympus Medical Systems, Tokyo, Japan) using the Soft coag (tissue effect 5 at 80 W) mode on the generator. Resected specimens were retrieved by a Nakao Spider-Net™ retrieval device (ConMed, Billerica, MA, USA).
For transplantation an overtube (Create Medic, Yokohama, Japan) was placed with the distal end positioned at the proximal border of the post-ESD defect. The support membrane with the attached cell sheet was grasped by an endoscopy forceps (Radial Jaw 4 Jumbo Forceps, Boston Scientific, Watertown, MA, USA) passed through the gastroscope and transported under vision to the transplantation site. Application was achieved by carefully pressing the membrane-borne cell sheet onto the submucosal bed. Slight pressure with the forceps on the support membrane was maintained for 5–10 min after which the membrane was left in situ. The procedure was repeated for each transplanted cell sheet.
Post-operative care
Before commencing ESD, patients received a bolus of esomeprazole 80 mg intravenous (IV) and thereafter a continuous infusion of 8 mg/h for 48 h. This was followed by esomeprazole 40 mg orally, twice daily for one month, after which proton pump inhibitor (PPI) treatment was continued as indicated for the underlying pathology. Post-intervention patients were kept fasting for two days, then started to drink water and followed a liquid diet until day 7. Patients were discharged when symptom-free and tolerating the fluid diet.
Post-intervention follow-up
Post-treatment EGD was performed weekly for four weeks. Healing of the mucosal defect was assessed and any stricture formation was documented. In patients that developed significant strictures, defined as inability to pass the treated area with a 9.2 mm gastroscope, dilatation was performed using three-stage single-use dilatation balloons (M00558410, M00558420, M00558430, M00558440, Boston Scientific, Watertown, MA, USA).
At gastroscopy probe-based confocal laser endomicroscopy (p-CLE) of the transplanted cell sheets and post-ESD defect was performed using a confocal mini-probe (GastroFlex UHD, Cellvizio; Mauna Kea Technologies, Paris, France) (magnification × 1000; field of view 240 μm; lateral resolution 1 μm; imaging depth 60 μm below tissue surface). Images were recorded with a frame rate of 12 per second. After IV administration of 2.5 ml 10% fluorescein, images were captured from transplanted surfaces and normal squamous epithelium proximal to the transplant area. Images were reviewed with a specially designed software package (Cellvizio Viewer), allowing image correction and stabilization. Special attention was paid to changes in the cell sheet morphology, specifically looking for signs of necrosis. Furthermore, the post-ESD surfaces were assessed for the presence of intrapapillary capillary loops (IPCLs) characterizing normal oesophageal epithelium. Quality of the images was rated as good, moderate or poor.
The QOL questionnaire (EORTC QLQ – OES18) was completed at the week 1 and week 4 EGD visits.
Statistical analysis
For analyses of the treatments and results only descriptive statistics were used.
The author and co-authors had access to data and had reviewed and approved the final manuscript.
Results
Between December 2012 and June 2014 five patients were treated. Demographic characteristics and clinical parameters are summarized in Table 3. The procedural aspects of the ESD procedures and cell sheet transplants are summarized in Table 4. ESD was circumferential in the majority of patients and 9–10 cm in length in two patients. Between two and six cell sheets were transplanted. Endoscopic images of the ESD and cell sheet transplantation of patient 3 are shown in Figure 1. No intra-procedural complications or early post-procedural complications occurred. Histology of the resected specimens showed carcinoma in three patients and HGD in two (Table 4).
Table 3.
Demographic characteristics and clinical parameters of patients
| Patient | Gender | Age (years) | Comorbidity | Previous endoscopic intervention | Pre-treatment histology | Paris classification | Prague class |
|---|---|---|---|---|---|---|---|
| 1 | Male | 70 | COPD, AF | No | Barrett HGD | 0-IIa | C4M5 |
| 2 | Male | 68 | Asthma, AF, IHD | ESD for HGD | Barrett HGD | 0-IIa + 0-IIc | C1M4 |
| 3 | Male | 55 | None | No | Barrett HGD | 0-IIa + 0-IIb | C6M10 |
| 4 | Male | 69 | IHD | No | Barrett HGD | 0-IIb | C5M7 |
| 5 | Male | 69 | HT, DM | ESD for T1a cancer | Barrett HGD | 0-IIa | C0M3 |
COPD: chronic obstructive pulmonary disease; AF: atrial fibrillation; IHD: ischaemic heart disease; HT: hypertension; DM: diabetes mellitus; ESD: endoscopic mucosal dissection; HGD: high-grade dysplasia
Table 4.
The procedural aspects of ESD and cell sheet transplant as well as final histology
| Patient | Mucosal defect after ESD (length/circumference) | Position of defect | Involvement of GE junction | Transplanted cell sheets (n) | Histology |
|---|---|---|---|---|---|
| 1 | 5 cm; 75% | Lower | Yes | 2 | Barrett’s mucosa HGD |
| 2 | 5 cm; 100% | Lower | Yes | 6 | Adenocarcinoma T1a |
| 3 | 10 cm 100% | Middle/lower | Yes | 5 | Adenocarcinoma T1b |
| 4 | 9 cm; 75% | Middle/lower | Yes | 5 | Adenocarcinoma T1a |
| 5 | 4 cm; 100% | Lower | Yes | 4 | Barrett’s mucosa HGD |
ESD: endoscopic mucosal dissection; GE, gastro-oesophageal; HGD, high-grade dysplasia
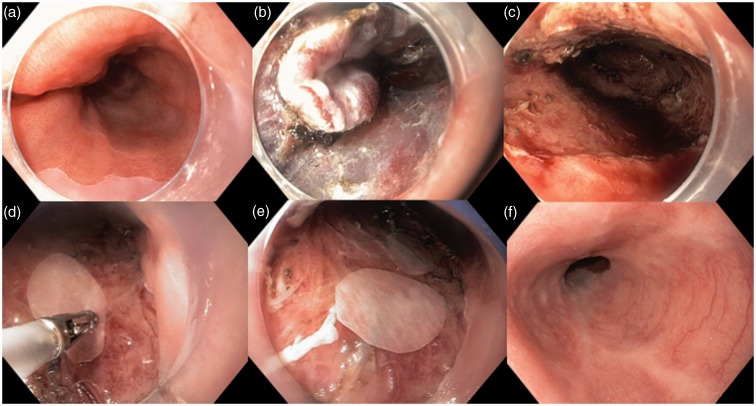
Figure 1.
Endoscopic images of patient 3 showing a 10 cm circumferential Barrett’s mucosa containing areas of high-grade dysplasia (a), the near-complete endoscopic mucosal dissection (ESD) (b), the mucosal defect after ESD (c), transplanted cell sheets with the support membranes clearly visible (d) and (e), the distal oesophagus at control gastroscopy four months after the procedure (f).
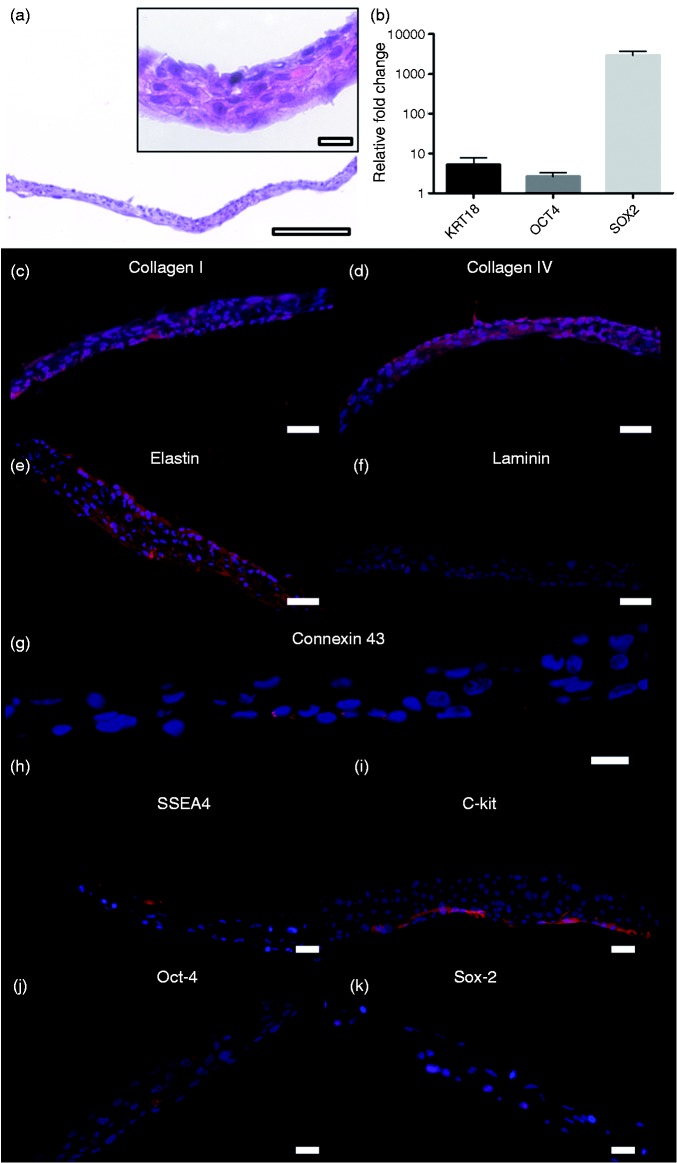
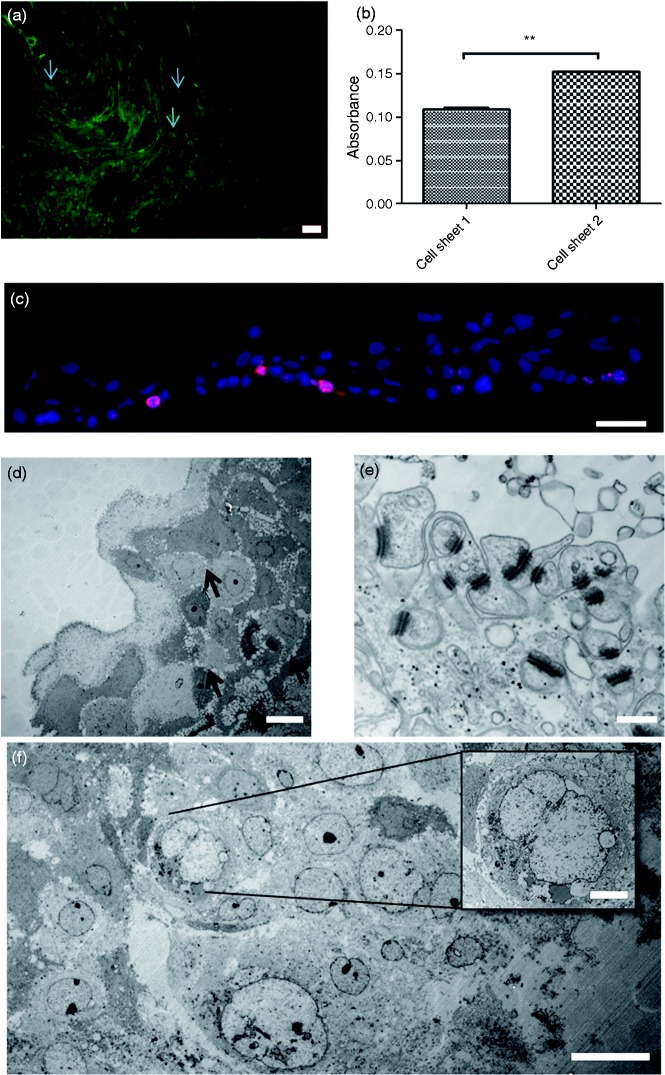
Between three and 12 cell sheets were produced per patient and all fulfilled the product specifications and were sterile. The average cell number was 1.67 ± 0.54 × 106 cells/cm2 biopsy. The harvested cell sheets consisted of cytokeratin-positive cells with average purity 94.55 ± 4.45%. We observed a non-disrupted cell sheet with 4–5 cell layers (Figure 2(a)) and found qPCR upregulation of genes for epithelial marker keratin 18 and pluripotency markers Oct-4, Sox-2 but no expression for NANOG (Figure 2(b)). A uniform deposition of collagen I, collagen IV and elastin was demonstrated (Figure 2(c) to (e)), while laminin was more localized to the basal parts of the sheets (Figure 2(f)), consistent with a normal epithelium. The gap junction protein connexin 43 was also localized to the basal part of the cell sheet (Figure 2(g)). Cells stained positive for pluripotency markers SSEA4, C-kit, Oct-4 and Sox-2 (Figure 2(h) to (k)). No expression of NANOG was found. After the non-enzymatic harvesting, cells were found to be highly viable, with only a few dead cells (Figure 3(a)). Cells were also metabolically active based on MTT assay (Figure 3(b)). Ki-67 staining indicated that 12 ± 7% of the cells were proliferating with proliferating cells mostly localized to a basal part of the sheet as in normal epithelium (Figure 3(c)). TEM revealed a multi-layered cell configuration with several desmosomes (Figure 3(d) and (e)). The majority of the cells looked healthy (Figure 3(f)).
Figure 2.
Histology and protein distribution in cell sheets. Hematoxylin and eosin (H&E) staining showing the oral mucosal epithelial cells detached as a continuous cell layer (a) (scale bar = 200 µm). In higher magnification the sheet is observed having 4–5 layers ((a) insert) (scale bar = 20 µm). Gene expression of cell sheets for keratin 18 (KRT18), Oct-4 and Sox-2 (b). Several extracellular matrix proteins were retained in the detached cell sheets: collagen I (c), collagen IV (d) and elastin (e). Laminin was found expressed on the basal side of the layer (f) (scale bar = 50 µm). Connexin 43 staining is suggesting gap-junctions in the lower parts of the sheet (scale bar = 20 µm) (g). Pluripotency markers were detected in the detached sheets, SSEA4 (h), C-kit (i), Oct-4 (j) and Sox-2 (k) (scale bar = 50 µm).
Figure 3.
Cell viability, metabolism, proliferation and transmission electron microscopy. The detached cell sheet showed a very low number of dead cells ((a) arrows) (scale bar = 200 µm) and the cells were found to be metabolically active (b). 12 ± 7% of cells were proliferating based on Ki-67-staining (c) (scale bar = 50 µm). Transmission electron microscopy reveals a multi-layered cell configuration of up to five layers, with several cell-to-cell contacts ((d) arrows) (scale bar = 20 µm). Higher magnification reveals abundance of desmosomes, indicating healthy epithelial cells (e) (scale bar = 500 nm). The majority of cells are healthy (f) (scale bar = 20 µm) but some are showing enlarged nuclei with loss of structure and cell-to-cell contact, indicating necrosis ((f) insert) (scale bar = 5 µm).
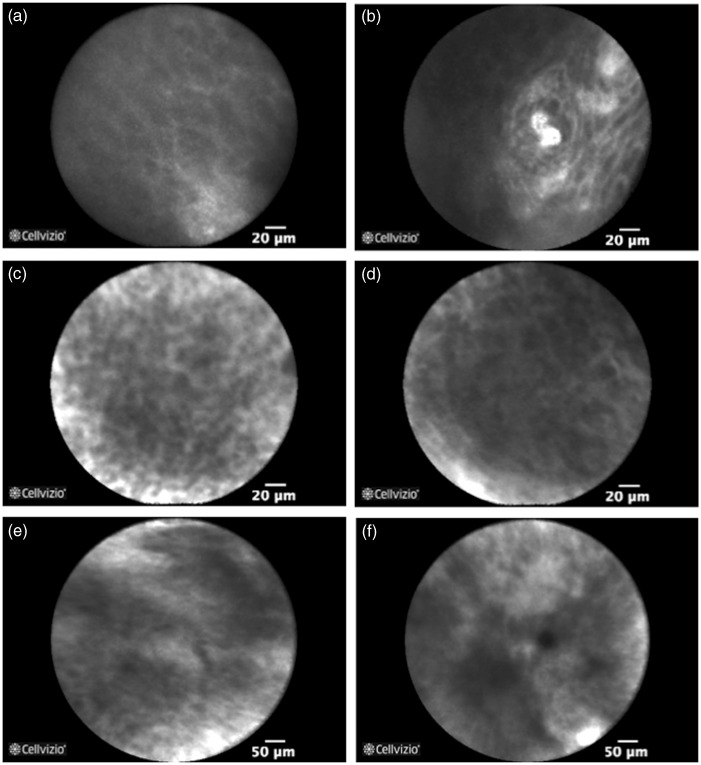
A total of 15 p-CLE examinations were performed (Table 5). Normal squamous epithelium with IPCL is shown in Figure 4(a) and (b). During the four-week follow-up no changes in the cell sheet morphology could be detected that would reflect overall necrosis. After one week squamous cells could be visualized in the transplanted areas (Figure 4(c) and (d)), whereas non-transplanted surfaces of the post-ESD defect were mostly covered by fibrin (Figure 4(e) and (f)). No signs of neo-vascularization in the form of IPCL, characterizing normal oesophageal epithelium, could be visualized in the transplanted surfaces at any of the time points.
Table 5.
Summary of probe-based confocal laser endomicroscopy examinations and findings after ESD
| Patient | Weeks after ESD | Examination quality | Number of images | IPCLs observed |
|---|---|---|---|---|
| 1 | 1 | Good | 2238 | No |
| 1 | 2 | Good | 1814 | No |
| 1 | 3 | Good | 796 | No |
| 1 | 4 | Good | 1005 | No |
| 2 | 1 | Good | 6990 | No |
| 2 | 2 | Moderate | 112 | No |
| 2 | 3 | Poor | 1044 | No |
| 3 | 1 | Poor | 525 | No |
| 3 | 3 | Moderate | 292 | No |
| 4 | 1 | Good | 499 | No |
| 4 | 2 | Good | 526 | No |
| 4 | 3 | Good | 847 | No |
| 4 | 4 | Moderate | 476 | No |
| 5 | 1 | Good | 560 | No |
| 5 | 2 | Moderate | 537 | No |
ESD: endoscopic mucosal dissection; IPCL: intrapapillary capillary loop
Figure 4.
Confocal laser endomicroscopy of normal squamous epithelium (a) with intrapapillary capillary loops (b). After one week squamous cells are visualized in the transplanted areas ((c) and (d)), whereas non-transplanted surfaces of the post-endoscopic mucosal dissection defect were mostly covered by fibrin and macrophages ((e) and (f)).
The results of endoscopic follow-up are shown in Table 6. The median time for endoscopically assessed complete re-epithelialization was three weeks. Three patients developed strictures requiring two, four and five dilatations respectively.
Table 6.
Endoscopic findings at follow-up and subsequent endoscopic intervention
| Patient | Time to re-epithelialization (weeks) | Stricture formation | Treatment |
|---|---|---|---|
| 1 | 2 | No | NA |
| 2 | 3 | Yes | Dilatation; 4 sessions |
| 3 | 3 | Yes | Dilatation; 5 sessions |
| 4 | 3 | No | NA |
| 5 | 2 | Yes | Dilatation; 2 sessions |
NA, not applicable
The results of the EORTC QLQ – OES18 questionnaire are shown in Figure 5. The difficulty in swallowing solid food and soft food reported at one week is probably a reflection of adherence to the dietary restrictions (a liquid diet until day 7) rather than true dysphagia. Some degree of difficulty in swallowing solid food and soft food was, however, reported at four weeks. Heartburn disappeared or improved in all patients during follow-up, probably due to the high-dose PPI administered for a duration of four weeks.
Figure 5.
Results of the EORTC QLQ – OES18 questionnaire. Values are shown as medians documented pre-interventionally and at week 1 and week 4 after endoscopic mucosal dissection with cell sheet transplantation.
Discussion
Stricture formation after ESD varies between 5% and 18%.12,13,16,17 It is related to the extent of the ESD, both in terms of length and of circumference.9,11 Ono et al. reported strictures in 90% of patients where ESD was performed for lesions of greater than three-quarters of the oesophageal circumference.16 A mucosal defect >30 mm in length has been reported as a risk factor for stricture development.8,16 The first-line treatment of post-ESD strictures is endoscopic balloon dilatation, often requiring multiple sessions. In a series of patients with circumferential oesophageal ESD Sato et al. reported a mean number of 13.8 and 33.5 dilatations in subjects with and without administration of post-dilatation steroids respectively.18 Isomoto et al. reported up to 48 dilatation sessions in patients undergoing twice weekly pre-emptive dilatations for eight weeks.10 Apart from the patient discomfort associated with multiple dilatations, there is a significant risk for perforation. Takahashi et al. reported perforation in 7/78 patients (9%) in patients undergoing dilatation for post-endoscopic resection strictures.19
A number of methods have been suggested for preventing stricture formation after endoscopic resection, including local and systemic post-treatment steroid administration, serial pre-emptive dilatations and prophylactic stent placement.8,20–22 Lately a number of tissue-engineered approaches to reducing the incidence of post-ESD strictures have been reported.23 Badylak et al. described placement of a biologic scaffold consisting of porcine extracellular matrix (ECM) to the wound bed after EMR.24 Although all five patients in their series developed strictures, they were treated easily. The technique for transplantable human oral mucosal epithelial cell sheet manufacturing and transplantation has previously been described.25,26 Ohki et al. reported nine cases of transplantation after ESD for squamous carcinoma.15 In four patients the post-ESD mucosal defect involved more than three-quarters of the oesophageal circumference and maximum specimen size was 11–70 mm. Complete re-epithelialization was observed at a median of 3.5 weeks. One patient developed a stricture after an almost complete circumferential resection extending to the gastro-oesophageal junction, requiring 21 dilatation sessions.
There are some important differences between patients in this report and the cohort reported by Okhi et al. All patients in our series had Barrett’s-associated lesions. Whereas the majority of lesions were situated in the upper or middle oesophagus in the Japanese study, lesions in the current study were located in the lower oesophagus in all patients and extended up to the middle oesophagus in two. Subsequent resection involved the gastro-oesophageal junction in all five patients and resections were more extensive with three having had circumferential mucosal defects and four with defects 5–10 cm in length.
The short time between intervention and healing of the defect reported by Okhi et al. was reproduced in our cohort with re-epithelialization complete at 2–3 weeks in all patients, compared with a mean of 3.5 weeks in the Japanese study. The enhanced healing may be due to a higher dosage of oral PPI used over a longer period after the initial IV administration (esomeprazole 80 mg daily for one month post-intervention versus rabeprazole 10 mg daily until epithelialization was completed). Three patients in our series developed post-ESD strictures. A possible explanation for the higher stricture rate in the presented cohort, compared with the Japanese study, could be the presence of GERD. Even though the acidity of the reflux content was reduced by administration of high-dose PPI, reflux of stomach contents, containing volatile molecules such as bile, continued unabated.27 All patients with strictures were successfully treated by dilatations. The number of dilatations needed to obtain a lasting result (two, four and five respectively) was considerably lower than would have been expected, given results reported in the literature, suggesting that strictures forming after cell sheet transplantation are less refractory to dilatation.8,10,18
In conclusion, in this second prospective report using autologous cell sheet transplantation after oesophageal ESD earlier results have been confirmed and the method shows to be safe and feasible in a Western reflux-prone population. Cell sheet production has been transferred successfully, establishing routines for manufacturing following GMP guidelines. Results suggest that cell sheet transplants decrease the risk for post-ESD stricture formation and that strictures that develop are less refractory to endoscopic balloon dilatation. These findings need to be tested in a controlled randomized trial.
Acknowledgements
The authors wish to thank Berit Sunde (RN) and Birgitta Holmgren (RN) for taking care of our patients, Heidi Pettersson, Maria Westberg and Charlotte Hållstrand, Vecura, for technical assistance in preparing the cell sheets and Mime Egami for support during the planning and execution phases of the study. PB and JML contributed equally to this work.
Declaration of conflicting interests
Teruo Okano is the founder and director of the board of CellSeed, Inc., which licenses technologies and patents from Tokyo Women’s Medical University. Teruo Okano and Masayuki Yamato are stakeholders in CellSeed, Inc.
Funding
This work was partially funded by the Knut and Alice Wallenberg Foundation (KAW 2009:0124), the Strategic Research Programme in Stem Cells and Regenerative Medicine at Karolinska Institutet (StratRegen) and Karolinska University Hospital.
References
- 1.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014; 371: 2499–2509. [DOI] [PubMed] [Google Scholar]
- 2.Dunst CM, Swanstrom LL. Minimally invasive esophagectomy. J Gastrointest Surg 2010; 14(Suppl. 1): S108–S114. [DOI] [PubMed] [Google Scholar]
- 3.Blom RL, van Heijl M, Bemelman WA, et al. Initial experiences of an enhanced recovery protocol in esophageal surgery. World J Surg 2013; 37: 2372–2378. [DOI] [PubMed] [Google Scholar]
- 4.Kumagai K, Rouvelas I, Tsai JA, et al. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg 2014; 101: 321–338. [DOI] [PubMed] [Google Scholar]
- 5.Shahbaz Sarwar CM, Luketich JD, Landreneau RJ, et al. Esophageal cancer: An update. Int J Surg 2010; 8: 417–422. [DOI] [PubMed] [Google Scholar]
- 6.Shah PM, Gerdes H. Endoscopic options for early stage esophageal cancer. J Gastrointest Oncol 2015; 6: 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg 2011; 253: 271–278. [DOI] [PubMed] [Google Scholar]
- 8.Ezoe Y, Muto M, Horimatsu T, et al. Efficacy of preventive endoscopic balloon dilation for esophageal stricture after endoscopic resection. J Clin Gastroenterol 2011; 45: 222–227. [DOI] [PubMed] [Google Scholar]
- 9.Funakawa K, Uto H, Sasaki F, et al. Effect of endoscopic submucosal dissection for superficial esophageal neoplasms and risk factors for postoperative stricture. Medicine 2015; 94: e373–e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isomoto H, Yamaguchi N, Nakayama T, et al. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol 2011; 11: 46–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim GH, Jee SR, Jang JY, et al. Stricture occurring after endoscopic submucosal dissection for esophageal and gastric tumors. Clin Endosc 2014; 47: 516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuta H, Nishimori I, Kuratani Y, et al. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus 2009; 22: 626–631. [DOI] [PubMed] [Google Scholar]
- 13.Ono S, Fujishiro M, Niimi K, et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy 2009; 41: 661–665. [DOI] [PubMed] [Google Scholar]
- 14.Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 2005; 3: S67–S70. [DOI] [PubMed] [Google Scholar]
- 15.Ohki T, Yamato M, Ota M, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 2012; 143: 582–588, e1–2. [DOI] [PubMed] [Google Scholar]
- 16.Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70: 860–866. [DOI] [PubMed] [Google Scholar]
- 17.Repici A, Hassan C, Carlino A, et al. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: Results from a prospective Western series. Gastrointest Endosc 2010; 71: 715–721. [DOI] [PubMed] [Google Scholar]
- 18.Sato H, Inoue H, Kobayashi Y, et al. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: Oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc 2013; 78: 250–257. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi H, Arimura Y, Okahara S, et al. Risk of perforation during dilation for esophageal strictures after endoscopic resection in patients with early squamous cell carcinoma. Endoscopy 2011; 43: 184–189. [DOI] [PubMed] [Google Scholar]
- 20.Wen J, Lu Z, Yang Y, et al. Preventing stricture formation by covered esophageal stent placement after endoscopic submucosal dissection for early esophageal cancer. Dig Dis Sci 2014; 59: 658–663. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73: 1115–1121. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H, Arimura Y, Okahara S, et al. A randomized controlled trial of endoscopic steroid injection for prophylaxis of esophageal stenoses after extensive endoscopic submucosal dissection. BMC Gastroenterol 2015; 15: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chian KS, Leong MF, Kono K. Regenerative medicine for oesophageal reconstruction after cancer treatment. Lancet Oncol 2015; 16: e84–e92. [DOI] [PubMed] [Google Scholar]
- 24.Badylak SF, Hoppo T, Nieponice A, et al. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: A regenerative medicine approach with a biologic scaffold. Tissue Eng Part A 2011; 17: 1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohki T, Yamato M, Murakami D, et al. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut 2006; 55: 1704–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takagi R, Murakami D, Kondo M, et al. Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest Endosc 2010; 72: 1253–1259. [DOI] [PubMed] [Google Scholar]
- 27.Tamhankar AP, Peters JH, Portale G, et al. Omeprazole does not reduce gastroesophageal reflux: New insights using multichannel intraluminal impedance technology. J Gastrointest Surg 2004; 8: 890–897. discussion 897–898. [DOI] [PubMed] [Google Scholar]