Abstract
With its airborne transmission and prolonged latency period, Mycobacterium tuberculosis spreads worldwide as one of the most successful bacterial pathogens and continues to kill millions of people every year. M. tuberculosis lineage 1 is inferred to originate ancestrally based on the presence of the 52-bp TbD1 sequence and analysis of single nucleotide polymorphisms. Previously, we briefly reported the complete genome sequencing of M. tuberculosis strains 96121 and 96075, which belong to the ancient Manila family and modern Beijing family respectively. Here we present the comprehensive genomic analyses of the Manila family in lineage 1 compared to complete genomes in lineages 2–4. Principal component analysis of the presence and absence of CRISPR spacers suggests that Manila isolate 96121 is distinctly distant from lineages 2–4. We further identify a truncated whiB5 gene and a putative operon consisting of genes encoding a putative serine/threonine kinase PknH and a putative ABC transporter, which are only found in the genomes of Manila family isolates. Six single nucleotide polymorphisms are uniquely conserved in 38 Manila strains. Moreover, when compared to M. tuberculosis H37Rv, 59 proteins are under positive selection in Manila family isolate 96121 but not in Beijing family isolate 96075. The unique features further serve as biomarkers for Manila strains and may shed light on the limited transmission of this ancestral lineage outside of its Filipino host population.
Introduction
The global epidemic tuberculosis (TB) accounts for millions of deaths worldwide every year, and it has been recognized as a World Health Organization (WHO) emergency since 1993 [1]. One-third of the world population is latently infected by tuberculosis-causing bacteria [2]. One major cause of death among human immunodeficiency virus (HIV) carrying populations is TB, and more than 10% of TB cases are associated with HIV [1]. In addition, multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) occur globally. Thus, identification of novel biomarkers of global TB-causing bacteria is needed for improving clinical detection and developing new treatments [3].
Mycobacterium tuberculosis is one pathogenic bacterial species in the Mycobacterium tuberculosis complex (MTBC). Seven human-adapted MTBC lineages are characterized based on the phylogenetic analysis; lineages 1–4 and 7 are M. tuberculosis strains, and lineages 5 and 6 are Mycobacterium africanum [4, 5]. Lineages 1, 5 and 6 are classified as ancient lineages due to the presence of a 52-bp region named TbD1, which is also identified in the cattle TB-causing bacterium Mycobacterium bovis, while the remaining lineages are classified as modern lineages due to the absence of TbD1 [6]. SNPs (single nucleotide polymorphisms) based phylogenetic analysis further supports this scenario and lineage 1 is estimated to diverge ~ 67,000 years ago [4, 5]. In addition, modern lineages are more successful than those ancient ones when compared in virulence and geographical spread [7].
The Manila family of M. tuberculosis was originally defined by investigating IS6110 polymorphism, spoligotyping, and three gene loci (oxyR, gyrA, and katG) in 48 M. tuberculosis strains, isolated from patients living in Manila, Republic of the Philippines [8]. Based on geographical distribution and our unpublished data, the Manila family belongs to lineage 1which includes M. tuberculosis strains circulating in the Philippines and around the rim of the Indian Ocean. In our previous work, we performed whole genome sequencing, de novo assembly, and gap closing of two drug sensitive M. tuberculosis strains from the Manila and Beijing families respectively [9]. However, the Manila family has not been fully characterized at the complete genome level. Here we present comparative analyses of the complete genomes of M. tuberculosis Manila family isolate 96121, strains in lineages 2–4, and two outgroup strains including M. bovis and Mycobacterium canettii. We also investigate whether the unique features in Manila family isolate 96121 are prevalent in 38 draft genomes of the Manila family. Our findings provide evidence to reveal the unique evolution of this ancestrally derived family and may shed light on the limited transmission of this family of M. tuberculosis.
Materials and methods
Genome sequencing, assembly, and data collection
Whole genome shotgun sequencing of M. tuberculosis Manila family isolate 11L4601 was carried out on the Ion Torrent PGM platform (Thermo Fisher Scientific, USA). The complete genome sequence of Manila family isolate 96121 was used as the reference genome to assemble PGM reads using 454 gsMapper software (Roche). This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession LSFJ00000000 (Table A in S1 File). The version described in this paper is version LSFJ01000000. MUMmer 3 package was used for whole genome alignments [10]. The Illumina reads sequenced for 37 Manila strains were downloaded from NCBI database (Table A in S1 File). Reads were trimmed using the ea-utils program [11] and then assembled using the reference assembly method as above. Additional genome sequences were downloaded from the NCBI FTP site as described in Table 1 and Table A in S1 File.
Table 1. Strain information list.
| Strain name | Accession number | Re-annotated protein coding gene number | Isolated place | TbD1 |
|---|---|---|---|---|
| UT205 | NC_016934 | 4109 | Colombia, South America | - |
| Erdman_ATCC35801 | NC_020559 | 4085 | Trudeau Mycobacterial Culture Collection | - |
| Beijing_96075 | NZ_CP009426 | 4054 | Beijing, China | - |
| Manila_96121 | NZ_CP009427 | 4088 | Manila, Philippines | + |
| CCDC5180 | NC_017522 | 4091 | Beijing, China | - |
| RGTB327 | NC_017026 | 4689 | Kerala, South India | - |
| CTRI-2 | NC_017524 | 4057 | Russia | - |
| NITR204 | NC_021193 | 5237 | Tamil Nadu, South India | - |
| CDC1551 | NC_002755 | 4067 | Kentucky/Tennessee, USA | - |
| KZN605 | NC_018078 | 4064 | KwaZulu-Natal, South Africa | - |
| CCDC5079 | NC_017523 | 4176 | Beijing, China | - |
| KZN4207 | NC_016768 | 4051 | KwaZulu-Natal, South Africa | - |
| KZN1435 | NC_012943 | 4064 | KwaZulu-Natal, South Africa | - |
| NITR202 | NC_021192 | - | Tamil Nadu, South India | - |
| RGTB423 | NC_017528 | 4793 | Kerala, South India | - |
| NITR206 | NC_021194 | 4137 | Tamil Nadu, South India | - |
| H37Rv | NC_000962 | 4069 | virulent lab strain | - |
| H37Ra | NC_009525 | 4079 | attenuated lab strain | - |
| NITR203 | NC_021054 | 4158 | Beijing, China | - |
| F11 | NC_009565 | 4068 | South Africa | - |
| 7199–99 | NC_020089 | 4061 | Europe | - |
Clustering of CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) spacers
CRISPR regions and spacers were identified using CRISPRFinder [12, 13]. A total of 716 spacer sequences from 21 complete genomes of M. tuberculosis were loaded for all-vs-all BLASTN-short to identify homologous spacer sequences. 90% and 95% were set as stringent cut-off values of minimum aligned length and sequence identity. The MCL program [14] was used to cluster homologous spacer sequences and identify 50 groups. The accumulation curve of spacers and principal component analysis were analyzed and visualized with R package.
Clustering of ortholog groups
Ortholog groups of proteins were identified using orthoMCL [15] with several additional steps to filter all-vs-all BLASTP results [16]. If the aligned length was less than 80% of the longer length of two protein sequences, the BLASTP result was filtered out. Then if the aligned length was equal to or above 150 amino acids, the BLASTP result with pairwise identity below 30% was filtered out; if the aligned length was less than 150 amino acids, the BLASTP result was filtered by the formula (100 × (0.06 + 4.8 × L^(-0.32 × (1+ e^(-L/1000))))), in which L stands for the aligned length [16]. The pipeline Prokka 1.11 [17] was used to re-annotate genomes and then the pan-genome was further analyzed using the Roary pipeline [18]. Gene accumulation curves were plotted using R package vegan or ggplot2 [19, 20].
Analysis of SNPs and synonymous/nonsynonymous substitution rates
Core-SNPs were identified using the kSNP v2.13 package [21]. Indels and SNPs were also identified using MUMer 3 package [10]. Synonymous substitution rate (Ks) and the ratio of nonsynonymous substitution rate to synonymous substitution rate (Ka/Ks ratio) were calculated by ParaAT and KaKs_Calculator packages [22, 23]. Density plots of Ka/Ks and Ks values were created using R commands and heat scatter plots of Ka and Ks values were generated by LSD package [24].
Functional annotation
RNA families were annotated using Rfam database [25]. The secondary structures of RNA sequences were calculated and predicted using the stand-alone ViennaRNA package [26]. The RNA structures were visualized using VARNA 3.92 [27].
Results
Genome sequencing, data collection, and assembly
We carried out whole genome shotgun sequencing of M. tuberculosis Manila family isolate 11L4601, which was collected from a female patient living in the Commonwealth of the Northern Mariana Islands in 2010. We generated 116.5 Mb and the assembly totaled 4,356,842 bases, covering 98.8% of the genome of Manila family isolate 96121 (Table B in S1 File). The assembled contigs aligned well with the genome sequence of Manila family 96121, only yielding random simple repeats located away from the plotted diagonal as seen in Fig A in S1 File. To obtain population information of genomes of Manila family, we downloaded Illumina reads sequenced for 37 Manila strains, which were mapped to the genome sequence of H37Rv strain for SNP-based subgrouping of Manila strains [28]. We trimmed the low-quality ends of Illumina reads using Phred quality score Q30 and performed reference assembly to obtain the 37 draft genomes of Manila strains provided in Table B in S1 File.
For comparative genomic analyses to unveil unique signatures of ancestral Manila family, 19 complete genomes of M. tuberculosis strains were downloaded from the NCBI database (Table 1). By BLASTN analysis, Manila family 96121 was the only complete genome containing the 52-bp TbD1 region which designated it as an ancestral strain, while the other 20 complete genomes only contained a partial region (~ 27 bp) of TbD1 which designated them as modern strains [6]. All the 38 draft genomes of Manila family strains contained 52-bp TbD1 region.
Gain and loss pattern of CRISPR spacers revealed that the Manila family was distinctly different from modern strains
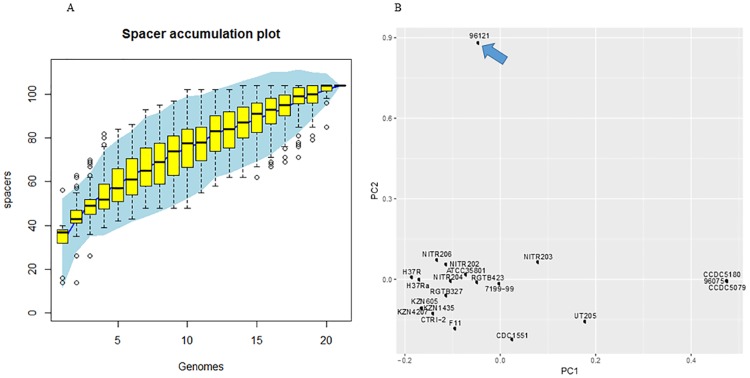
CRISPR is a repetitive region identified in bacterial and archaeal genomes, and it is proposed as immune system resistant to horizontal gene transfer (HGT) in prokaryotes [29, 30]. We used CRISPRFinder to identify CRISPR regions in 21 complete genomes of M. tuberculosis and 2 genomes of outgroup strains. The number of CRISPR arrays in M. tuberculosis genomes ranged from 1 to 3. Two CRISPR regions were identified in Manila family isolate 96121, and one CRISPR region was found in Beijing family isolate 96075. The presence and absence of homologous spacer sequences of each strain were counted from 50 clustered groups and recorded in a binary matrix. The spacer accumulation curve of 21 strains indicated that the pan-genome spacer number for 21 strains was 104 and there was a potentially larger pan-genome spacer size (Fig 1A). Using the Eigenvector method, principal component analysis (PCA) indicated that Manila family isolate 96121 was obviously different from the other 20 strains (Fig 1B). Adding CRISPR spacers of Manila family 11L4601, T17, T92, and outgroup species for PCA, we found that Manila family 11L4601 located closely to Manila family 96121. Furthermore, T17, T92, and M. bovis located at points between Manila family 96121 and lineages 2–4 (Fig B in S1 File).
Fig 1. Evolution of CRISPR spacers in complete genomes of 21 M. tuberculosis strains.
(A) Accumulation curve of spacers in complete genomes of 21 M. tuberculosis strains. (B) PCA of presence and absence of CRISPR spacers in complete genomes of 21 M. tuberculosis strains. The blue arrow indicates the position of Manila family 96121, illustrating a dramatic difference in the composition of spacers in Manila family 96121.
We further compared spacer region rearrangements among Manila family isolate 96121, Beijing family isolate 96075, and H37Rv to reveal the spacer birth-death pattern of M. tuberculosis strains isolated from various niches (Fig 2). CRISPR array 1 in Manila family 96121 lacked the acquisition of five spacers in H37Rv, of which the acquisition of spacer 7 occurred in Beijing family isolate 96075. However, it maintained eight homologous spacers with high similarity to those of other species in MTBC including M. bovis, Mycobacterium microti, and M. africanum. Four spacers in CRISPR array 1 of Manila family isolate 96121 were strain-specific. Comparison of CRISPR array 2 between Manila family isolate 96121 and H37Rv also suggested that eight spacers shared with MTBC were maintained in Manila family 96121 but absent from H37Rv.
Fig 2. CRISPR spacer rearrangements in Manila family 96121, Beijing family 96075, and H37Rv.
Green highlights spacers conserved with other MTBC species but lost in modern Beijing family isolate 96075 and H37Rv. Red highlights spacers specific in Manila family isolate 96121. Blue highlights spacers present in H37Rv but absent in Manila family isolate 96121 and Beijing family isolate 96075.
Core-genome and pan-genome analysis of complete genomes revealed genetic diversity in the Manila family
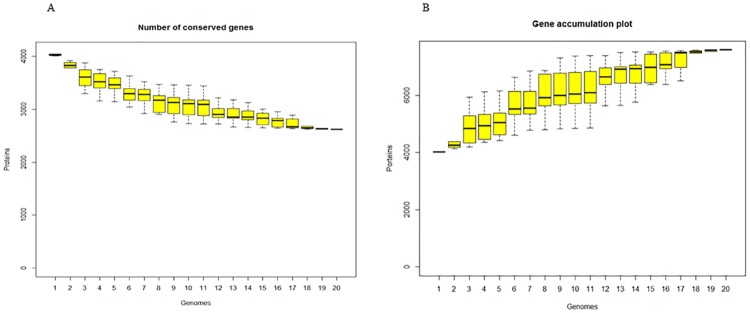
We only considered complete genomes of M. tuberculosis for core and pan-genome analysis in this study. A total of 82,395 protein sequences from 21 complete genomes of M. tuberculosis were loaded for all-vs-all BLASTP analysis to identify homologous protein sequences. OrthoMCL [15] was further used to cluster 4,899 groups among strains. Only 736 ortholog groups were identified to contain core genes across the 21 complete genomes of strains, of which 711 orthologous groups contained single-copy orthologous genes from each strain.
Considering extremely high identities of genome nucleotides of M. tuberculosis strains, we further questioned whether various gene prediction algorithms caused artificial genetic diversity or whether indels/mutations caused frameshift, premature stop codon, or stop codon readthrough. We used the stand-alone Prokka program [17] to re-annotate 20 complete genome sequences (Table 1), excluding NITR202 strain due to unexpected nucleotide designations. Based on Roary results [18], the core genome included 2,623 orthologous genes and the pan-genome included 7,591 genes (Fig 3A and 3B), indicating that different annotation processes affected core and pan-genome analysis and that considerable genetic diversity within M. tuberculosis existed. 264 genes in Manila family isolate 96121 were not clustered with genes in H37Rv by Roary. Furthermore, 58 genes in Manila family isolate 96121 were not clustered with genes in 19 re-annotated genomes (Table 1), in which 46 genes were annotated as hypothetical proteins and 12 genes were assigned with functions. We further retrieved coding DNA sequences (CDS) of the 58 genes and ran BLASTN against H37Rv genome sequence to double check whether they were singletons. We manually confirmed that 13 singletons were formed by frameshift or stop codon formation/readthrough and 2 singletons were absent in the 19 complete genomes.
Fig 3. Core-genome and pan-genome of M. tuberculosis in lineages 1–4, based on analysis of 20 completed genomes.
(A) Accumulation curve for conserved genes in 20 re-annotated genomes. (B) Accumulation curve for total gene numbers in 20 re-annotated genomes.
Comparing the genome sequence of Manila isolate 96121 to H37Rv, we identified 983 indels and 2,155 SNPs. 83 genes in the Manila isolate 96121 were identified to contain indels. We suggest that though genome sequences are relatively conserved with high identity, encoded functional proteomes are re-shaped by indels and SNPs, and these microevolutionary changes result in the dynamics and diversity of mycobacterial genomes.
A truncated WhiB5 protein in the Manila family
We identified one C to T mutation in Manila family isolate 96121 that caused the formation of the stop codon TAG in the whiB5 gene. This C to T mutation was also present in 38 draft genomes of Manila family, leading to a truncated N-terminal sensor domain that contained four conserved cysteine residues to coordinate the Fe-S cluster but lost the C-terminal alpha helixes that may function to bind DNA or interact with other proteins. The WhiB5 protein in H37Rv is required for expression of 58 genes and is required for progressive and chronic mouse infections [31]. The full CDS of the whiB5 gene was present in M. canettii, M. bovis, and the other 20 M. tuberculosis complete genomes by BLASTN searches.
Coupled ancestral ABC transporter and STPK PknH in the Manila family
We identified a unique MTM_01342 gene in the complete genome of Manila family isolate 96121, which was absent from 19 complete genomes of modern strains. MTM_01342 gene was the paralog of MTM_01852 gene, which encoded the forkhead-associated (FHA) domain containing ATP-binding cassette (ABC) transporter in the Manila family isolate 96121 (Table C in S1 File). The CDS of MTM_01342 had no homologous DNA sequence in 19 complete genomes of M. tuberculosis by BLASTN searches. In contrast, M. canettii contained the homologous CDS of MTM_01342 gene with 99% identity. We further confirmed that the homologous CDS of MTM_01342 gene was present in 37 Manila strains with 100% identity and that its absence in one Manila strain was due to the fragmented assembly of the draft genome. The above data suggest the loss of the ortholog of putative MTM_01342 gene in modern strains.
In H37Rv, Rv1747 (the ortholog of MTM_01852), the FHA domain-containing ABC transporter, is phosphorylated by Ser/Thr protein kinase (STPK) PknF, and its encoding gene, Rv1747, locates adjacent to pknF in the genome [32–34]. In the genome of Manila family isolate 96121, MTM_01342 located at ~ 561 kb upstream of MTM_01852, and it was flanked by two pknH genes (Table C in S1 File). Coupling the loss of the ortholog of MTM_01342, the paralogous pknH gene (MTM_01343) was also absent in most of the modern strains except NITR203 and RGTB423. The intergenic region between MTM_01342 and MTM_01343 contained only 10 bases, suggesting that these two genes may be co-transcribed and form an operon. STPK PknH was reported to phosphorylate the FHA domain of EmbR and helix α10 of DosR, both of which are transcriptional regulators [35, 36]. Deletion of the pknH gene in M. tuberculosis caused hypervirulence in BALB/c mice [37]. Taken together, the genomic locations suggest that the putative FHA domain-containing ABC transporter may be phosphorylated by the putative STPK PknH, and they may function as an additional independent signaling transducer and receptor to regulate virulence in the Manila family.
SNPs identified in functional coding regions
To investigate SNPs in M. tuberculosis strains, we used the kSNP v2.13 package [21] to identify SNPs in 21 complete genomes of M. tuberculosis and 7 draft genomes of Beijing family strains, using the k-mer counting analysis method. After optimization, 11-mers were chosen and generated for SNPs analysis. 158 core SNPs and 2386 non-core SNPs were identified through 28 genomes. Out of 158 core SNPs, 118 SNPs were annotated to be located in protein coding regions of H37Rv, and an additional 17 SNPs were annotated to be located in protein coding regions of strains other than H37Rv. Searching against H37Rv annotation in intergenic regions, we found only two SNPs with functions. To explore SNPs in RNA families, we further annotated 28 genomes with the Rfam database [25]. SNPs in a cobalamin riboswitch and the ASpks small RNA were identified in Manila family isolate 96121. We further used the stand-alone RNAfold program in ViennaRNA package [26] to predict the SNPs’ effects on RNA structure folding. A single-base G to A mutation in the cobalamin riboswitch of Manila family isolate 96121 may cause a local confirmation change (Fig C in S1 File). It was also found in Indian strains RGTB423 and NITR206. A single-base G to C mutation in the ASpks small RNA of Manila family isolate 96121 may cause a global confirmation change, causing a double stem-loop structure to form a single stem-loop (Fig C in S1 File). Again, this mutation was also in Indian strain RGTB423 and NITR206. ASpks was identified as an antisense regulator of pks12 mRNA, which is involved in polyketide biosynthesis and pathogenesis [38].
Compared to H37Rv, 29 out of 158 core SNPs were identified in Manila strain 96121, and 20 SNPs were located in protein coding regions of H37Rv. 8 SNPs were synonymous mutations, while 12 SNPs were nonsynonymous. Annotated functional genes with these nonsynonymous SNPs included the possible protein exporter SecE, methyltransferase, oxidoreductase, polyketide synthase pks15, and others (Table 2).
Table 2. List of Manila 96121 nonsynonymous SNPs in protein coding genes.
| SNP ID | H37Rv Location | H37Rv base | H37Rv amino acid | Manila 96121 Location | Manila 96121 base | Manila 96121 amino acid | H37Rv name | H37Rv locus tag |
|---|---|---|---|---|---|---|---|---|
| T189 | 3504418 | G | P | 3510068 | A | L | pflA | Rv3138 |
| T337 | 4013388 | G | M | 4014984 | A | I | kshB | Rv3571 |
| T444 | 734116 | T | M | 732013 | C | T | secE1 | Rv0638 |
| T530 | 1689349 | G | R | 1687692 | A | H | Rv1498c | Rv1498c |
| T678 | 2569593 | T | H | 2584176 | C | R | Rv2298 | Rv2298 |
| T857 | 3296721 | G | G | 3305059 | C | R | pks15 | Rv2947c |
| T907 | 15117 | C | I | 15117 | G | M | trpG | Rv0013 |
| T1565 | 895082 | A | F | 890641 | G | L | Rv0802c | Rv0802c |
| T1594 | 885689 | C | T | 884148 | A | K | Rv0791c | Rv0791c |
| T2042 | 3299413 | G | A | 3307751 | A | V | fadD22 | Rv2948c |
| T2046 | 3035533 | G | D | 3044018 | A | N | Rv2723 | Rv2723 |
| T2264 | 1113290 | G | E | 1108916 | C | Q | Rv0996 | Rv0996 |
Six unique SNPs were identified in the complete genome of Manila family isolate 96121 and draft genomes of 38 Manila family isolates. Four SNPs were in protein coding genes, including pflA, kshB, Rv2723 (alx), and Rv0925c, while the other two SNPs were in intergenic regions. These unique SNPs may serve as additional biomarkers for Manila family strains. Interestingly, another SNP in the treZ gene was in Manila family isolate 96121 and 6 other (15.8%) Manila strains, but not in the remaining 32 Manila strains.
Purifying and positive selection
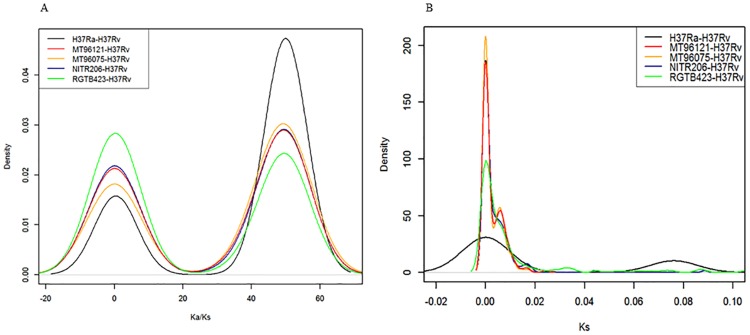
Both purifying selection and positive selection have been reported in M. tuberculosis genome-wide studies [39–41]. To examine natural selection directions in Manila family isolate 96121 and Beijing family isolate 96075, we scanned 711 single-copy orthologs to calculate and plot densities of Ka/Ks ratios (the ratio of nonsynonymous substitution rate (Ka) to synonymous substitution rate (Ks)), comparing each strain to H37Rv (Fig 4A and 4B, Fig D in S1 File). The densities of Ka/Ks values showed two peaks, one centered at less than 1 and the other centered above 1, indicating that one set of genes are under purifying selection, while the other set of genes are under positive selection. Density plot of the synonymous substitution rate Ks indicated a different pattern for the attenuated strain H37Ra.
Fig 4. Distributions of Ka/Ks and Ks values for single-copy orthologs among M. tuberculosis strains.
(A) The black, red, yellow, blue, and green lines represent Ka/Ks distributions of single-copy orthologous gene pairs in H37Ra-H37Rv, Manila family isolate 96121-H37Rv, Beijing family isolate 96075-H37Rv, NITR206-H37Rv and RGTB423-H37Rv, respectively. (B) The black, red, yellow, blue, and green lines represent Ks distributions of single-copy orthologous gene pairs in H37Ra-H37Rv, Manila family isolate 96121-H37Rv, Beijing family isolate 96075-H37Rv, NITR206-H37Rv and RGTB423-H37Rv, respectively.
A total of 108 proteins were under positive selection in Manila family isolate 96121. 16 proteins were annotated as hypothetical proteins by the NCBI Prokaryotic Genome Annotation Pipeline (PGAP). 59 proteins under positive selection were identified in Manila family isolate 96121 but not in Beijing family isolate 96075 (Table 3). These proteins were encoded by genes involved in diverse functions. These nonsynonymous substitutions may cause changes in protein structure and functional activity. Compared to strain H37Rv, 11 amino acids were mutated from nonpolar amino acids to polar amino acids in Manila family isolate 96121, including the ribosomal protein S9 and the PhoU family transcription/regulation protein.
Table 3. List of proteins under positive selection in Manila family 96121 but not Beijing family 96075.
| Protein ID | Start | End | Strand | Product |
|---|---|---|---|---|
| AIQ06604.1 | 5240 | 7267 | + | DNA gyrase subunit B |
| AIQ06635.1 | 39871 | 41196 | - | MFS transporter |
| AIQ06685.1 | 92390 | 93340 | + | formate hydrogenlyase |
| AIQ06723.1 | 147972 | 148406 | - | F420-dependent protein |
| AIQ06735.1 | 160933 | 161538 | + | acetyltransferase |
| AIQ06768.1 | 193734 | 194174 | + | cyclase |
| AIQ06771.1 | 196926 | 197723 | + | ABC transporter permease |
| AIQ06915.1 | 377093 | 377749 | + | hypothetical protein |
| AIQ06932.1 | 391858 | 393207 | - | cytochrome P450 |
| AIQ06965.1 | 438910 | 440292 | + | magnesium transporter |
| AIQ07080.1 | 562596 | 563966 | + | membrane protein |
| AIQ07164.1 | 643362 | 644150 | + | bromoperoxidase |
| AIQ07252.1 | 731634 | 732119 | + | preprotein translocase subunit SecE |
| AIQ07264.1 | 745467 | 746375 | + | ROK family transcriptional regulator |
| AIQ07313.1 | 795363 | 796802 | + | dehydrogenase |
| AIQ07411.1 | 882531 | 883259 | - | transglutaminase-like enzyme |
| AIQ07419.1 | 890531 | 891187 | - | succinyl-CoA transferase |
| AIQ07421.1 | 893640 | 894269 | + | abortive phage infection protein |
| AIQ07440.1 | 909816 | 911870 | - | LytR family transcriptional regulator |
| AIQ07471.1 | 944559 | 945386 | - | short-chain dehydrogenase |
| AIQ07554.1 | 1036753 | 1037865 | - | phosphate-binding protein |
| AIQ07573.1 | 1058781 | 1059944 | + | succinyl-CoA synthetase subunit beta |
| AIQ07602.1 | 1092448 | 1093134 | + | transcriptional regulator |
| AIQ07613.1 | 1104204 | 1104797 | - | 5-formyltetrahydrofolate cyclo-ligase |
| AIQ07936.1 | 1459675 | 1460427 | + | ATP synthase F0F1 subunit A |
| AIQ07961.1 | 1493984 | 1496575 | + | glycogen phosphorylase |
| AIQ08030.1 | 1572174 | 1572575 | - | ribonuclease |
| AIQ08344.1 | 1934517 | 1935977 | + | sulfate transporter |
| AIQ08345.1 | 1936088 | 1936951 | + | chromosome partitioning protein ParA |
| AIQ08437.1 | 2038829 | 2040049 | + | secretion protein EccC |
| AIQ08693.1 | 2298175 | 2299170 | + | NAD(P)H nitroreductase |
| AIQ08698.1 | 2301793 | 2302758 | - | membrane protein |
| AIQ08731.1 | 2350042 | 2350449 | + | hypothetical protein |
| AIQ08827.1 | 2449181 | 2449840 | + | conserved lipoprotein LppM |
| AIQ08912.1 | 2544083 | 2544586 | + | hypothetical protein |
| AIQ08916.1 | 2546103 | 2546921 | - | beta-lactamase |
| AIQ08928.1 | 2560636 | 2561163 | + | lipoprotein LppN |
| AIQ08953.1 | 2583665 | 2584636 | + | oxidoreductase |
| AIQ08998.1 | 2627687 | 2628454 | - | molybdopterin biosynthesis protein moeW |
| AIQ09020.1 | 2654411 | 2654803 | + | Fur family transcriptional regulator |
| AIQ09095.1 | 2739621 | 2740154 | + | alkyl hydroperoxide reductase |
| AIQ09342.1 | 3000837 | 3001532 | - | hypothetical protein |
| AIQ09443.1 | 3094683 | 3095222 | - | AsnC family transcriptional regulator |
| AIQ09460.1 | 3114882 | 3115445 | - | hypothetical protein |
| AIQ09573.1 | 3226665 | 3227540 | + | D-alanyl-D-alanine carboxypeptidase |
| AIQ09609.1 | 3306175 | 3308292 | - | acyl-CoA synthetase |
| AIQ09658.1 | 3360602 | 3361612 | - | 3-isopropylmalate dehydrogenase |
| AIQ09670.1 | 3372790 | 3373974 | + | lipoprotein LppZ |
| AIQ09799.1 | 3509845 | 3510933 | + | pyruvate formate lyase-activating protein |
| AIQ09869.1 | 3585653 | 3586507 | - | aminoglycoside phosphotransferase |
| AIQ09965.1 | 3690872 | 3691537 | - | PhoU family transcriptional regulator |
| AIQ09974.1 | 3701158 | 3702057 | + | acid phosphatase |
| AIQ10101.1 | 3864414 | 3864869 | - | 30S ribosomal protein S9 |
| AIQ10155.1 | 3920827 | 3921669 | - | ABC transporter permease |
| AIQ10191.1 | 3979655 | 3981094 | + | hypothetical protein |
| AIQ10406.1 | 4199060 | 4199581 | - | tRNA adenosine deaminase |
| AIQ10452.1 | 4249217 | 4250344 | + | metal dependent hydrolase |
| AIQ10466.1 | 4275887 | 4277401 | + | hypothetical protein |
| AIQ10528.1 | 4350498 | 4351604 | + | hypothetical protein |
Discussion
Using comprehensive comparative-genomic analysis methods, we identified genomic features of the M. tuberculosis Manila family isolate 96121. Our analysis suggested that the pan-genome derived from 20 complete M. tuberculosis genomes was estimated to contain more than 7000 genes, which is much less than the reported pan-genome of E. coli [42]. This difference may be due to the longer evolution time of E. coli strains, which has a more rapid growth rate and has taken tens of millions of years [43] to accumulate mutations, indels, and recombinations, and due to the more diversified ecological niches of E. coli strains that lead to higher odds of acquisition of xenologs via HGT.
The CRISPR regions were fully conserved in Manila family isolates 96121 and 11L4601, and maintained 16 spacer sequences which were lost in modern lineages but shared high identities with other ancient MTBC species (M. bovis, M. microti, and M. africanum). The international standard method of spoligotyping identifies the presence or absence of CRISPR spacers against a set of preselected oliogos through reverse-line hybridization, but cannot detect mutations or indels. Genomic analysis can identify 57 spacers in the complete genome of Manila family isolate 96121 and show the accurate similarity between spacers from different strains.
Positive selections have been reported in viruses, pathogenic bacteria, and other M. tuberculosis strains [40, 41, 44]. In this work, the distribution of genome-wide Ka/Ks ratios suggested that one group (75 orthologous genes in Manila 96121-H37Rv) was under purifying selection (Ka/Ks <1) and another group (108 orthologous genes in Manila 96121-H37Rv) was under positive selection (Ka/Ks >1). For decades, synonymous substitution was regarded as silent and neutral and was used to calculate species diversification time. However, recent discoveries suggest that synonymous mutations can cause genetic code bias and affect mRNA stability, and may also be under evolutionary pressure instead of only under neutral evolution [45]. Thus, proteins defined in the purifying selection group may also be the result of natural adaptation.
In this study, we compared Manila family genomes to complete genomes in lineages 2–4 and two outgroup species. We identified the truncated transcription factor whiB5 gene and an additional potential operon containing the STPK pknH and ABC transporter gene. The WhiB5 regulon contains 58 genes, including the alternative sigma factor sigM and genes in type VII secretion systems [31]. We hypothesize that the truncated WhiB5 protein may contribute to the Manila family’s limited transmission outside of the Philippines. Gagneux proposed that less virulent MTB strains were selected for during humanity’s hunter–gatherer period, when low population densities meant that more virulent strains would quickly decimate their susceptible hosts, while less virulent strains could be transmitted to a new generation of hosts by reemerging after a period of latency in their current hosts [7]. As human population density increased, more virulent strains could have seen higher transmission and greater spread. Thus, Gagneux proposed that the ancient lineage 1 (including the Manila family) evolved towards lower virulence, while the modern lineages evolved increased virulence. This may partially explain the Manila family’s limited transmission outside of the Philippines. However, more data is still needed to support Gagneux’s suggestion of lower virulence for ancestral MTB lineages. While it has been shown that MTB transmission is more likely to occur in sympatric host populations [46], reduced virulence associated with the Manila family has not been demonstrated. Furthermore, while complete deletion of whiB5 has been shown to result in attenuated progressive and chronic infections in mice [31], the same study showed that their whiB5 mutant was unable to resume growth after latency, a characteristic not supported by our data on Manila family latency in humans. From our unpublished data of the 267 TB cases belonging to the Manila family in Hawaii from 2002 to 2013, 249 of the cases were in foreign-born persons. Of those 249 foreign-born cases, 200 of them (80.3%) occurred in patients who had resided in Hawaii for at least two years prior to diagnosis, demonstrating that the Manila family is fully able to cause active disease after a period of latency. Considering about 67,000 ~ 70,000 years of independent evolution of lineage 1 [4, 5], during which the last ice age occurred from ~ 60,000 to 20,000 before present, the C to T mutation in the whiB5 gene may have been selected during this time by Darwinian selection due to the dramatic decline of human population; later it may have been fixed in the Manila family population by founder effect.
MTM_01342 and MTM_01343 encode the putative FHA domain-containing ABC transporter and STPK PknH separately. MTM_01342 was lost in the modern strains, while MTM_01343 was lost in most of the modern strains except NITR203 and RGTB423. The paralog of MTM_01342 gene in H37Rv, Rv1747, is required for in vivo growth in mice model but not for in vitro growth [32], suggesting that it plays a role in stress response during host-pathogen interactions. We hypothesize that the retention of MTM_01342 gene in the ancestral Manila family may contribute to the host adaptation and limited transmission when Manila family strains compete with the modern strains. Experimental characterization of functions of MTM_01342 will help understand why it is present in Manila family genomes.
Taken together, our findings in this study provide additional biomarkers to identify Manila family strains and may shed light on the limited transmission of this ancestral lineage outside of its Filipino host population.
Supporting information
Table A. Accession numbers of additional strains used in this study. Table B. Summary of genome assemblies of M. tuberculosis Manila family strains. Table C. Gene list of STPK coupled ABC transporters. Fig A. Alignment of genome sequence of Manila family isolate 11L4601 with 96121 reference sequence. X axis presents complete genome of Manila family 96121, and y axis presents contigs of Manila family 11L4601. Fig B. PCA of presence and absence of CRISPR spacers in Manila family 96121 and 11L4601, T17, T92, M. bovis, M. canettii and strains in lineages 2–4. Fig C. Predicted secondary structures of cobalamin riboswitch and ASpks small RNA. (A) Predicted secondary structure of cobalamin riboswitch in Manila family 96121. (B) Predicted secondary structure of cobalamin riboswitch in H37Rv. (C) Predicted secondary structure of ASpks small RNA in Manila family 96121. (D) Predicted secondary structure of ASpks small RNA in H37Rv. Fig D. Relationship between Ka, Ks, Ka/Ks values in Manila family 96121-H37Rv (A, B, C) and Beijing family 96075-H37Rv (D, E, F).
(PDF)
Acknowledgments
We thank Shuli Kang for advice regarding Roary pipeline, and acknowledge the support of The Leahi Trust of the Hawaii Community Foundation and The University of Hawaii.
Data Availability
All relevant data and accession numbers are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the University of Hawai'i Office of Research Services and the Leahi Trust of the Hawai'i Community Foundation, Honolulu, Hawaii. Partial funding for the work described here was provided by the Dean of the College of Natural Sciences, Dr. William Ditto, to JTD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. 2014. Global tuberculosis report.
- 2.World Health Organization. 2016. Tuberculosis fact sheet.
- 3.World Health Organization. 2015. Global tuberculosis report.
- 4.Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, et al. 2013. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet 45: 1176–1182. 10.1038/ng.2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galagan JE. 2014. Genomic insights into tuberculosis. Nat Rev Genet 15:307–320. 10.1038/nrg3664 [DOI] [PubMed] [Google Scholar]
- 6.Brosch R, Gordon SV, Marmiesse M., Brodin P, Buchrieser C, Eiglmeier K, et al. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A 99:3684–3689. 10.1073/pnas.052548299 10.1073/pnas.052548299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagneux S. 2012. Host-pathogen coevolution in human tuberculosis. Philos Trans R Soc B 367:850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas JT, Qian L, Montoya JC, Musser JM, Van Embden JDA, Van Soolingen D, et al. 2003. Characterization of the Manila family of Mycobacterium tuberculosis. Journal of Clinical Microbiology, 41(6): 2723–2726. 10.1128/JCM.41.6.2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan X, Qian L, Hou S, Drees KP, Foster JT, Douglas JT. 2014. Complete genome sequences of Beijing and Manila family strains of Mycobacterium tuberculosis. Genome Announc 2(6):e01135–14. 10.1128/genomeA.01135-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, et al. 2004. Versatile and open software for comparing large genomes. Genome Biol 5:R12 10.1186/gb-2004-5-2-r12 10.1186/gb-2004-5-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronesty E. 2011. Ea-utils: “command-line tools for processing biological sequencing data”. http://code.google.com/p/ea-utils.
- 12.Grissa I, Vergnaud G, Pourcel C. 2007. The CRISPRdb database and tools to display CRISPRs and to generate dictionaries of spacers and repeats. BMC bioinformatics 8:172 10.1186/1471-2105-8-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grissa I, Vergnaud G, Pourcel C. 2007. CRISPRFinder: a web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res 35:W52–7. 10.1093/nar/gkm360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stijn van Dongen. 2000. A cluster algorithm for graphs. Technical Report INS-R0010, National Research Institute for Mathematics and Computer Science in the Netherlands, Amsterdam
- 15.Li L, Stoeckert CJ Jr, Roos DS. 2003. OrthoMCL: Identification of Ortholog Groups for Eukaryotic Genomes. Genome Res 13(9):2178–2189. 10.1101/gr.1224503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rost B. 1999. Twilight zone of protein sequence alignments. Protein Eng 12(2):85–94. 10.1093/protein/12.2.85 [DOI] [PubMed] [Google Scholar]
- 17.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30(14):2068–9. 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 18.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, et al. 2015. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31(22):3691–3693. 10.1093/bioinformatics/btv421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, et al. 2015. vegan: Community Ecology Package. R package version 2.3–0. http://CRAN.R-project.org/package=vegan
- 20.Wickham H. 2009. ggplot2: elegant graphics for data analysis. Springer; New York [Google Scholar]
- 21.Gardner SN, Hall BG. 2013. When whole-genome alignments just won’t work: KSNP v2 software for alignment-free SNP discovery and phylogenetics of hundreds of microbial genomes. PLoS ONE 8(12). 10.1371/journal.pone.0081760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Li J, Zhao XQ, Wang J, Wong GK, Yu J. 2006. KaKs Calculator: Calculating Ka and Ks through model selection and model averaging. Genomics Proteomics Bioinformatics 4:259–263. 10.1016/S1672-0229(07)60007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Xiao J, Wu J, Zhang H, Liu G, Wang X, et al. 2012. ParaAT: A parallel tool for constructing multiple protein-coding DNA alignments. Biochem Biophys Res Commun 419(4):779–781. 10.1016/j.bbrc.2012.02.101 [DOI] [PubMed] [Google Scholar]
- 24.Schwalb B, Tresch A, Torkler P, Duemcke S, Demel C. 2015. LSD: Lots of Superior Depictions. R package version 3.0. http://CRAN.R-project.org/package=LSD
- 25.Burge SW, Daub J, Eberhardt R, Tate J, Barquist L, Nawrocki EP, et al. 2012. Rfam 11.0: 10 years of RNA families. Nucleic Acids Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenz R, Bernhart SH, Höner zu Siederdissen C, Tafer H, Flamm C, Stadler PF, et al. 2011. ViennaRNA Package 2.0. Algorithms Mol Biol 6(1):26 10.1186/1748-7188-6-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darty K, Denise A, Ponty Y. 2009. VARNA: Interactive drawing and editing of the RNA secondary structure. Bioinformatics 25(15):1974–1975. 10.1093/bioinformatics/btp250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamieson FB, Teatero S, Guthrie JL, Neemuchwala A, Fittipaldi N, Mehaffy C. 2014. Whole-Genome Sequencing of the Mycobacterium tuberculosis Manila Sublineage Results in Less Clustering and Better Resolution than Mycobacterial Interspersed Repetitive-Unit-Variable-Number Tandem-Repeat (MIRU-VNTR) Typing and Spoligotyping. J Clin Microbiol 52(10):3795–3798. 10.1128/JCM.01726-14 10.1128/JCM.01726-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, et al. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712. 10.1126/science.1138140 [DOI] [PubMed] [Google Scholar]
- 30.Marraffini LA, Sontheimer EJ. 2008. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322(5909):1843–1845. 10.1126/science.1165771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casonato S, Cervantes Sánchez A, Haruki H, Rengifo González M, Provvedi R, Dainese E, et al. 2012. WhiB5, a Transcriptional Regulator That Contributes to Mycobacterium tuberculosis Virulence and Reactivation. Infect Immun 80(9):3132–3144. 10.1128/IAI.06328-11 10.1128/IAI.06328-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curry JM, Whalan R, Hunt DM, Strom M, Rickman L, Colston MJ, et al. 2005. An ABC Transporter Containing a Forkhead-Associated Domain Interacts with a Serine-Threonine Protein Kinase and Is Required for Growth of Mycobacterium tuberculosis in Mice. Infect Immun 73(8):4471–4477. 10.1128/IAI.73.8.4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molle V, Soulat D, Jault JM, Grangeasse C, Cozzone AJ, Prost JF. 2004. Two FHA domains on an ABC transporter, Rv1747, mediate its phosphorylation by PknF, a Ser/Thr protein kinase from Mycobacterium tuberculosis. FEMS Microbiol Lett 234(2):215–223. 10.1016/j.femsle.2004.03.033 10.1016/j.femsle.2004.03.033 [DOI] [PubMed] [Google Scholar]
- 34.Spivey VL, Molle V, Whalan RH, Rodgers A, Leiba J, Stach L, et al. 2011. Forkhead-associated (FHA) domain containing ABC transporter Rv1747 is positively regulated by Ser/Thr phosphorylation in Mycobacterium tuberculosis. J Biol Chem 286(29):26198–209. 10.1074/jbc.M111.246132 10.1074/jbc.M111.246132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molle V, Kremer L, Girard-Blanc C, Besra GS, Cozzone AJ, Prost JF. 2003. An FHA phosphoprotein recognition domain mediates protein EmbR phosphorylation by PknH, a Ser/Thr protein kinase from Mycobacterium tuberculosis. Biochemistry 42(51):15300–15309. 10.1021/bi035150b 10.1021/bi035150b [DOI] [PubMed] [Google Scholar]
- 36.Chao JD, Papavinasasundaram KG, Zheng X, Chávez-Steenbock A, Wang X, Lee GQ, et al. 2010. Convergence of Ser/Thr and two-component signaling to coordinate expression of the dormancy regulon in Mycobacterium tuberculosis. J Biol Chem 285:29239–29246. 10.1074/jbc.M110.132894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papavinasasundaram KG, Chan B, Chung JH, Colston MJ, Davis EO, Av-Gay Y. 2005. Deletion of the Mycobacterium tuberculosis pknH gene confers a higher bacillary load during the chronic phase of infection in BALB/c mice. J Bacteriol 187:5751–5760. 10.1128/JB.187.16.5751-5760.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnvig KB, Young DB. 2009. Identification of small RNAs in Mycobacterium tuberculosis. Mol Microbiol 73(3):397–408. 10.1111/j.1365-2958.2009.06777.x 10.1111/j.1365-2958.2009.06777.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleischmann RD, Alland D, Eisen JA, Carpenter L, White O, Peterson J, et al. 2002. Whole-genome comparison of Mycobacterium tuberculosis clinical and laboratory strains. J Bacteriol 184:5479–5490. 10.1128/JB.184.19.5479-5490.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Li D, Zhao L, Fleming J, Lin N, Wang T, et al. 2013. Genome sequencing of 161 Mycobacterium tuberculosis isolates from China identifies genes and intergenic regions associated with drug resistance. Nat Genet 45(10):1255–60. 10.1038/ng.2735 10.1038/ng.2735 [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Zhang H, Zhou T, Zhong Y, Jin Q. 2011. Genes under positive selection in Mycobacterium tuberculosis. Comput Biol Chem 35(5):319–322. 10.1016/j.compbiolchem.2011.08.001 10.1016/j.compbiolchem.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 42.Rasko DA, Rosovitz MJ, Myers GSA, Mongodin EF, Fricke WF, Gajer P, et al. 2008. The Pangenome Structure of Escherichia coli: Comparative Genomic Analysis of E. coli Commensal and Pathogenic Isolates. J Bacteriol 190(20):6881–6893. 10.1128/JB.00619-08 10.1128/JB.00619-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Retchless AC, Lawrence JG. 2007. Temporal fragmentation of speciation in bacteria. Science 317(5841):1093–1096. 10.1126/science.1144876 [DOI] [PubMed] [Google Scholar]
- 44.Duffy S, Shackelton LA, Holmes EC. 2008. Rates of evolutionary change in viruses: patterns and determinants. Nat Rev Genet 9(4):267–276. 10.1038/nrg2323 10.1038/nrg2323 [DOI] [PubMed] [Google Scholar]
- 45.Sauna ZE, Kimchi-Sarfaty C. 2011. Understanding the contribution of synonymous mutations to human disease. Nat Rev Genet 12(10):683–691. 10.1038/nrg3051 10.1038/nrg3051 [DOI] [PubMed] [Google Scholar]
- 46.Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, et al. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 103:2869–2873. 10.1073/pnas.0511240103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A. Accession numbers of additional strains used in this study. Table B. Summary of genome assemblies of M. tuberculosis Manila family strains. Table C. Gene list of STPK coupled ABC transporters. Fig A. Alignment of genome sequence of Manila family isolate 11L4601 with 96121 reference sequence. X axis presents complete genome of Manila family 96121, and y axis presents contigs of Manila family 11L4601. Fig B. PCA of presence and absence of CRISPR spacers in Manila family 96121 and 11L4601, T17, T92, M. bovis, M. canettii and strains in lineages 2–4. Fig C. Predicted secondary structures of cobalamin riboswitch and ASpks small RNA. (A) Predicted secondary structure of cobalamin riboswitch in Manila family 96121. (B) Predicted secondary structure of cobalamin riboswitch in H37Rv. (C) Predicted secondary structure of ASpks small RNA in Manila family 96121. (D) Predicted secondary structure of ASpks small RNA in H37Rv. Fig D. Relationship between Ka, Ks, Ka/Ks values in Manila family 96121-H37Rv (A, B, C) and Beijing family 96075-H37Rv (D, E, F).
(PDF)
Data Availability Statement
All relevant data and accession numbers are within the paper and its Supporting Information files.