Abstract
The potential prognostic value of GATA binding protein 3 (GATA3) in breast cancer has recently increased, although the evidence is inconclusive. This meta-analysis of 10 articles involving 5,080 breast cancer patients explored the prognostic and clinicopathological value of GATA3 in breast cancer. Time to tumor progression (TTP) and overall survival (OS) were primary endpoints. Pooled hazard ratio (HR), pooled risk ratio (RR), and 95% confidence interval (CI) were calculated to evaluate the association between GATA3, prognosis, and clinicopathological parameters. High GATA3 expression predicts breast cancer, with a HR (HR = 0.671; 95% CI = 0.475–0.947; P = 0.023) of TTP, but is not associated with OS (HR = 0.889; 95% CI = 0.789–1.001; P = 0.052). GATA3 overexpression is associated with positive ER (RR = 3.155; 95% CI = 1.680–5.923; P = 0.000), positive PR (RR = 3.949; 95% CI = 1.567–9.954, P = 0.004), lower nuclear grade (RR = 0.435; 95% CI = 0.369–0.514; P = 0.000), and smaller tumor size (RR = 0.816; 95% CI = 0.709–0.940; P = 0.005). High GATA3 expression may predict TTP in breast cancer, and such patients may show better clinicopathological features.
Introduction
Transcription factors or trans-acting factors are often organized in multigene families and play essential roles in activating target genes of specific cell fates by binding to their cognate DNA sequence to aid (and sometimes inhibit) RNA polymerase II (pol II) in locating the proper initiation site for transcription [1, 2]. The GATA family of transcription factors, which is composed of six highly conserved transcription factors, binds a consensus DNA sequence (A/T)GATA(A/G) in the promoters of target genes via two zinc-finger domains with the consensus sequence CX2CX17CX2C to directly activate or repress target gene expression [3].
GATA transcription factors play a wide role in determination of cell differentiation and control of cell proliferation and movement. GATA1, GATA2, and GATA3 are expressed primarily in hematopoietic cells and are linked to their specification, while GATA4, GATA5, and GATA6 play key roles in the specification of mesoderm and endoderm-derived tissues including the heart, intestines, and lungs [4]. In particular, GATA3 is also present in non-hematopoietic tissues including the kidneys [5], central nervous system [6], endothelial cells [7], and mammary gland [8], regulating their specification and differentiation.
During puberty, pregnancy, lactation, and involution in women, the mammary gland undergoes morphologic changes including cellular proliferation, differentiation, and apoptosis [9]. GATA3 plays a vital role in orchestrating the lineage determination and maturation of these cells by directing mammogenesis towards a luminal cell fate [10]. Even though GATA3 is so important to the mammary gland, how GATA3 influences survival of breast carcinoma patients remains an important question. To our knowledge, no meta-analyses have been performed on this topic thus far. Here, we performed a meta-analysis to explore determine the prognostic and clinicopathological value of GATA3 in breast cancer.
Materials and methods
Data sources and keywords
We searched studies without language restrictions using the bibliographic databases PubMed/MEDLINE, Web of Science, EMBASE, and the Cochrane Library databases from inception to July 29, 2016. Both Medical Subject Headings (MeSH) terms and free-text words were used in the search strategy to increase sensitivity. The key terms were “GATA3,” “HDR,” “HDRS,” “GATA binding protein 3,”; “breast cancer,” “breast carcinoma,” or “breast tumor”; and “survival,” “outcome,” or “prognosis.” Additional relevant papers were obtained through extensive crosschecking of the reference lists of all retrieved articles, if necessary.
Inclusion and exclusion criteria
Studies were selected for meta-analysis if they fulfilled the following criteria: (1) studies must have focused on breast cancer; (2) all patients must have had breast cancer diagnoses confirmed via pathological examination; (3) the expression level of GATA3 protein must be analyzed in tumor tissues by immunohistochemical staining (IHC); (4) adequate data of pooled hazard ratios (HRs) and 95% confidence intervals (CIs); and (5) the correlation between GATA3 and clinicopathological features was discussed. Exclusion criteria were as follows: (1) duplicate publication; (2) non-English papers where non-human experiments were performed; (3) conference abstract; (4) letters, reviews, case reports, editorials, and comments; (5) not a prognostic study about breast cancer; (6) insufficient data regarding 95% CI and HRs provided, and a Kaplan-Meier curve could not be extracted. In addition, for studies where more than one article was published from a single center, the article with the information most relevant to this study was included [11].
Data extraction
Data extraction was conducted independently by two investigators (Yawen Guo and Pan Yu) from selected studies according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [12]. For each relevant parameter, data were extracted including the following: surname of first author, year of publication, country, number of patients, specimen, detection method for GATA3 protein expression, cut-off value for the rates of GATA3 positive/high expression, duration of follow-up after surgery, and end-point. To evaluate the quality of the studies included we used the Newcastle-Ottawa Scale (NOS) for each study, and a score ≥ 6 was considered a high quality study [13]. Disagreement between investigators was resolved by consensus. Engauge Digitizer software, version 4.1 (http://digitizer.sourceforge.net) was utilized to reconstruct the HRs estimate and its variance. For articles that only provided survival data in a Kaplan Meier curve, we assumed that patients were censored at a constant rate during follow-up.
Statistical analyses
Statistical analysis was performed according to the guidelines proposed by the MetaAnalysis of Observational Studies in Epidemiology (MOOSE) group [14]. Heterogeneity analysis was carried out by calculating the I2, which was interpreted as low (25%), moderate (50%), or high (75%); a P value of > 0.10 indicated a lack of heterogeneity among studies [12]. Pooled HRs and 95% CIs were obtained for the two outcome endpoints overall survival (OS) and time to tumor progression (TTP), which is a unified prognostic parameter combining disease-free survival (DFS) and relapse-free survival (RFS), cause they all means that the disease progression, via a fixed effects model if there was an absence of statistical heterogeneity among the studies. A random-effects model was adopted if heterogeneity was observed. An HR > 1 indicated worse survival for patients with high GATA3 expression, whereas an HR < 1 implied a survival benefit. What is more, pooled risk ratios (RRs) and their 95% CIs were used to assess the correlations between GATA3 expression and several clinicopathological features of breast cancer including the expression of ER, PR, Her-2, and Ki67; tumor size; lymph node metastasis; and nuclear grade. Forest plots were sorted according to the name of the first author, year, and country to illustrate HRs and RRs. Begg’s funnel plots and the Egger’s test were employed to estimate possible publication bias. We also performed sensitivity analysis by omitting each study or specific studies to find potential outliers. All these statistical analyses were performed using Stata version 12.0 (StataCorp, College Station, Texas, USA). P values for all comparisons were two-tailed and statistical significance was defined as P < 0.05.
Results
Study characteristics
A total of 587 articles were identified after the literature search from PubMed/MEDLINE, Web of Science, EMBASE, and the Cochrane Library databases utilizing different combinations of key terms. After reviewing titles and abstracts, 317 articles were excluded for being a non-English, non-human study (n = 1); a review, book, letter, or case report (n = 17); a conference abstract (n = 149); or not a prognostic study (n = 150). Additionally, 74 articles were excluded after screening the complete text because they were reviews (n = 1), not a prognostic study (n = 52), or had insufficient data (n = 21). The remaining 10 articles involving 5080 patients fulfilled our inclusion criteria [15–24]. The process of study selection is shown as a flow diagram (Fig 1). In addition, after evaluating the quality of all selected articles with the Newcastle-Ottawa Scale (NOS), each showed a high score (Table 1).
Fig 1. Flow chart of study selection.
Table 1. Basic characteristics of the studies included in this meta-analysis evaluating survival in breast cancer patients.
| Author | Year | Country | Number of patients | Specimen | Detection method | Cut-off (positive/high expression) | Follow up (months) | End-point | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Mehra | 2005 | America | 139 | Tissue | IHC | a median intensity of > 2.5 | 106.8 (1.5–204) | DFS/OS | 7 |
| Voduc | 2008 | Canada | 3119 | Tissue | IHC | > 5% positive nuclear staining | 151.2 (1–222) | BCSS/RFS/DFS/DRFS/OS | 8 |
| Albergaria | 2009 | Portugal | 249 | Tissue | IHC | scores ≥ 4 | 32(2–129) | DFS | 7 |
| Ciocca | 2009 | America | 166 | Tissue | IHC | ≥ 20% tumor cells staining | 115(4–133) | RFS/OS | 7 |
| Yoon | 2010 | America | 86 | Tissue | IHC | weighted scores ≥ 1.8 | NA | DFS | 6 |
| Gulbahce | 2013 | America | 516 | Tissue | IHC | ≥ 1% tumor cells staining | 81(3–140) | DFS | 7 |
| Hosoda | 2014 | Japan | 289 | Tissue | IHC | ≥ 10% positive nuclear staining | 66.2(3–114) | DFS | 7 |
| Hisamatsu | 2015 | Japan | 214 | Tissue | IHC | ≥ 77.4% median value | 58.1 | DFS/OS | 8 |
| McCleskey | 2015 | America | 72 | Tissue | IHC | H-score > 210 | 55.2 | RFS/OS | 7 |
| Min | 2016 | Korea | 230 | Tissue | IHC | immunoreactive score > 5 | NA | RFS/OS | 6 |
NOS score, Newcastle-Ottawa Scale score; NA, not available; H-score, Histo-score; DFS, disease-free survival; OS, overall survival; RFS, relapse-free survival; BCSS, breast cancer-specific survival; DRFS, distant relapse-free survival.
The baseline characteristics of each article are summarized in Table 1. All 10 studies were published between 2005 and 2016. Seven included Caucasian patient populations (five from America, one from Canada, and one from Portugal) and three included Asian patient populations (two from Japan and one from Korea). IHC was used to measure the protein expression level of GATA3 in breast cancer tissues in all studies, but each study has its own cut-off value to identify whether the expression level of GATA3 was high or not. Follow-up after surgery ranged from 1 to 222 months. TTP was used as an endpoint in all studies, while five also used OS as an endpoint. Moreover, several included studies mentioned a connection between expression of GATA3 with clinicopathological features; among them, four focused on the expression of ER, PR, Her-2 and nuclear grade, while three focused on tumor size and lymph node metastasis.
Effect of GATA3 protein expression level on survival and pathological features of breast cancer.
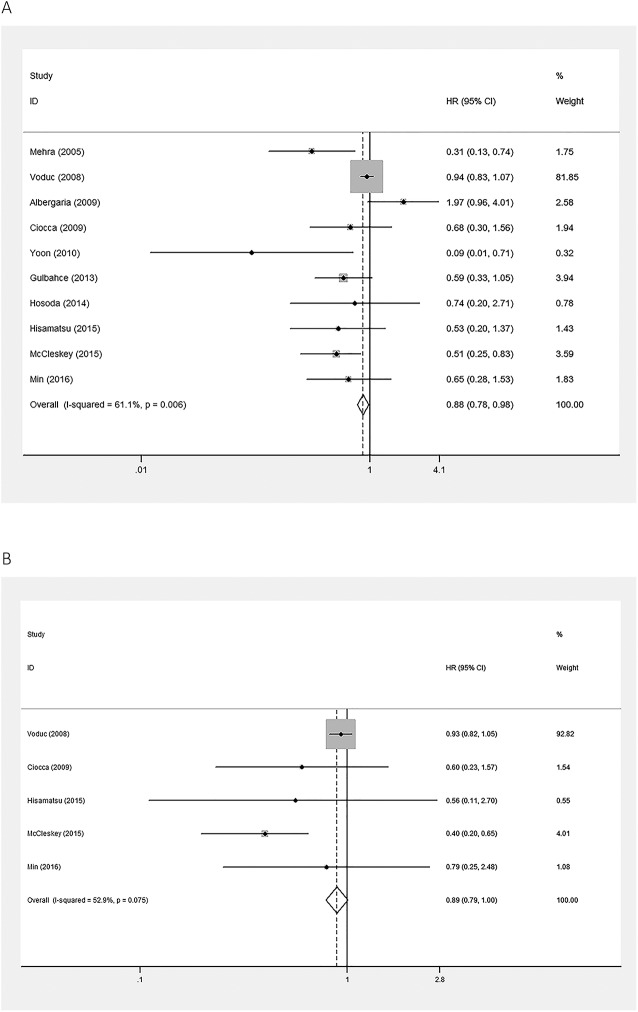
Pooled HRs of OS and TTP were used to illustrate survival of breast cancer patients in this meta-analysis. A random effects model was applied to calculate the pooled HR and 95% CI of TTP, which included 10 articles, as the heterogeneity test reported a P value of 0.006 and an I2 value of 61.1%. High expression of GATA3 was associated with better TTP of breast cancer (pooled HR = 0.671; 95% CI = 0.475–0.947; P = 0.023; Fig 2A). A fixed effects model was used to calculate the pooled HR and 95% CI of OS, which included five studies, as the heterogeneity test reported a P value of 0.075 and an I2 value of 52.9%. There was no association between expression of GATA3 and OS in breast cancer (pooled HR = 0.889; 95% CI = 0.789–1.001; P = 0.052; Fig 2B).
Fig 2. Forest plots of meta-analysis of the association between GATA3 expression and the prognoses of patients with breast cancer.
A. Time to tumor progression (TTP); B. Overall survival (OS). Abbreviations: HR, hazard ratio; CI, confidence interval.
Pooled RRs were used to illustrate the association between GATA3 protein expression level and pathological features of breast cancer. Four studies were included in the meta-analysis of ER, PR, and HER-2 expression. Random effects models were used to calculate the pooled RR and 95% CI for existent heterogeneity (I2 = 93.1%, P = 0.000; I2 = 93.0%, P = 0.000; I2 = 80.5%, P = 0.002). High expression of GATA3 was associated with positive expression of ER and PR, but had no association with HER-2 (pooled RR = 3.155, 95% CI = 1.680–5.923, P = 0.000; pooled RR = 3.949, 95% CI = 1.567–9.954, P = 0.004; pooled RR = 0.659, 95% CI = 0.304–1.427, P = 0.290; Fig 3A, 3B and 3C).
Fig 3. Forest plots of meta-analysis of the association between GATA3 expression and pathological features of patients with breast cancer.
A. ER (+ vs.—); B. PR (+ vs.—); C. HER-2 (+ vs.—); D. nuclear grade (1 or 2 vs. 3); E. Tumor size (≤ 2 vs. > 2); F. Lymph node metastasis (negative vs. positive). Abbreviation: RR, risk ratio.
Four studies were included in the meta-analysis of nuclear grade (1 or 2 vs. 3). A fixed effects model was used to calculate the pooled RR and 95% CI, as the heterogeneity test reported a P value of 0.107 and an I2 value of 50.8%. High expression of GATA3 was associated with lower nuclear grade (pooled RR = 0.435; 95% CI = 0.369–0.514; P = 0.000; Fig 3D).
Three studies were included in the meta-analysis of tumor size (≤2 vs. >2) and lymph node metastasis (negative vs. positive). A fixed effects model was used for tumor size and a random effects model was used for lymph node metastasis to calculate the pooled RR and 95% CI for the heterogeneity test report as follows: I2 = 20.4%, P = 0.285; I2 = 57.6%, P = 0.094. High expression of GATA3 was associated with smaller tumor size, but was unrelated to lymph node metastasis (pooled RR = 0.816, 95% CI = 0.709–0.940, P = 0.005; pooled RR = 0.907, 95% CI = 0.808–1.017, P = 0.611; Fig 3E and 3F)
Publication bias and sensitivity analysis
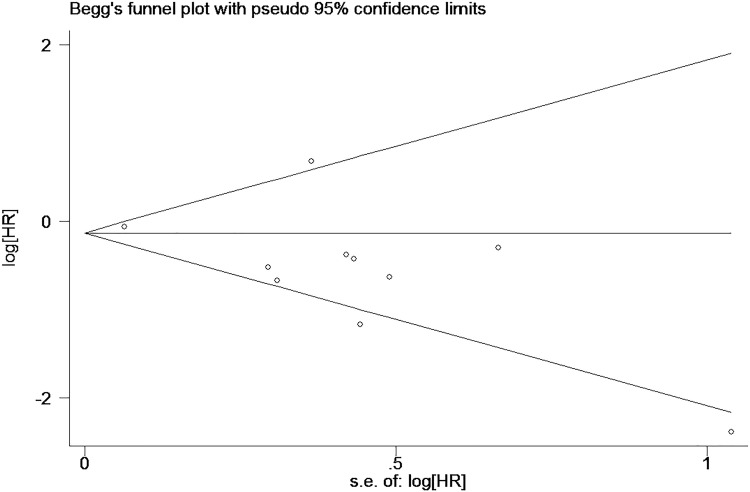
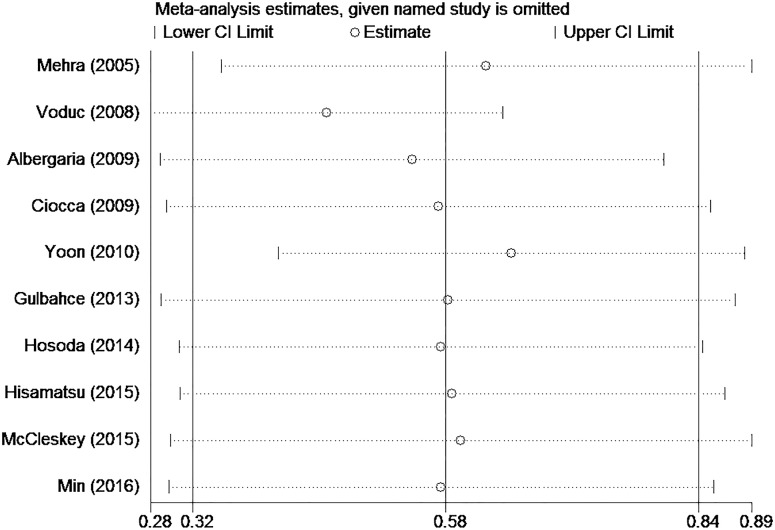
We used Egger’s tests to estimate publication bias of the included studies for each meta-analysis that was statistically significant (TTP, expression of ER and PR, tumor size, and nuclear grade). Egger’s tests revealed non-significant values (all P > 0.05). The graphical output of the Begg’s funnel plots of the 10 included studies showed symmetry (Fig 4). In addition, sensitivity analysis was performed to assess the influence of individual studies on the meta-analyses. The significance of the pooled HRs was not affected by omitting any single study, which highlighted the lack of publication bias and supports the credibility of the results (Fig 5).
Fig 4. Begg’s funnel plots for the relationship between GATA3 expression and breast cancer.
Fig 5. Sensitivity analysis to investigate the association between GATA3 expression and breast cancer prognosis.
Discussion
GATA3 is associated with a less aggressive phenotype and a better prognosis in patients with breast cancer [15–22, 24]. However, until now, no meta-analyses have been performed to evaluate the prognostic value of GATA3 protein expression in breast cancer patients. To our knowledge, this meta-analysis is the first comprehensive assessment of the literature focused on this topic. Our results indicate that high expression of GATA3 is associated with an unfavorable prognosis and is a predictive factor associated with TTP in breast cancer patients (pooled HR = 0.671; 95% CI = 0.475–0.947; P = 0.023) but is not significantly associated with OS (pooled HR = 0.889; 95% CI = 0.789–1.001; P = 0.052). To further explore the role of GATA3 in breast cancer patients, we performed subgroup analysis between GATA3 and clinicopathological parameters, finding that overexpression of GATA3 is associated with positive ER (pooled RR = 3.155; 95% CI = 1.680–5.923; P = 0.000), positive PR (pooled RR = 3.949; 95% CI = 1.567–9.954; P = 0.004), lower nuclear grade (pooled RR = 0.435; 95% CI = 0.369–0.514; P = 0.000), and smaller tumor size (pooled RR = 0.816; 95% CI = 0.709–0.940; P = 0.005), which is consistent with several studies that have been published [25–27].
To avoid bias from the method used to detect GATA3 protein expression, we excluded one study that used immunofluorescence staining [28]. Meanwhile, three studies that focused on GATA3 mRNA expression or GATA3 mutation by PCR or RT-PCR were excluded from this meta-analysis [29–31]. There is no doubt that comparing IHC-based protein expression with microarray-based gene-expression levels can lead to quite distinct conclusions. However, even at the gene level, the connection between GATA3 expression and survival of breast cancer patients exists. Thakkar et al. found that high expression of GATA3 mRNA improves prediction of RFS in estrogen receptor-positive and node-positive breast tumors [31] and Jiang et al. suggested that GATA3 mutations were correlated with improved OS in the entire population [29]; however, Liu et al. indicated that GATA3 mRNA expression, but not GATA3 mutation, is an independent predictor of prolonged progression-free survival (PFS) in ER-positive breast cancer patients who received first-line tamoxifen for recurrent disease [30]. The differences in results between these studies may be mainly due to differences in the detection method used, cut off value, and the subtype of breast cancer. Although there were differences in their findings, all found an association between GATA3 and survival at the gene level; however, this hypothesis requires further study.
Our meta-analysis suggests that GATA3 is a critical biomarker for predicting improved survival in breast cancer patients, which is the major finding of this study. Nevertheless, there are some limitations of this study that should be taken into consideration when interpreting patient outcomes. First, although we used a random-effects model when there was heterogeneity among the included studies, we could not avoid the effect of heterogeneity on the results. This heterogeneity may have been caused by varying patient characteristics or differences in the specific study designs in the different subtypes of breast cancer. Second, although we did not detect significant asymmetry via Begg’s funnel plots and found no evidence of publication bias via an Egger’s test, publication bias may have been inevitable as studies are more likely to be published if they have positive results than if they have negative results. Thus, the pooled results may be an overestimate. Third, the cut-off value of GATA3 protein expression varied between studies and the results are necessarily influenced by individual variations in interpretation by different observers or antibody, which is a major drawback and limits immediate clinical application. However, there is no standard cut-off value that is agreed upon, or used, by every one. Thus, to achieve a more convincing conclusion, further analysis using a larger sample size, a unified detection method, and adjusted individual data will be required.
In conclusion, this meta-analysis revealed that high expression of GATA3 in breast cancer is associated with improved TTP. Meanwhile, high GATA3 expression may be associated with positive ER, positive PR, smaller tumor size, and lower nuclear grade, all of which lead to improved survival.
Supporting information
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the the National Natural Science Foundation of China: NSFC 81550009 (http://www.nsfc.gov.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Fang SH, Chen Y, Weigel RJ. GATA-3 as a marker of hormone response in breast cancer. The Journal of surgical research. 2009;157(2):290–5. Epub 2008/12/09. doi: 10.1016/j.jss.2008.07.015 [DOI] [PubMed] [Google Scholar]
- 2.Chou J, Provot S, Werb Z. GATA3 in Development and Cancer Differentiation: Cells GATA Have It! Journal of cellular physiology. 2010;222(1):42–9. doi: 10.1002/jcp.21943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kouros-Mehr H, Kim JW, Bechis SK, Werb Z. GATA-3 and the regulation of the mammary luminal cell fate. Curr Opin Cell Biol. 2008;20(2):164–70. PubMed Central PMCID: PMCPMC2397451. doi: 10.1016/j.ceb.2008.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tevosian SG. Transgenic mouse models in the study of reproduction: insights into GATA protein function. Reproduction. 2014;148(1):R1–R14. doi: 10.1530/REP-14-0086 [DOI] [PubMed] [Google Scholar]
- 5.Grote D, Souabni A, Busslinger M, Bouchard M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133(1):53–61. doi: 10.1242/dev.02184 [DOI] [PubMed] [Google Scholar]
- 6.Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nature genetics. 2000;25(2):209–12. doi: 10.1038/76080 [DOI] [PubMed] [Google Scholar]
- 7.Song H, Suehiro J, Kanki Y, Kawai Y, Inoue K, Daida H, et al. Critical role for GATA3 in mediating Tie2 expression and function in large vessel endothelial cells. The Journal of biological chemistry. 2009;284(42):29109–24. PubMed Central PMCID: PMCPMC2781456. doi: 10.1074/jbc.M109.041145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127(5):1041–55. PubMed Central PMCID: PMCPMC2646406. doi: 10.1016/j.cell.2006.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naylor MJ, Ormandy CJ. Gata-3 and mammary cell fate. Breast cancer research: BCR. 2007;9(2):302 PubMed Central PMCID: PMCPMC1868924. doi: 10.1186/bcr1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ, et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer cell. 2008;13(2):141–52. PubMed Central PMCID: PMCPMC2262951. doi: 10.1016/j.ccr.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y, Yu P, Liu Z, Maimaiti Y, Wang S, Yin X, et al. Prognostic and Clinicopathological Value of Programmed Death Ligand-1 in Breast Cancer: A Meta-Analysis. PloS one. 2016;11(5):e0156323 doi: 10.1371/journal.pone.0156323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 14.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16 PubMed Central PMCID: PMCPMC1920534. doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albergaria A, Paredes J, Sousa B, Milanezi F, Carneiro V, Bastos J, et al. Expression of FOXA1 and GATA-3 in breast cancer: the prognostic significance in hormone receptor-negative tumours. Breast cancer research: BCR. 2009;11(3):R40 Epub 2009/06/25. PubMed Central PMCID: PMCPMC2716509. doi: 10.1186/bcr2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciocca V, Daskalakis C, Ciocca RM, Ruiz-Orrico A, Palazzo JP. The significance of GATA3 expression in breast cancer: a 10-year follow-up study. Human pathology. 2009;40(4):489–95. doi: 10.1016/j.humpath.2008.09.010 [DOI] [PubMed] [Google Scholar]
- 17.Gulbahce HE, Sweeney C, Surowiecka M, Knapp D, Varghese L, Blair CK. Significance of GATA-3 expression in outcomes of patients with breast cancer who received systemic chemotherapy and/or hormonal therapy and clinicopathologic features of GATA-3-positive tumors. Human pathology. 2013;44(11):2427–31. Epub 2013/09/04. doi: 10.1016/j.humpath.2013.05.022 [DOI] [PubMed] [Google Scholar]
- 18.Hisamatsu Y, Tokunaga E, Yamashita N, Akiyoshi S, Okada S, Nakashima Y, et al. Impact of GATA-3 and FOXA1 expression in patients with hormone receptor-positive/HER2-negative breast cancer. Breast cancer (Tokyo, Japan). 2015;22(5):520–8. Epub 2014/01/15. [DOI] [PubMed] [Google Scholar]
- 19.Hosoda M, Yamamoto M, Nakano K, Hatanaka KC, Takakuwa E, Hatanaka Y, et al. Differential expression of progesterone receptor, FOXA1, GATA3, and p53 between pre- and postmenopausal women with estrogen receptor-positive breast cancer. Breast cancer research and treatment. 2014;144(2):249–61. doi: 10.1007/s10549-014-2867-0 [DOI] [PubMed] [Google Scholar]
- 20.McCleskey BC, Penedo TL, Zhang K, Hameed O, Siegal GP, Wei S. GATA3 Expression in Advanced Breast Cancer: Prognostic Value and Organ-Specific Relapse. American journal of clinical pathology. 2015;144(5):756–63. doi: 10.1309/AJCP5MMR1FJVVTPK [DOI] [PubMed] [Google Scholar]
- 21.Mehra R, Varambally S, Ding L, Shen R, Sabel MS, Ghosh D, et al. Identification of GATA3 as a breast cancer prognostic marker by global gene expression meta-analysis. Cancer research. 2005;65(24):11259–64. Epub 2005/12/17. doi: 10.1158/0008-5472.CAN-05-2495 [DOI] [PubMed] [Google Scholar]
- 22.Min KW, Kim DH, Do SI, Chae SW, Kim K, Sohn JH, et al. Negative association between GATA3 and fascin could predict relapse-free and overall survival in patients with breast cancer. Virchows Archiv. 2016;468(4):409–16. doi: 10.1007/s00428-015-1894-5 [DOI] [PubMed] [Google Scholar]
- 23.Voduc D, Cheang M, Nielsen T. GATA-3 expression in breast cancer has a strong association with estrogen receptor but lacks independent prognostic value. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(2):365–73. Epub 2008/02/13. [DOI] [PubMed] [Google Scholar]
- 24.Yoon NK, Maresh EL, Shen D, Elshimali Y, Apple S, Horvath S, et al. Higher levels of GATA3 predict better survival in women with breast cancer. Human pathology. 2010;41(12):1794–801. Epub 2010/11/17. PubMed Central PMCID: PMCPMC2983489. doi: 10.1016/j.humpath.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivyna Bong PN, Zakaria Z, Muhammad R, Abdullah N, Ibrahim N, Emran NA, et al. Expression and mutational analysis of GATA3 in Malaysian breast carcinomas. Malaysian Journal of Pathology. 2010;32(2):117–22. [PubMed] [Google Scholar]
- 26.Izzo F, Mercogliano F, Venturutti L, Tkach M, Inurrigarro G, Schillaci R, et al. Progesterone receptor activation downregulates GATA3 by transcriptional repression and increased protein turnover promoting breast tumor growth. Breast Cancer Research. 2014;16(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demir H, Turna H, Can G, Ilvan S. Clinicopathologic and prognostic evaluation of invasive breast carcinoma molecular subtypes and GATA3 expression. Journal of Buon. 2010;15(4):774–82. [PubMed] [Google Scholar]
- 28.Dolled-Filhart M, Ryden L, Cregger M, Jirstrom K, Harigopal M, Camp RL, et al. Classification of breast cancer using genetic algorithms and tissue microarrays. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12(21):6459–68. Epub 2006/11/07. [DOI] [PubMed] [Google Scholar]
- 29.Jiang YZ, Yu KD, Zuo WJ, Peng WT, Shao ZM. GATA3 mutations define a unique subtype of luminal-like breast cancer with improved survival. Cancer. 2014;120(9):1329–37. doi: 10.1002/cncr.28566 [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Prager-van der Smissen WJ, Look MP, Sieuwerts AM, Smid M, Meijer-van Gelder ME, et al. GATA3 mRNA expression, but not mutation, associates with longer progression-free survival in ER-positive breast cancer patients treated with first-line tamoxifen for recurrent disease. Cancer letters. 2016;376(1):104–9. Epub 2016/03/29. doi: 10.1016/j.canlet.2016.03.038 [DOI] [PubMed] [Google Scholar]
- 31.Thakkar A, Raj H, Ravishankar, Muthuvelan B, Balakrishnan A, Padigaru M. High Expression of Three-Gene Signature Improves Prediction of Relapse-Free Survival in Estrogen Receptor-Positive and Node-Positive Breast Tumors. Biomarker insights. 2015;10:103–12. doi: 10.4137/BMI.S30559 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.