Abstract
Prostaglandin D2 (PGD2) is one of the most potent endogenous sleep promoting substances. PGD2 activates the PGD2 receptor (DPR) and increases the extracellular level of adenosine in wild-type (WT) mice but not DPR knockout (KO) mice, suggesting that PGD2-induced sleep is DPR-dependent, and adenosine may be the signaling molecule that mediates the somnogenic effect of PGD2. The aim of this study was to determine the involvement of the adenosine A2A receptor (A2AR) in PGD2-induced sleep. We infused PGD2 into the lateral ventricle of WT and A2AR KO mice between 20:00 and 2:00 for 6 h, and electroencephalograms and electromyograms were simultaneously recorded. In WT mice, PGD2 infusion dose-dependently increased non-rapid eye movement (non-REM, NREM) sleep, which was 139.1%, 145.0% and 202.7% as large as that of vehicle-treated mice at doses of 10, 20 and 50 pmol/min, respectively. PGD2 infusion at doses of 20 and 50 pmol/min also increased REM sleep during the 6-h PGD2 infusion and 4-h post-dosing periods in WT mice to 148.9% and 166.7%, respectively. In A2AR KO mice, however, PGD2 infusion at 10 pmol/min did not change the sleep profile, whereas higher doses at 20 and 50 pmol/min increased the NREM sleep during the 6-h PGD2 infusion to 117.5% and 155.6%, respectively, but did not change the sleep in the post-dosing period. Moreover, PGD2 infusion at 50 pmol/min significantly increased the episode number in both genotypes but only enhanced the episode duration in WT mice. The results demonstrate that PGD2-induced sleep in mice is mediated by both adenosine A2AR-dependent and -independent systems.
Keywords: PGD2, adenosine A2A receptor, A2AR KO mice, NREM sleep, REM sleep, electroencephalogram
Introduction
Prostaglandin (PG) D2 is the most abundant prostanoid produced in the central nervous system of mammals1 and one of the most potent sleep-inducing substances2,3,4,5. Clinical observations show that excessive endogenous production of PGD2 is responsible for sleep in humans under certain pathological conditions such as systemic mastocytosis6 and African sleeping sickness7. Under physiological conditions, the PGD2 concentration in the cerebrospinal fluid (CSF) of rats exhibits a circadian fluctuation coupled with the sleep-wake cycle in which it is increased in the light period when rodents mainly sleep8. The infusion of PGD2 through a microdialysis probe showed that PGD2 did not induce sleep in most parts of the brain parenchyma but effectively promoted sleep when it was infused into the subarachnoid space underlying the rostral basal forebrain of rats9,10 where the DP receptor (DPR) is predominantly localized11. PGD2 promoted sleep and increased the extracellular level of adenosine in wild-type (WT) mice but not in DPR knockout (KO) mice11, indicating that PGD2-induced sleep is DPR-dependent, and adenosine may be a signaling molecule that mediates the somnogenic effect of PGD2.
Adenosine is a modulator of the sleepiness associated with prolonged wakefulness12. During prolonged wakefulness, extracellular adenosine accumulates selectively in the basal forebrain and cortex and promotes the transition from wakefulness to non-rapid eye movement (non-REM, NREM) sleep by inhibiting cholinergic and non-cholinergic wakefulness-promoting neurons of the basal forebrain through the adenosine A1 receptor (A1R)2,3,13. Moreover, Satoh et al found that the adenosine A2A receptor (A2AR) agonist could mimic the somnogenic activity of PGD2. Administration of CGS21680, a selective A2AR agonist, into the subarachnoid space induced NREM and REM sleep14,15,16, and PGD2-induced sleep was completely inhibited by pretreatment with KF17837, an A2AR-selective antagonist in rats14. In contrast, an A1R agonist did not produce any significant increases in the total amount of either type of sleep when administered into the lateral ventricle or subarachnoid space in mice and rats15,17, suggesting that PGD2-induced sleep may be mediated by adenosine through the A2AR system. A2AR KO mice generated by Chen et al18 offer complete and specific deletion of the A2AR system in vivo and thereby avoid the problems of non-specific and partial A2AR antagonists. Therefore, mice lacking the A2AR provided a unique model to elucidate the impact of A2AR deficiency on the sleep-wake regulation of PGD2 through the combination of microinfusion of PGD2 into the lateral ventricle of mice and electroencephalogram (EEG) and electromyogram (EMG) recordings.
In the present study, we investigated the sleep-wake profiles after microinfusion of PGD2 into the lateral ventricle of the brain in WT and A2AR KO mice. The results showed that PGD2 increased sleep in both WT and A2AR-KO mice in a dose-dependent manner, yet the somnogenic effect was observed in A2AR KO mice when much higher doses of PGD2 were given. The increase in PGD2-induced sleep in A2AR KO mice was much less than that in WT mice, indicating that PGD2-induced sleep is mediated by both adenosine A2AR-dependent and -independent systems.
Materials and methods
Animals and chemicals
Male WT and A2AR KO mice of the inbred C57BL/6 strain18 (weighing 23–27 g, 11–13 weeks old) were maintained at Oriental Bioservice Ltd (Kyoto, Japan) and used in these experiments. They were housed at a constant temperature (24±0.5 °C) with a relative humidity of 60%±2% on an automatically controlled 12 h:12 h light/dark cycle (lights on at 8:00 AM) and had free access to food and water. The experimental protocols were approved by the Animal Care Committee of Osaka Bioscience Institute and were in complete compliance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. All attempts were made to minimize the number of animals used in the study and their suffering.
PGD2 (Cayman Chemical Co, Ann Arbor, MI, USA) was dissolved in sterile artificial CSF (aCSF) containing (in mmol/L, pH 7.4) 140 NaCl, 3 KCl, 1.0 MgCl2, 1.3 CaCl2, 2 Na2HPO4, and 0.2 NaH2PO4, stored in aliquots at -20 °C, and diluted to the final concentration immediately before use.
Implantation of electrodes for EEG/EMG recordings and cannulae for PGD2 microinfusion
Mice were anesthetized with sodium pentobarbital (50 mg/kg, ip) and then mounted on the stereotaxic instrument (SR-5R, Narishige, Tokyo, Japan). The incisor bar was adjusted to achieve equal heights of lambda and bregma. EEG and EMG electrodes for polysomnographic recordings were implanted as previously described19,20. Briefly, for monitoring EEG signals, two stainless steel EEG recording screws were positioned 1 mm anterior to bregma or lambda and 1.5 mm lateral to the midline. EMG activity was monitored by stainless steel, Teflon-coated wires bilaterally placed into both trapezius muscles.
For continuous infusion of solution, one 33-gauge stainless-steel cannula (Plastics One, Roanoke, VA, USA) was implanted stereotaxically into the left lateral ventricle 2 mm lateral from bregma and 2.2 mm ventral from the surface of the cortex at an angle of 25° to the midsagittal plane according to the brain atlas of Franklin and Paxinos21. A dummy cannula, protruding 0.1 mm below the tip of the cannula, was placed to prevent clogging before microinfusion. The cannula and electrodes were fixed to the skull with dental cement. When an experiment was complete, mice were euthanized with an overdose of pentobarbital sodium. The positions of the cannulae were histologically verified.
EEG and EMG recordings with microinfusion of PGD2 into the lateral ventricle of the mouse brain
After a 10-day recovery period, the mice were transferred to an experimental system for adaptation. The dummy cannulae were removed. The cannula was connected to a 100-μL microsyringe (Hamilton, Reno, Nevada, USA) with PE20 polyethylene tubing (Becton Dickinson, Sparks, MD, USA). The chronic microinjection into the lateral ventricle of the mouse was operated by a micropump (Harvard apparatus, South Natick, MT, USA) at a speed of 1 μL/h. PGD2 infusion and EEG/EMG recordings were carried out via a swivel and slip-ring designed so that behavioral movement of the mouse was not restricted.
After an acclimation period of 4 days, sleep-wakefulness states were monitored for 48 h, which comprised control and experimental days. The EEG/EMG signals were amplified, filtered (EEG: 0.5–30 Hz, EMG: 20–200 Hz), and then digitized at a sampling rate of 128 Hz and recorded using SleepSign (Kissei Comtec, Nagoya, Japan) as previously described22,23,24. The EEG power spectrum was computed for 4-s epochs by fast Fourier transform within the frequency range of 0–24.75 Hz. Baseline recordings with continuous aCSF infusion were taken in each animal for 24 h, beginning at 20:00, which served as the within-animal control. On the next day, PGD2(5, 10, 20, or 50 pmol/min) was infused into the lateral ventricle of mice for 6 h between 20:00 and 2:00, respectively. Each animal received only 1 dose of PGD2.
Vigilance state analysis
The vigilance states were automatically classified off-line in 4-s epochs into 3 stages of wakefulness, NREM and REM sleep by SleepSign, according to the standard criteria23,24. Then, defined sleep-wake stages were examined visually and corrected by hand.
Statistical analysis
The data are presented as the mean±SEM. The animal number is n=5–7 for each group. The number of the different sleep-wake states was expressed in minutes for the vigilance studies. Comparisons between vehicle and PGD2 infusion were conducted with the paired Student's t-test. The time-course data were analyzed by the repeated measures analysis of variance (ANOVA) followed by Tukey's test. The changes in sleep for 10 h after PGD2 infusion in WT and A2AR KO mice were analyzed by one-way ANOVA followed by Fisher's PLSD test. In all cases, P<0.05 was taken as the level of significance.
Results
Sleep-wake profiles induced by PGD2 in A2AR KO mice
Under basal conditions, both WT and A2AR KO mice had the following clear circadian variations on EEG: decreased sleep during the dark period and increased during the light period. Quantitative analysis of the time spent in NREM and REM sleep within 24 h showed that there was no difference between WT mice and A2AR KO mice. However, during the first 6 h of the light-off period, A2AR KO mice spent 91.9±9.2 min in NREM sleep, the duration of which was 1.5-fold of that in WT mice (61.0±8.2 min), indicating that the genetic deficiency of A2AR resulted in an increase in the total amount of NREM sleep during the first 6 h of the light-off period.
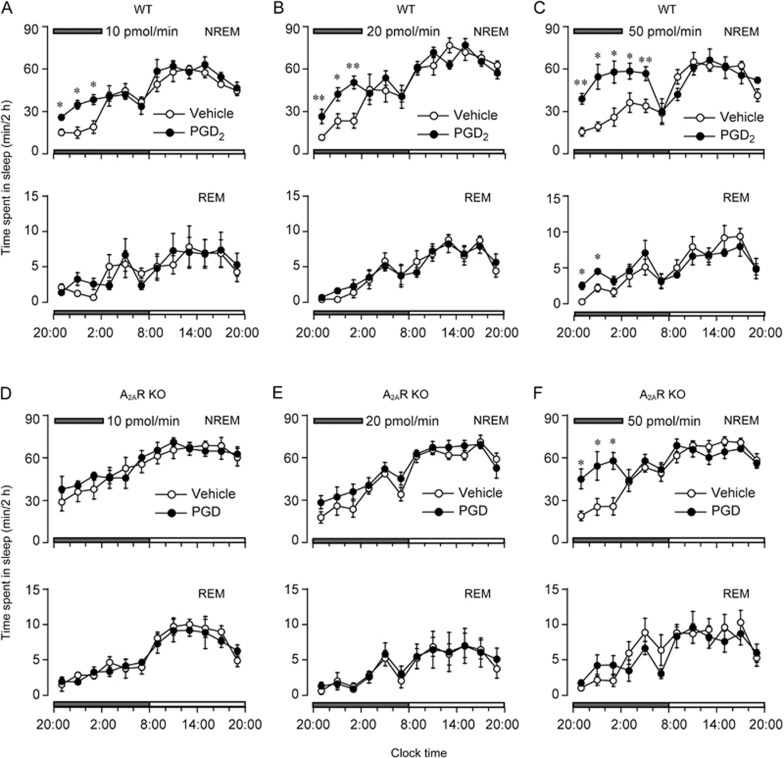
To investigate the role of adenosine A2AR in the somnogenic effect of PGD2, PGD2 was infused into the lateral ventricle of WT and A2AR KO mice during 20:00–2:00 for 6 h. As shown in Figure 1A-C, PGD2 increased sleep in WT mice. PGD2 at 20 pmol/min significantly increased NREM sleep during the 12-h dark period (F1,50=6.94, P<0.05, n=6). During the first, second and third 2-h periods of PGD2 infusion, NREM sleep was increased to 227.6% (vehicle, 11.6±1.9 min; PGD2, 26.4±5.0 min, n=6, P<0.01), 181.5% (vehicle, 23.3±5.1 min; PGD2, 42.3±4.6 min, n=6, P<0.05), and 216.2% (vehicle, 23.4±5.2 min; PGD2, 50.6±4.4 min, n=6, P<0.01) of the duration of NREM sleep in vehicle control, respectively. In contrast, there was no significant difference in REM sleep during infusion of PGD2. This enhancement of NREM sleep was concomitant with a decrease in wakefulness. PGD2 decreased the wakefulness by 13.8% (vehicle, 108.0±1.9 min; PGD2, 93.1±4.9 min, n=6, P<0.05), 20.7% (vehicle, 96.3±5.1 min; PGD2, 76.4±4.6 min, n=6. P<0.05), and 29.4% (vehicle, 95.2±4.7 min; PGD2, 67.2±4.4 min, n=6, P<0.01) during the first, second and third 2-h periods of infusion, respectively. There was no further disruption in the sleep architecture during the subsequent period. Similar time course profiles were observed with a low dose of PGD2 (10 pmol/min, Figure 1A) (F1,50=5.412, P<0.05, n=6). PGD2 at 5 pmol/min did not affect the sleep-wake distribution (data not shown). However, A2AR KO mice displayed a tendency to show an increase in NREM sleep during the 6-h infusion of PGD2 (10 or 20 pmol/min) (Figure 1D, 1E). There was no significant difference between the NREM sleep duration of each 2-h period during the experimental day and that during the baseline day.
Figure 1.
Sleep-stage distribution produced by PGD2 (10, 20, and 50 pmol/min) infusion into the lateral ventricle in WT mice (A–C) and A2AR KO mice (D– F) between 20:00 and 2:00 indicated by horizontal shaded bars on the experimental day. Each circle represents 2-h amounts of NREM and REM sleep. Open and closed circles signify the vehicle control and PGD2 treatment, respectively. Values are expressed as the mean±SEM (n=5–7). Time course changes in hourly amounts of each stage between vehicle and PGD2 infusion were compared with repeated measures ANOVA followed by Tukey's test and paired t-test. *P<0.05, **P<0.01.
When the dosage of PGD2 was increased to 50 pmol/min, NREM sleep was significantly increased in WT mice during the 6-h infusion and even during the 4 h after the infusion ended (Figure 1C, F1,50=14.923, P<0.05, n=6). The amount of NREM sleep during these 10 h was almost comparable to that observed during the day time, which is an inactive period for mice. PGD2 also increased REM sleep during the first and second 2-h periods after PGD2 infusion. On the other hand, PGD2 (50 pmol/min) increased NREM sleep in A2AR KO mice during the first, second and third 2-h periods to 237.4% (vehicle, 19.0±3.4 min; PGD2, 45.1±7.0 min, n=7, P<0.05), 213.8% (vehicle, 25.4±5.9 min; PGD2, 54.3±10.0 min, n=7, P<0.05) and 225.3% (vehicle, 25.7±6.2 min; PGD2, 57.9±5.8 min, n=7, P<0.05) of that in vehicle control, respectively (Figure 1F, F1,60=10.165, P<0.05, n=7). However, PGD2 did not increase the NREM sleep during the 4 h post-dosing.
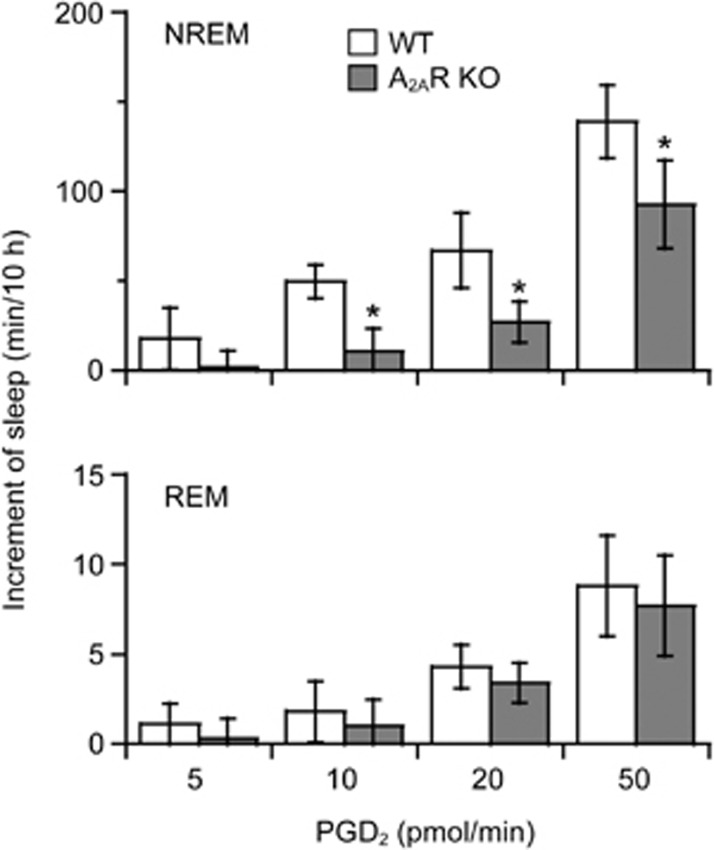
We calculated the total increase in NREM and REM sleep for 10 h, during the 6-h PGD2 infusion and 4-h post-dosing periods, as the value during an experimental day minus that of the baseline day. In WT mice, PGD2 given at 5 pmol/min had little effect on sleep-wake profiles (Figure 2). PGD2 at doses of 10, 20, and 50 pmol/min significantly increased NREM sleep to 139.1% (vehicle, 134.9±7.5 min; PGD2, 187.7±7.8 min, n=6, P<0.05), 145.0% (vehicle, 148.9±25.2 min; PGD2, 215.9±11.4 min, n=6, P<0.05), and 202.7% (vehicle, 135.4±14.7 min; PGD2, 274.5±24.6 min, n=6, P<0.01) (F3,20=7.405, P<0.01, n=6 in each group) of that in vehicle control, respectively. PGD2 at doses of 20 and 50 pmol/min also increased REM sleep to 148.9% (vehicle, 8.8±2.1 min; PGD2, 13.1±1.5 min, n=6, P<0.05) and 166.7% (vehicle, 13.2±2.4; PGD2, 22.0±2.4 min, n=6, P<0.05) (F3,20=4.552, P<0.05, n=6 in each group), respectively. However, PGD2 at 5 and 10 pmol/min did not change the sleep duration in A2AR KO mice (Figure 2). PGD2 infusion at 20 and 50 pmol/min increased NREM sleep in A2AR KO mice to 117.5% (vehicle, 154.7±17.1 min; PGD2, 181.8±11.4 min, n=5, P<0.05) and 155.6% (vehicle, 166.7±11.0 min; PGD2, 259.4±26.7 min, n=7, P<0.01) of that in vehicle control mice, respectively (F3,18=5.942, P<0.01, n=5–7). The increases in NREM sleep in A2AR KO mice were significantly less than those of WT mice with administration of PGD2 at 10, 20, and 50 pmol/min by 78.7% (WT, 49.7±9.2 min, n=6; A2AR KO, 10.6±13.1 min, n=5, P<0.05), 47.0% (WT, 67.0±21.1 min, n=6; A2AR KO, 27.1±11.6 min, n=5, P<0.05), and 41.0% (WT, 139.1± 20.2 min, n=6; A2AR KO, 92.7±24.6 min, n=7, P<0.05), respectively. The somnogenic effect of PGD2 at 10 pmol/min was abolished in A2AR KO mice, and the increase in sleep induced by PGD2 at higher doses was significantly lower in A2AR KO mice than in WT mice, indicating that PGD2-induced NREM sleep is mediated by both A2AR-dependent and -independent mechanisms.
Figure 2.
Increase in sleep for 10 h during PGD2 5, 10, 20, and 50 pmol/min infusion for 6 h and the 4-h period post-dosing. Open and shaded columns show the profiles in WT and A2AR KO mice, respectively. Values are expressed as the mean±SEM (n=5–7). *P<0.05 by one-way ANOVA followed by Fisher's PLSD test and unpaired t-test.
Analysis of power spectra and episode numbers and durations
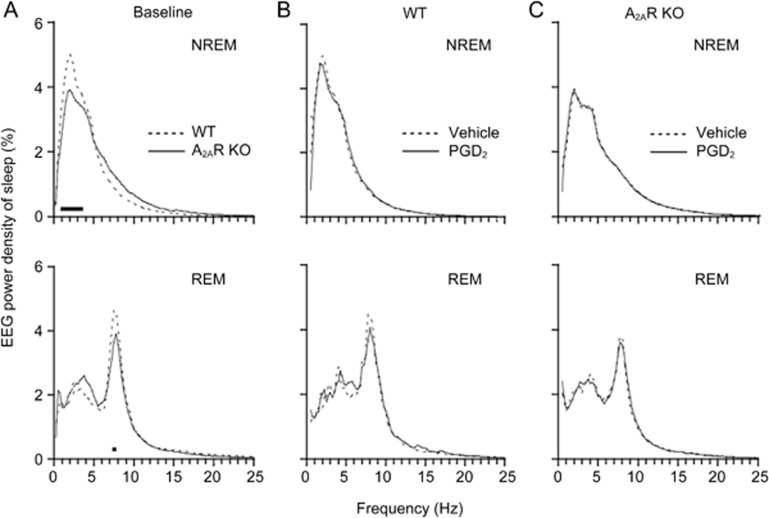
Under basal conditions, the EEG power density of NREM sleep in frequencies 1–3.75 Hz was significantly lower in A2AR KO mice than in WT mice during the first 6 h in the dark period. The peak values appeared in the 2-Hz frequency, in which the EEG power of NREM sleep was 5.0% in WT mice and 3.8% in A2AR KO mice (Figure 3A, upper). The EEG power density of REM sleep was also smaller in A2AR KO mice at 7.25–7.75 Hz during the first 6 h in the dark period than that in the WT mice (Figure 3A, lower). There was no significant change in EEG power densities of NREM or REM sleep during the other periods. These results indicate that A2AR is essential for increases in NREM and REM sleep components in those EEG ranges during the first half of the active period of mice.
Figure 3.
Mean percentage power density in the basal condition (A) and PGD2 (50 pmol/min) infusion for the first 6 h in WT (B) and A2AR KO (C) mice. Mean percentage power density calculated as the mean power (in square microvolts) in each 0.25 Hz frequency bin divided by the total power (0–24.75 Hz) in the same epoch. The horizontal bands indicate the frequency bins that significantly differed between A2AR KO and WT mice (P<0.05) by unpaired t-test in the baseline group and paired t-test in the other groups. n=5–7.
When PGD2 was given at 50 pmol/min, it did not change the EEG power density of NREM or REM sleep during the 6-h infusion in WT and A2AR KO mice (Figure 3B and 3C), suggesting that PGD2 did not change the EEG components in WT or A2AR KO mice and that it did not disturb their physiological sleep.
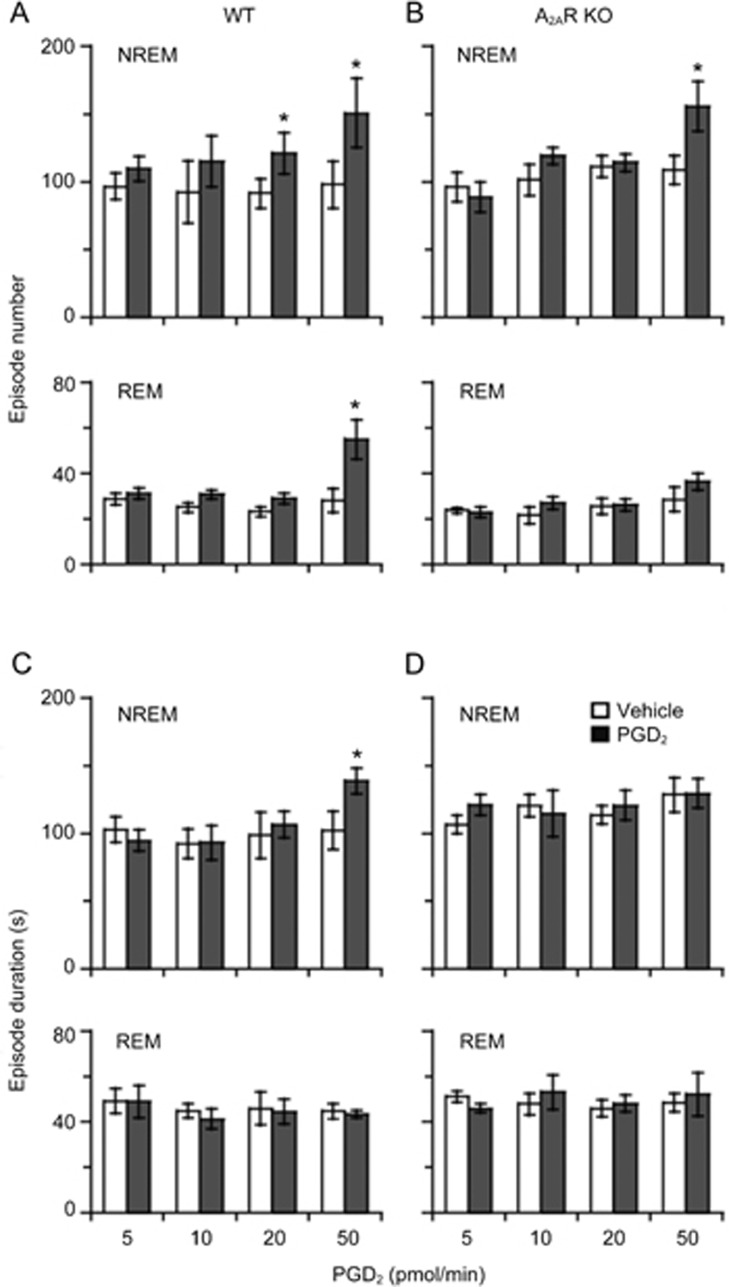
We then analyzed the episode number and duration of NREM and REM sleep in WT and A2AR KO mice during the 6-h PGD2 infusion and 4-h post-doing periods (Figure 4). PGD2 given at 20 pmol/min only increased NREM episode numbers to 132.3% (vehicle, 91.7±11.0, PGD2, 121.3±15.1, n=6, P<0.05) in WT mice (Figure 4A, upper), while PGD2 given at 50 pmol/min increased NREM episode numbers in WT to 153.8% (vehicle, 98.2±17.4; PGD2, 151.0±25.7, n=6, P<0.05) and those in A2AR KO mice to 143.4% (vehicle, 108.8±10.5; PGD2, 156.0±18.2, n=7, P<0.05) (Figure 4A and B, upper). With respect to REM sleep, the number of REM episodes was increased with 50 pmol/min of PGD2 to 195.0% (vehicle, 28.2±5.1; PGD2, 55.0±8.6, n=6, P<0.05) in WT mice (Figure 4A, lower), but there was no increase observed in A2AR KO mice (Figure 4B, lower). In addition, WT mice also displayed a significant increase in episode duration of NREM sleep with 50 pmol/min of PGD2 to 135.5% (vehicle, 102.4±18.7; PGD2, 138.8±9.4, n=6, P<0.05) (Figure 4C, upper), but A2AR KO mice did not (Figure 4D, upper). These changes resulted in more NREM sleep in WT than in A2AR KO mice after administration of PGD2 at 50 pmol/min. When the concentration of PGD2 was decreased to 10 and 5 pmol/min, PGD2 did not affect the episode number or duration in both genotypes. These analyses indicated that A2AR is crucial for the increase in episode numbers of NREM sleep by low doses of PGD2 and the increase in episode numbers of REM sleep and the elongation of NREM episodes by high doses of PGD2.
Figure 4.
Episode number (A, B) and episode duration (C, D) for 10 h during PGD2 5, 10, 20, and 50 pmol/min infusion for 6 h and the 4-h period post-dosing in WT and A2AR KO mice. Open and shaded columns show the vehicle control and PGD2 treatment, respectively. Values are expressed as the mean±SEM. n=5–7. *P<0.05 by paired t-test between vehicle and PGD2 infusion.
Discussion
In this study, we demonstrated that PGD2 induces sleep by a combination of A2AR-dependent and -independent mechanisms using A2AR KO mice. We previously demonstrated that intracerebroventricular infusion of PGD2 induces sleep and increases the extracellular levels of adenosine in the brain of rats and mice by activation of DPR, as these two effects were completely absent in DPR KO mice11. Adenosine receptors are pharmacologically and structurally classified into A1, A2A, A2B, and A3 subtypes25. Both the A1R and A2AR subtypes are reported to be involved in sleep regulation (see review)26,27. Therefore, we predicted that released adenosine is involved in PGD2-induced sleep by activating these receptors. Because A2AR deficiency attenuated the somnogenic effect of PGD2 in mice, as shown in the present study, it appeared that the adenosine A2AR system is, at least in part, involved in PGD2-induced sleep. However, PGD2-induced sleep was also observed in A2AR KO mice to various extents depending on the dose of PGD2, indicating that a part of PGD2-induced sleep is independent of A2AR.
When PGD2 was infused into the subarachnoid space just anterior to the ventrolateral preoptic area (VLPO), which has been identified as a distinct nucleus that contains a cluster of sleep-active neurons28, it promotes sleep more effectively than injections in a variety of other sites within the brain of rats9. In addition, PGD2 and an adenosine A2AR agonist induce Fos-immunoreactivity in the VLPO that is proportional to the production of sleep10,29, thereby suggesting that PGD2 and an adenosine A2AR agonist may stimulate sleep-active VLPO neurons through the same pathway. These sleep-active cells are both galaninergic and GABAergic and send inhibitory projections to waking-related neurons in the tuberomammillary nucleus (TMN)30, the sole source of histaminergic innervation of the mammalian brain, as well as the locus coeruleus and raphe nuclei31. It is known that both galanin and GABA inhibit neurons in the TMN and locus coeruleus32,33, implying that the descending projection from the VLPO is inhibitory in nature (see review)34. However, the VLPO receives reciprocal inputs from several monoaminergic systems, including the arousal-related histaminergic projections from the TMN, noradrenergic inputs from the locus coeruleus, and serotonergic inputs from the midbrain raphe nuclei35. VLPO neurons from acute hypothalamic slices are inhibited by noradrenaline, 5-hydroxytryptamine36 and histamine37, and TMN neurons also contain GABA and galanin, which may inhibit the VLPO37. The reciprocity of projections of the sleep- and wake-promoting brain regions, the VLPO and TMN, respectively, may work like a “flip-flop” circuit in electrical engineering terms. Briefly stated, when VLPO neurons are active during sleep and firing rapidly, they inhibit the monoaminergic cell groups, allowing for their own disinhibition and reinforced firing. Conversely, during wakefulness, monoaminergic neurons fire at a high rate, thus inhibiting the VLPO and resulting in the disinhibition of their own firing.
Although Ribeiro reported the importance of adenosine A1R on neurotransmitter release and the existence of a balance between adenosine activation at A1R and A2AR at many synapses38, A1R expression was indistinguishable in the cerebral cortex and striatum between WT and A2AR KO mice39. N6-Cyclopentyladenosine, a highly selective A1R agonist, did not induce sleep in WT and A2AR KO mice17, suggesting that the A1R system may not compensate for the genetic A2AR deficiency. Moreover, total sleep deprivation for 6 h induced an increase in NREM and REM sleep during the recovery period in WT mice but not in A2AR KO mice17, whereas sleep deprivation induced a strong NREM rebound in WT, heterozygotes and A1R KO mice40, suggesting that A2AR but not A1R plays an important role in the homeostatic regulation of sleep.
Activation of adenosine A2AR has been shown to promote sleep. Therefore, mice that lack A2AR should be expected to spend more time in wakefulness. However, quantitative analysis of sleep profiles during the light and dark periods showed increased spontaneous sleep and, concomitantly, decreased wakefulness during the dark period in A2AR KO mice (Figure 1). A2ARs are particularly abundant in the striatum, although they are also expressed at a much lower level in other brain areas41. A2AR is colocalized with dopamine D2 receptors (D2R) in GABAergic striatopallidal neurons. Formation of functional A2AR-D2R heteromeric complexes was proposed as a morphological framework for the existence of an A2AR-D2R interaction at the biochemical level42,43,44. Dopamine has been implicated in inducing wakefulness45. We reported that D2R KO mice displayed an increase in NREM sleep and a decrease in wakefulness23. In addition, microdialysis in the striatum revealed a 45% reduction in extracellular dopamine concentration in A2AR KO mice39. Therefore, the increase in NREM sleep (Figure 1) coupled to the decrease in the delta wave (1–3.75 Hz) component of NREM sleep (Figure 3A) is probably due to functional striatal hypodopaminergic activity in A2AR KO mice.
PGD2-induced sleep has been reported to be completely inhibited by an A2AR antagonist in rats14. However, we found that PGD2 at a high dose also increased NREM sleep in A2AR KO mice. The difference in the PGD2-induced sleep between pharmacological A2AR antagonist treatment and receptor KO mice remains to be clarified. Koyama and Hayaishi46 used head-restrained unanesthetized rats to examine the effects of PGD2 on the activity of neurons in the preoptic/anterior hypothalamic areas and found that PGD2 had an excitatory effect on approximately one-third of the NREM related-neurons examined, thereby suggesting that another possibility may be that PGD2 directly stimulates sleep-active neurons to induce sleep. Those neurons may be involved in A2AR-independent PGD2-induced sleep. On the other hand, Matsumura9 et al discovered that the PGD2-sensitive sleep promoting zone was in the ventral surface of the rostral basal forebrain; thus, region-specific microinfusion of PGD2 should be in the subarachnoid space underlying the rostral basal forebrain instead of the lateral ventricle. These differences may result in the lack of an effect of PGD2 at low doses in A2AR KO mice.
In the present study, PGD2 given at doses of 10 and 20 pmol/min induced sleep only in WT mice but not in A2AR KO mice. With an increase in dosage, PGD2 also promoted sleep in A2AR KO mice, but the amounts of sleep were much lower than that in the WT mice, suggesting that A2AR signal transduction is one of the pathways for PGD2 somnogenic effects. In summary, PGD2 promotes sleep in a manner partially dependent on A2AR, and adenosine A2AR deficiency attenuates PGD2-induced sleep.
Author contribution
Wei-min QU, Zhi-li HUANG, and Yoshihiro URADE designed this study; Bin-jia ZHANG and Wei-min QU performed the experiments and analyzed the data; Jiang-fan CHEN provided the A2AR KO mice, reviewed the manuscript and gave suggestions. All authors prepared the manuscript and approved the final version.
Abbreviations
A2AR, adenosine A2A receptor; aCSF, artificial cerebrospinal fluid; A1R, adenosine A1 receptor; DPR, PGD2 receptor; EEG, electroencephalogram; EMG, electromyogram; KO, knockout; PGD2, prostaglandin D2; REM, rapid eye movement; NREM, non-REM; VLPO, ventrolateral preoptic area; TMN, tuberomammillary nucleus; WT, wild-type.
Acknowledgments
This study was supported in part by Grants-in-Aid for scientific research from the National Natural Science Foundation of China (No 31530035, 31471064, 31271164, 81571295, and 81420108015), the National Basic Research Program of China (No 2015CB856401 and 2011CB711000), and the Shanghai Committee of Science and Technology (No 14JC1400900).
References
- Narumiya S, Ogorochi T, Nakao K, Hayaishi O. Prostaglandin D2 in rat brain, spinal cord and pituitary: basal level and regional distribution. Life Sci 1982; 31: 2093–103. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Zhang Z, Qu WM. Roles of adenosine and its receptors in sleep-wake regulation. Int Rev Neurobiol 2014; 119: 349–71. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Urade Y, Hayaishi O. The role of adenosine in the regulation of sleep. Curr Top Med Chem 2011; 11: 1047–57. [DOI] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Aritake K, Eguchi N, Nambu F, et al. Lipocalin-type prostaglandin D synthase produces prostaglandin D2 involved in regulation of physiological sleep. Proc Natl Acad Sci U S A 2006; 103: 17949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaishi O, Urade Y. Prostaglandin D2 in sleep-wake regulation: recent progress and perspectives. Neuroscientist 2002; 8: 12–5. [DOI] [PubMed] [Google Scholar]
- Roberts LJ 2nd, Sweetman BJ, Lewis RA, Austen KF, Oates JA. Increased production of prostaglandin D2 in patients with systemic mastocytosis. N Engl J Med 1980; 303: 1400–4. [DOI] [PubMed] [Google Scholar]
- Pentreath VW, Rees K, Owolabi OA, Philip KA, Doua F. The somnogenic T lymphocyte suppressor prostaglandin D2 is selectively elevated in cerebrospinal fluid of advanced sleeping sickness patients. Trans R Soc Trop Med Hyg 1990; 84: 795–9. [DOI] [PubMed] [Google Scholar]
- Pandey HP, Ram A, Matsumura H, Hayaishi O. Concentration of prostaglandin D2 in cerebrospinal fluid exhibits a circadian alteration in conscious rats. Biochem Mol Biol Int 1995; 37: 431–7. [PubMed] [Google Scholar]
- Matsumura H, Nakajima T, Osaka T, Satoh S, Kawase K, Kubo E, et al. Prostaglandin D2-sensitive, sleep-promoting zone defined in the ventral surface of the rostral basal forebrain. Proc Natl Acad Sci U S A 1994; 91: 11998–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell T, Gerashchenko D, Urade Y, Onoe H, Saper C, Hayaishi O. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2. Proc Natl Acad Sci U S A 1998; 95: 7754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A, Eguchi N, Kimura K, Kiyohara Y, Qu WM, Huang ZL, et al. Dominant localization of prostaglandin D receptors on arachnoid trabecular cells in mouse basal forebrain and their involvement in the regulation of non-rapid eye movement sleep. Proc Natl Acad Sci U S A 2001; 98: 11674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science 1997; 276: 1265–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Kalinchuk AV. Adenosine, energy metabolism and sleep homeostasis. Sleep Med Rev 2011; 15: 123–35. [DOI] [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Suzuki F, Hayaishi O. Promotion of sleep mediated by the A2a-adenosine receptor and possible involvement of this receptor in the sleep induced by prostaglandin D2 in rats. Proc Natl Acad Sci U S A 1996; 93: 5980–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Hayaishi O. Involvement of adenosine A2A receptor in sleep promotion. Eur J Pharmacol 1998; 351: 155–62. [DOI] [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Koike N, Tokunaga Y, Maeda T, Hayaishi O. Region-dependent difference in the sleep-promoting potency of an adenosine A2A receptor agonist. Eur J Neurosci 1999; 11: 1587–97. [DOI] [PubMed] [Google Scholar]
- Urade Y, Eguchi N, Qu WM, Sakata M, Huang ZL, Chen JF, et al. Sleep regulation in adenosine A2A receptor-deficient mice. Neurology 2003; 61: S94–6. [DOI] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, et al. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci 1999; 19: 9192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XH, Qiu MH, Dong H, Qu WM, Urade Y, Huang ZL. GABA transporter-1 inhibitor NO-711 alters the EEG power spectra and enhances non-rapid eye movement sleep during the active phase in mice. Eur Neuropsychopharmacol 2014; 24: 585–94. [DOI] [PubMed] [Google Scholar]
- Qu WM, Yue XF, Sun Y, Fan K, Chen CR, Hou YP, et al. Honokiol promotes non-rapid eye movement sleep via the benzodiazepine site of the GABA(A) receptor in mice. Br J Pharmacol 2012; 167: 587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinatess. San Diego; 1997.
- Wang Q, Yue XF, Qu WM, Tan R, Zheng P, Urade Y, et al. Morphine inhibits sleep-promoting neurons in the ventrolateral preoptic area via mu receptors and induces wakefulness in rats. Neuropsychopharmacology 2013; 38: 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WM, Xu XH, Yan MM, Wang YQ, Urade Y, Huang ZL. Essential role of dopamine D2 receptor in the maintenance of wakefulness, but not in homeostatic regulation of sleep, in mice. J Neurosci 2010; 30: 4382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci 2008; 28: 8462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau JL, Huber G. Central adenosine A2A receptors: an overview. Brain Res Brain Res Rev 1999; 31: 65–82. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol 2004; 73: 379–96. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Urade Y, Hayaishi O. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Curr Opin Pharmacol 2007; 7: 33–8. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science 1996; 271: 216–9. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, et al. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience 2001; 107: 653–63. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci 1998; 18: 4705–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger TL, Gong H, McGinty D, Szymusiak R. Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. J Comp Neurol 2001; 429: 638–53. [PubMed] [Google Scholar]
- Seutin V, Verbanck P, Massotte L, Dresse A. Galanin decreases the activity of locus coeruleus neurons in vitro. Eur J Pharmacol 1989; 164: 373–6. [DOI] [PubMed] [Google Scholar]
- Yang QZ, Hatton GI. Electrophysiology of excitatory and inhibitory afferents to rat histaminergic tuberomammillary nucleus neurons from hypothalamic and forebrain sites. Brain Res Brain Res Rev 1997; 773: 162–72. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 2001; 24: 726–31. [DOI] [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci 2002; 22: 977–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, et al. Identification of sleep-promoting neurons in vitro. Nature 2000; 404: 992–5. [DOI] [PubMed] [Google Scholar]
- Liu YW, Li J, Ye JH. Histamine regulates activities of neurons in the ventrolateral preoptic nucleus. J Physiol 2010; 588: 4103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JA. Adenosine A2A receptor interactions with receptors for other neurotransmitters and neuromodulators. Eur J Pharmacol 1999; 375: 101–13. [DOI] [PubMed] [Google Scholar]
- Dassesse D, Massie A, Ferrari R, Ledent C, Parmentier M, Arckens L, et al. Functional striatal hypodopaminergic activity in mice lacking adenosine A(2A) receptors. J Neurochem 2001; 78: 183–98. [DOI] [PubMed] [Google Scholar]
- Stenberg D, Litonius E, Halldner L, Johansson B, Fredholm BB, Porkka-Heiskanen T. Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J Sleep Res 2003; 12: 283–90. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol 1998; 401: 163–86. [PubMed] [Google Scholar]
- Schiffmann SN, Vanderhaeghen JJ. Adenosine A2 receptors regulate the gene expression of striatopallidal and striatonigral neurons. J Neurosci 1993; 13: 1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, et al. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem 2002; 277: 18091–7. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Ledent C, Parmentier M, Greengard P, Fredholm BB, et al. Regulation of the phosphorylation of the dopamine- and cAMP-regulated phosphoprotein of 32 kDa in vivo by dopamine D1, dopamine D2, and adenosine A2A receptors. Proc Natl Acad Sci U S A 2000; 97: 1856–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci 2001; 21: 1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Hayaishi O. Modulation by prostaglandins of activity of sleep-related neurons in the preoptic/anterior hypothalamic areas in rats. Brain Res Bull 1994; 33: 367–72. [DOI] [PubMed] [Google Scholar]