Abstract
Aspirin (ASA) is a cardioprotective drug with anti-cardiac fibrosis action in vivo. This study was aimed to clarify the anti-cardiac fibrosis action of ASA and the underlying mechanisms. Two heart injury models (injection of isoproterenol and ligation of the left anterior descending branch) were used in mice to induce cardiac fibrosis. The animals were treated with ASA (10 mg·kg−1·d−1, ig) for 21 and 14 d, respectively. ASA administration significantly improved cardiac function, and ameliorated heart damage and fibrosis in the mice. The mechanisms underlying ASA's anti-fibrotic effect were further analyzed in neonatal cardiac fibroblasts (CFs) exposed to hypoxia in vitro. ASA (0.5–5 mmol/L) dose-dependently inhibited the proliferation and Akt phosphorylation in the CFs. In addition, ASA significantly inhibited CF apoptosis, and decreased the levels of apoptosis markers (cleaved caspase 3 and Parp1), which might serve as a side effect of anti-fibrotic effect of ASA. Furthermore, ASA dose-dependently inhibited the autophagy in the CFs, as evidenced by the reduced levels of autophagy marker LC3-II. The autophagy inhibitor Pepstatin A (PepA) promoted the inhibitory effect of ASA on CF proliferation, whereas the autophagy inducer rapamycin rescued ASA-caused inhibition of CF proliferation, suggesting an autophagy-dependent anti-proliferative effect of ASA. Both p38 inhibitor SB203580 and ROS scavenger N-acetyl-cysteine (NAC) significantly decreased Akt phosphorylation in CFs in the basal and hypoxic situations, but they both significantly increased LC3-II levels in the CFs. Our results suggest that an autophagy- and p38/ROS-dependent pathway mediates the anti-cardiac fibrosis effect of ASA in CFs. As PepA and SB203580 did not affect ASA-caused inhibition of CF apoptosis, the drug combination will enhance ASA's therapeutic effects.
Keywords: aspirin, cardiac fibrosis, cardiac fibroblasts, autophagy, p38 MAPK, ROS, Pepstatin A, rapamycin, SB203580, N-acetyl-cysteine
Introduction
Cardiac fibrosis is a common pathological feature of many heart diseases1. Fibrosis leads to reduced cardiac pumping capacity and abnormal heart rhythms through elevated myocardial stiffness and impaired cardiac function, and it eventually leads to heart failure2. Cardiac fibrosis has been acknowledged as an early warning sign of sudden death for patients with heart failure1. However, few reports of medications that alleviate cardiac fibrosis have been published.
Aspirin, also known as acetylsalicylic acid (ASA), is a widely used medicine that prevents heart attack, stroke, and blood clot formation in people at high risk of developing blood clots3,4. It is reported that ASA inhibits angiotensin II (Ang II)-activated fibroblast growth, collagen synthesis and reactive oxygen species (ROS) generation5. ASA also inhibits IL-18-induced fibroblast migration6, suggesting that ASA plays an anti-cardiac fibrosis role, but ASA's function in cardiac fibrosis in vivo and the underlying mechanisms of this function remain unclear.
Autophagy is a conserved process for the bulk degradation of long-lived proteins and organelles. Accumulating evidence suggests that autophagy plays an essential role in maintaining cardiac function with cardiac fibrosis7. ASA has been reported to alleviate heart diseases through its anti-platelet and anti-inflammatory functions8. The role of ASA on autophagy is cell specific; ASA inhibits epithelial autophagy through the activated PI3K/Akt-GSK3-mTOR pathway9, while it induces autophagy in colorectal cancer cells through inhibiting mTOR signaling. Until now, its effect on autophagy in cardiac fibroblasts is not clear.
p38 MAPK (p38), one of three distinct families of MAPKs (ERK, JNK and p38 kinase), is a vital factor mediating ASA functions, such as ASA-induced anti-inflammatory and anti-cancer effects10,11. In addition, p38 activation is involved in the fibrosis of various organs. Inhibition of p38 can attenuate renal fibrosis, and the activation of p38 can promote liver fibrosis12,13, although whether p38 participates in the ASA-induced anti-fibrosis process and its relationship with autophagy remain unknown.
Reactive oxygen species (ROS) are an important factor in regulating cell fate. As an anti-oxidation agent, ASA can widely attenuate the generation of ROS14,15,16. Thus, ROS are another vital factor mediated by ASA. The pathogenesis of cardiac fibrosis is thought to involve the ROS-dependent induction of inflammatory cytokines and matrix metalloproteinases (MMPs) and the activation and migration of cardiac fibroblasts (CF)17,18. Furthermore, many reports suggest that ROS and the p38 axis play important roles in cardiac fibrosis18. Whether and how ROS participate in ASA-induced anti-fibrosis processes still requires clarification.
CFs undergo phenotypic transformation to myofibroblasts following myocardial injury. Myofibroblasts are highly active cells that express α-SMA and exhibit improved proliferation, migration and collagen protein secretion19. It has been reported that hypoxia can upregulate α-SMA, increase the collagen content and enhance the migration abilities of CFs, suggesting that hypoxia induces the transformation of CFs into myofibroblasts and might be used as a model to study cardiac fibrosis in vitro19. We therefore used CFs exposed to hypoxia to study the mechanisms of ASA on fibrosis.
Here, we report that ASA ameliorated heart damage and fibrosis. ASA performed its functions through inhibiting CF proliferation, which is regulated by autophagy and p38/ROS.
Materials and methods
Reagents
The ASA was purchased from Sigma (St Louis, MO, USA). The Dulbecco's modified Eagle's medium (DMEM) was from Gibco (Grand Island, NY, USA). The fetal bovine serum (FBS) was from HyClone Lab (Logan, UT, USA). The antibodies, including LC3, caspase 3, PARP 1, total and phosphorylated Akt (Ser 473), JNK (Thr183/Tyr185), p38 MAPK (Thr180/Tyr182) and ERK (Thr202/Tyr204) were all from Cell Signaling Technology (Beverly, MA, USA). The antibodies for β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The p38 inhibitor SB203580 and the ROS scavenger N-acetyl-cysteine (NAC) were from Selleck Chemicals (Houston, USA).
Animal protocol
Ten-week-old Balb/c mice and C57bl/6 mice were purchased from the animal center of Shandong University. The animal care and experimental procedures involved in this study were approved by the Institutional Animal Care and Use Committee of Shandong University and adhered to the American Physiological Society's “Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training”.
ISO injection associated cardiac fibrosis model
We divided the Balb/c mice into 4 groups: vehicle, isoproterenol (ISO), ISO plus ASA, and ISO plus captopril. The vehicle group was treated with saline. Captopril is a positive control for ASA. Cardiac fibrosis was induced by subcutaneous ISO injection daily (3 mg·kg−1·d−1 for 3 d and 6 mg·kg−1·d−1 for 18 d), and ASA (10 mg·kg−1·d−1 for 21 d) was administered by oral gavage according to a published report20. After 14 d, animal echocardiography was used to preliminarily evaluate the success of the cardiac fibrosis model, and the mice were sacrificed by cervical dislocation under deep anesthesia with 2% isoflurane (Ruiwode Corporation, Shenzhen, China) at the end of the experiment.
Myocardial infarction (MI) model
C57BL/6 male mice were divided into 3 groups: sham, MI and MI plus ASA. Every group had 6 surviving mice. MI was induced by permanent ligation of the left anterior descending (LAD) coronary artery for 14 d as described16. ASA (10 mg·kg−1·d−1 for 14 d) was administered by oral gavage according to a published report beginning on the second day of LAD17. At the end of the experiment, cardiac function was analyzed, and then the mice were sacrificed.
Animal echocardiography
The mice were anesthetized with intraperitoneal 4% chloral hydrate by weight, and the heart rate was controlled at 360±30 beat/min. Echocardiographic assessments of the left ventricular (LV) anatomy and function were performed using a Sonos 5500 Imaging System (Philips, Philips Healthcare, Andover, MA, USA) with a 12 MHz transducer. Systolic and diastolic functions were evaluated individually using echocardiography by left ventricular ejection fraction (EF) and left ventricular fractional shortening (FS).
Histology
The mouse hearts were fixed in 4% formalin for a minimum of 24 h. The fixed tissues were embedded in paraffin and sectioned (5 μm) for staining. The heart sections were stained with Masson's trichrome and picrosirius red to evaluate interstitial collagen deposition. The results were analyzed by Image J software (National Institutes of Health, USA).
Cell culture and treatment
Neonatal cardiac fibroblasts (CFs) were isolated from the hearts of 1- to 3-d-old Sprague-Dawley rats. Briefly, the hearts were quickly excised and immediately embedded in PBS solution. CFs were isolated from the rat hearts by enzymatic digestion with pancreatin and collagenase type II, as described previously21; grown to 80% confluence and then cultured in serum-free medium and exposed to hypoxia (95% N2, 5% CO2)22 and treated with ASA for 12 h.
Cell survival assay
The CFs were seeded in 96-well cell culture plates for sub-confluency, then they were exposed to hypoxia and treated with ASA at the indicated concentrations for 12 h. Next, the cells were fixed and studied to estimate the numbers of cells using the sulforhodamine B assay (SRB).
Western blot assay
Total protein was extracted in RIPA buffer (CxBio, Shanghai, China) supplemented with phenylmethanesulfonyl fluoride (PMSF, CxBio). The protein concentrations were measured using the BCA Protein Assay (Applygen, Beijing, China). The samples were separated on a 10% SDS-polyacrylamide gel and then transferred to a nitrocellulose membrane, which was blocked in 5% skim milk for 1 h and then incubated with primary antibodies at 4 °C overnight. After washing, the membranes were incubated with horseradish peroxidase-labeled secondary antibodies for 1 h at room temperature. The bands were visualized using a chemiluminescence detection system, and the densitometric results were analyzed with Image J software.
ROS detection
CFs were treated with different doses of ASA as described above. Intracellular ROS levels were measured using a fluorescent probe, 2,7-dichlorodihydrofluorescein (DCHF), as previously described23. After incubation with the probe for 30 min, the CFs were washed with PBS. A region of interest was randomly selected, then zoomed into the same frame; the relative fluorescent intensity per cell was the total value of the sample in the zoom scan divided by the total number of cells (at least 200 cells) in the same scan. A clear and representative result from three similar experiments is shown. The data are the mean±SEM from all independent experiments.
Statistical analysis
All of the experiments were performed at least three times. The data are expressed as the means±SEM and were analyzed by ANOVA and a post hoc Tukey analysis or by a t-test, as appropriate. A P value of 0.05 or less was considered significant.
Results
ASA inhibits heart fibrosis in ISO injected mouse models
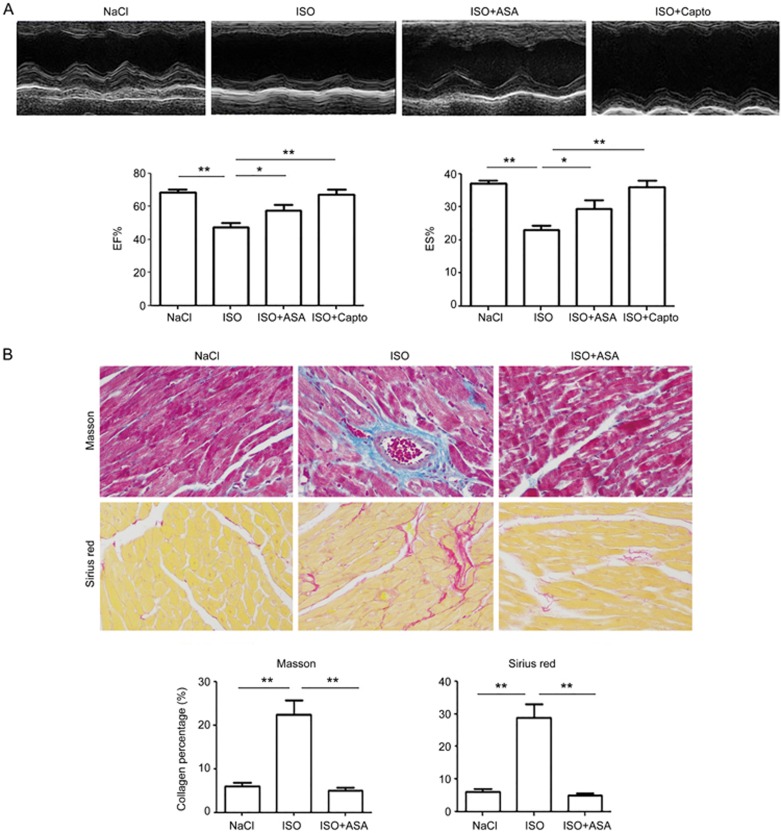
The mice were injected with ISO or ISO combined with ASA for 21 d. Cardiac function analyses showed that the mice had decreased EF and FS. Masson's trichrome and picrosirius red staining showed that fibrosis increased when the myocardium was injured, and ASA ameliorated the heart damage and fibrosis (Figure 1A and 1B).
Figure 1.
ASA protects the heart and inhibits fibrosis. Mice were injected with ISO or ISO combined with ASA for 21 days. (A) Animal echocardiography was conducted to analyze cardiac function at 14 d. The results showed that ASA rescued EF and FS significantly. (B) Masson's and Sirius red staining showed that ASA rescued the ISO-induced fibrosis. Magnification is 200×. Mean±SEM. n=6. **P<0.01. Captopril was used as positive control.
ASA protects the heart against damage caused by MI and inhibits heart fibrosis
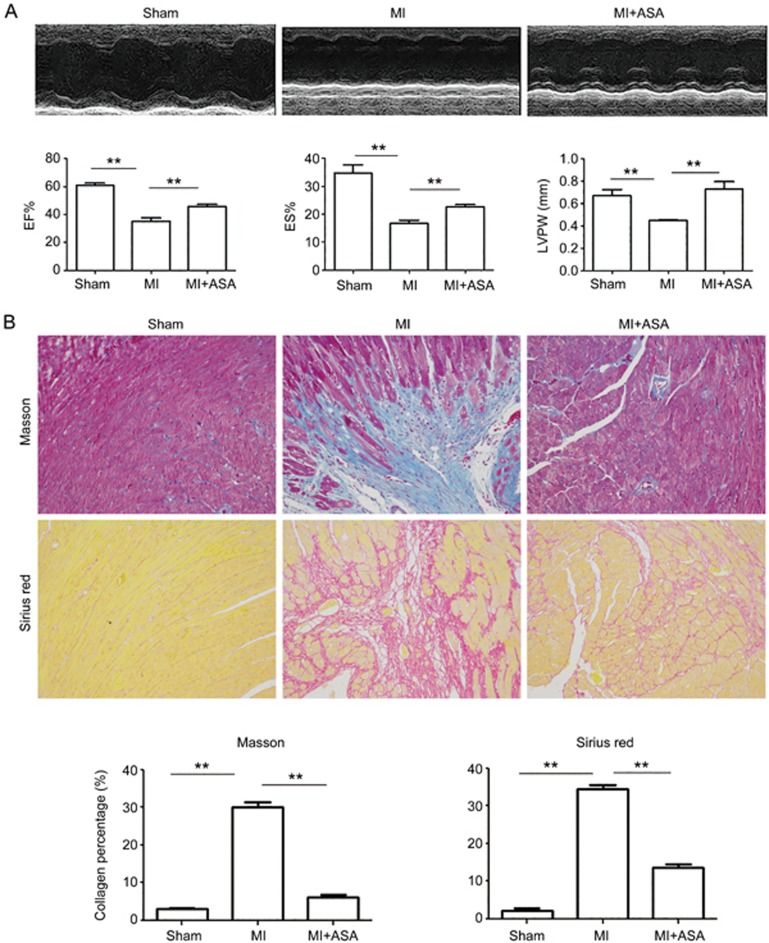
We also used the MI model to verify the anti-fibrotic function of ASA in vivo. MI models were generated as reported24, and ASA was injected into the MI mice beginning on the second day of MI and continued for 14 d. The heart function analysis showed that ASA rescued the decreased EF, FS and LVPW (left ventricular posterior wall thickness). The heart sections stained with Masson's trichrome and picrosirius red staining showed that ASA inhibited the fibrosis caused by the MI (Figure 2A and 2B).
Figure 2.
ASA protects the heart against MI-induced heart damage and inhibits heart fibrosis. (A) ASA was injected into the MI mice starting on the second day of MI and continued for 14 d. Heart function was analyzed by echocardiography, and the changes of EF, FS, and LVPW were revealed. (B) Heart sections stained with Masson's and Sirius red staining were used to analyze the fibrosis. Representative images are shown. Magnification is 100×. Mean±SEM. n=6. **P<0.01.
ASA inhibits CF proliferation and apoptosis
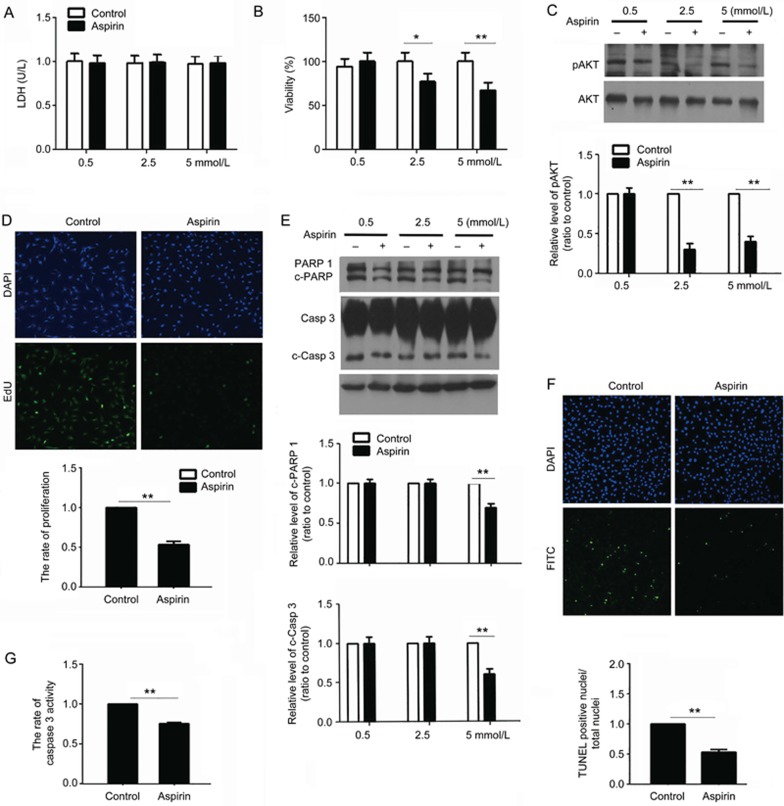
CF exposure to hypoxia is a widely accepted model for researching fibrosis in vitro19. CFs cultured in vitro were exposed to hypoxia and treated with 0.5, 2.5, or 5 mmol/L ASA for 12 h. LDH results showed that there was no necrosis (Figure 3A).
Figure 3.
ASA inhibits CF proliferation and apoptosis. CFs were cultured in a hypoxia situation and treated with 0.5, 2.5, or 5 mmol/L ASA for 12 h. (A) The culture supernatant was collected and used in LDH detection. (B) An SRB assay was performed to detect the changes in CF survival. (C) The proteins were collected for Western blot detection. The changes in phosphorylated Akt were shown. (D-G) CFs were cultured in a hypoxia situation and treated with 2.5 mmol/L ASA for 12 h. (D) The EdU assay showed the proliferation of CFs. (E) Western blot results showed the changes of apoptosis markers, including cleaved PARP 1 and caspase 3. (F) TUNEL assay showed the apoptosis of CFs. (G) Caspase 3 activity was detected using the caspase 3 fluorescence metric assay kit. Mean±SEM. n=3. *P<0.05, **P<0.01.
Because a report showed that ASA could inhibit CF proliferation5, we detected the changes in CF survival using the SRB assay. The results showed that ASA inhibited CF survival (Figure 3B) and the phosphorylation of Akt (Figure 3C), a key factor in the proliferation pathway. The proliferation inhibition by ASA was also confirmed by the EdU assay, in which fewer CFs were labeled by EdU (Figure 3D). Our data suggest that ASA inhibited fibrosis through the inhibition of proliferation. We used the phosphorylation of Akt to show the changes in CF proliferation in the followed experiments.
CF apoptosis may be important for restoring the balance impaired by fibroblast activation. We thus deduced that ASA could induce CF apoptosis. In contrast, the Western blot results showed that ASA inhibited the cleavage of caspase 3 and PARP 1 (Figure 3E), suggesting an anti-apoptotic role of ASA. The anti-apoptotic role of ASA was further confirmed by TUNEL and caspase 3 activity assays (Figure 3F, 3G).
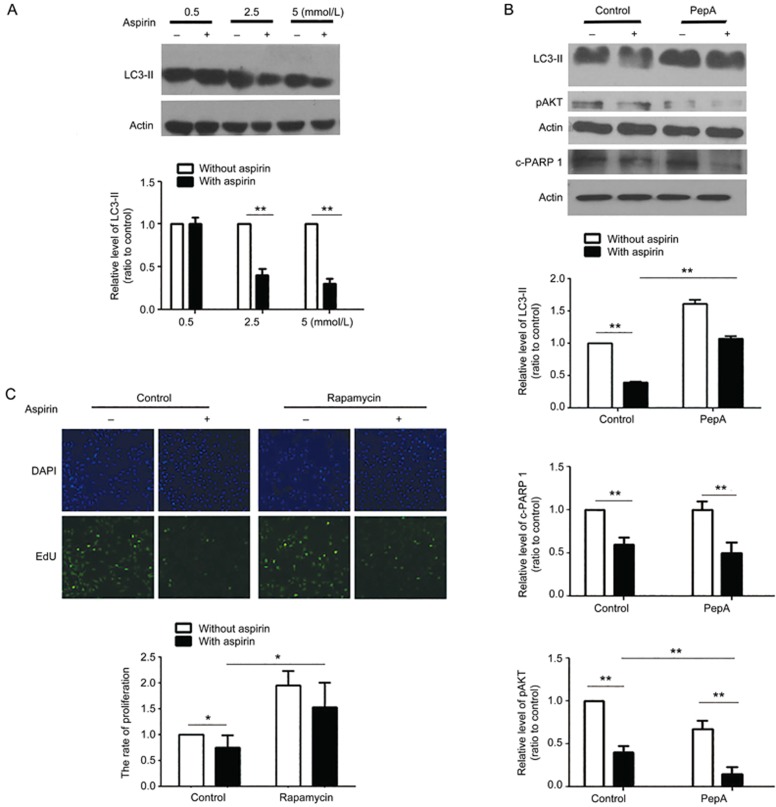
ASA alleviates CF proliferation by inhibiting autophagy
Autophagy is a key mechanism affecting fibrosis25, and in order to know its roles in ASA-inhibited fibrosis, we detected levels of LC3-II, a well-known marker of autophagy26. The autophagy in CFs exposed to hypoxia was inhibited by ASA, as was demonstrated by reduced LC3-II levels (Figure 4A). Pepstatin A (PepA) could block the autophagy flux by impairing lysosome functions to inhibit autophagy27. As a result, PepA increased LC3-II levels in ASA-treated cells when it inhibited autophagy. Furthermore, PepA reduced the phosphorylation of Akt (Figure 4B), as did ASA, and enhanced ASA functions, which suggested that ASA performed its anti-fibrotic function through inhibiting autophagy. Importantly, PepA did not affect apoptosis, as was evidenced by no changes in cleaved PARP 1 (Figure 4B). We then enhanced autophagy by rapamycin when the CF cells were treated with hypoxia, and the results showed that the proliferation of CFs increased (Figure 4C), which further confirmed that ASA-inhibited CF proliferation was autophagy-dependent.
Figure 4.
ASA inhibits CF proliferation and apoptosis by impairing autophagy. (A) CFs exposed to hypoxia were treated with 0.5, 2.5, or 5 mmol/L ASA for 12 h, then the changes in LC3-II were detected by Western blot. (B) CFs were treated with 2.5 mmol/L ASA combined with the lysosome inhibitor PepA for 12 h, then the changes of LC3-II, phosphorylated Akt and PARP 1 were all detected by Western blot. (C) CFs were treated with 2.5 mmol/L ASA and the autophagy inducer rapamycin for 12 h, then the proliferation of CFs was analyzed by EdU. Mean±SEM. n=3. **P<0.01.
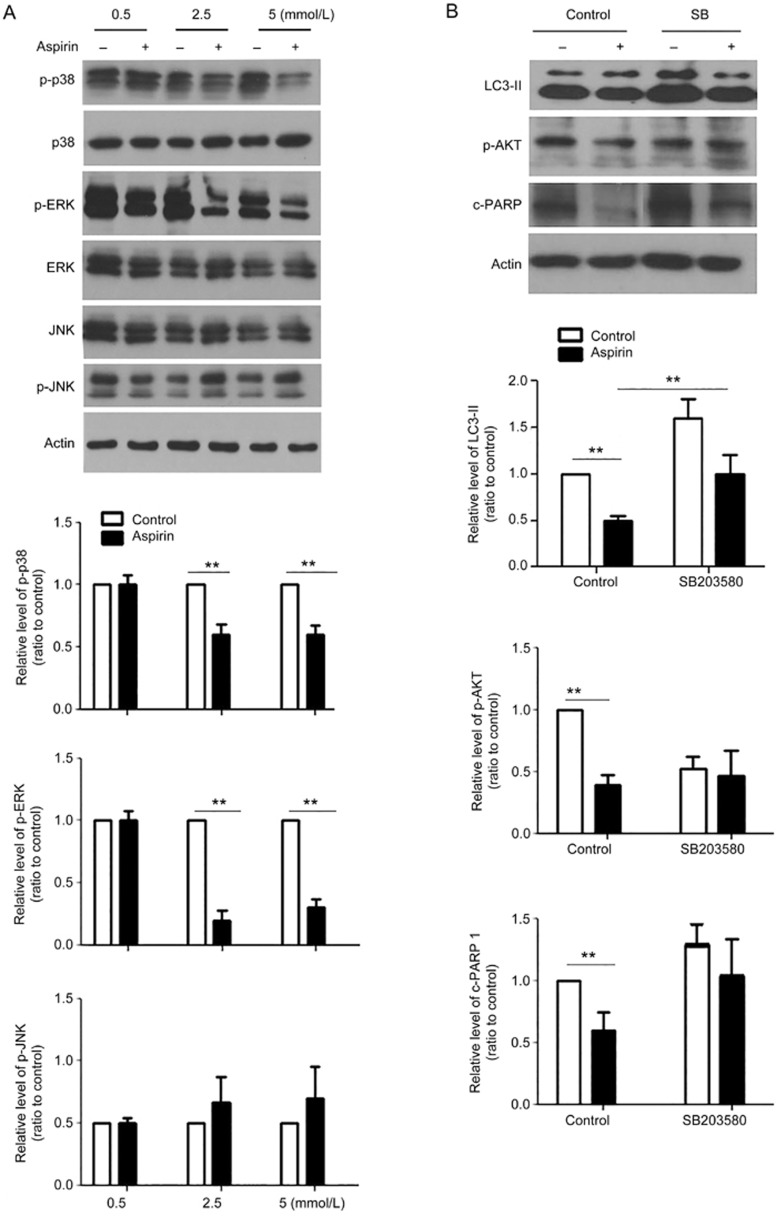
p38 MAPK induced an autophagy-dependent pathway in ASA-impaired CF proliferation
To understand the molecular mechanisms of ASA inhibition of autophagy, we detected changes in MAPK members, including p38, JNK, and ERK. ASA decreased the phosphorylation of p38 and ERK, but it did not affect the phosphorylation of JNK (Figure 5A). We are interested in the role of p38 in ASA inhibiting autophagy because of its importance in fibrosis12,13. The p38 inhibitor SB203580 increased LC3-II levels in basal and ASA-treated CFs (Figure 5B), which suggested that p38 regulated autophagy. At the same time, SB203580 inhibited the ASA-mediated reduction of Akt phosphorylation (Figure 5B). Therefore, p38 signaling is necessary for the anti-fibrotic effect of ASA. Furthermore, SB203580 did not affect apoptosis, as was evidenced by no changes in PARP 1 (Figure 5B).
Figure 5.
p38 MAPK induced an autophagy-dependent pathway in ASA-regulated CF proliferation. (A) CFs exposed to hypoxia were treated with doses of ASA at 0.5, 2.5, and 5 mmol/L for 12 h, and then the changes of phosphorylated p38, ERK, JNK were detected by Western blot. (B) Cardiac fibroblasts were pre-treated with the p38 inhibitor SB203580 (10 μmol/L) for 1 h and were then treated with 2.5 mmol/L ASA for 12 h. Western blot results showed the changes of LC3-II, phosphorylated Akt and cleaved PARP 1. Mean±SEM. n=3, **P<0.01.
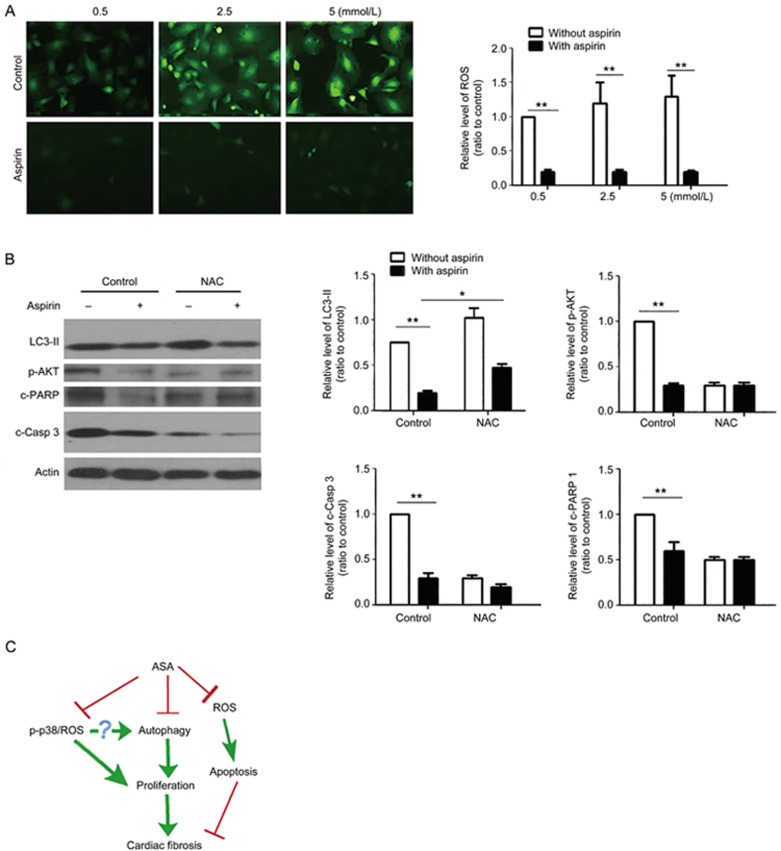
The function of ROS in ASA-mediated inhibition of CF proliferation and apoptosis
Many ASA functions have been associated with inhibited ROS17,18. Here, our data showed that ASA inhibited ROS in CFs (Figure 6A). NAC upregulated the level of LC3-II and inhibited the phosphorylation of Akt (Figure 6B), similar to the effects of ASA and SB203580. Therefore, ROS participated in the anti-fibrotic functions of ASA through regulating autophagy. NAC also inhibited the cleavage of caspase3 (Figure 6B), which suggested an autophagy-independent but ROS-dependent mechanism of ASA inhibited apoptosis.
Figure 6.
Roles of ROS in ASA-inhibited CF proliferation and apoptosis. (A) CFs exposed to hypoxia were treated with 0.5, 2.5, or 5 mmol/L ASA for 12 h. Then, the changes in ROS were detected by an ROS probe using a fluorescence microscope. (B) Cardiac fibroblasts were pre-treated with the ROS scavenger NAC for 1 h and then treated with 2.5 mmol/L ASA for 12 h. Western blot results showed the changes of LC3-II, phosphorylated Akt, cleaved PARP 1, and caspase 3. Mean±SEM. n=3. *P<0.05, **P<0.01. (C) The graphic shows that ASA plays anti-proliferative and anti-apoptotic roles in CFs exposed to hypoxia. The autophagy- and p38/ROS-dependent pathways are responsible for ASA's anti-proliferation function in CFs. ROS participated in the ASA-inhibited apoptosis of CFs, which might aggravate cardiac fibrosis.
Discussion
Cardiac fibrosis is a basic pathological characteristic of the post-infarct myocardium and plays a pivotal role in the development of other cardiovascular diseases such as heart failure and atrial fibrillation1. Although ASA was reported to affect CF proliferation and migration in some situations stimulators, its function in the circumstance of cardiac fibrosis is not clear. In this study, we used two classical models of cardiac injury to verify the anti-fibrotic role of ASA in vivo. Our results showed that early administration of ASA could ameliorate heart damage by inhibiting myocardial fibrosis. Therefore, we revealed an anti-fibrotic pharmacological activity of ASA against cardiac injury.
The importance of autophagy in cardiac fibrosis was realized recently, when many reports suggested that autophagy is required for the fibrosis response25,28,29, although the role of ASA on CF autophagy is not clear. In this study, we found that ASA performed its anti-fibrotic function at least partly though inhibiting autophagy. Our data support a potential to treat fibrosis by altering the mechanisms of autophagy. In this study, upregulated LC3-II in SB203580- or NAC-treated CFs were accompanied by decreased phosphorylation of Akt, but whether autophagy flux inhibition or autophagy promotion caused increases in LC3-II requires further research.
It has been reported that an increase in ROS levels is accompanied by p38 activation, fibrosis, apoptosis and cardiac dysfunction18,30. In this study, ASA inhibited levels of ROS and phosphorylated p38 when inhibiting fibrosis and apoptosis. This result is in accordance with a previous report14 and confirmed the importance of the ROS-p38 axis in fibrosis. Our results provide new insights into the mechanisms underlying the anti-fibrotic effects of ASA in the heart.
CF proliferation and adaptive collagen synthesis are major sources of cardiac fibrosis31, and apoptosis may be important for restoring the cell population balance in the context of fibroblast proliferation to alleviate cardiac fibrosis. Because of this, ASA-inhibited apoptosis is a side effect of its anti-fibrotic effects. Drug combination is a novel method that enhances useful effects and avoids side effects. In our study, the autophagy inhibitors PepA and SB203580 did not affect apoptosis when decreasing the phosphorylation of Akt, and they enhanced the effect of ASA in fibrosis therapy. Our data support a novel method that enhances the useful effects and avoids the side effects of ASA.
In summary, we report that ASA ameliorates heart damage and fibrosis in vivo. ASA performs its functions through an autophagy- and p38/ROS-dependent pathway. Our data reveal an anti-fibrotic pharmacological activity of ASA against cardiac injury and support autophagy targeting for fibrosis therapy.
Author contribution
Jing ZHAO designed research; Ping-ping LIU and Honghong LIU performed research; Xing-xing SHI and Wan-cheng YANG helped preform the research; Shu-hong SUN and Guohai SU contributed new analytical tools and reagents; Pingping LIU and Hong-hong LIU analyzed data; Jing ZHAO wrote the paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No 31671180; 31070999; 31371158).
References
- Deb A, Ubil E. Cardiac fibroblast in development and wound healing. J Mol Cell Cardiol 2014; 70: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong PP, Christia NG. Frangogiannis, The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014; 71: 549–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankey GJ. The benefits of aspirin in early secondary stroke prevention. Lancet 2016; 388: 312–4. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Algra A, Chen Z, Diener HC, Norrving B, Mehta Z, et al. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet 2016; 388: 365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu J, Khaidakov M, Mitra S, Ding Z, Raina S, et al. Aspirin suppresses cardiac fibroblast proliferation and collagen formation through downregulation of angiotensin type 1 receptor transcription. Toxicol Appl Pharmacol 2012; 259: 346–54. [DOI] [PubMed] [Google Scholar]
- Siddesha JM, Valente AJ, Sakamuri SS, Gardner JD, Delafontaine P, Noda M, et al. Acetylsalicylic acid inhibits IL-18-induced cardiac fibroblast migration through the induction of RECK. J Cell Physiol 2014; 229: 845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Otsu K. Autophagy during cardiac remodeling. J Mol Cell Cardiol 2016. 95: 11–8. [DOI] [PubMed] [Google Scholar]
- Yasuda O, Takemura Y, Kawamoto H, Rakugi H. Aspirin: recent developments. Cell Mol Life Sci 2008; 65: 354–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández C, Barrachina MD, Vallecillo-Hernández J, Álvarez Á, Ortiz-Masiá D, Cosín-Roger J, et al. Aspirin-induced gastrointestinal damage is associated with an inhibition of epithelial cell autophagy. J Gastroenterol 2016; 51: 691–701. [DOI] [PubMed] [Google Scholar]
- Zhang C, Shi J, Mao SY, Xu YS, Zhang D, Feng LY, et al. Role of p38 MAPK in enhanced human cancer cells killing by the combination of aspirin and ABT-737. J Cell Mol Med 2015; 19: 408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JM, Rui BB, Chen C, Chen H, Xu TJ, Xu WP, et al. Acetylsalicylic acid enhances the anti-inflammatory effect of fluoxetine through inhibition of NF-kappaB, p38-MAPK and ERK1/2 activation in lipopolysaccharide-induced BV-2 microglia cells. Neuroscience 2014; 275: 296–304. [DOI] [PubMed] [Google Scholar]
- Mao ZM, Shen SM, Wan YG, Sun W, Chen HL, Huang MM, et al. Huangkui capsule attenuates renal fibrosis in diabetic nephropathy rats through regulating oxidative stress and p38MAPK/Akt pathways, compared to alpha-lipoic acid. J Ethnopharmacol 2015; 173: 256–65. [DOI] [PubMed] [Google Scholar]
- Li ZL, Shi Y, Le G, Ding Y, Zhao Q. 24-Week exposure to oxidized tyrosine induces hepatic fibrosis involving activation of the MAPK/TGF-beta1 signaling pathway in sprague-dawley rats model. Oxid Med Cell Longev 2016; 2016: 3123294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Lee WJ, Wu CM, Chen JF, Sheu WH. Aspirin prevents resistin-induced endothelial dysfunction by modulating AMPK, ROS, and Akt/eNOS signaling. J Vasc Surg 2012; 55: 1104–15. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhai H, Wang Y, Li L, Wu J, Wang F, et al. Aspirin-triggered lipoxin A(4) attenuates lipopolysaccharide-induced intracellular ROS in BV2 microglia cells by inhibiting the function of NADPH oxidase. Neurochem Res 2012; 37: 1690–6. [DOI] [PubMed] [Google Scholar]
- Zhu X, Li Z, Li C, Zhang J, Zou Z, Wang J. Ginkgo biloba extract and aspirin synergistically attenuate activated platelet-induced ROS production and LOX-1 expression in human coronary artery endothelial cells. Phytomedicine 2013; 20: 114–9. [DOI] [PubMed] [Google Scholar]
- Essick EE, Ouchi N, Wilson RM, Ohashi K, Ghobrial J, Shibata R, et al. Adiponectin mediates cardioprotection in oxidative stress-induced cardiac myocyte remodeling. Am J Physiol Heart Circ Physiol 2011; 301: H984–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Tian A, Wu J, Yang C, Xing R, Jia P, et al. Danshensu inhibits beta-adrenergic receptors-mediated cardiac fibrosis by ROS/p38 MAPK axis. Biol Pharm Bull 2014; 37: 961–7. [DOI] [PubMed] [Google Scholar]
- Wang YS, Li SH, Guo J, Mihic A, Wu J, Sun L, et al. Role of miR-145 in cardiac myofibroblast differentiation. J Mol Cell Cardiol 2014; 66: 94–105. [DOI] [PubMed] [Google Scholar]
- Carlson LM, Rasmuson A, Idborg H, Segerström L, Jakobsson PJ, Sveinbjörnsson B, et al. Low-dose aspirin delays an inflammatory tumor progression in vivo in a transgenic mouse model of neuroblastoma. Carcinogenesis 2013; 34: 1081–8. [DOI] [PubMed] [Google Scholar]
- Xiao J, Sheng X, Zhang X, Guo M, Ji X. Curcumin protects against myocardial infarction-induced cardiac fibrosis via SIRT1 activation in vivo and in vitro. Drug Des Devel Ther 2016; 10: 1267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Li D, Zhang X, Hermonat PL, Mehta JL. Anoxia-reoxygenation stimulates collagen type-I and MMP-1 expression in cardiac fibroblasts: modulation by the PPAR-gamma ligand pioglitazone. J Cardiovasc Pharmacol 2004; 44: 682–7. [DOI] [PubMed] [Google Scholar]
- Zhao J, He Q, Cheng Y, Zhao B, Zhang Y, Zhang S, et al. A benzoxazine derivative induces vascular endothelial cell apoptosis in the presence of fibroblast growth factor-2 by elevating NADPH oxidase activity and reactive oxygen species levels. Toxicol In Vitro 2009; 23: 1039–46. [DOI] [PubMed] [Google Scholar]
- Zhao J, Feng Y, Yan H, Chen Y, Wang J, Chua B, et al. beta-arrestin2/miR-155/GSK3beta regulates transition of 5'-azacytizine-induced Sca-1-positive cells to cardiomyocytes. J Cell Mol Med 2014; 18: 1562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou M, Wang F, Gao R, Wu J, Ou Y, Chen X, et al. Autophagy inhibition of hsa-miR-19a-3p/19b-3p by targeting TGF-beta R II during TGF-beta1-induced fibrogenesis in human cardiac fibroblasts. Sci Rep 2016; 6: 24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008; 4: 151–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue H, Li W, Liu P, Gao J, Miao J, Zhao J. Inhibition of autophagy promoted sphingosylphosphorylcholine induced cell death in non-small cell lung cancer cells. Biochem Biophys Res Commun 2014; 453: 502–7. [DOI] [PubMed] [Google Scholar]
- Shirakabe A, Ikeda Y, Sciarretta S, Zablocki DK, Sadoshima J. Aging and autophagy in the heart. Circ Res 2016; 118: 1563–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavami S, Cunnington RH, Gupta S, Yeganeh B, Filomeno KL, Freed DH, et al. Autophagy is a regulator of TGF-beta1-induced fibrogenesis in primary human atrial myofibroblasts. Cell Death Dis 2015; 6: e1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F, Kaludercic N, Paolocci N. beta(2)-Adrenoceptors, NADPH oxidase, ROS and p38 MAPK: another 'radical' road to heart failure? Br J Pharmacol 2011; 162: 1009–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Yu H, Zhang Y, Li Z, Gao W. MicroRNA-214 mediates isoproterenol-induced proliferation and collagen synthesis in cardiac fibroblasts. Sci Rep 2015; 5: 18351. [DOI] [PMC free article] [PubMed] [Google Scholar]