Abstract
Evidence suggests that neuroinflammation is involved in depression and that the cysteinyl leukotriene receptor 1 (CysLT1R) plays a potential pathophysiological role in several types of CNS disorders. Our previous study has shown that knockdown of hippocampal CysLT1R in mice prevents the depressive-like phenotype and neuroinflammation induced by chronic mild stress (CMS). Here, we examined the effects of hippocampal CysLT1R knockdown and CysLT1R blockade on LPS-induced depressive-like behavior in mice. We found that injection of LPS (0.5 mg/kg, ip) caused marked increase in hippocampal CysLT1R expression, which was reversed by pretreatment with fluoxetine (20 mg·kg−1·d−1 for 7 d, ig). Knockdown of hippocampal CysLT1R or blockade of CysLT1R by pretreatment with pranlukast (0.5 mg/kg, ip) significantly suppressed LPS-induced depressive behaviors, as evidenced by decreases in mouse immobility time in the forced swimming test (FST) and tail suspension test (TST) and latency to feed in the novelty-suppressed feeding (NSF) test. Moreover, both CysLT1R knockdown and CysLT1R blockade markedly prevented LPS-induced neuroinflammation, as shown by the suppressed activation of microglia and NF-κB signaling as well as the hippocampal levels of TNF-α and IL-1β in mice. Our results suggest that CysLT1R may be involved in LPS-induced depressive-like behaviors and neuroinflammation, and that downregulation of CysLT1R could be a novel and potential therapeutic strategy for the treatment of depression, at least partially due to its role in neuroinflammation.
Keywords: depression, cysteinyl leukotriene receptor 1, lipopolysaccharide, fluoxetine, pranlukast, hippocampus, neuroinflammation
Introduction
Depression, a common multi-causal psychiatric disorder and a significant contributor to the social burden of disease, is characterized by a lack of interest, enduring sadness, sleep disturbances, poor concentration and suicidal tendency1. The accepted monoamine-deficiency theory represents an important aspect of depression pathophysiology. However, only 30% of depressive patients achieve full remission (Hamilton rating score ≤7), and a considerable proportion of them fail to respond to the antidepressants that are based on the monoaminergic system2. Therefore, developing new targets based on the understandings of the pathophysiological mechanisms is urgently required for the study of novel antidepressants.
Accumulating data have revealed that neuroinflammatory responses play a vital role in the pathogenesis of depression. Patients with depressive disorder display a marked rise in pro-inflammatory mediators, such as IL-1, IL-6 and TNF-α, in the serum and cerebrospinal fluid3, while administration of antidepressants suppresses the elevation of these cytokines4,5. In addition, clinical observations show that cancer sufferers treated with IL-2 and IFN-α, a certain group of pro-inflammatory cytokines, frequently develop major depressive symptoms, whereas withdrawal of such treatments can reverse the behavioral effects6. In rodents, depressive symptoms can be induced by treatment with cytokines and cytokine-inducers, such as lipopolysaccharide (LPS), and by chronic mild stress (CMS) exposure7,8. Consistently, anti-inflammatory drugs9 and antidepressants have been shown to suppress depressive behaviors and alterations in the levels of pro-inflammatory cytokines in response to LPS. These studies strongly suggest that inflammation is associated with depressive symptoms.
Cysteinyl leukotrienes (CysLTs), including leukotriene C4, D4 and E4, are inflammatory eicosanoids generated by the activation of the lipoxygenase pathway of arachidonic acid metabolism10. They exert effects through cell surface receptors, such as cysteinyl leukotriene receptor 1 (CysLT1R). Leukotriene pathway modifiers, including 5-lipoxygenase inhibitor (zileuton) and CysLT1R antagonists (montelukast, zafirlukast, and pranlukast), have been used as anti-asthmatic drugs11,12,13. Recently, several studies on CysLT1R have focused on its novel pathophysiological role in CNS disorders, such as cognitive impairment14, cerebral ischemia15, brain trauma16 and experimental autoimmune encephalomyelitis17. We recently reported that CysLT1R knockdown in the mouse hippocampus prevented the CMS-induced depressive-like phenotype and neuroinflammation18. To further confirm the role of CysLT1R in depression, we investigated whether hippocampal CysLT1R knockdown or blockade repressed depressive-like behaviors and neuroinflammatory responses in LPS-treated mice.
Materials and methods
Mice
Male ICR mice (2 months old, 22–25 g) were supplied by the Yangzhou University Medical Center (Yangzhou, China). Mice were maintained under controlled laboratory conditions (12:12 h light-dark schedule; 22±2 °C; humidity 40%–60%) with available food and water. Mouse experimental protocols were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and permitted by the guidelines of the Institutional Animal Care and Use Committee of China Pharmaceutical University.
Drugs and reagents
LPS (055:B5) was obtained from Sigma-Aldrich (St Louis, MO). Pranlukast (Pran) and fluoxetine (FLX) were from Jiangsu Hengrui Medicine Co, Ltd (Lianyungang, Jiangsu, China) and Eli Lilly Company (Indianapolis, USA), respectively. Antibodies were from different companies: anti-NF-κB p65 was from Cell Signaling Technology, Inc (Massachusetts, USA); anti-CysLT1R, anti-TNF-α and anti-IL-1β were from Santa Cruz Biotechnology, Inc (Heidelberg, Germany); anti-β-actin, anti-Histone H3, anti-Iba1 were from Wako Pure Chemical Industries, Ltd (Osaka, Japan); and secondary antibodies were from Bioworld Technology Co Ltd (Minnesota, USA). The nucleoprotein extraction kit and streptavidin-biotin complex (SABC) immunohistochemistry kit were from Sangon Biotech Co, Ltd (Shanghai, China) and Boster Biotechnology Co, Ltd (Wuhan, China), respectively. All chemicals used were of analytical grade and purchased from approved sources. Drugs for ig treatment were dissolved in 0.5% sodium carboxymethyl cellulose solution (0.5% CMC-Na), and LPS for ip injection was dissolved in PBS.
CysLT1R knockdown experiments
The lentivirus generation was performed according to the previously described protocol18. All surgeries were conducted under aseptic conditions. After mice were anesthetized with trichloroacetaldehyde hydrate (350 mg/kg, ip), they were immobilized on a stereotaxic apparatus. LV-CysLT1R-shRNA-EGFP or LV-EGFP (8×108 TU/μL, 1 μL/site) was infused into the dentate gyrus (DG) (AP, -3.0 mm, ML, ±2.0 mm, DV, -1.9 mm)19 at a rate of 0.25 μL/min with a glass micropipette connected to a micro-injection pump (CMA402 Suringo Pump). Injectors were kept in place for 10 min after injection to minimize backflow of the liquid.
Behavioral tests
Open field test
The open field test (OFT) was applied to assess the mouse motor and behavioral changes. The arena was a rectangular plastic chamber (50 cm×50 cm×40 cm) and was illuminated by 450 lux fluorescent lights at the center (200 cm above field). The test arena was divided into 144 squares, and the 36 squares in the center were referred to as inner squares and the others as outer squares. The mouse was gently placed into a corner square of the field for a 6-min period of recording. Locomotor activity was measured by counting the number of squares crossed with the paws (line crossings) during the 6-min session. The apparatus was then cleaned with 70% ethanol after each trial. The data were automatically calculated using ANY-MAZE software.
Novelty-suppressed feeding
The novelty-suppressed feeding (NSF) test in mice was conducted as described elsewhere20. The test apparatus was constructed of a 50 cm×50 cm×20 cm plastic box, illuminated by a fluorescent light (450 lux). The floor was covered in sawdust with a single chow pellet on a circular white platform at the center. After 24 h of food deprivation but not water deprivation, mice were individually placed into the corner and allowed a maximum of 6 min to begin feeding. Latency to feed (defined as animals sitting on their haunches and food held in forepaws while eating) was manually timed. As soon as the animal began eating, it was transferred to an individual home cage containing a pre-weighed single pellet of food, and home cage food consumption (defined as the weight of food consumed within 6 min) was recorded.
Forced swimming test
The forced swimming test (FST) was carried out using a previously described method21. Each animal was placed into a transparent cylinder (25 cm in height, 12 cm in diameter), containing 15 cm of water at 25±2 °C. During the 6-min session, immobility time (considered as mice floating motionless with only small movement essential to keep the head above water) throughout the last 4 min was recorded.
Tail suspension test
The tail suspension test (TST) was conducted using a previously described method22. Briefly, mice were permitted to adapt to the testing environment for 1 h before testing. The mouse was suspended upside-down for 6 min by its tail with adhesive tape on the top of apparatus. The immobility duration was recorded by ANY-MAZE software within the last 4 min of the 6-min mission and statistically analyzed.
Western blot analysis
Mice were euthanized, and the hippocampus was quickly separated, minced and homogenized in 0.5 mL ice-cold RIPA buffer [50 mmol/L Tris-HCl (pH 7.4), 1 mmol/L EDTA, 0.1% SDS, 150 nmol/L NaCl, 1% sodium deoxycholate, 1% Triton X-100] containing 0.1% PMSF. The homogenate was centrifuged at 12 000×g for 15 min at 4 °C, and the supernatant was then collected. Proteins were separated by SDS-10% polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. After blocking with 5% nonfat milk, membranes were incubated with primary antibodies (anti-CysLT1R, 1:500; anti-TNF-α, 1:800; anti-IL-1β, 1:600 and anti-β-actin, 1:5000) overnight at 4 °C. On the second day, membranes were washed in Tris-buffered saline-Tween 20 (TBST) and incubated for 2 h at room temperature with secondary antibodies (1:8000) diluted in TBST. Imaging was performed using enhanced chemiluminescence detection reagents and detected with a gel imaging system (Tanon Science & Technology Co, Ltd, Shanghai, China).
Nuclear extracts were performed using a nucleoprotein extraction kit. Briefly, the mouse hippocampus was minced, homogenized in ice-cold hypotonic buffer (containing 0.1% DL-dithiothreitol, 0.5% phosphatase inhibitor and 1% phenylmethylsulfonyl fluoride) and then centrifuged at 3000×g for 5 min at 4 °C. The precipitate was harvested and washed with hypotonic buffer and centrifuged at 5000×g for 5 min at 4 °C. Finally, the precipitate was added to 0.2 mL lysis buffer (containing 0.1% DL-dithiothreitol, 0.5% phosphatase inhibitor and 1% phenylmethylsulfonyl fluoride), chilled for 20 min and centrifuged at 15 000×g for 10 min at 4 °C. The supernatant nuclear protein extract was then subjected to Western blot assay for determination of NF-κB p65 (1:1000), and histone H3 (1:1000) was used as a loading control.
Immunohistochemistry
Immunohistochemistry staining was conducted using the streptavidin-biotin complex (SABC) immunohistochemistry kit in accordance with the manufacturer's recommendations. Mice were anesthetized and transcardially perfused with PBS, followed by 4% formaldehyde. Samples were dissected, post-fixed in 4% paraformaldehyde for 24 h at 4 °C and then cryoprotected in a 30% sucrose solution for an additional 24 h. Samples were embedded into an optimal cutting temperature compound (Tissue-Tek, Torrance, CA) and cryosectioned (30 μm). After washing with PBS (3×5 min), sections were blocked with 0.3% Triton X-100 for 4 h at 60 °C and then treated with 3% H2O2 at room temperature for 30 min. After washing with PBS (3×5 min), sections were blocked with 5% BSA for 30 min and incubated in anti-Iba1 (1:1000) primary antibody diluted in 5% BSA overnight at 4 °C. Sections were washed with PBS (3×5 min), further incubated with biotinylated mouse anti-rabbit IgG (40 min, 37 °C) and then washed again (3×5 min). Sections were incubated with streptavidin-biotin complex (20 min, 37 °C) and washed with PBS (4×5 min). Diaminobenzidine (DAB) was applied as the final chromogen for protein detection. After gradient dehydration (70% ethanol, 5 min; 95% ethanol, 5 min; 100% ethanol, 5 min; xylene, 5 min), imaging (200× and 400×) was performed using a Nikon DS-Fi2 camera connected to a Nikon Eclipse Ti microscope and quantified using Image-Pro Plus software. The number of microglia in the hippocampus was measured, followed by the microglial-positive area to generate the ratio of microglial staining to hippocampal area (% area occupied). The mean values from 5 sections analyzed in each animal were used for statistical analysis.
Statistical analysis
The data are presented as the mean±SEM, and significant differences were analyzed using a one-way ANOVA followed by Scheffe's post hoc test for inter-group comparisons. The data normality was calculated by Kolmogorov-Smirnov test using SPSS software, version 20.0. P<0.05 was considered to be statistically significant.
Experimental setup
Experiment 1
Effect of hippocampal CysLT1R knockdown on LPS-induced depressive-like behaviors and the neuroinflammatory response in mice. LV-CysLT1R-shRNA-EGFP or LV-EGFP was bilaterally injected into the DG of the mouse hippocampus to chronically inhibit CysLT1R expression. EGFP-positive cells in the DG were observed on the 6th d after infection. On the 7th d, mice were treated with saline or LPS (0.5 mg/kg, ip). Behavioral tests (OFT, NSF, FST, and TST) were performed in turn at 24 h after the LPS challenge. In the meantime, the hippocampal CysLT1R, NF-κB p65, TNF-α, and IL-1β were determined using Western blot assay, and Iba1 was analyzed using immunohistochemistry staining.
Experiment 2
Effect of pranlukast on LPS-induced depressive-like behaviors and the inflammatory response. Four groups of mice were pre-treated with saline or pranlukast (0.3 or 0.6 mg/kg, ig) and, 30 min later, with LPS (0.5 mg/kg, ip). On the second day, mice were subjected to behavioral tests 30 min after administration with pranlukast, and hippocampal CysLT1R, NF-κB p65, TNF-α, IL-1β, and Iba1 were determined.
Results
Hippocampal CysLT1R expression was up-regulated by LPS exposure in mice and reversed by FLX
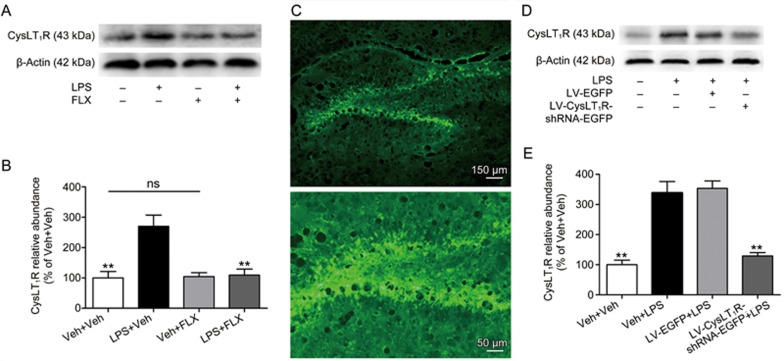
Inflammation challenge by LPS administration has long been applied to induce depressive-like and anxiety-like behaviors in animal models23,24. Accordingly, we explored whether LPS exposure changed CysLT1R expression in the mouse hippocampus using a Western blot assay. Surprisingly, peripheral injection of LPS significantly increased the expression of CysLT1R in the mouse hippocampus (F(3, 12)=11.384, P=0.003; Figure 1A, 1B). To further examine the effect of antidepressants on the LPS-induced increase in CysLT1R, mice were pre-treated with FLX (20 mg/kg, ig) once daily for 7 consecutive days. On the 7th day, LPS (0.5 mg/kg, ip) or PBS was injected 30 min after drug administration. Hippocampal CysLT1R expression was determined 24 h after LPS exposure. As shown in Figure 1A, the LPS-induced increase in the mouse hippocampal CysLT1R level was reversed by FLX treatment (P=0.005; Figure 1A, 1B), while treatment with FLX alone did not affect the expression of CysLT1R in the mouse hippocampus (Veh+Veh vs Veh+FLX: P=0.864; Figure 1A, 1B). These results suggested that CysLT1R might be involved in LPS-induced depressive-like alterations.
Figure 1.
Hippocampal CysLT1R expression is up-regulated in LPS-treated mice and reversed by FLX and that knockdown of hippocampal CysLT1R prevents LPS-induced CysLT1R alternation. (A, B) Mice were pre-treated with FLX (20 mg/kg, ig) once daily for 7 consecutive days. On the 7th day, mice were injected with LPS (0.5 mg/kg, ip) or PBS. After 24 h, hippocampal expression of CysLT1R was detected by Western blot and quantified as the ratio (in percentage) of Veh+Veh group. (C) Representative DG area with LV-CysLT1R-shRNA-EGFP transfection. Original magnifications are 100× (Scale bar=150 μm) and 300× (Scale bar=50 μm). (D, E) Shown are CysLT1R expressions in mouse hippocampus and quantification of CysLT1R as the ratio (in percentage) of Veh+Veh group after CysLT1R knockdown. Data shown are expressed as mean±SEM. n=4. *P<0.05, **P<0.01 vs Veh+LPS.
We then generated a lentiviral vector tagged with EGFP that selectively knocked down CysLT1R, namely LV-CysLT1R-shRNA-EGFP. We also generated a lentiviral vector expressing EGFP (LV-EGFP) alone as a control. LV-CysLT1R-shRNA-EGFP or LV-EGFP was microinfused into the mouse DG by stereotaxic injection. On the 6th day after transfection, the DG region displayed numerous EGFP-positive cells (Figure 1C). One week after infusion, mice were treated with a single LPS administration and then subjected to Western blot assay. Quantification of Western blot showed that infusion of LV-CysLT1R-shRNA-EGFP but not LV-EGFP significantly suppressed the LPS-induced increase in CysLT1R expression in the mouse hippocampus (F(3, 12)=30.01, Veh+Veh vs Veh+LPS: P=0.0013; Veh+LPS vs LV-EGFP+LPS: P=0.747; Veh+LPS vs LV-CysLT1R-shRNA-EGFP+LPS: P<0.001; Figure 1D, 1E).
Hippocampal CysLT1R knockdown or blockade ameliorated LPS-induced depressive behaviors
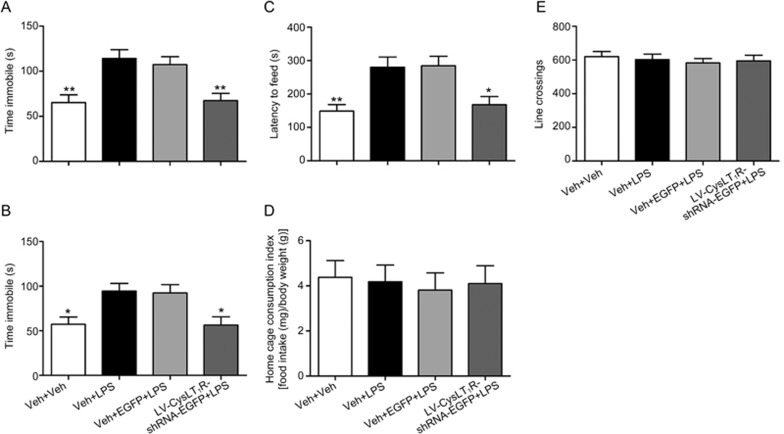
To evaluate the role of CysLT1R in depressive behaviors in response to LPS exposure, mice treated with lentivirus infusion were subjected to behavior tests. We found that LPS-treated mice displayed a significant increase in the immobility time in the FST (F(3, 40)=10.86, P=0.001; Figure 2A) and TST (F(3, 40)=5.688, P=0.014; Figure 2B). Hippocampal CysLT1R knockdown prevented the LPS-induced prolongation of the immobility time in the FST (P=0.003; Figure 2A) and TST (P=0.011; Figure 2B). The NSF test was performed to assess the effect of CysLT1R knockdown on anxiety-like behaviors in LPS-treated mice. Compared to LPS-treated mice, LV-CysLT1R-shRNA-EGFP microinjection inhibited the LPS-induced latency to feed (F(3, 40)=6.276, P=0.043; Figure 2C), but no difference was observed in the home cage consumption index (F(3, 40)=0.097; P=0.999; Figure 2D). The line crossings in the OFT were indistinguishable from all groups (F(3, 40)=0.272; P=0.997; Figure 2E), indicating that our findings were not confounded by a difference in locomotor activity. Taken together, hippocampal CysLT1R knockdown can significantly prevent LPS-induced depressive behaviors.
Figure 2.
Hippocampal CysLT1R knockdown prevents LPS-induced depressive-like behaviors. (A, B) Immobility time in the FST and TST, (C) latency to feed and (D) home cage consumption index in the NSF test, and (E) line crossings in the OFT in lentiviruse-treated mice, following a single injection of LPS after one week. Data shown are expressed as mean±SEM. n=11. *P<0.05, **P<0.01 vs Veh+LPS.
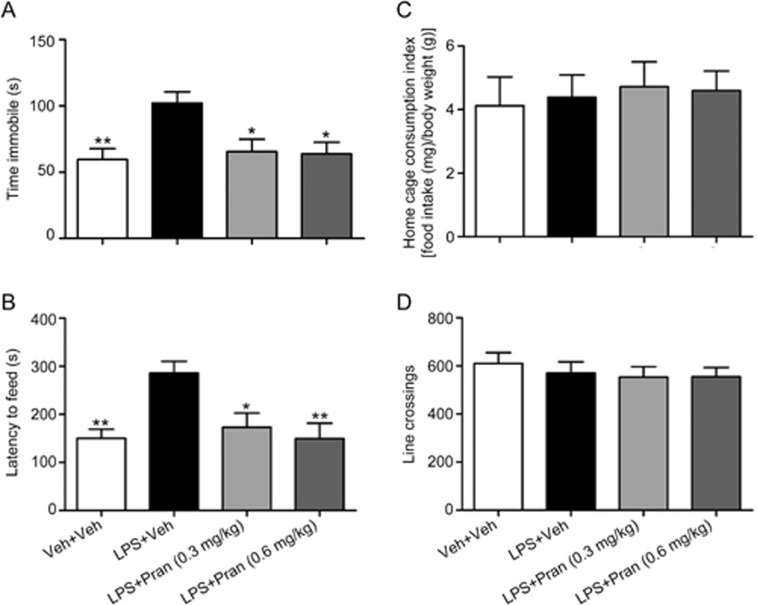
To further verify the effects of CysLT1R in regulating depressive-like behaviors, we then explored the antidepressive effect of pranlukast, a CysLT1R antagonist, in mice exposed to LPS. Pranlukast administration (0.3 or 0.6 mg/kg, ig) completely blocked the LPS-induced prolongation of the immobility time in the TST (F(3, 40)=5.124; P=0.014 for Pran 0.3 mg/kg; P=0.01 for Pran 0.6 mg/kg; Figure 3A). In the NSF test, pranlukast notably reduced the latency to feed compared to that in the LPS-treated mice [F(3, 40)=5.992; P=0.013 for Pran 0.3 mg/kg; P=0.002 for Pran 0.6 mg/kg; Figure 3B] without changing the home cage consumption index of the mice (F(3, 40)=0.118; P=0.981 for Pran 0.3 mg/kg; P=0.995 for Pran 0.6 mg/kg; Figure 3C). No effect of pranlukast administration on locomotor activity was observed (F(3, 40)=0.373; P=0.986 for Pran 0.3 mg/kg; P=0.989 for Pran 0.6 mg/kg; Figure 3D). Therefore, CysLT1R blockade by pranlukast may ameliorate LPS-induced depressive behaviors.
Figure 3.
Blockade of CysLT1R by Pran ameliorates LPS-induced depressive-like behaviors. (A) Immobility time in the TST and (B) latency to feed and (C) home cage consumption index in the NSF test and (D) line crossings in the OFT were examined at 30 min after drug treatments. Data shown are expressed as mean±SEM. n=11. *P<0.05, **P<0.01 vs LPS+Veh.
Hippocampal CysLT1R knockdown or blockade inhibited LPS-induced activation of the NF-κB pathway and production of pro-inflammatory cytokines
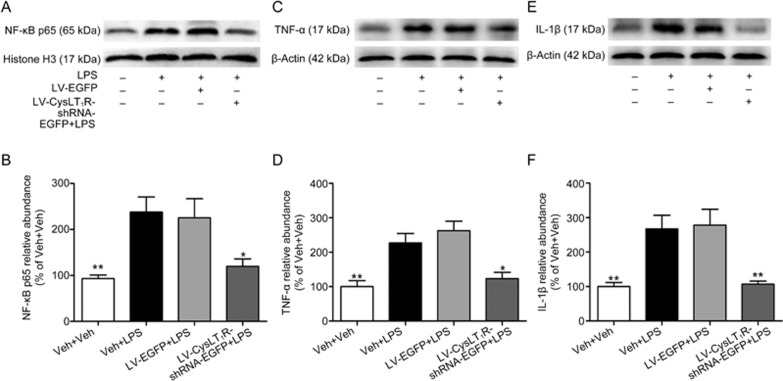
CysLT1R has been reported to be implicated in neuroinflammation by regulating the NF-κB signaling pathway23,24,25,26, and LPS exposure activates NF-κB signaling by inducing the nuclear translocation of p6527. To explore whether CysLT1R is involved in LPS-activated NF-κB signaling, the hippocampal level of NF-κB p65 in the nuclear fraction was detected by Western blot analysis. As shown in Figure 4A, the LPS challenge exerted a significant increase in the nuclear level of NF-κB p65 in the mouse hippocampus, which was prevented by pretreatment with LV-CysLT1R-shRNA-EGFP (F(3, 12)=6.938; Veh+Veh vs Veh+LPS: P=0.008; Veh+LPS vs LV-EGFP+LPS: P=0.978; Veh+Veh vs LV-CysLT1R-shRNA-EGFP+LPS: P=0.028; Figure 4A, 4B).
Figure 4.
Hippocampal CysLT1R knockdown inhibits LPS-activated NF-κB signaling and production of pro-inflammatory cytokines. (A, B) Nuclear extracts were prepared from hippocampus, and nuclear NF-κB p65 and histone H3 as loading control were examined by Western blot using respective antibodies (1:1000). (C, D) TNF-α and (E, F) IL-1β and β-actin as loading control were examined by Western blot by respective antibodies, and quantification of TNF-α and IL-1β were presented as the ratio (in percentage) of Veh+Veh group. Data shown are expressed as mean±SEM. n=4. *P<0.05, **P<0.01 vs Veh+LPS.
To determine the effect of CysLT1R downregulation on LPS-induced pro-inflammatory cytokine production in the mouse hippocampus, we examined the expression of TNF-α and IL-1β by Western blot assay. We found that LPS-treated mice exhibited marked increases in hippocampal TNF-α (F(3, 12)=10.849; P=0.013; Figure 4C, 4D) and IL-1β (F(3, 12)=9.807; P=0.007; Figure 4E, 4F), which were significantly suppressed by LV-CysLT1R-shRNA-EGFP microinjection (P=0.048 for TNF-α P=0.009 for IL-1β Figure 4C–4F). These findings revealed that CysLT1R knockdown in the hippocampus prevented LPS-induced activation of the NF-κB pathway and overproduction of pro-inflammatory cytokines.
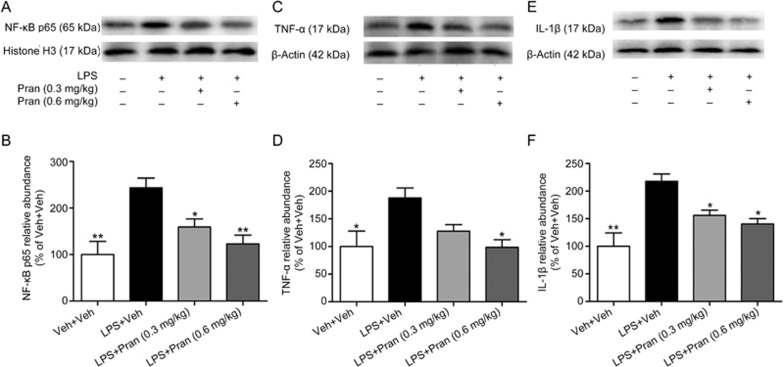
To further confirm the effect of CysLT1R in mediating the neuroinflammation process in depression, we investigated the anti-inflammatory effect of pranlukast in LPS-treated mice. Consistently, LPS treatment activated the NF-κB pathway in the mouse hippocampus, as demonstrated by an increase in nuclear p65. Interestingly, administration of pranlukast (0.3 or 0.6 mg/kg) not only blocked LPS activation of the NF-κB pathway (F(3, 12)=8.338; P=0.045 for Pran 0.3 mg/kg; P=0.006 for Pran 0.6 mg/kg; Figure 5A, B) but also suppressed the increase of TNF-α (F(3, 12)=4.836; P=0.107 for Pran 0.3 mg/kg; P=0.017 for Pran 0.6 mg/kg; Figure 5C, 5D) and IL-1β (F(3, 12)=9.949; P=0.039 for Pran 0.3 mg/kg; P=0.011 for Pran 0.6 mg/kg; Figure 5E, 5F) induced by LPS, which further confirmed the role of CysLT1R in mediating LPS-induced neuroinflammatory responses.
Figure 5.
Blockade of CysLT1R suppresses LPS-activated NF-κB pathway and expression of pro-inflammatory cytokines. (A, B) NF-κB p65 and histone H3 as loading control were determined by Western blot using respective antibodies (1:1000). (C, D) TNF-α and (E, F) IL-1β and β-actin as loading control were examined by Western blot by respective antibodies, and quantification of TNF-α and IL-1β were presented as the ratio (in percentage) of Veh+Veh group. Data shown are expressed as mean±SEM. n=4. *P<0.05, **P<0.01 vs LPS+Veh.
Hippocampal CysLT1R knockdown or blockade prevented LPS-induced microglial activation
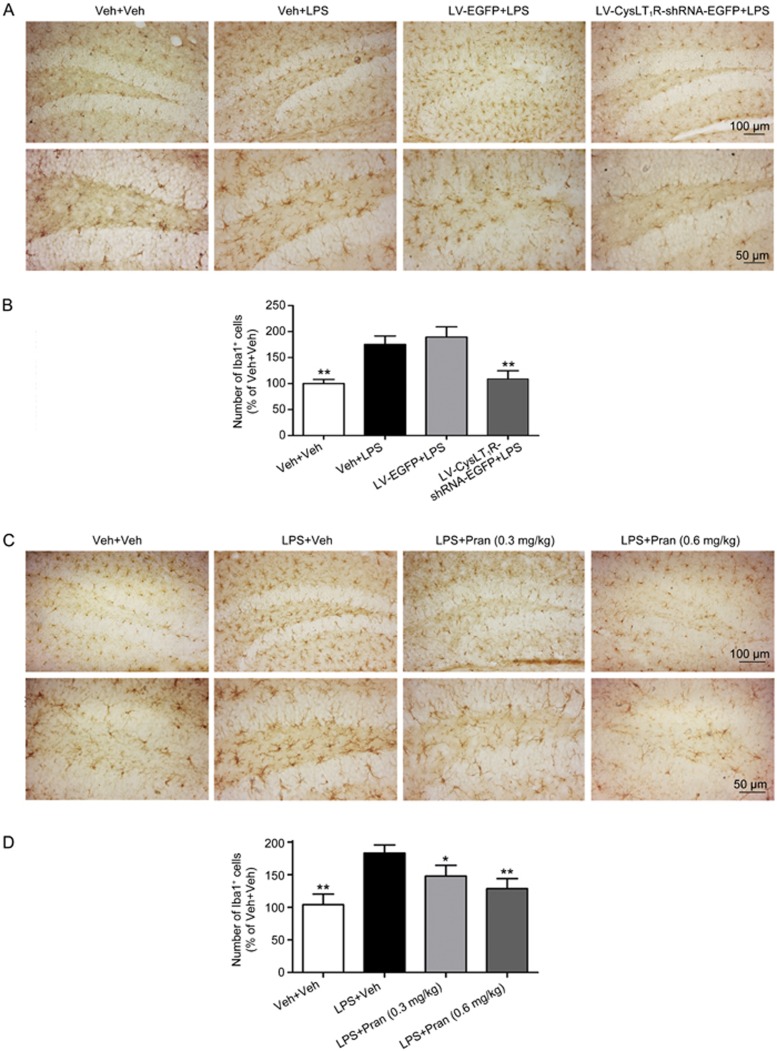
The depressive-like behavioral effects of LPS are believed to be related to microglial activation, causing an increase in the number of activated microglia as well as morphological changes, including shorter, thicker processes and size enlargement28. Immunohistochemical detection of brain sections revealed a marked increase in the number of Iba1-positive cells in the mouse hippocampus after LPS challenge (F(4, 25)=8.988; P=0.004; Figure 6A, 6B), indicating a massive activation of microglia. Pre-treatment with LV-CysLT1R-shRNA-EGFP significantly prevented the increase in Iba1-positive cells (P=0.009; Figure 6A, 6B). In addition, treatment with pranlukast (0.3 or 0.6 mg/kg) also notably decreased the number of Iba1-positive cells (F(4, 25)=7.174; LPS+Veh vs LPS+Pran (0.3 mg/kg): P=0.011; LPS+Veh vs LPS+Pran (0.6 mg/kg): P=0.004; Figure 6C, 6D).
Figure 6.
Iba1 immunohistochemistry. Mice were sacrificed and brain sections were immunohistochemically stained for the microglial marker Iba1. (A, C) Representative microphotographs show Iba1 immuno-stained microglia of the mouse hippocampus. (B, D) The number of Iba1 antibody-stained microglia was normalized in the corresponding same area, as the ratio (in percentage) of the Veh+Veh are shown. Original magnifications are 200× (Scale bar=150 μm) and 400× (Scale bar=50 μm). Data shown are expressed as mean±SEM. n=6. *P<0.05, **P<0.01 vs LPS+Veh.
Discussion
Increasing evidence indicates a close link between neuroinflammation and depression, which is manifested by elevated levels of pro-inflammatory cytokines and chemokines in the central nervous system29,30,31,32. We previously identified CysLT1R signaling as a novel pro-inflammatory pathway in mice and suggested it as a potential therapeutic target for depression18. Here, we demonstrated that a single injection of LPS (0.5 mg/kg, ip) produced depressive-like behaviors accompanied by neuroinflammation. Both knockdown and pharmacological blockade of CysLT1R inhibited LPS-induced depressive behaviors. Moreover, these treatments also suppressed LPS-activated conformational changes of microglia and pro-inflammatory cytokine production in the mouse hippocampus, suggesting that the antidepressive effect of CysLT1R downregulation is accompanied by reduced neuroinflammation.
Numerous reports have supported a strong association between LPS exposure and depression, and acute administration of LPS has been frequently used to model inflammation-associated depressive disorder33. A single injection of LPS in animals induces sickness behaviors34, characterized by sleep disturbances, lethargy, decreased food intake and increased sensitivity to pain, some of which are similar to depressive symptoms in humans. Generally, peripheral administration of low-dose LPS is a well-established model for studying the behavioral responses that are accompanied by the acute activation of the peripheral immune system35. The ensuing depressive-like behaviors peak at 24 h post-treatment33, without the confounding effect of sickness36. Therefore, depressive behaviors were tested at 24 h after LPS exposure in the current study. As previously reported, the LPS dosage of 0.5 mg/kg8,37,38 was selected for its ability to successfully induce depressive-like behaviors and neurochemical alterations in mice.
Intriguingly, basal hippocampal expression of CysLT1R was pretty weak, but LPS administration resulted in a marked increase in CysLT1R expression, which was reversed by the antidepressant FLX. These results suggested that CysLT1R might be involved in the depressive-like alterations induced by LPS. We also found that infusion of LV-CysLT1R-shRNA-EGFP into the DG of the hippocampus downregulated hippocampal CysLT1R expression and produced a marked antidepressive effect in the behavioral tests, as demonstrated by suppressing the LPS-induced increase in immobility time in the FST and TST, without changing the locomotor activity of the mice. A similar effect was observed in mice treated with the CysLT1R antagonist pranlukast. In addition, depression often co-exists with anxiety, as seen by the frequent intermixture of symptoms of depression and anxiety in humans. We therefore performed the NSF test, which applied the conflict between the mice's drive to eat and fear in a new paradigm, to test anxiety behavior. LV-CysLT1R-shRNA-EGFP or pranlukast treatment significantly suppressed anxiety-like behaviors in depressive-like mice, characterized by inhibiting the LPS-induced increase in the latency to feed, without affecting the home cage consumption index. Taken together, CysLT1R knockdown or blockade showed a potential antidepressant effect.
It has been widely reported that the expression of inflammatory cytokines in the periphery and the brain of depressive patients is altered, which contributes to depressive symptoms, and antidepressants can suppress these effects of inflammatory cytokines5. Among these cytokines, TNF-α and IL-1β, induced by injury, infection and psychological stress, are two important inflammatory markers involved in the etiology and treatment of depression3,39,40 and have been implicated in depressive-like behaviors in rodent models. Interestingly, the hippocampus, as one of the depression-related limbic brain structures, displays high levels of receptors for these inflammatory mediators. One possible signaling cascade for mediating the expression of TNF-α and IL-1β is the NF-κB pathway. The NF-κB signaling pathway is known to regulate the expression of cytokines, chemokines, chemokine enzymes, adhesion molecules and pro- and anti-apoptotic genes involved in immunity and inflammation. Activation of NF-κB signaling might be a central event in pro-inflammatory signal transduction, and suppression of NF-κB activation can inhibit the expression of various genes41,42, including IL-1β, IL-6, TNF-α, and COX-2. We thus speculate that the anti-inflammatory effect of CysLT1R downregulation might be mediated by the NF-κB pathway. The pathophysiological role of CysLT1R, a high-affinity G protein-coupled receptor, is well documented in several inflammatory conditions. CysLT1R-mediated signaling is related to the NF-κB pathway in some peripheral cells25,43,44. Recently, the CysLT1R-mediated NF-κB pathway was also observed in neurons22. Consistently, our study showed that knockdown or blockade of hippocampal CysLT1R inhibited not only LPS-induced CysLT1R expression but also NF-κB signaling and expression of pro-inflammatory cytokines (TNF-α and IL-1β) in the mouse hippocampus.
Microglia, as key cellular elements of the acute neuroinflammatory response, are the main source of pro-inflammatory mediators in the mouse brain28. Peripheral treatment with LPS promotes the neuroinflammatory response through microglial activation and overproduction of inflammatory cytokines45. Notably, microglia were activated after LPS exposure in the current study, which could be prevented by CysLT1R knockdown or blockade. It has been reported that CysLT1R expression was predominantly observed in microglia, astrocytes and some oligodendroglial cells46, and inhibition of NF-κB transcriptional activity in the microglial nucleus suppresses pro-inflammatory cytokine expression47,48. Therefore, we speculate that LPS-induced neuroinflammation could be involved in microglia activation via CysLT1R-mediated NF-κB signaling.
In summary, our study indicated that downregulation of hippocampal CysLT1R by genetic transfection or pharmacological antagonism displayed an antidepressive effect in LPS-treated mice. The effects were at least partially mediated by reducing LPS-induced microglial activation and the following production of pro-inflammatory cytokines via the NF-κB pathway. Our findings provide new insight into the mechanism of depression and highlight CysLT1R as a novel and promising target for the prevention or treatment of depression.
Author contribution
Hao HONG, Mei HU, Yan LONG, Hong-bin SUN, and Ling-yi KONG designed the study and wrote the protocols; Jing-ran LIN, Shun-chang FANG, Su-su TANG, and Arijit GHOSH managed the literature retrieval and performed the experimental work and data analysis; Jing-ran LIN and Shun-chang FANG wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No 81273497; 81573413), the Program for Changjiang Scholars and Innovative Research Team in University (No IRT1193), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci 2001; 2: 343–51. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Osuntokun O, Heinloth AN, Corya SA. Therapeutic options for treatment-resistant depression. CNS Drugs 2010; 24: 131–61. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager WL, Reim EK, Lanctôt KL. Efficacy and tolerability of antidepressants for treatment of depression in coronary artery disease: a meta-analysis. Can J Psychiatry 2010; 55: 91–9. [DOI] [PubMed] [Google Scholar]
- Babcock TA, Carlin JM. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine 2000; 12: 588–94. [DOI] [PubMed] [Google Scholar]
- Hannestad J, Dellagioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 2011; 36: 2452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun 2004; 18: 205–13. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Gadotti VM, Calixto JB, Santos AR, Rodrigues AL. Depressive-like behavior induced by tumor necrosis factor-α in mice. Neuropharmacology 2012; 62: 419–26. [DOI] [PubMed] [Google Scholar]
- Mello BS, Monte AS, Mcintyre RS, Soczynska JK, Custódio CS, Cordeiro RC, et al. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J Psychiatr Res 2013; 47: 1521–9. [DOI] [PubMed] [Google Scholar]
- De Paiva VN, Lima SN, Fernandes MM, Soncini R, Andrade CA, Giusti-Paiva A. Prostaglandins mediate depressive-like behaviour induced by endotoxin in mice. Behav Brain Res 2010; 215: 146–51. [DOI] [PubMed] [Google Scholar]
- Singh RK, Gupta S, Dastidar S, Ray A. Cysteinyl leukotrienes and their receptors: molecular and functional characteristics. Pharmacology 2010; 85: 336–49. [DOI] [PubMed] [Google Scholar]
- Nishio H, Hayashi Y, Terashima S, Takeuchi K. Protective effect of pranlukast, a cysteinyl-leukotriene receptor 1 antagonist, on indomethacin-induced small intestinal damage in rats. Inflammopharmacology 2008; 15: 266–72. [DOI] [PubMed] [Google Scholar]
- Rao NL, Dunford PJ, Xue X, Jiang X, Lundeen KA, Coles F, et al. Anti-inflammatory activity of a potent, selective leukotriene A4 hydrolase inhibitor in comparison with the 5-lipoxygenase inhibitor zileuton. J Pharmacol Exp Ther 2007; 321: 1154–60. [DOI] [PubMed] [Google Scholar]
- Schelfhout V, Van De Velde V, Pauwels R, Joos G. The effect of the leukotriene receptor antagonist zafirlukast on neurokinin A-induced bronchoconstriction in patients with asthma — A comparison with leukotriene D4 induced broncoconstriction. Pulm Pharmacol Ther 2008; 21: 276–84. [DOI] [PubMed] [Google Scholar]
- Tang SS, Ji MJ, Chen L, Hu M, Long Y, Li YQ, et al. Protective effect of pranlukast on Aβ1-42-induced cognitive deficits associated with downregulation of cysteinyl leukotriene receptor 1. Int J Neuropsychopharmacol 2014; 17: 581–92. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Zhang L, Ye YL, Fang SH, Zhou Y, Zhang WP, et al. Cysteinyl leukotriene receptors CysLT1 and CysLT2 are upregulated in acute neuronal injury after focal cerebral ischemia in mice. Acta Pharmacol Sin 2006; 27: 1553–60. [DOI] [PubMed] [Google Scholar]
- Zhang WP, Hu H, Zhang L, Ding W, Yao HT, Chen KD, et al. Expression of cysteinyl leukotriene receptor 1 in human traumatic brain injury and brain tumors. Neurosci Lett 2004; 363: 247–51. [DOI] [PubMed] [Google Scholar]
- Wang L, Du C, Lv J, Wei W, Cui Y, Xie X. Antiasthmatic drugs targeting the cysteinyl leukotriene receptor 1 alleviate central nervous system inflammatory cell infiltration and pathogenesis of experimental autoimmune encephalomyelitis. J Immunol 2011; 187: 2336–45. [DOI] [PubMed] [Google Scholar]
- Yu XB, Dong RR, Wang H, Lin JR, An YQ, Du Y, et al. Knockdown of hippocampal cysteinyl leukotriene receptor 1 prevents depressive behavior and neuroinflammation induced by chronic mild stress in mice. Psychopharmacology (Berl) 2016; 233: 1739–49. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burqhardt NS, Costantini DO, Tannenholz L, Ahmari SE, et al. Differential control of learning and anxiety along the dorsalventral axis of the dentate gyrus. Neuron 2013; 77: 955–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Stephanie D, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301: 805–9. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 1977; 229: 327–36. [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 2005; 29: 571–625. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ichiyama T, Hasegawa M, Hasegawa S, Matsubara T, Furukawa S. Cysteinyl leukotrienes induce monocyte chemoattractant protein-1 in human monocyte/macrophages via mitogen-activated protein kinase and nuclear factor-κB pathways. Int Arch Allergy Immunol 2009; 149: 275–82. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 2009; 14: 511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Tang SS, Hu M, Long Y, Li YQ, Liao MX, et al. Leukotriene D4 induces amyloid-β generation via CysLT(1)R-mediated NF-κB pathways in primary neurons. Neurochem Int 2013, 62: 340–7. [DOI] [PubMed] [Google Scholar]
- Kawano T, Matsuse H, Kondo Y, Machida I, Saeki S, Tomari S, et al. Cysteinyl leukotrienes induce nuclear factor kappaB activation and rantes production in a murine model of asthma. J Allergy Clin Immunol 2003; 112: 369–74. [DOI] [PubMed] [Google Scholar]
- Santa-Cecília FV, Socias B, Ouidja MO, Sepulveda-Diaz JE, Acuña L, Silva RL, et al. Doxycycline suppresses microglial activation by inhibiting the p38 MAPK and NF-κB signaling pathways. Neurotox Res 2016; 29: 447–59. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol 2010; 119: 89–105. [DOI] [PubMed] [Google Scholar]
- Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry 2012; 83: 495–502. [DOI] [PubMed] [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, et al. Grand challenges in global mental health. Nature 2011; 475: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Chen F, Thakur A, Hong H. Cysteinyl leukotrienes and their receptors: emerging therapeutic targets in central nervous system disorders. CNS Neurosci Ther 2016. doi:10.1111/cns.12596. [DOI] [PMC free article] [PubMed]
- Zhang JQ, Wu XH, Feng Y, Xie XF, Fan YH, Yan S, et al. Salvianolic acid B ameliorates depressive-like behaviors in chronic mild stress-treated mice: involvement of the neuroinflammatory pathway. Acta Pharmacol Sin 2016; 37: 1141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 1988; 12: 123–37. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav 2005; 81: 688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation 2008; 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C, et al. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol 2014; 18. pii: pyu077. doi:10.1093/ijnp/pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaz VS, Cordeiro RC, Costa AM, de Lucena DF, Nobre Júnior HV, de Sousa FC, et al. Antidepressant-like effect of nitric oxide synthase inhibitors and sildenafil against lipopolysaccharide-induced depressive-like behavior in mice. Neuroscience 2014; 268: 236–46. [DOI] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shlik J, Maron E. Effects of bupropion augmentation on pro-inflammatory cytokines in escitalopram-resistant patients with major depressive disorder. J Psychopharmacol 2008; 23: 854–8. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho CM, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord 2012; 139: 230–9. [DOI] [PubMed] [Google Scholar]
- Sethi G, Sung B, Aggarwal BB. Nuclear factor-kappaB activation: from bench to bedside. Exp Biol Med (Maywood) 2008; 233: 21–31. [DOI] [PubMed] [Google Scholar]
- Qiao L, Zhao TJ, Wang FZ, Shan CL, Ye LH, Zhang XD. NF-kappaB downregulation may be involved in the depression of tumor cell proliferation mediated by human mesenchymal stem cells. Acta Pharmacol Sin 2008; 29: 333–40. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Takeuchi K, Ishinaga H, Basbaum C, Majima Y. Leukotriene D4 upregulates MUC2 gene transcription in human epithelial cells. Pharmacology 2008; 81: 221–8. [DOI] [PubMed] [Google Scholar]
- Thompson C, Cloutier A, Bossé Y, Thivierge M, Gouill CL, Larivée P, et al. CysLT1 receptor engagement induces activator protein-1- and NF-kappaB-dependent IL-8 expression. Am J Respir Cell Mol Biol 2006; 35: 697–704. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007; 55: 453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschallinger J, Schäffner I, Klein B, Gelfert R, Rivera FJ, Illes S, et al. Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat Commun 2015; 6: 8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Chen X, Wang LY, Gao W, Zhu MJ. Rho kinase inhibitor fasudil protects against β-amyloid-induced hippocampal neurodegeneration in rats. CNS Neurosci Ther 2013; 19: 603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor kappaB. J Neuroimmunol 2001; 117: 87–96. [DOI] [PubMed] [Google Scholar]