Abstract
The endothelial surface layer is covered with abundant proteoglycans, of which syndecans and glycosaminoglycans are major constituents. Among the endothelial glycocalyx (eGC) constituents, syndecan-1 (sdc1) is a main component, and an elevated serum level of sdc1 may indicate the degradation of eGC. In patients with ischemic heart disease or heart failure, elevation of serum sdc1 has been associated with worsening cardiac and renal function; however the causal relationship between degradation of eGC and clinical outcomes is unclear. Herein, we review the previous literature on eGC in cardiovascular and non-cardiovascular diseases and their clinical implications.
Keywords: endothelial glycocalyx, Cardiovascular Diseases, syndecan-1 (sdc1)
INTRODUCTION
Endothelial cells are covered with abundant proteoglycan complexes known as “endothelial glycocalyx (eGC),” which is comprised of transmembrane core proteins known as syndecans, and polymers of glycosaminoglycans such as heparin sulfate, chondroitin sulfate, and hyaluronans that branch from the highly sulfated and negatively-charged syndecan cores. Endothelial glycocalyx maintains the integrity of the interface between blood flow and endothelial surface, and prevents extravasation of salt, water and proteins. It also functions as a barrier to keep proteins or cytokines of untoward effect from direct contact to the endothelium. Adhesion of leukocytes and platelets to endothelium are attenuated in the presence of the eGC, diminishing blood coagulation [1, 2]. The eGC controls nitric-oxide mediated vasodilation by transducing shear stress to the cytoskeleton in the endothelial cells [3, 4]. Deformation of cortical cytoskeleton by such mechanotransduction is assumed to initiate the various chains of intracellular signaling as well as nitric oxide production [5–7]. Although a growing body of evidence shows degradation of eGC leads to adverse cardiovascular events, the studies on eGC in the clinical setting remain limited. The following is a review of studies evaluating the clinical impact of measured eGC in cardiovascular and non-cardiovascular diseases and endeavor to illuminate the future direction of investigation.
Endothelial glycocalyx in non-cardiovascular disease
Inflammation
In addition to increased porosity of the eGC during mild inflammation, proteases from activated neutrophils and severely traumatized tissue promote breakdown of the eGC. The shedding of the eGC exposes intercellular adhesion molecules (ICAMs) and P-selectin underneath the glycocalyx layer. Consequently, the eGC shedding facilitates more adhesion of inflammatory cells to the endothelium, which is followed by increased endothelial permeability and propagation of inflammation [8, 9]. On the other hand, the soluble fragments from eGC appear to have opposing actions, which are spread between inflammation and contribution to immunosuppression [10]. Syndecan-1 (sdc1) is the most investigated constituent of the cell surface glycocalyx in the area of cancer prognosis and trauma related inflammation [11]. In wild-type mice, the level of inflammation due to Pseudomonas aeruginosa infection was more severe with a greater amount of shed sdc1, while sdc1-null mice had attenuated lung-injury. Interestingly, the protective phenotype in sdc-1-null mice was lost when isolated sdc1 ectodomains were administered, suggesting that shed sdc1 enhances bacterial virulence by a probable way of altered innate immunity [12]. In contrast, the presence of soluble sdc1 in blood accelerated removal of the tissue-bound inflammatory chemokines and resolution of the accumulated neutrophils, while multi-organ injury and lethality were attenuated with infused heparin sulfate [2, 13, 14]. Additionally, absence of sdc1 in sdc1-null mice delayed wound healing due to defected migration of keratinocytes [11, 15]. Those results suggested that soluble sdc1 shed from the cells helped recovery from systemic inflammation.
Tumors
In most tumors, reduced expression of sdc1 in epithelial-origin tumor cells was associated with less adhesion of the cells to the extracellular matrix, leading to increased cell migration/invasion, and eventually poor clinical outcomes [16, 17]. Elevated serum sdc1 in hematologic malignancies was also associated with poor prognosis [18–21]. Overexpression of sdc1 in stromal fibroblasts or shedding of sdc1 from the cells into tumor microenvironments was associated with accelerated angiogenesis and cancer progression. [16, 22–26]. Contrary to the general nature of tumor behaviors, several cancers (e.g. breast cancer, gallbladder cancer, glioma and squamous cell carcinoma of the head and neck) showed better prognosis when sdc1 was overexpressed on the cells [22]. Therefore, cellular integral membrane proteins, in conjunction with properties of the extracellular matrix and the amount of sdc1 shed in the tumor microenvironments, each interactively affect the modulation mechanism of cell migration [11].
Soluble sdc1 is thought to be a biologically active mediator of interaction between extracellular matrix and cancer cells, and may enhance migration and invasion of malignant cells [22]. Shed sdc1 can bind the same ligands of intact ectodomain and even compete with intact ectodomain to downregulate signal transduction [27]. Interestingly, soluble sdc1 may be a potential pro-angiogenic factor. In Hodgkin’s lymphoma, with high expression of heparinase, soluble sdc1 stimulated vascular endothelial growth factor (VEGF) receptors on the adjacent endothelial cells by forming a complex with VEGF [28]. In an animal study, high expression of sdc1 in stromal fibroblasts increased the density of microvasculature in the tumors, which was explained by sequestration of pro-angiogenic factors in the tumor microenvironment [29]. These findings suggested that soluble sdc1 may both promote angiogenesis [14] and may be used to treat cardiovascular diseases.
Although soluble sdc1 from the cells may switch malignant cells from proliferative to invasive phenotypes [27], it is unclear whether a high level of soluble sdc1 resulted from overproduction of sdc1 or excessive shedding from the cells. While study investigators primarily used antibodies to sdc1 ectodomains on the cell surface, antibodies to both the ectodomains and the carboxy-terminal-fragments of sdc1 are warranted to answer the question.
Endothelial glycocalyx in cardiovascular disease
Preclinical studies
Oxidized low density lipoprotein cholesterol (ox-LDL) degrades eGC and enhances adherence of leukocytes to the endothelial surface in mouse cremaster venules, [1] corresponding to the notion of endothelial dysfunction in patients with dyslipidemia. Among four subtypes of syndecan, sdc1 is the most prominent on the surface of endothelial cells [30], and the function of membrane-bound or soluble sdc1 ectodomain is increasingly investigated. Sdc1-null mice developed larger neointimal hyperplasia in injured carotid arteries, and had increased proliferation of smooth muscle cells compared to wild type mice. [31]. Reduced motility of macrophages in sdc1-null mice also appear to contribute to augmented atherosclerosis, as lymphatic clearance of sdc1 −/− macrophages was significantly delayed [32]. This data suggested that sdc1 restricted intimal thickening in healing arteries by inhibiting smooth muscle cell proliferation and modulation of macrophage motility.
Contradictory results regarding the influence of sdc1 on matrix remodeling of injured animal hearts also exist. In sdc1-null mice with induced myocardial infarction, enhanced endothelial adhesion and trans-endothelial migration of the inflammatory cells, as well as exaggerated adverse matrix remodeling and fibrosis, were observed [33]. The absence of sdc1 also attenuated angiotensin II-induced cardiac dysfunction and fibrosis in mice [34]. The Langendorf model of isolated guinea pig hearts demonstrated that degradation of the eGC led to reduced endothelial barrier function and extravasation of administered starch, which seems analogous to the fluid shift to the third space as observed in patients with heart failure [35–37].
Clinical studies
Various clinical studies demonstrate different effects of eGC glycosaminoglycans, depending on arteries and subfamilies of glycosaminoglycans. Heparin sulfate, the major glycosaminoglycan molecule in the eGC, decreased in human aorta as age and cholesterol contents in the aorta increased [38]. The concentration of hyaluronic acid, a minor constituent, showed a negative gradient from normal areas to atheromatous areas in the human aorta, with its biological function being a negative regulator on vascular smooth muscle cell (VSMC) proliferation and a positive regulator of the migration [39]. One study reported that the progression of atherosclerosis was favored by an increase in chondroitin sulfate in the arterial walls [40], while another reported that serum soluble sdc1 was significantly elevated in patients with acute coronary syndrome, suggesting a contributory effect of eGC damage to atherosclerotic plaque vulnerability [41].
In a study by Ostrowski et al., with acute ST segment elevation myocardial infarction, sdc1 was weakly correlated with circulating catecholamine level, with marginal association with development of heart failure (p=0.069) and no association with 30-day mortality [42]. In the same study, adrenaline was associated with both 30-day mortality and heart failure development [42]. Interestingly, there was no reported relationship between serum soluble sdc1 with peak troponin I, which could infer that eGC damage does not correlate with size of infarction, but it potentially develops prior to coronary arterial occlusion.
Several studies investigated the clinical implication of sdc1 in patients with heart failure. Bielecka-Dabrowa et al. assigned 120 hypertensive patients to two groups, based on the presence of heart failure and investigated C-statistics of various combinations of biomarkers for the diagnosis of heart failure [43]. In that cross-sectional analysis, syndecan-4 showed only 0.781 of area under the curve (AUC) in the receiver operation characteristics (ROC) for the diagnosis with heart failure, whereas N-terminal pro-brain type natriuretic peptide (NT-proBNP) showed 0.873 of AUC [43]. In another study by the same investigators, serum soluble syndecan-4 levels showed significant correlation with left ventricular dilation and reduced left ventricular systolic function in patients with dilated cardiomyopathy, but their causal relationship was not explained [44].
Longitudinal observational studies of patients with acute or chronic heart failure also suggest correlations to serum soluble sdc1 [45–47]. Neves et al. studied 201 patients with acute decompensated heart failure [46]. The ratio of patients with sdc1 >125 ng/ml and <125 ng/ml was about 1:3, and the risk of 6 month-mortality was 22.4% and 8.5%, respectively. Higher serum soluble sdc1 was also associated with more frequent renal dysfunction during hospitalization [46].
Tromp et al. and Meyer et al. both studied 567 patients with acute heart failure, by measuring sdc1 following stabilization and prior to discharge [47, 48]. The ratio of patients with sdc1 >27.7 ng/mL and sdc1 < 27.6 ng/mL was about 1:4, suggesting a rapid decrease of serum soluble sdc1 levels when compared to the previous study [46]. Through univariable analysis, doubling of sdc1 significantly increased the risk of mortality (HR=1.2) in patients with preserved ejection fraction. Interestingly, the association between sdc1 and mortality was significant in women and marginal in men [47, 48].
Demissei et al. also prospectively observed 2,033 patients with acute heart failure, after stabilization during hospital stay, of which 17.6% of patients died within 180 days of the study [45]. They evaluated the diagnostic accuracy of 44 biomarkers for heart failure, including sdc1. Although differences in hazard ratios of sdc1 for 30-day and 180-day mortality were significant at 1.43 and 1.27 per 1 standard deviation, their C-statistics were not as high (0.767 for 30-day mortality and 0.706 for 180-day mortality), and continuous net reclassification improvements (cNRI) were only 0.38 and 0.24, when sdc1 was added to the conventional model [45].
Areas awaiting further investigation
Evaluation of the amount of endothelial glycocalyx in situ
Due to optical transparency and fragility of eGC, in vivo virtual characteristics were primarily inferred through existence of the “exclusion zone” of erythrocytes in the blood-perfused microvessels, which is observed with light microscopy [49, 50]. Although the elevated eGC constituents in serum (i.e. syndecan or heparan sulfate) appear to represent degradation of the eGC, methods to directly measure the actual amount of eGC were not sufficiently investigated, which may relate to the lack of clinical studies. Despite minimal investigation, several novel methods were devised to measure the amount of eGC on the endothelial surface layer in situ.
Direct visualization of harvested microvessels with electron microscopy is being performed. Although the ex vivo measurements of vessel thickness is extremely variable (0.3 μm ~ 60 μm) between studies and vessels, due to fragility and potential eGC structural loss [51], microscopic imaging modalities (confocal, multiphoton fluorescence or transmission electron microscopy) are still being used for direct visualization. In particular, rapid freezing of endothelial cells and use of a novel fixation solution, such as lanthanium (III)-nitrate/glutaraldehyde, revealed a thicker layer of eGC ex vivo, which was more efficacious compared to preparation with a conventional fixation method for microscopic analysis [35, 52].
Another method, known as intravital microscopy, enables observation of capillary blood flow in living animals. Bright-field and fluorescent microscopy can demonstrate the virtual diameter of capillaries in vivo, which are comparable to anatomical diameters [53]. Functional diameter of capillaries can also be estimated by measuring the diameter of deformed erythrocytes flowing through capillaries. Considering that capillary functional diameters are smaller than anatomical diameters, the thickness of the endothelial surface layer can be calculated from the difference. Endothelial surface layer thickness can then be used as a true interface between blood flow and the luminal surface [49]. The resolution may be enhanced with molecules that have high affinity to the glycosaminoglycan chains. [54] Using this method, significant relationships were determined between thickness of eGC in human sublingual microvasculature and cardiovascular risk factors [55, 56].
Lastly, the double tracer dilution method uses two tracers to quantify virtual volume of eGC in the circulatory system [57]. Erythrocytes labeled with sodium fluorescein, which is impermeable to the eGC, are used to quantify circulating plasma volume not included in the eGC layer. eGC-permeable tracers (e.g. dextran 40) are used to quantify total intravascular volume, which includes the eGC layer [49]. The difference between circulating plasma volume and total intravascular volume was assumed to represent the volume of the eGC [55]. Nieuwdorp et al. used the double tracer method to demonstrate that acute normo-insulinemic hyperglycemia reduced the systemic total eGC volume from 1.75 L to 0.8 L [58]. Despite debates on the precision [59], this method is worthy of further investigation because of limited quantitative methods for in vivo evaluation of total human eGC volume.
While the techniques mentioned above may be beneficial for quantitative and qualitative measurement of eGC, they do not completely represent the eGC functional aspect. The permeation rate of intravenous substance into extravascular space through endothelial layer, flow mediated vasodilation (indicative of mechano-transduction function of eGC), or grade of inflammatory reaction dependent on syndecan level, may provide more direct information regarding the functional aspect of the eGC. In general, further investigation of eGC quantitative and qualitative structural measures, in conjunction with analytical methods of function, are required.
Shedding of syndecan-1 and endothelial dysfunction in heart failure
Although new validation tests are gaining popularity in studies exploring new biomarkers, the studies mentioned above did not demonstrate high C-statistics, and the improvement of net reclassification was only moderate. [45, 47] In addition, the interpretation of the tests requires special attention because the application of C-statistics often eliminates established risk factors from new models [60, 61], and net reclassification is often positive for even weak markers, leading to overestimation of the real risk. [62–64] Conversely, the clinical value of sdc1 as a prognosticator of cardiovascular events is still unestablished. In fact, none of the novel biomarkers alone showed superiority to natriuretic peptide (e.g. BNP or NT-proBNP), although addition of novel biomarkers to the traditional diagnostic model may improve discriminative ability. [43] Therefore, considering cost-effectiveness, additional measurements of serum sdc1 may not be plausible. Perhaps, it may be worth focusing on the mechanism of how the degradation of the eGC, represented by elevated serum soluble sdc1, leads to adverse cardiovascular events or vice versa, rather than solely investigating sdc1 outcomes predictability.
The endothelial dysfunction and vascular permeability can be identified using flow-mediated vascular dilation (FMD) and non-invasive measurement of microvascular leakage with strain gauge plethysmography [65, 66]. Flux forces applied to core proteins on the glycocalyx are transferred and amplified in both the cells and deformed cortical cytoskeleton. This mechano-transduction is assumed to initiate the chain of intracellular signaling, such as nitric oxide production, and can be estimated by FMD [5, 6]. In a study that evaluated the relationship between FMD and serum soluble sdc1, 49 patients with untreated nephrotic syndrome and 25 healthy controls were compared. After adjustment for several clinical variables and endothelial function biomarkers (ICAM-1 and e-selectin), only sdc1 was independently associated with FMD [67]. Hyperglycemia induced reduction of the eGC volume was accompanied by impairment of FMD and activation of coagulation system, which were attenuated by infusion of N-acetylcysteine [58, 68]. These findings support the notion that damaged eGC causes endothelial dysfunction in the peripheral arteries. Additionally, previous studies demonstrate that peripheral artery vasomotor dysfunction is prevalent in patients with heart failure [65, 66, 69, 70] and that degradation of eGC appeared to contribute to worse cardiovascular outcomes in patients with heart failure [45–47]. If a temporal/causal relationship can be established among increased sdc1, endothelial dysfunction, vascular permeability, and adverse cardiovascular events in this population, then eGC will become a promising therapeutic target of heart failure.
Endothelial glycocalyx as a potential salt reservoir in heart failure
An area that requires more evaluation is the role of eGC in sodium storage. Accumulation of sodium in interstitial tissue does not necessarily require commensurate water retention [71–73]. Skin is abundant with glycosaminoglycan polymerization [74], and the modulation of intracutaneous tonicity and lymph capillary network enables skin to be a sizable reservoir of sodium without commensurate water [73]. Considering the similarities in composition of eGC to the cutaneous glycosaminoglycans and abundance on the endothelial surface, the eGC may be able to buffer excess sodium and contribute to homeostasis of the systemic volume status. Moreover, the negative charge of sulfated glycosaminoglycan polymers in eGC and their direct contact to sodium in circulating blood confers more weight to the idea of sodium buffering [75]. Siegel et al. used ex vivo human coronary arteries to suggest that blood flow causes conformational changes of negatively charged glycosaminoglycan chains from random coils to a filamentous state. The conformational change exposes intramolecular cation-binding sites to sodium ions, which allows increased sodium binding in glycosaminoglycan chains [76, 77]. Despite doubts on the accuracy, the calculated amount of sodium that can be buffered by total endothelial glycocalyx was estimated to range from 0.7 g to as much as 14 g, when the thickness of eGC is assumed to range from 0.3 μm to 60 μm [51, 78].
Additionally, the increase in endothelial permeability is another important area to be explored. Even if the buffer capacity of eGC for sodium is minimal, its role as a sodium buffer is still significant such that eGC can smoothen sharp fluctuation of intravascular sodium concentration and osmolality after a meal by binding sodium with its abundant, free negative charges. Contrary to the classical principle of Ernest Starling, in which the oncotic pressure difference between the intravascular and the interstitial tissue matters, the thin protein-free zone of eGC was suggested to play greater role in the control of vascular permeability [37]. When eGC are shed, sodium can readily enter into the intracellular space of the endothelial cells and eventually extravasate into interstitial space [3, 22, 35, 79]. Furthermore, there is evidence showing that increased permeability by perturbed eGC allows leakage of other intravascular constituents such as albumin in other organs [80–82].
Shedding and signaling mechanism of syndecan-1
So far, multiple shedding mechanisms were suggested. When colloids were infused during major surgery, the majority of infused colloids extravasated, and the eGC volume was significantly reduced regardless of the type of colloids. Although the authors suggested “wash-out” of the eGC as a shedding mechanism, it was not clarified whether the “wash-out” was induced by volume expansion or trauma from surgery [57]. In another study, patients who underwent major surgery had elevated serum sdc1. Increased production of natriuretic peptide induced by overloaded volume was thought to degrade the eGC. [35, 83].
Sodium overload per se can accelerate shedding of eGC without mediators. When the stiffness of eGC from a human umbilical cord artery was directly measured ex vivo using atomic force microscopy, increase in sodium concentration induced eGC stiffening and reduced heparin sulfate residues by 68% [84]. Meanwhile, hyperglycemia-induced reduction of the eGC volume was accompanied by impairment of flow mediated vascular diameter and activation of the coagulation system, which were attenuated by infusion of N-acetylcysteine. Therefore, reactive oxygen generated under hyperglycemic conditions was suggested as a cause of eGC destruction, where a Toll-like receptor gene appeared to mediate the signaling [58, 68].
Syndecan, heparan sulfate and hyaluronan in plasma are consistently increased in cardiopulmonary bypass or post-cardiac arrest syndrome where ischemia and reperfusion ensue. [85, 86] Hypoxia/reoxygenation in experimental models cause similar deterioration of eGC as found in ischemia/reperfusion in humans. Hypoxia and reoxygenation lead to degradation of eGC, aided by mast cells. Tissue deoxygenation produces nucleoside adenosine and inosine from the adenine nucleotide, which stimulates mast cells to release heparinase in the granular stores [87–89].
Clinically, shedding of the eGC is well reported in systemic inflammation syndrome (i.e., sepsis, major surgery, trauma, etc.), and degraded eGC are associated with altered vascular permeability and resistance, systemic edema, hypovolemia and multi-organ dysfunction, leading to poor clinical outcomes [90, 91]. Bacterial endotoxin in sepsis is thought to initiate the endothelial perturbation in a TNF-α mediated manner [89], and reduced thickness and stiffness of eGC was demonstrated in experimental models exposed to lipopolysaccharide or TNF-α [92]. Mice deficient of the TNF-α receptor did not show reduced thickness in the endothelial surface layer, and inhibition of heparinase prevented endotoxemia-induced degradation of eGC [2].
Apart from the potential shedding mechanism mentioned above, there are many potential “sheddases,” which includes tryptase, elastase, proteinase-3, thrombin, tissue-type plasminogen activator, plasmin, cathepsin B, hyaluronidase, etc. Nevertheless, more research is required to determine which enzymes are the main degraders of eGC [30, 93]. An effective inhibitor of eGC sheddases also requires further investigation.
Potential restorer of endothelial glycocalyx
Albumin-containing solutions elicit shear stress-dependent vasodilation, consequently lower fluid flux across the vessel walls more than other types of colloid fluids. Other studies have shown that human albumin affords protection of the glycocalyx in experimental models and possesses a high affinity for the glycocalyx [36]. This was probably due to the high negative charge on the albumin molecules. The glycocalyx in vivo is highly enriched with cations, which are attracted by the negative charges of heparin sulfate. Hypothetically, the calcium ions on top of the endothelial surface layer, which are divalent cations with dense positive charges, attract albumin more efficiently than other cations [30, 36]. In addition to the electrostatic interaction between albumin and eGC, sphingosine-1 phosphate (S1P)-mediated restoration of the eGC was suggested. The majority of S1P is carried in serum albumin, and endothelial cells contain abundant S1P receptors. Adding S1P to the culture media of the endothelial cells inhibited shedding of sdc1 and induced recovery of the eGC [94, 95].
Apart from albumin, blood-borne proteins and glycosaminoglycan in fresh frozen plasma protected or restored eGC. Animal models with hemorrhagic shock demonstrated that infusion of fresh frozen plasma inhibited shedding of the hemorrhagic shock-induced eGC, preserving microvascular perfusion [96–98]. Steroids also appear to have a protective effect. Preliminary data have shown that hydrocortisone could help in sustaining vascular barrier function and possibly abrogate damage of the glycocalyx, in the acute inflammatory and proteolytic situation of a myocardial infarction. [93, 99, 100]. In mouse cremaster venules, destruction of eGC by infusion of heparinase or oxidized low density lipoprotein cholesterol (ox-LDL) leads to increase in the number of adherent leukocytes to the endothelial surface, which was partially reversed by infusion of heparin sulfate. In the study, circulating heparin sulfate was suggested to reconstitute eGC [1]. Circulating shed heparin sulfate may be re-integrated into the eGC and can reconstitute the endothelial surface. Sulodexide, a mixture of natural porcine heparin and dermatan sulfates, may be also be administered to restore the eGC. In rats or mice, the restitution of damaged eGC takes several days [101], but the duration required for eGC restitution requires further clarification. In patients with heart failure, slowing of the incremental elevation in serum sdc1 may provide information on the dynamics of the eGC replenishment.
The synthesis of glycocalyx components is also affected by mechano-transduction of the fluid shear stress on the cell surface. In cultured endothelial cells, expression of heparin sulfate on the cell surface was accelerated with atheroprotective shear stress waveforms, which has relatively “high mean shear” and “no shear reversal”, whereas it was reduced with atherosclerotically prone waveform, which has “low mean shear” and “shear reversal” [102–104]. These findings may be confirmed in patients with severe left ventricular systolic dysfunction, severe aortic regurgitation, or those who are supported with extracorporeal mechanical life support, where low shear stress waveforms with shear reversal would be observed.
CONCLUSION
Although many studies proposed that endothelial dysfunction, particularly concerning the eGC, might have intervened between cardiovascular diseases and development of adverse outcomes, the number of studies on this topic are limited. The technical difficulties in evaluating the endothelial dysfunction may have created a vicious cycle of poor understanding and poor attention. Despite difficulties with visualization and volume quantification of eGC, progress in laboratory techniques has increased awareness of eGC roles in the control of endothelial dysfunction by way of shear stress transduction, control of endothelial permeability, and protection of endothelium from inflammatory cells, enzymes, or cytokines with untoward effects.
As increased serum-soluble syndecan in patients with heart failure was associated with adverse clinical outcomes, preservation of eGC is thought to have a protective effect on cardiovascular systems. However, the complexity of eGC constituents mediating a poor prognosis in this population is not well-understood. With the increasing prevalence of heart failure among ageing populations and necessity for developing diverse diagnostic and treatment methods, the role of the eGC is a fascinating and potentially beneficial area that requires further investigation. Currently, the limited number of clinical studies evaluating benefits of eGC protection urges investigators to increase exploration efforts into this emerging field.
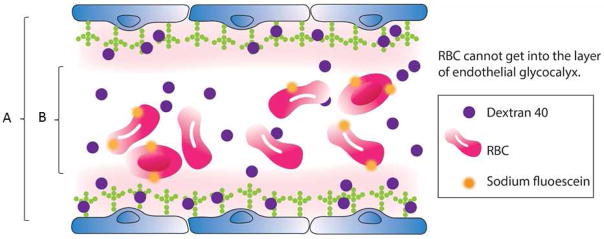
Figure 1.
Double tracer dilution method. The endothelial glycocalyx volume can be obtained by subtracting the circulating volume measured by labeled erythrocytes from the total circulation volume measured using dextran (or other tracer permeable to the glycocalyx layer).
Volume of endothelial glycocalyx = Total circulation volume measured using dextran 40 (A) – Circulation volume measured by labeled RBCs (B)
*RBC, red blood cell
Table 1.
Clinical studies that evaluated syndecan-1 as a diagnostic/prognostic factor in patients with cardiovascular disease.
| Authors | Study Subject | Methods | Measurement | Outcomes | Results | Weak points |
|---|---|---|---|---|---|---|
| Oliveira neves et al. [46] | Brazil 201 patients, LVEF 41.5+-14.4%, ADHF | Retrospective cohort, comparison among syndecan-1, GFR and mortality | During ED admission, syndecan-1, hsCRP, ICAM-1, NO, BNP | In-hospital mortality, 6-months mortality | Syndecan-1 is associated with AKI development, associated with 6 month mortality | No functional data (e.g. FMD, association with permeability, no temporal relationship data btw syndecan and HF aggravation |
| Tromp J et al. [47] | Netherland, 567 patients, chronic HF | Nested cohort study of a prospective randomized study (COACH). | Before discharge, syndecan- 1NTproBNP, hsCRP, IL-6, ST-2, galectin- 3, periostin, TGFbeta | primary: all-cause mortality + rehospitalization at 18 months, Secondary: all-cause mortality at 3 yrs. | Doubling of syndecan was associated with pri/sec end points only in HFpEF (n=107), not in HFrEF(n=353), syndecan- 1 associated with cardiac fibrosis markers. | Only chronic stabilized patients. Associated with only HFpEF patients. No functional data. No temporal/causal relationship |
| Meyer S et al [48] | Netherland, 567 patients, chronic HF | Nested cohort study of a prospective randomized study (COACH). | Before discharge, syndecan- 1NTproBNP, hsCRP, IL-6, ST-2, galectin- 3, periostin, TGFbeta | primary: all cause mortality at 3 yrs, Secondary: all-cause mortality+ rehospitalization at 18months. | Differential sex association between biomarkers and mortality, syndecan was significantly associated with mortality in women, marginally in men. | Only chronic stabilized patients. Associated with only women. No functional data. No temporal/causal relationship |
| Bielecka - Dabrow a et al [44] | Poland, 68 stable DCM patients | Nested cohort study of a prospective randomized study | proBNP, cystatin C, syndecan-4 | Association with further cardiac function assessed by echo. | syndecan-4 are associated with future LVEF, LV dimension. | No data for CV outcomes. |
| Demissei et al. [45] | Netheland, 2033 acute heart failure | Prospective cohort | During admission, 44 biomarkers including syndecan-1q | time to death, rehospitalization through 30 and 180days | syndecan-1 showed significant HR of adverse events, but was not best biomarker. | No functional data. No temporal/causal relationship |
| Bielecka - Dabrow a et al. [43] | Poland, 120 hypertensive patients. (60 HF vs. 60 non- HF.) | Cross- sectional | Outpatients, syndecan-4, NTproBNP used for confirmation. | Accuracy of each biomarkers for diagnosis with HF. | AUC for syndecan- 4=0.781 | Cross sectional. No causal relationship. No data for CV outcomes. |
| Ostrowsk et al. [42] | Denmark, 571 patients, STEMI | Prospective cohort | During primary PCI, adrenalin, noradrenalin, syndecan-1, soluble thrombomodulin | 30day all-cause mortality CV mortality, Re-MI, HF | adrenalin was associated with 30day mortality, syndecan was marginally associated with only 30days HF admission (p=0.069) |
Footnotes
COMPLIANCE WITH ETHICS GUIDELINES
Conflict of Interest
Youn-Hyun Kim, Petra Nijst, and Kathryn Kiefer declare that they have no conflict of interest
W. H. Wilson Tang has received grants from the National Health Institutes (R01HL103931) outside of the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arteriosclerosis, thrombosis, and vascular biology. 2003;23(9):1541–1547. doi: 10.1161/01.ATV.0000085630.24353.3D. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nature medicine. 2012;18(8):1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korte S, Wiesinger A, Straeter AS, Peters W, Oberleithner H, Kusche-Vihrog K. Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflugers Archiv: European journal of physiology. 2012;463(2):269–278. doi: 10.1007/s00424-011-1038-y. [DOI] [PubMed] [Google Scholar]
- 4.Siegel G, Malmsten M, Ermilov E. Anionic biopolyelectrolytes of the syndecan/perlecan superfamily: physicochemical properties and medical significance. Advances in colloid and interface science. 2014;205:275–318. doi: 10.1016/j.cis.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Maksimenko AV, Turashev AD. No-reflow phenomenon and endothelial glycocalyx of microcirculation. Biochemistry research international. 2012;2012:859231. doi: 10.1155/2012/859231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(13):7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circulation research. 2003;93(10):e136–142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 8.Chignalia AZ, Yetimakman F, Christiaans SC, et al. Shock. 2015. The glycocalyx and Trauma: A Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Annals of surgery. 2011;254(2):194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 10.Darwiche SS, Ruan X, Hoffman MK, et al. Selective roles for toll-like receptors 2, 4, and 9 in systemic inflammation and immune dysfunction following peripheral tissue injury. The journal of trauma and acute care surgery. 2013;74(6):1454–1461. doi: 10.1097/TA.0b013e3182905ed2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stepp MA, Pal-Ghosh S, Tadvalkar G, Pajoohesh-Ganji A. Syndecan-1 and Its Expanding List of Contacts. Advances in wound care. 2015;4(4):235–249. doi: 10.1089/wound.2014.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park PW, Pier GB, Hinkes MT, Bernfield M. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature. 2001;411(6833):98–102. doi: 10.1038/35075100. [DOI] [PubMed] [Google Scholar]
- 13.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114(14):3033–3043. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teng YH, Aquino RS, Park PW. Molecular functions of syndecan-1 in disease. Matrix biology: journal of the International Society for Matrix Biology. 2012;31(1):3–16. doi: 10.1016/j.matbio.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stepp MA, Gibson HE, Gala PH, et al. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. Journal of cell science. 2002;115(Pt 23):4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- 16.Szarvas T, Reis H, Kramer G, et al. Enhanced stromal syndecan-1 expression is an independent risk factor for poor survival in bladder cancer. Human pathology. 2014;45(4):674–682. doi: 10.1016/j.humpath.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Nault JC, Guyot E, Laguillier C, et al. Serum proteoglycans as prognostic biomarkers of hepatocellular carcinoma in patients with alcoholic cirrhosis. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(8):1343–1352. doi: 10.1158/1055-9965.EPI-13-0179. [DOI] [PubMed] [Google Scholar]
- 18.Mahtouk K, Hose D, Raynaud P, et al. Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood. 2007;109(11):4914–4923. doi: 10.1182/blood-2006-08-043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jilani I, Wei C, Bekele BN, et al. Soluble syndecan-1 (sCD138) as a prognostic factor independent of mutation status in patients with chronic lymphocytic leukemia. International journal of laboratory hematology. 2009;31(1):97–105. doi: 10.1111/j.1751-553X.2007.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen NF, Kristensen IB, Preiss BS, Christensen JH, Abildgaard N. Upregulation of Syndecan-1 in the bone marrow microenvironment in multiple myeloma is associated with angiogenesis. European journal of haematology. 2015;95(3):211–217. doi: 10.1111/ejh.12473. [DOI] [PubMed] [Google Scholar]
- 21.Larsen AM, Leinoe EB, Johansson PI, Birgens H, Ostrowski SR. High syndecan-1 levels in acute myeloid leukemia are associated with bleeding, thrombocytopathy, endothelial cell damage, and leukocytosis. Leukemia research. 2013;37(7):777–783. doi: 10.1016/j.leukres.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Akl MR, Nagpal P, Ayoub NM, et al. Molecular and clinical profiles of syndecan-1 in solid and hematological cancer for prognosis and precision medicine. Oncotarget. 2015;6(30):28693–28715. doi: 10.18632/oncotarget.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinyo Y, Kodama J, Hasengaowa, Kusumoto T, Hiramatsu Y. Loss of cell-surface heparan sulfate expression in both cervical intraepithelial neoplasm and invasive cervical cancer. Gynecologic oncology. 2005;96(3):776–783. doi: 10.1016/j.ygyno.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Zuo D, Chen Y, et al. Shed Syndecan-1 is involved in chemotherapy resistance via the EGFR pathway in colorectal cancer. British journal of cancer. 2014;111(10):1965–1976. doi: 10.1038/bjc.2014.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashimoto Y, Skacel M, Adams JC. Association of loss of epithelial syndecan-1 with stage and local metastasis of colorectal adenocarcinomas: an immunohistochemical study of clinically annotated tumors. BMC cancer. 2008;8:185. doi: 10.1186/1471-2407-8-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langford JK, Stanley MJ, Cao D, Sanderson RD. Multiple heparan sulfate chains are required for optimal syndecan-1 function. The Journal of biological chemistry. 1998;273(45):29965–29971. doi: 10.1074/jbc.273.45.29965. [DOI] [PubMed] [Google Scholar]
- 27.Nikolova V, Koo CY, Ibrahim SA, et al. Differential roles for membrane-bound and soluble syndecan-1 (CD138) in breast cancer progression. Carcinogenesis. 2009;30(3):397–407. doi: 10.1093/carcin/bgp001. [DOI] [PubMed] [Google Scholar]
- 28.Purushothaman A, Uyama T, Kobayashi F, et al. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood. 2010;115(12):2449–2457. doi: 10.1182/blood-2009-07-234757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda T, Desouky J, Friedl A. Syndecan-1 expression by stromal fibroblasts promotes breast carcinoma growth in vivo and stimulates tumor angiogenesis. Oncogene. 2006;25(9):1408–1412. doi: 10.1038/sj.onc.1209168. [DOI] [PubMed] [Google Scholar]
- 30.Becker BF, Jacob M, Leipert S, Salmon AH, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. British journal of clinical pharmacology. 2015;80(3):389–402. doi: 10.1111/bcp.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukai N, Kenagy RD, Chen L, Gao L, Daum G, Clowes AW. Syndecan-1: an inhibitor of arterial smooth muscle cell growth and intimal hyperplasia. Arteriosclerosis, thrombosis, and vascular biology. 2009;29(9):1356–1362. doi: 10.1161/ATVBAHA.109.190132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angsana J, Chen J, Smith S, et al. Syndecan-1 modulates the motility and resolution responses of macrophages. Arteriosclerosis, thrombosis, and vascular biology. 2015;35(2):332–340. doi: 10.1161/ATVBAHA.114.304720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanhoutte D, Schellings MW, Gotte M, et al. Increased expression of syndecan-1 protects against cardiac dilatation and dysfunction after myocardial infarction. Circulation. 2007;115(4):475–482. doi: 10.1161/CIRCULATIONAHA.106.644609. [DOI] [PubMed] [Google Scholar]
- 34.Schellings MW, Vanhoutte D, van Almen GC, et al. Syndecan-1 amplifies angiotensin II-induced cardiac fibrosis. Hypertension. 2010;55(2):249–256. doi: 10.1161/HYPERTENSIONAHA.109.137885. [DOI] [PubMed] [Google Scholar]
- 35.Jacob M, Saller T, Chappell D, Rehm M, Welsch U, Becker BF. Physiological levels of A-, B- and C-type natriuretic peptide shed the endothelial glycocalyx and enhance vascular permeability. Basic research in cardiology. 2013;108(3):347. doi: 10.1007/s00395-013-0347-z. [DOI] [PubMed] [Google Scholar]
- 36.Jacob M, Rehm M, Loetsch M, et al. The endothelial glycocalyx prefers albumin for evoking shear stress-induced, nitric oxide-mediated coronary dilatation. Journal of vascular research. 2007;44(6):435–443. doi: 10.1159/000104871. [DOI] [PubMed] [Google Scholar]
- 37.Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic research in cardiology. 2010;105(6):687–701. doi: 10.1007/s00395-010-0118-z. [DOI] [PubMed] [Google Scholar]
- 38.Hollmann J, Schmidt A, von Bassewitz DB, Buddecke E. Relationship of sulfated glycosaminoglycans and cholesterol content in normal and arteriosclerotic human aorta. Arteriosclerosis. 1989;9(2):154–158. doi: 10.1161/01.atv.9.2.154. [DOI] [PubMed] [Google Scholar]
- 39.Papakonstantinou E, Roth M, Block LH, Mirtsou-Fidani V, Argiriadis P, Karakiulakis G. The differential distribution of hyaluronic acid in the layers of human atheromatic aortas is associated with vascular smooth muscle cell proliferation and migration. Atherosclerosis. 1998;138(1):79–89. doi: 10.1016/s0021-9150(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 40.Soto Y, Mesa N, Alfonso Y, et al. Targeting arterial wall sulfated glycosaminoglycans in rabbit atherosclerosis with a mouse/human chimeric antibody. mAbs. 2014;6(5):1340–1346. doi: 10.4161/mabs.29970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miranda CH, de Carvalho Borges M, Schmidt A, Marin-Neto JA, Pazin-Filho A. Evaluation of the endothelial glycocalyx damage in patients with acute coronary syndrome. Atherosclerosis. 2016;247:184–188. doi: 10.1016/j.atherosclerosis.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 42.Ostrowski SR, Pedersen SH, Jensen JS, Mogelvang R, Johansson PI. Acute myocardial infarction is associated with endothelial glycocalyx and cell damage and a parallel increase in circulating catecholamines. Critical care. 2013;17(1):R32. doi: 10.1186/cc12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bielecka-Dabrowa A, Gluba-Brzozka A, Michalska-Kasiczak M, Misztal M, Rysz J, Banach M. The multi-biomarker approach for heart failure in patients with hypertension. International journal of molecular sciences. 2015;16(5):10715–10733. doi: 10.3390/ijms160510715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bielecka-Dabrowa A, von Haehling S, Aronow WS, Ahmed MI, Rysz J, Banach M. Heart failure biomarkers in patients with dilated cardiomyopathy. International journal of cardiology. 2013;168(3):2404–2410. doi: 10.1016/j.ijcard.2013.01.157. [DOI] [PubMed] [Google Scholar]
- 45.Demissei BG, Valente MA, Cleland JG, et al. Optimizing clinical use of biomarkers in high-risk acute heart failure patients. European journal of heart failure. 2015 doi: 10.1002/ejhf.443. [DOI] [PubMed] [Google Scholar]
- 46.Neves FM, Meneses GC, Sousa NE, et al. Syndecan-1 in Acute Decompensated Heart Failure--Association With Renal Function and Mortality. Circulation journal: official journal of the Japanese Circulation Society. 2015;79(7):1511–1519. doi: 10.1253/circj.CJ-14-1195. [DOI] [PubMed] [Google Scholar]
- 47.Tromp J, van der Pol A, Klip IT, et al. Fibrosis marker syndecan-1 and outcome in patients with heart failure with reduced and preserved ejection fraction. Circulation Heart failure. 2014;7(3):457–462. doi: 10.1161/CIRCHEARTFAILURE.113.000846. [DOI] [PubMed] [Google Scholar]
- 48.Meyer S, van der Meer P, van Deursen VM, et al. Neurohormonal and clinical sex differences in heart failure. European heart journal. 2013;34(32):2538–2547. doi: 10.1093/eurheartj/eht152. [DOI] [PubMed] [Google Scholar]
- 49.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circulation research. 1996;79(3):581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- 50.Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. American journal of physiology Heart and circulatory physiology. 2000;278(1):H285–289. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]
- 51.Oberleithner H. Two barriers for sodium in vascular endothelium? Annals of medicine. 2012;44(Suppl 1):S143–148. doi: 10.3109/07853890.2011.653397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebong EE, Macaluso FP, Spray DC, Tarbell JM. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arteriosclerosis, thrombosis, and vascular biology. 2011;31(8):1908–1915. doi: 10.1161/ATVBAHA.111.225268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gretz JE, Duling BR. Measurement uncertainties associated with the use of bright-field and fluorescence microscopy in the microcirculation. Microvascular research. 1995;49(1):134–140. doi: 10.1006/mvre.1995.1011. [DOI] [PubMed] [Google Scholar]
- 54.Kataoka H, Ushiyama A, Kawakami H, Akimoto Y, Matsubara S, Iijima T. Fluorescent imaging of endothelial glycocalyx layer with wheat germ agglutinin using intravital microscopy. Microscopy research and technique. 2016;79(1):31–37. doi: 10.1002/jemt.22602. [DOI] [PubMed] [Google Scholar]
- 55.Nieuwdorp M, Meuwese MC, Mooij HL, et al. Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. Journal of applied physiology. 2008;104(3):845–852. doi: 10.1152/japplphysiol.00440.2007. [DOI] [PubMed] [Google Scholar]
- 56.Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. Journal of the American Society of Nephrology: JASN. 2012;23(11):1900–1908. doi: 10.1681/ASN.2011121181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rehm M, Haller M, Orth V, et al. Changes in blood volume and hematocrit during acute preoperative volume loading with 5% albumin or 6% hetastarch solutions in patients before radical hysterectomy. Anesthesiology. 2001;95(4):849–856. doi: 10.1097/00000542-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Nieuwdorp M, van Haeften TW, Gouverneur MC, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55(2):480–486. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- 59.Michel CC, Curry FR. Glycocalyx volume: a critical review of tracer dilution methods for its measurement. Microcirculation. 2009;16(3):213–219. doi: 10.1080/10739680802527404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115(7):928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 61.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in medicine. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 62.Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician’s guide. Annals of internal medicine. 2014;160(2):122–131. doi: 10.7326/M13-1522. [DOI] [PubMed] [Google Scholar]
- 63.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statistics in medicine. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 65.Bethell DB, Gamble J, Pham PL, et al. Noninvasive measurement of microvascular leakage in patients with dengue hemorrhagic fever. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2001;32(2):243–253. doi: 10.1086/318453. [DOI] [PubMed] [Google Scholar]
- 66.Takishima I, Nakamura T, Hirano M, et al. Predictive value of serial assessment of endothelial function in chronic heart failure. International journal of cardiology. 2012;158(3):417–422. doi: 10.1016/j.ijcard.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 67.Salmito FT, de Oliveira Neves FM, Meneses GC, de Almeida Leitao R, Martins AM, Liborio AB. Glycocalyx injury in adults with nephrotic syndrome: Association with endothelial function. Clinica chimica acta; international journal of clinical chemistry. 2015;447:55–58. doi: 10.1016/j.cca.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 68.Pahwa R, Nallasamy P, Jialal I. Toll-like receptors 2 and 4 mediate hyperglycemia induced macrovascular aortic endothelial cell inflammation and perturbation of the endothelial glycocalyx. Journal of diabetes and its complications. 2016 doi: 10.1016/j.jdiacomp.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 69.Lee JF, Barrett-O’Keefe Z, Garten RS, et al. Evidence of microvascular dysfunction in heart failure with preserved ejection fraction. Heart. 2015 doi: 10.1136/heartjnl-2015-308403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marechaux S, Samson R, van Belle E, et al. Vascular and Microvascular Endothelial Function in Heart Failure With Preserved Ejection Fraction. Journal of cardiac failure. 2016;22(1):3–11. doi: 10.1016/j.cardfail.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 71.Titze J, Machnik A. Sodium sensing in the interstitium and relationship to hypertension. Current opinion in nephrology and hypertension. 2010;19(4):385–392. doi: 10.1097/MNH.0b013e32833aeb3b. [DOI] [PubMed] [Google Scholar]
- 72.Heer M, Baisch F, Kropp J, Gerzer R, Drummer C. High dietary sodium chloride consumption may not induce body fluid retention in humans. American journal of physiology Renal physiology. 2000;278(4):F585–595. doi: 10.1152/ajprenal.2000.278.4.F585. [DOI] [PubMed] [Google Scholar]
- 73.Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nature medicine. 2009;15(5):545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 74.Titze J, Shakibaei M, Schafflhuber M, et al. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. American journal of physiology Heart and circulatory physiology. 2004;287(1):H203–208. doi: 10.1152/ajpheart.01237.2003. [DOI] [PubMed] [Google Scholar]
- 75.Olde Engberink RH, Rorije NM, Homan van der Heide JJ, van den Born BJ, Vogt L. Role of the vascular wall in sodium homeostasis and salt sensitivity. Journal of the American Society of Nephrology: JASN. 2015;26(4):777–783. doi: 10.1681/ASN.2014050430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siegel G, Walter A, Kauschmann A, Malmsten M, Buddecke E. Anionic biopolymers as blood flow sensors. Biosensors & bioelectronics. 1996;11(3):281–294. doi: 10.1016/0956-5663(96)88415-6. [DOI] [PubMed] [Google Scholar]
- 77.Siegel G, Malmsten M, Klussendorf D, Walter A, Schnalke F, Kauschmann A. Blood-flow sensing by anionic biopolymers. Journal of the autonomic nervous system. 1996;57(3):207–213. doi: 10.1016/0165-1838(95)00071-2. [DOI] [PubMed] [Google Scholar]
- 78.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Archiv: European journal of physiology. 2000;440(5):653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 79.Jeansson M, Haraldsson B. Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. American journal of physiology Renal physiology. 2006;290(1):F111–116. doi: 10.1152/ajprenal.00173.2005. [DOI] [PubMed] [Google Scholar]
- 80.Xu C, Wu X, Hack BK, Bao L, Cunningham PN. TNF causes changes in glomerular endothelial permeability and morphology through a Rho and myosin light chain kinase-dependent mechanism. Physiological reports. 2015;3(12) doi: 10.14814/phy2.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adamson RH. Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. The Journal of physiology. 1990;428:1–13. doi: 10.1113/jphysiol.1990.sp018197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nieuwdorp M, Mooij HL, Kroon J, et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55(4):1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 83.Chappell D, Bruegger D, Potzel J, et al. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Critical care. 2014;18(5):538. doi: 10.1186/s13054-014-0538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oberleithner H, Peters W, Kusche-Vihrog K, et al. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Archiv: European journal of physiology. 2011;462(4):519–528. doi: 10.1007/s00424-011-0999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rehm M, Bruegger D, Christ F, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116(17):1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 86.Grundmann S, Fink K, Rabadzhieva L, et al. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation. 2012;83(6):715–720. doi: 10.1016/j.resuscitation.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 87.Annecke T, Fischer J, Hartmann H, et al. Shedding of the coronary endothelial glycocalyx: effects of hypoxia/reoxygenation vs ischaemia/reperfusion. British journal of anaesthesia. 2011;107(5):679–686. doi: 10.1093/bja/aer269. [DOI] [PubMed] [Google Scholar]
- 88.Becker BF, Fischer J, Hartmann H, et al. Inosine, not adenosine, initiates endothelial glycocalyx degradation in cardiac ischemia and hypoxia. Nucleosides, nucleotides & nucleic acids. 2011;30(12):1161–1167. doi: 10.1080/15257770.2011.605089. [DOI] [PubMed] [Google Scholar]
- 89.Gilles S, Zahler S, Welsch U, Sommerhoff CP, Becker BF. Release of TNF-alpha during myocardial reperfusion depends on oxidative stress and is prevented by mast cell stabilizers. Cardiovascular research. 2003;60(3):608–616. doi: 10.1016/j.cardiores.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 90.Donati A, Domizi R, Damiani E, Adrario E, Pelaia P, Ince C. From macrohemodynamic to the microcirculation. Crit Care Res Pract. 2013;2013:892710. doi: 10.1155/2013/892710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32(9):1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 92.Wiesinger A, Peters W, Chappell D, et al. Nanomechanics of the endothelial glycocalyx in experimental sepsis. PLoS One. 2013;8(11):e80905. doi: 10.1371/journal.pone.0080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chappell D, Jacob M, Hofmann-Kiefer K, et al. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology. 2007;107(5):776–784. doi: 10.1097/01.anes.0000286984.39328.96. [DOI] [PubMed] [Google Scholar]
- 94.Zeng Y, Liu XH, Tarbell J, Fu B. Sphingosine 1-phosphate induced synthesis of glycocalyx on endothelial cells. Experimental cell research. 2015;339(1):90–95. doi: 10.1016/j.yexcr.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 95.Zeng Y, Adamson RH, Curry FR, Tarbell JM. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. American journal of physiology Heart and circulatory physiology. 2014;306(3):H363–372. doi: 10.1152/ajpheart.00687.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Torres LN, Sondeen JL, Ji L, Dubick MA, Torres Filho I. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. The journal of trauma and acute care surgery. 2013;75(5):759–766. doi: 10.1097/TA.0b013e3182a92514. [DOI] [PubMed] [Google Scholar]
- 97.Torres Filho I, Torres LN, Sondeen JL, Polykratis IA, Dubick MA. In vivo evaluation of venular glycocalyx during hemorrhagic shock in rats using intravital microscopy. Microvascular research. 2013;85:128–133. doi: 10.1016/j.mvr.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 98.Straat M, Muller MC, Meijers JC, et al. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: a prospective substudy of a randomized trial. Critical care. 2015;19:163. doi: 10.1186/s13054-015-0828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chappell D, Hofmann-Kiefer K, Jacob M, et al. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic research in cardiology. 2009;104(1):78–89. doi: 10.1007/s00395-008-0749-5. [DOI] [PubMed] [Google Scholar]
- 100.Chappell D, Dorfler N, Jacob M, et al. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock. 2010;34(2):133–139. doi: 10.1097/SHK.0b013e3181cdc363. [DOI] [PubMed] [Google Scholar]
- 101.Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circulation research. 2009;104(11):1318–1325. doi: 10.1161/CIRCRESAHA.108.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Giantsos-Adams KM, Koo AJ, Song S, et al. Heparan Sulfate Regrowth Profiles Under Laminar Shear Flow Following Enzymatic Degradation. Cellular and molecular bioengineering. 2013;6(2):160–174. doi: 10.1007/s12195-013-0273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koo A, Dewey CF, Jr, Garcia-Cardena G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. American journal of physiology Cell physiology. 2013;304(2):C137–146. doi: 10.1152/ajpcell.00187.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Voyvodic PL, Min D, Liu R, et al. Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. The Journal of biological chemistry. 2014;289(14):9547–9559. doi: 10.1074/jbc.M113.541573. [DOI] [PMC free article] [PubMed] [Google Scholar]