Abstract
Purpose
The goal of this RCT was to examine the efficacy and safety of a web-based program to improve cardiovascular and bone health outcomes, among 35 BRCA1/2+ breast cancer survivors who underwent prophylactic oophorectomy and thus, experienced premature surgical menopause.
Methods
A 12-month commercially available web-based lifestyle modification program (Precision Nutrition Coaching) was utilized. Cardiovascular fitness, dietary intake, leisure-time activity, body composition, bone mineral density, bone structure, and muscle strength were assessed.
Results
Average adherence to all program components was 74.8%. Women in the intervention group maintained their cardiovascular fitness level over the 12 months (1.1 ± 7.9%), while the control group significantly decreased fitness capacity (−4.0 ± 7.5%). There was a significant difference between groups in percent change of whole body bone area (−0.8 ± 2.5 control and 0.5 ± 1.30 intervention). We also observed decreased BMI (−4.7 ± 6.2%) and fat mass (−8.6 ± 12.7%) in the intervention group due to significant concomitant decreases in caloric intake and increases in caloric expenditure. The control group demonstrated decreased caloric intake, and decreased lean tissue mass.
Conclusions
In this population at high risk for detrimental cardiovascular and bone outcomes, a commercially available lifestyle intervention program mitigated a decline in cardiovascular health, improved bone health, and decreased weight through fat loss.
Implications for Cancer Survivors
Precision Nutrition Coaching has shown benefit in breast cancer survivors for reduced risk of deleterious cardiovascular and bone outcomes.
Keywords: Neoplasm, exercise, heart, bone, weight loss, BRCA1/2
Introduction
Breast cancer patients often present at diagnosis with co-morbidities and risk factors for cardiovascular disease (CVD). The direct effect of cancer treatment, such as cardiotoxic chemotherapies, immunotherapies, and radiation, paired with indirect effects such as aging, deconditioning and sedentary lifestyle, as well as weight gain, all combine to decrease cardiovascular reserve. These direct and indirect effects are components of what has been called the “multiple hit hypothesis” [1]. The effects of aging, comorbid conditions, and cardiotoxic treatments combine to contribute to the 50% increased risk of CVD seen in breast cancer survivors and the trend for CVD to be the leading cause of death in breast cancer survivors nine years following diagnosis [2, 3].
Women with BRCA1 or BRCA2 mutations account for 5-10% of all breast cancers and 15% of all ovarian cancers [4, 5]. The loss of BRCA function results in impaired ability to repair DNA double strand breaks, and predisposes BRCA1/2+ women for malignancies. However, loss of BRCA function is not only linked to cancer. After breast cancer treatment, BRCA1/2+ women face a 2-fold increase in risk of diabetes compared to non-BRCA1/2+ breast cancer survivors [6]. BRCA1 or BRCA2 mutation carriers also have an elevated risk of overall mortality compared to non-carriers which is beyond the increased risk for cancers such as breast, ovarian, prostate, pancreatic, and melanoma [7].
The risk for CVD, as well as osteoporosis, in BRCA1/2+ breast cancer survivors is further complicated in that BRCA mutation carriers often turn to preventative surgery to decrease their enhanced risk for ovarian cancer (39% elevated risk in BRCA1 and 11-17% in BRCA2) [8, 9]. Risk-reducing salpingo-oophrectomy (RRSO) in women who carry BRCA1 or BRCA2 gene mutations is associated with 50 to 80% reductions in the risk of breast cancer and ovarian cancer, respectively [10-12]. Given the substantive elevated lifetime cancer risk in BRCA1/2 mutation carriers, this is a reasonable and advisable course of action. However, it is not without consequence, particularly among women who undergo RRSO before the age of 45 and without hormone replacement therapy (HRT) [13-15]. The precipitous loss of ovarian hormones due to RRSO may pose particular risks for long term CVD and bone health in younger women. As such, interventions to improve these outcomes are worth pursuing.
Aerobic exercise training is well established to improve cardiorespiratory fitness [16]. Cardiorespiratory fitness is highly predictive of overall and cardiovascular specific mortality in women [17, 18]. More specifically, after adjusting for age, coronary disease risk factors, and the Charlson index, a 1 MET increase of workload on a treadmill exercise test has been associated with a 23% reduction in risk for cardiac events [19]. Weight loss also improves cardiovascular outcomes among breast cancer survivors, as measured by changes in blood levels of glucose, insulin, cholesterol and lipids [20]. However, weight loss can result in bone loss due to the decrease in weight bearing load [21]. Fortunately, both aerobic and resistance exercise training has been shown to protect bone mass from weight loss-related decreases [22, 23].
Therefore, a randomized controlled lifestyle modification trial was conducted for breast cancer survivors who were BRCA1 or BRCA2 mutation carriers, had undergone RRSO by age 45, had not taken HRT for two years, and who were, as a result, at increased risk for negative long term cardiovascular and bone health outcomes. The intervention was a commercially available web-based weight loss program called Precision Nutrition Coaching, from Precision Nutrition Inc. The 12 month intervention included multiple elements previously shown to be efficacious for improving cardiovascular and metabolic outcomes in women who’ve experienced natural menopause. The impact of these interventions, in this population, packaged as a commercially available lifestyle modification program, was unknown as weight loss may exacerbate bone loss. The primary outcome was maximal cardiovascular fitness, an established surrogate for cardiovascular morbidity and mortality. This was an innovative use of a web-based behavioral intervention for the primary prevention of CVD and osteoporosis in an understudied, high-risk group of cancer survivors.
Methods
Study Design
The trial was coined Project HOPE (Heart disease Osteoporosis Prevention and Efficacy). It was a phase II, two-armed, randomized controlled trial. Participant recruitment took place over 12 weeks between October and December 2013 and the study commenced in January 2014. Interested participants were screened via telephone. All testing was completed at the University of Pennsylvania and took place at two time points: prerandomization (baseline) and after the 12 month intervention (follow up). Participants randomized to the intervention group took part in Precision Nutrition?s Lean Eating for Women Program for 12 months. Participants randomized to the control group were waitlisted and enrolled in the program following study activities. To ensure equal representation across BMI and age categories, participants were randomized by the study biostatistician within one of four strata using a random sequence assignment: 1-- BMI < 30, age 18-35; 2-- BMI ≥ 30, age 18-35; 3-- BMI < 30, age 36-55; 4-- BMI ≥ 30, age 36-55. Allocation was concealed prior to assignment.
Study population and recruitment
BRCA1/2+ breast cancer survivors, aged 18-55, who underwent prophylactic oophorectomy two or more years prior to study initiation, were age ≤ 45 at date of oophorectomy, completed breast cancer treatment at least 4 months prior to study initiation, did not use hormone replacement therapy for 2 years prior to study initiation, received physician clearance to participate in the weight loss and exercise program, were weight stable over the past year (e.g. no changes greater than 10% in the past 12 months), and had a BMI ≥ 23 kg/m2 were recruited. Other eligibility criteria included being cancer free, having access to the internet and a computer, having access to basic fitness equipment (dumbbells, resistance bands) or willingness to join a fitness facility. Women were identified through the WISER Sister trial screening database [24], the Basser Center for BRCA patient databases, and, as with our prior work recruiting high risk women, emails sent out through the national advocacy organization called „FORCE? (Facing Our Risk Of Cancer Empowered). This study was approved by the Human Subjects Review Committee at the University of Pennsylvania and informed consent was obtained from all subjects in writing prior to beginning study activities. All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Pennsylvania and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Intervention
The intervention included 3 daily activities: Workout - completing relative and progressive strength-training and aerobic exercises (described in print and demonstrated by online video) at home or at a local fitness facility; Habit - completing a nutritional/lifestyle habit (new habits followed every 2 weeks, Table 1); Lesson - reading lessons on health, nutrition, fitness, or behavior change. All participants were assigned to a level 1 Precision Nutrition trained female coach whom was also additionally trained in motivational interviewing and certified through the National Academy of Sports Medicine. The role of the Precision Nutrition coach is to support and guide the participant by building an individualized relationship. Participants can request pre-set accountability calls or reach out at the coach as needed. Further, contacts are made over the online program platform, by email, phone, of video conference call. These interactions are not standardized, but tailored to the needs of the participant. Further, the exercise program was customized to women based on the equipment they had available to them (at home or at the gym) and their exercise capabilities (previous or new injuries were taken into account).
Table 1.
Intervention lifestyle modification habits
| Habit title |
|---|
| Weeks 1-2: Do a 5 minute action |
| Weeks 3-4: Eat slowly |
| Weeks 5-6: Stop when 80% full |
| Weeks 7-8: Eat lean protein with every meal |
| Weeks 9-10: Eat at least 5 servings of veggies |
| Weeks 11-12: Make smart carb choices |
| Weeks 13-14: Plan PN-friendly meals |
| Weeks 15-16: Record what you eat |
| Weeks 17-18: Create and use a sleep ritual |
| Weeks 19-20: Drink only calorie free beverages |
| Weeks 21-22: Use a targeted recovery strategy |
| Weeks 23-24: Take a break |
| Weeks 24-26: Eat only whole foods |
| Weeks 27-28: Do a little more a little better |
| Weeks 29-30: Eat veggies and protein at each meal |
| Weeks 31-32: Practice eating to 80% full |
| Weeks 33-34: Modify carb intake |
| Weeks 35-36: Do a 5min mind body scan |
| Weeks 37-38: Do a fitness media fast |
| Weeks 39-40: Do 20 minutes of de-stressing |
| Weeks 41-42: Create a fitness mission statement |
| Weeks 43-44: Pick your own habit |
| Weeks 45-46: Prepare for final photo |
| Weeks 47-48: Celebrate progress |
| Weeks 49-50 Pay it forward |
Women completed the program at home or at a local gym, and recorded online whether they completed each of the 3 activities daily. The web-based program incorporates suggestions from literature, social cognitive theory, and elements from prior successful clinic and internet-based interventions, including tailored feedback, physical activity logging, goal setting,[25] more than 5 participant contacts,[26] and peer support [27]. The exercise component required 160 min/wk of exercise (3 days/week of progressive resistance exercise, 2 days/week of interval aerobic exercise, and 1 day/week of active recovery aerobic exercise). Control group participants were not prohibited from exercise or eating a healthy diet, as it would be unethical to do so. Prior research by our team has indicated that allowing for such health behavior changes but not supporting participants in these changes results in no substantial physiologic changes over 12 months or more [28, 29]. The intervention group paid $10 per month for the program and women were reimbursed ($120) at the end of the 12 months if they were 80% adherent to the program. Additionally, both the intervention and control groups were contacted bi-weekly via email by study staff to document any physical activity related injuries.
Adherence
An overall adherence score was calculated by averaging the percentage of time each of the 3 activities was completed over the 12 month intervention. Adherence to habit formation was calculated by dividing the number of days the participant was asked to perform a habit (350 days in the program) by the number of days the participant said “yes I did my habit”). Adherence to lesson reading was calculated by dividing the number of days the participant was asked to read a lesson (350 days in the program) by the number of days the participant said “yes I read my lesson”). Adherence to the exercise program was calculated by dividing the number of days the participant was assigned a workout (300 days in the program) by the number of days the participant said “yes I did my workout”).
Measurements
Measurement staff was trained to conduct all assessments in a standardized fashion, at the University of Pennsylvania. Cardiovascular fitness level was determined using the modified Bruce protocol [30]. This protocol increases speed and incline in regular three minute intervals. Total time on the treadmill before volitional fatigue is representative of fitness capacity. Heart rate was measured using 12 lead ECG monitoring, and blood pressure was measured following JNC IIIV guidelines [31]. Dietary intake was measured through self-reported logging on 3-day dietary records and analyzed using Nutrition Data Systems for Research v2014 (University of Minnesota, MN). Anthropometric measures were assessed via digital scale and wall mounted stadiometer (Scale-tronix 5005 stand-on digital scale, Scale-tronix, White Plains, NY) for weight and height, respectively. Demographic characteristics (age, race, ethnicity, and education) as well as cancer treatment modalities were self-reported at baseline. Leisure time physical activity was assessed using the interviewer administered Modifiable Activity Questionnaire [32]. Lymphedema was assessed by inter-limb arm volume difference as measured by perometry (Optoelectronic Perometer®, Juzo USA, Cuyahoga Falls, OH) and the Norman Lymphedema Survey.[33, 34]
Dual-energy x-ray absorptiometry (DXA) (Hologic, Bedford MA) was used to measure body composition and whole body bone area, content, and density (including head). All scans were analyzed by APEX v13.3. Body composition z-scores were calculated for fat mass index (fat mass (kg)/ ht (m)2) and lean body mass index (lean mass (kg)/ ht (m)2) using NHANES data. Cortical volumetric bone was assessed in the left tibia by peripheral quantitative CT (pQCT; Stratec XCT2000 12-detector unit, Orthometrix, Inc.) with a voxel size of 0.4 mm, slice thickness of 2.3 mm, and scan speed of 25 mm/s. All scans were analyzed with the Stratec software version 6.00. A scout view was obtained to place the reference line at the proximal border of the distal endplate. Cortical bone measurements were obtained at 38% of tibia length proximal to the reference line. At the 38% diaphyseal site, scans were analyzed for cortical volumetric BMD (mg/cm3), cortical area (cm2), and periosteal and endosteal circumference (mm). Quality control was monitored daily using a phantom. The in vivo coefficient of variation (CV) ranged from 0.5 to 1.6% for pQCT outcomes.
Muscle strength testing was performed to determine one-repetition maximum (1 RM) values for lower body strength using a leg press machine, and upper body strength was assessed by a bench press using a flat bench and Olympic bar. All metrics were completed at baseline and follow up.
Statistical analysis
The study was powered to detect a 12.5% net increase in cardiopulmonary fitness, the primary outcome variable. Data was assessed for normality using the Shapiro-Wilk test. Normally distributed variables were examined using Student?s T-test to test for differences between control and intervention groups at baseline. A paired T-test was used to assess changes within group. Percent change of variables was calculated and between group differences were assessed using Student?s T-test. Non parametric data was only observed for the following variables: percent change of caloric intake, percent change of caloric expenditure, percent change of whole body bone area, percent change of periosteal circumference, and percent change of endosteal circumference. These variables were examined using the Wilcoxon rank-sum test. Statistical analyses were conducted using STATA version 12 (Stata Corp., College Station, TX) and statistical significance was set at an alpha level of p < 0.05.
Results
Participant characteristics
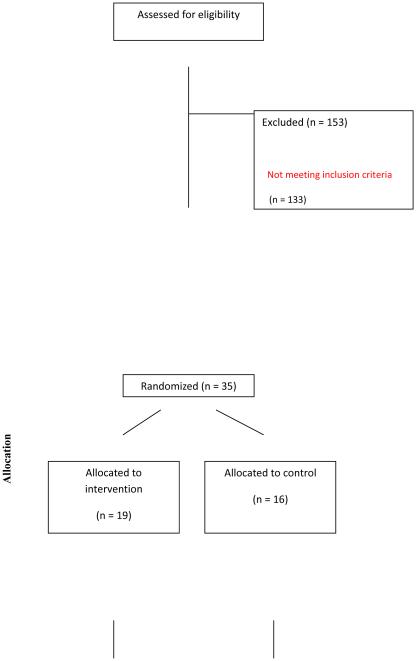
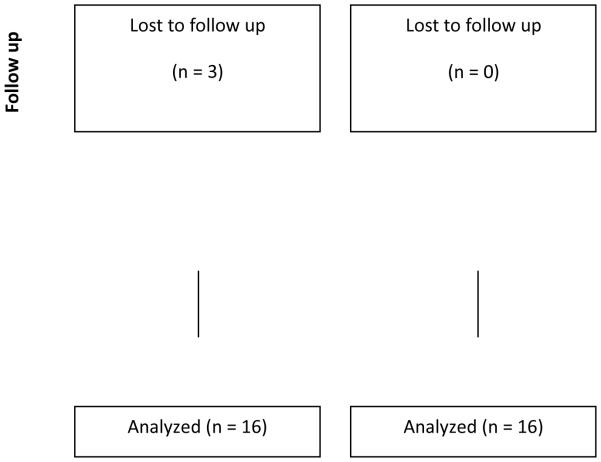
Figure 1 presents the flow of participants through the study. Two-hundred and fifty-four women responded to advertisements and physician letters. One-hundred and eighty-eight women were screened. One-hundred and thirty-three women did not meet the full inclusion criteria. Common reasons for not progressing to consent included: loss of interest and other known obligations that would inhibit participation. Three women were lost to follow up and did not return for final testing. Table 2 presents demographics and breast cancer treatment of the cohort. The cohort was middle-aged (46.1 ± 4.0 years of age), white, and well-educated.
Fig. 1.
CONSORT diagram. Project HOPE enrolled 35 women into the trial and 32 completed final testing.
Table 2.
Demographics and breast cancer treatment characteristics
| Characteristics | Total Sample (n=35) |
Control (n=16) |
Intervention (n=19) |
|---|---|---|---|
| Age at intervention (yr) | 46.1 ± 4.0 | 47.2 ± 3.8 | 45.1 ± 4.0 |
| Age at oophorectomy (yr) | 40.4 ± 4.0 | 40.7 ± 3.5 | 40.1 ± 4.4 |
| Time since diagnosis (yr) | 7.5 ± 4.6 | 9.4 ± 5.6 | 5.8 ± 2.5 |
| Race, no. (%) | |||
| White | 35 (100%) | 16 (100%) | 19 (100%) |
| Ethnicity, no. (%) | |||
| Hispanic | 2 (6%) | 2 (13%) | 0 (0%) |
| Non-Hispanic | 33 (94%) | 14 (87%) | 19 (100%) |
| Education, no. (%) | |||
| High school or less | 1 (3%) | 0 (0%) | 1 (5%) |
| Some college | 2 (6%) | 1 (6%) | 1 (5%) |
| College or more | 32 (91%) | 15 (94%) | 17 (90%) |
| Radiation (overall) | 14 (40%) | 7 (44%) | 7 (37%) |
| Radiation - Left | 7 (50%) | 2 (29%) | 5 (72%) |
| Radiation - Right | 4 (29%) | 3 (42%) | 1 (14%) |
| Radiation - Both | 3 (21%) | 2 (29%) | 1 (14%) |
| Chemotherapy (overall) | 29 (83%) | 15 (94%) | 14 (74%) |
| Anthracyclines | 21 (72%) | 10 (66%) | 11 (78%) |
| Immunotherapy (overall) | 4 (14%) | 2 (13%) | 2 (10%) |
| Avastin | 2 (50%) | 1 (50%) | 1 (50%) |
| Herceptin | 2 (50%) | 1 (50%) | 1 (50%) |
Values are presented as mean ± SD.
The intervention group demonstrated an average of 74.8% ± 30.0 adherence to the program. This can be compared to the rate of 68% overall adherence to the program among individuals in the general public who had paid to participate in Precision Nutrition as a commercially available program. These individuals were not a part of the study. Sixty-two % of the intervention group maintained at least 80% adherence to the workout component of the program, 75% of the intervention group maintained at least 80% adherence to the habit component of the program, and 68% of the intervention group maintained at least 80% adherence to the reading component of the program. No differences in reported major medical issues (control: 25%, intervention: 25%), musculoskeletal injuries (joint pain: control: 25%, intervention: 31.25%; soft tissue injury: control: 37.5%, intervention: 25%), or side effects caused by physical activity (headaches: control: 0%, intervention: 0%; nausea: control: 0%, intervention: 6.25%); dizziness (control: 12.5%, intervention: 12.5%); abdominal pain (control: 0%, intervention: 0%) between groups were observed. Additionally, there were no differences in the onset of, or progression of, lymphedema between groups (data not shown).
Body composition and cardiovascular fitness
As anticipated with randomization, no statistically significant between-group differences were observed at baseline for caloric intake, daily caloric expenditure, self-reported physical activity, BMI, or body composition (Table 3). At the 12-month follow up visit we observed that both the control and intervention groups reported a decrease in their caloric intake relative to their baseline caloric intake levels (P = 0.03 control, P = 0.0004 intervention). However, only the intervention group demonstrated a significant decrease in BMI (P = 0.007), weight (P = 0.007), fat mass (P = 0.02), and % body fat (P = 0.04) compared to baseline. There was a significant between group difference for daily caloric expenditure with the intervention group increasing physical activity more by the end of the intervention period (P = 0.04). While the intervention group decreased fat mass and % body fat compared to a year earlier, the control group lost lean body mass compared to a year earlier (P = 0.02). These differences remained when body composition indexes were calculated as population adjusted z-scores (a trend, P = 0.07 also remained for between group differences in fat mass and % body fat).
Table 3.
Caloric balance and body composition.
| Variable | Control | Intervention | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline (n=16) |
Final (n=16) |
Change | Baseline (n=19) |
Final (n=16) |
Change | |
| Caloric intake (kcal/day) | 1911.6 ± 479.8 |
1632.9 ± 391.5a |
−380.7 ± 663.4 |
1902.7 ± 423.9 |
1489.8 ± 329.7a |
−675.8 ± 729.2 |
| Caloric expenditure (kcal/day) |
559.6 ± 656.6 |
425.2 ± 325.6 |
−134.4 ± 448.8 |
483.7 ± 292.2 |
740.6 ± 330.2 | 207.1 ± 449.4b |
|
| ||||||
| % Change | % Change | |||||
| BMI (kg/m2) | 29.6 ± 5.1 | 29.0 ± 4.9 | −1.8 ± 4.0 | 30.2 ± 4.6 | 28.0 ± 4.7a | −4.7 ± 6.2 |
| Body weight (lbs) | 161.3 ± 35.6 | 158.2 ± 34.5 | −1.8 ± 4.0 | 167.0 ± 24.6 | 157.3 ± 26.2a | −4.7 ± 6.8 |
| Lean tissue (lbs) | 96.1 ± 16.7 | 94.4 ± 16.5a | −2.0 ± 3.0 | 101.6 ± 13.0 | 97.7 ± 10.8 | −2.2 ± 5.6 |
| Lean mass index (z- score) |
0.7 ± 0.9 | 0.5 ± 0.9a | −85.8 ± 226.4 | 0.7 ± 0.9 | 0.5 ± 0.9 | −32.9 ± 103.7 |
| Fat tissue (lbs) | 64.9 ± 20.7 | 63.8 ± 20.0 | −1.3 ± 9.0 | 65.3 ± 15.4 | 59.4 ± 18.5a | −8.6 ± 12.7 |
| Fat mass index (z-score) | 1.2 ± 0.6 | 1.2 ± 0.6 | −2.7 ± 26.1 | 1.2 ± 0.5 | 0.9 ± 0.65a | −31.3 ± 53.5 |
| Body fat (%) | 39.6 ± 4.9 | 39.7 ± 4.8 | 0.4 ± 6.1 | 38.1 ± 4.7 | 36.4 ± 5.3a | −4.5 ± 8.2 |
Body Mass Index (BMI); values are presented as mean ± SD;
P < 0.05 within group,
P< 0.05 between groups.
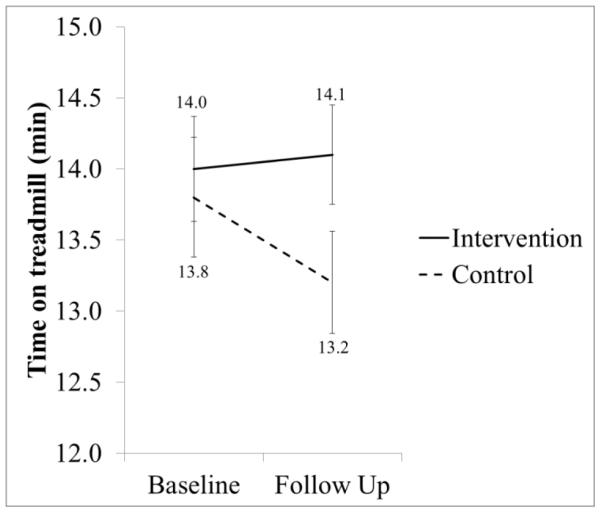
Cardiovascular fitness declined in the control group over one year (−4.0% ± 7.7, P = 0.04) compared to baseline values while the intervention group maintained cardiovascular fitness levels (1.1% ± 8.1) (Figure 2). We did not observe any differences at baseline or at follow up for heart rate recovery following the test, or resting systolic or diastolic blood pressure (data not shown). Average blood pressure for the cohort at baseline was (SBP: 114.2 ± 13.5, DBP: 74.0 ± 10.9), and follow up (SBP: 111.9 ± 10.9, DBP: 75.2 ± 11.9).
Fig. 2.
Aerobic fitness capacity. Total time on the treadmill during a modified Bruce protocol is presented at baseline and at 12 month follow up for the control (n=16) and intervention (n=16) groups. The control group demonstrated a significant decrease in aerobic fitness between baseline and follow up (aP < 0.05) and there was a trend (P = 0.07) for differences between groups in the percent change of aerobic capacity; values are presented as mean ± SEM.
Muscle strength and bone quality
At baseline, the intervention group displayed greater upper body strength as measured by bench press (P = 0.02) (Table 4). Twelve months later, the intervention group bench-pressed, on average, 6.6 more pounds (P = 0.06) while the control group bench pressed, on average, 3.7 pounds more. The intervention group demonstrated enhanced whole body bone area (P = 0.04) compared to the control group that lost whole body bone area. Cortical bone density increased in the intervention group compared to the control group (P = 0.02). Bone modeling was also observed in the control group, as both endosteal circumference and periosteal circumference increased (P = 0.01 and 0.04, respectively). However, the intervention group did demonstrate greater periosteal circumference at baseline (P = 0.04).
Table 4.
Bone quality and muscle strength
| Parameter | Control | Intervention | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline (n=16) |
Final (n=16) |
% Change | Baseline (n=19) |
Final (n=16) |
% Change | |
| Whole Body Bone | ||||||
| Area (cm2) | 55.8 ± 5.6 | 55.4 ± 6.1 | −0.8 ± 2.5 | 58.6 ± 3.7 | 58.9 ± 3.9 | 0.5 ± 1.3b |
| BMC (g) | 52.5 ± 9.1 | 51.9 ± 9.3 | −1.4 ± 3.7 | 54.6 ± 7.5 | 54.6 ± 8.0 | −0.1 ± 5.4 |
| BMD (g/cm2) | 0.9 ± 0.1 | 0.9 ± 0.1 | −0.6 ± 2.4 | 0.9 ± 0.1 | 0.9 ± 0.1 | −0.6 ± 4.8 |
|
| ||||||
| Cortical Bone | ||||||
| Density (mg/cm3) | 1159.9 ± 38.5 | 1153.6 ± 37.8a | −0.4 ± 0.7 | 1153.2 ± 32.0 | 1163.4 ± 32.6 | 0.3 ± 0.8b |
| Area (mm2) | 262.6 ± 31.3 | 262.3 ± 31.5 | 0.3 ± 1.4 | 283.9 ± 30.4 | 282.2 ± 31.9 | −0.4 ± 0.8 |
| Periosteal circ. (mm) | 66.1 ± 4.1 | 66.4 ± 3.9a | 0.4 ± 0.7 | 68.9 ± 3.6b | 68.9 ± 3.6 | 0.07 ± 0.3 |
| Endosteal circ. (mm) | 32.9 ± 3.8 | 33.3 ± 3.7a | 1.2 ± 1.6 | 34.6 ± 4.1 | 34.7 ± 4.2 | 0.3 ± 0.9 |
|
| ||||||
| Muscle Strength | ||||||
| Leg Press (lbs) | 235.9 ± 47.1 | 252.2 ± 60.4 | 7.8 ± 21.7 | 261.0 ± 64.3 | 266.2 ± 68.2 | 5.7 ± 11.6 |
| Bench Press (lbs) | 60.0 ± 17.8 | 63.7 ± 21.4 | 9.2 ± 35.2 | 75.3 ± 19.7b | 81.9 ± 21.9 | 12.5 ± 23.1 |
Bone Mineral content (BMC), Bone Mineral Density (BMD), circumference (circ); values are presented as mean ± SD;
P < 0.05 within group,
P < 0.05 between groups.
Discussion
A commercially available lifestyle modification program improved the body composition and bone health of the intervention group while preventing significant decline in cardiovascular fitness capacity over one year. This population of women is at high risk for cardiovascular and osteoporotic complications related to their RRSO and BRCA1/2 mutation status. The Precision Nutrition program resulted in significant fat loss and preservation of lean body tissue in the intervention group compared to baseline levels. Further, as bone loss has been associated with weight loss, it was unknown if this could be prevented given the unique biology of this high-risk population. There was an improvement in bone health for the intervention group as indicated by a critical increase in whole body bone area, cortical bone density, and the absence of periosteal and endosteal circumference modeling observed in the control group. Lastly, a clinically significant 4.0% decrease in fitness capacity over 12 months in the control group was noted. The Precision Nutrition program was able to prevent this decrement in the intervention group compared to baseline levels.
BRCA1 and BRCA2 proteins have important roles in DNA damage repair and genomic stability. DNA damage occurs from exogenous and endogenous sources. Endogenous sources of DNA damage include replication errors and free radical species. Exercise training has consistently been associated with increased antioxidant capacity in many tissues including the heart and skeletal muscle [35, 36]. Decreased levels of reactive oxygen species through elevated antioxidant capacity may be one mechanism by which the Precision Nutrition program prevented a decline in fitness capacity in BRCA1/2+ women. Of further importance to this population, enhanced physical activity has also been previously reported to improve DNA repair capacity.[37]
An exogenous source of DNA damage is Adriamycin, a cardiotoxic chemotherapy that 83% of the women in our study received during their breast cancer treatment. Left side chest radiation is another source of exogenous DNA damage and is associated with cardiotoxicity. Twenty-nine % of women in Project HOPE also received left side radiation as part of their breast cancer treatment. The combined effects of cardiotoxic breast cancer treatment and primary aging in BRCA1/2+ women may be responsible for the above normal decline in yearly fitness capacity seen in the control group [38, 39]. Fleg et al report that between 40 and 50 years of age, the average % change in peak VO2 for females over this decade is −10.9% [40]. An average % change of −4.0% (peak time on treadmill) in one year for BRCA1/2 mutation carriers in the control group was observed in this study.
Not only do BRCA1/2+ breast cancer survivors appear to have accelerated decline in fitness capacity, but they also have other complications associated with risk-reduction procedures such as RRSO. Surgically-induced menopause without subsequent hormone replacement therapy has been shown to increase cardiovascular mortality [41], and early menopause is an important predictor of osteopenia, osteoporosis, and fractures [42, 43]. However, multi-purpose exercise programs have shown long-term efficacy (16 years post intervention) in mitigating age related declines in bone mineral density compared to the control group [44]. Significant differences in bone density, specific to cortical bone, with the control group losing bone density and the intervention group increasing bone density was noted. The increase seen in the intervention group (0.3%) is below what has been observed in other post-menopausal women (0.9%) [45]. Further, the control group not only lost bone density, but also whole body bone area, and demonstrated cortical drift as resorption increased on the endosteal surface, while bone was deposited on the periosteal surface.
The maintenance of bone health in the intervention group is important as weight loss can cause bone loss due to decreased mechanical loading stress. A decrease in both BMI and fat tissue mass, as well as caloric intake, from baseline to follow up in the intervention group was observed. Additionally, the intervention group had a significant increase in caloric expenditure compared to the control group. These changes reflect the benefits of a tailored lifestyle modification program. Precision Nutrition is designed to engage women with educational readings and the tools to implement health behaviors into individual circumstances. Further, it utilizes relative and progressive exercise training goals to apply basic exercise physiology principles such as overload, adaptation, individuality, and specificity. The 4.6% weight loss we observed in this 12 month online program is comparable to the 6.0% weight loss the ENERGY Trial observed at 12 months following weekly, then monthly, group dietary and physical activity guidance sessions.[46] A weight loss of 5% or more is believed to be clinically meaningful by the United States Preventative Services Taskforce, though most weight loss intervention studies in breast cancer survivors achieve over 5% from baseline [47, 48].
Project HOPE has demonstrated both effectiveness and clinical utility in a high-risk population of breast cancer patients. Many breast cancer survivors seek guidance on weight management and risk mitigation for cancer recurrence and other chronic diseases. The improvements observed in this trial indicate that a commercially available lifestyle modification program may be a viable option in survivorship care plans. Despite awareness regarding the need for survivorship programming, there is a lack of movement at the individual level for scalable programs to address many health concerns reported by breast cancer survivors.
Above average adherence to the Precision Nutrition program (74.8%) compared to women whom enrolled with Precision Nutrition on their own (68.0%) was noted. This could be due to the inclusion criteria which yielded a study sample that was white, non-Hispanic, and college-educated. Because our population did not include minorities or persons of lower education status there are limits to the generalizability of this study given the homogenous study population. Further, in order to complete the Precision Nutrition program, participants needed to have regular access to the internet. The intervention is also intensive for the commitment to interact within the online portal daily for check-ins and readings. Thus, due to the known study requirements of consistent internet access and the time burden of the study our recruitment was biased for individuals with higher socio-economic status.
An interesting potential limitation of the study is the packaged nature of the Precision Nutrition program. The program utilized evidence based approaches to lifestyle modification such as motivational coaching, self-education, multiple contacts, etc. However, this also makes it difficult to un-package what component(s) were most influential in behavior change that led to the differences seen between groups. A logical next step would be to extend this trial into a multi-site setting to address some of the limitations of this study such as 1) examining clinical utility in a more generalizable population, and 2) utilizing an attention control experimental group.
Overall, in the Project HOPE trial, a commercially available lifestyle intervention program was safe and efficacious in inducing weight loss from fat, improving bone health, and preventing decline in cardiovascular fitness. The population was uniquely at risk for cardiovascular and bone health issues due to BRCA1/2 mutation status, RRSO, and cardiotoxic treatment for breast cancer. The Precision Nutrition program delivers clinically significant results and scalability will be tested in future multi-site studies.
Acknowledgements
The project described was supported by the Basser Research Center for BRCA and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000003.
Funding
The project described was supported by the Basser Research Center for BRCA and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest
David Sarwer discloses that he has consulting relationships with BARONova, Enteromedics, Kythera, Medtronic, and Neothetics. These relationships have no relationship to this study.
Compliance with Ethical Standards:
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50(15):1435–41. doi: 10.1016/j.jacc.2007.06.037. doi:S0735-1097(07)02240-1 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Khan NF, Mant D, Carpenter L, Forman D, Rose PW. Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: a database study. Br J Cancer. 2011;105(Suppl 1):S29–37. doi: 10.1038/bjc.2011.420. doi:10.1038/bjc.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. doi: 10.1186/bcr2901. doi:10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campeau PM, Foulkes WD, Tischkowitz MD. Hereditary breast cancer: new genetic developments, new therapeutic avenues. Hum Genet. 2008;124(1):31–42. doi: 10.1007/s00439-008-0529-1. doi:10.1007/s00439-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 5.Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104(12):2807–16. doi: 10.1002/cncr.21536. doi:10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 6.Bordeleau L, Lipscombe L, Lubinski J, Ghadirian P, Foulkes WD, Neuhausen S, et al. Diabetes and breast cancer among women with BRCA1 and BRCA2 mutations. Cancer. 2011;117(9):1812–8. doi: 10.1002/cncr.25595. doi:10.1002/cncr.25595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mai PL, Chatterjee N, Hartge P, Tucker M, Brody L, Struewing JP, et al. Potential excess mortality in BRCA1/2 mutation carriers beyond breast, ovarian, prostate, and pancreatic cancers, and melanoma. PLoS One. 2009;4(3):e4812. doi: 10.1371/journal.pone.0004812. doi:10.1371/journal.pone.0004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–30. doi: 10.1086/375033. doi:S0002-9297(07)60640-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–33. doi: 10.1200/JCO.2006.09.1066. doi:25/11/1329 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebbeck TR, Levin AM, Eisen A, Snyder C, Watson P, Cannon-Albright L, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91(17):1475–9. doi: 10.1093/jnci/91.17.1475. [DOI] [PubMed] [Google Scholar]
- 11.Rebbeck TR. Prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers. Eur J Cancer. 2002;38(Suppl 6):S15–7. doi: 10.1016/s0959-8049(02)00269-1. doi:S0959804902002691 [pii]. [DOI] [PubMed] [Google Scholar]
- 12.Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–7. doi: 10.1200/JCO.2007.13.9626. doi:10.1200/JCO.2007.13.9626 JCO.2007.13.9626 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ., 3rd Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7(10):821–8. doi: 10.1016/S1470-2045(06)70869-5. doi:S1470-2045(06)70869-5 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Michelsen TM, Pripp AH, Tonstad S, Trope CG, Dorum A. Metabolic syndrome after risk-reducing salpingo-oophorectomy in women at high risk for hereditary breast ovarian cancer: a controlled observational study. Eur J Cancer. 2009;45(1):82–9. doi: 10.1016/j.ejca.2008.09.028. doi:10.1016/j.ejca.2008.09.028 S0959-8049(08)00786-7 [pii]. [DOI] [PubMed] [Google Scholar]
- 15.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13(2):265–79. doi: 10.1097/01.gme.0000218683.97338.ea. doi:10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 16.Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, et al. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2015;4(7) doi: 10.1161/JAHA.115.002014. doi:10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr., et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–10. [PubMed] [Google Scholar]
- 18.Vigen R, Ayers C, Willis B, DeFina L, Berry JD. Association of cardiorespiratory fitness with total, cardiovascular, and noncardiovascular mortality across 3 decades of follow-up in men and women. Circ Cardiovasc Qual Outcomes. 2012;5(3):358–64. doi: 10.1161/CIRCOUTCOMES.111.963181. doi:10.1161/CIRCOUTCOMES.111.963181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation. 1998;98(25):2836–41. doi: 10.1161/01.cir.98.25.2836. [DOI] [PubMed] [Google Scholar]
- 20.Campbell KL, Van Patten CL, Neil SE, Kirkham AA, Gotay CC, Gelmon KA, et al. Feasibility of a lifestyle intervention on body weight and serum biomarkers in breast cancer survivors with overweight and obesity. J Acad Nutr Diet. 2012;112(4):559–67. doi: 10.1016/j.jada.2011.10.022. doi:10.1016/j.jada.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Hunter GR, Plaisance EP, Fisher G. Weight loss and bone mineral density. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):358–62. doi: 10.1097/MED.0000000000000087. doi:10.1097/MED.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah K, Armamento-Villareal R, Parimi N, Chode S, Sinacore DR, Hilton TN, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26(12):2851–9. doi: 10.1002/jbmr.475. doi:10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–29. doi: 10.1056/NEJMoa1008234. doi:10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz KH, Williams NI, Kontos D, Kurzer MS, Schnall M, Domchek S, et al. Women In Steady Exercise Research (WISER) Sister: study design and methods. Contemp Clin Trials. 2015;41:17–30. doi: 10.1016/j.cct.2014.12.016. doi:10.1016/j.cct.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Lewis B, Williams D, Dunsiger S, Sciamanna C, Whiteley J, Napolitano M, et al. User attitudes towards physical activity websites in a randomized controlled trial. Prev Med. 2008;47(5):508–13. doi: 10.1016/j.ypmed.2008.07.020. doi:10.1016/j.ypmed.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vandelanotte C, Spathonis KM, Eakin EG, Owen N. Website-delivered physical activity interventions a review of the literature. Am J Prev Med. 2007;33(1):54–64. doi: 10.1016/j.amepre.2007.02.041. doi:S0749-3797(07)00164-X. [DOI] [PubMed] [Google Scholar]
- 27.Norman GJ, Zabinski MF, Adams MA, Rosenberg DE, Yaroch AL, Atienza AA. A review of eHealth interventions for physical activity and dietary behavior change. Am J Prev Med. 2007;33(4):336–45. doi: 10.1016/j.amepre.2007.05.007. doi:S0749-3797(07)00363-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361(7):664–73. doi: 10.1056/NEJMoa0810118. doi:10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz KH, Hannan PJ, Stovitz SD, Bryan CJ, Warren M, Jensen MD. Strength training and adiposity in premenopausal women: strong, healthy, and empowered study. Am J Clin Nutr. 2007;86(3):566–72. doi: 10.1093/ajcn/86.3.566. doi:86/3/566 [pii]. [DOI] [PubMed] [Google Scholar]
- 30.Foster C, Jackson AS, Pollock ML, Taylor MM, Hare J, Sennett SM, et al. Generalized equations for predicting functional capacity from treadmill performance. Am Heart J. 1984;107(6):1229–34. doi: 10.1016/0002-8703(84)90282-5. [DOI] [PubMed] [Google Scholar]
- 31.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. doi:10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 32.Gagnon JF, Sanschagrin F, Jacob S, Tremblay AA, Provencher L, Robert J, et al. Quantitative DNA methylation analysis of laser capture microdissected formalin-fixed and paraffin-embedded tissues. Experimental and molecular pathology. 2010;88(1):184–9. doi: 10.1016/j.yexmp.2009.09.020. doi:10.1016/j.yexmp.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Tierney S, Aslam M, Rennie K, Grace P. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg. 1996;12(4):412–7. doi: 10.1016/s1078-5884(96)80005-0. [DOI] [PubMed] [Google Scholar]
- 34.Norman SA, Miller LT, Erikson HB, Norman MF, McCorkle R. Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Phys Ther. 2001;81(6):1192–205. [PubMed] [Google Scholar]
- 35.Powers SK, Sollanek KJ, Wiggs MP, Demirel HA, Smuder AJ. Exercise-induced improvements in myocardial antioxidant capacity: the antioxidant players and cardioprotection. Free Radic Res. 2014;48(1):43–51. doi: 10.3109/10715762.2013.825371. doi:10.3109/10715762.2013.825371. [DOI] [PubMed] [Google Scholar]
- 36.Powers SK, Ji LL, Leeuwenburgh C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc. 1999;31(7):987–97. doi: 10.1097/00005768-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Cash SW, Beresford SA, Vaughan TL, Heagerty PJ, Bernstein L, White E, et al. Recent physical activity in relation to DNA damage and repair using the comet assay. J Phys Act Health. 2014;11(4):770–6. doi: 10.1123/jpah.2012-0278. doi:10.1123/jpah.2012-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shukla PC, Singh KK, Quan A, Al-Omran M, Teoh H, Lovren F, et al. BRCA1 is an essential regulator of heart function and survival following myocardial infarction. Nat Commun. 2011;2:593. doi: 10.1038/ncomms1601. doi:10.1038/ncomms1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh KK, Shukla PC, Quan A, Desjardins JF, Lovren F, Pan Y, et al. BRCA2 protein deficiency exaggerates doxorubicin-induced cardiomyocyte apoptosis and cardiac failure. J Biol Chem. 2012;287(9):6604–14. doi: 10.1074/jbc.M111.292664. doi:10.1074/jbc.M111.292664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–82. doi: 10.1161/CIRCULATIONAHA.105.545459. doi:CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 41.Rivera CM, Grossardt BR, Rhodes DJ, Brown RD, Jr., Roger VL, Melton LJ, 3rd, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16(1):15–23. doi: 10.1097/gme.0b013e31818888f7. doi:10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Der Voort DJ, van Der Weijer PH, Barentsen R. Early menopause: increased fracture risk at older age. Osteoporos Int. 2003;14(6):525–30. doi: 10.1007/s00198-003-1408-1. doi:10.1007/s00198-003-1408-1. [DOI] [PubMed] [Google Scholar]
- 43.Cohen JV, Chiel L, Boghossian L, Jones M, Stopfer JE, Powers J, et al. Non-cancer endpoints in BRCA1/2 carriers after risk-reducing salpingo-oophorectomy. Fam Cancer. 2012;11(1):69–75. doi: 10.1007/s10689-011-9480-8. doi:10.1007/s10689-011-9480-8. [DOI] [PubMed] [Google Scholar]
- 44.Kemmler W, Bebenek M, Kohl M, von Stengel S. Exercise and fractures in postmenopausal women. Final results of the controlled Erlangen Fitness and Osteoporosis Prevention Study (EFOPS) Osteoporos Int. 2015;26(10):2491–9. doi: 10.1007/s00198-015-3165-3. doi:10.1007/s00198-015-3165-3. [DOI] [PubMed] [Google Scholar]
- 45.Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nature reviews Genetics. 2001;2(1):21–32. doi: 10.1038/35047554. doi:10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- 46.Rock CL, Flatt SW, Byers TE, Colditz GA, Demark-Wahnefried W, Ganz PA, et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial: A Behavioral Weight Loss Intervention in Overweight or Obese Breast Cancer Survivors. J Clin Oncol. 2015;33(28):3169–76. doi: 10.1200/JCO.2015.61.1095. doi:10.1200/JCO.2015.61.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolin KY, Schwartz AL, Matthews CE, Courneya KS, Schmitz KH. Implementing the exercise guidelines for cancer survivors. The journal of supportive oncology. 2012;10(5):171–7. doi: 10.1016/j.suponc.2012.02.001. doi:10.1016/j.suponc.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biro FM, Greenspan LC, Galvez MP. Puberty in girls of the 21st century. J Pediatr Adolesc Gynecol. 2012;25(5):289–94. doi: 10.1016/j.jpag.2012.05.009. doi:10.1016/j.jpag.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]