Abstract
Intravenous immunoglobulin (IVIg) has been a candidate as a potential anti-amyloid immunotherapy for Alzheimer disease (AD) because it contains anti-amyloid β (Aβ) antibodies. Although several studies with IVIg in AD have been published, changing levels of Aβ efflux from the brain, or disaggregation of Aβ species induced by immunotherapy, have not been properly investigated. Here, we carried out an open label study of therapy with IVIg in five patients with AD. We collected plasma from a peripheral vein (peripheral-plasma) and from the internal jugular vein (jugular-plasma) to estimate directly the efflux of soluble Aβ from the brain. We also measured high molecular weight (HMW) Aβ oligomers in CSF as a marker to detect disaggregated Aβ. IVIg infusions were well tolerated in the majority of cases. However, one study subject had epileptic seizures after IVIg. Levels of HMW CSF Aβ oligomers in all participants were significantly increased after IVIg. Aβ40 and Aβ42 levels in jugular-plasma were continuously or temporarily elevated after treatment in three of five patients who showed preserved cognitive function, whereas levels of those in peripheral-plasma did not correlate with reactivity to the treatment. Other conventional biomarkers including 11C-Pittsburgh compound B retention were not altered after the treatment. These findings imply that HMW Aβ oligomer levels could be a better biomarker to reflect the anti-amyloid effects of IVIg than conventional Aβ species; moreover, Aβ in jugular-plasma seems to be a more direct and precise biomarker to estimate clearance of Aβ from the brain rather than Aβ in peripheral-plasma.
Trial registration: UMIN000022319
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia in elderly people but the available symptomatic drug treatments for this disease do not have any long-term effect [1]. Over the last decade, passive immunization using anti-amyloid β (Aβ) antibodies has held great promise as a potential new disease modifying therapy for AD. The principle of passive immunotherapy in AD is to reduce the levels of toxic Aβ species in the brain. Three molecular mechanisms for immunotherapy in AD have been generally postulated: increased efflux of Aβ from the brain by a ‘peripheral sink’ mechanism etc. [2]; the disaggregation of fibrillar and/or oligomeric Aβ in the brain [3]; and inhibition of Aβ aggregation[4]. Several studies suggest that passive immunization reverses cognitive deficits and reduces the load of cerebral Aβ in transgenic mouse models of AD [2, 5, 6] but no phase 3 trial of passive immunotherapy with positive results has been reported in human AD [7, 8]. This difference in response to immunotherapy between transgenic mice and humans could be caused by cerebrovascular aging, including atherosclerosis, which is seldom observed in mice, even in aged transgenic mouse models. Such cerebrovascular dysfunction could disturb the efflux of soluble Aβ from the brain and hinder the effects of immunotherapy. The other reason for the failure of clinical trials may be lack of good surrogate biomarkers measuring the anti-amyloid effects of drugs. It has been scarcely investigated whether anti-Aβ antibodies are sufficient to dissolve or to remove amyloid from the humans. If such a biomarker were available in the trial to exclude poor responders, the trial might have shown some success. Consequently, we propose that the following two considerations are important in pursuing clinical trials of passive immunization for AD. The first is that relatively young patients with AD should be included in these studies to avoid any possible interference due to cerebrovascular disease. The second is that the performance of the solubilization, mobilization and clearance of aggregated Aβ species induced by immunotherapy should be directly and precisely measured.
Intravenous immunoglobulin (IVIg) is a fractionated blood product that has been used to treat several medical conditions [9]and contains naturally occurring antibodies directed against Aβ [10, 11]. Therefore, IVIg has been a promising candidate as an anti-amyloid passive immunotherapy for AD, having high safety [12–14]. Although this treatment did not significantly slow the rate of cognitive decline in patients with AD in recent phase 3 trials, treatment with IVIg earlier in the course of the disease could still be beneficial [15]. Here, we conducted an open-label study of add on therapy with IVIg in five patients with AD. As mentioned above, we enrolled relatively young AD patients to avoid interference by atherosclerosis on Aß clearance. Then, we drew blood samples not only from a peripheral vein but also from the internal jugular vein to directly estimate brain Aβ clearance by IVIg. Moreover, we also collected cerebrospinal fluid (CSF) to measure disaggregated Aß species. We measured high molecular weight (HMW) Aβ oligomers in CSF as a more suitable biomarker to detect disaggregated Aβ species than conventional markers of Aβ[16]. The primary objectives of this study are, firstly, to compare the levels of Aβ (Aβ40 and Aβ42) in plasma from a peripheral vein (peripheral-plasma) and from the internal jugular vein (jugular-plasma) during the treatment; secondly, to assess the change in fibrillar Aβ load in patients with AD treated with IVIg: and thirdly to examine changes in levels of HMW Aβ oligomers in CSF during the treatment. The outcomes are investigating changes of conventional biomarkers for AD: Aβ42, total tau (t-tau), and 181-phosphorylated tau (p-tau) in CSF; amyloid deposition as measured by 11C-Pittsburgh compound B positron emission tomography (PIB-PET): and HMW Aβ oligomers as measured with our originally developed ELISA [16]. Neuropsychological tests, including mini-mental state examination (MMSE), were administered throughout the observational period to identify any changes in cognitive function.
Material and methods
Study design, ethics statement, and subject recruitment
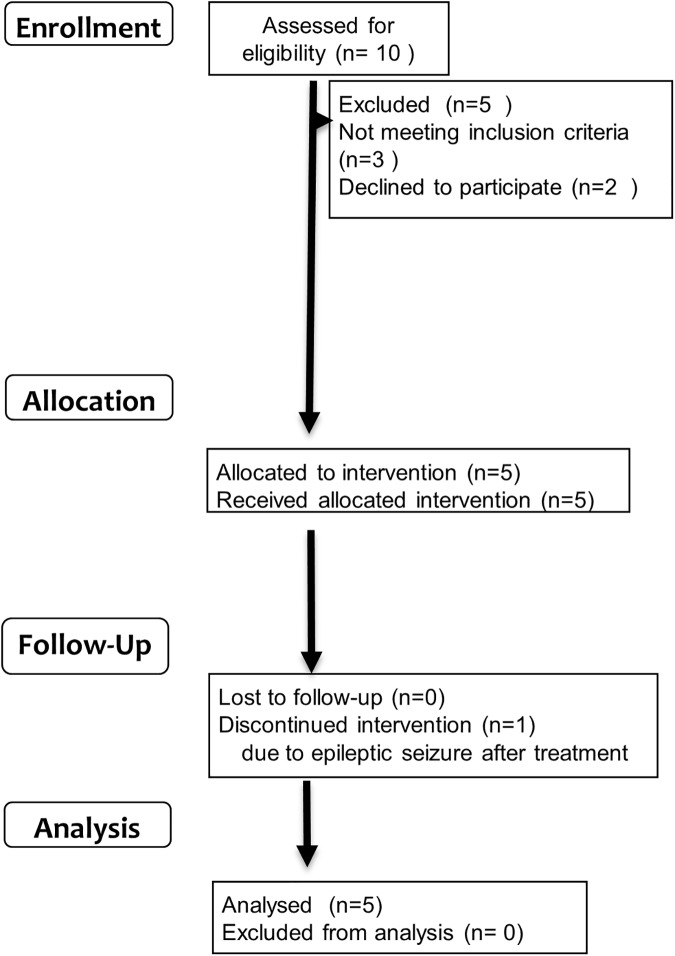
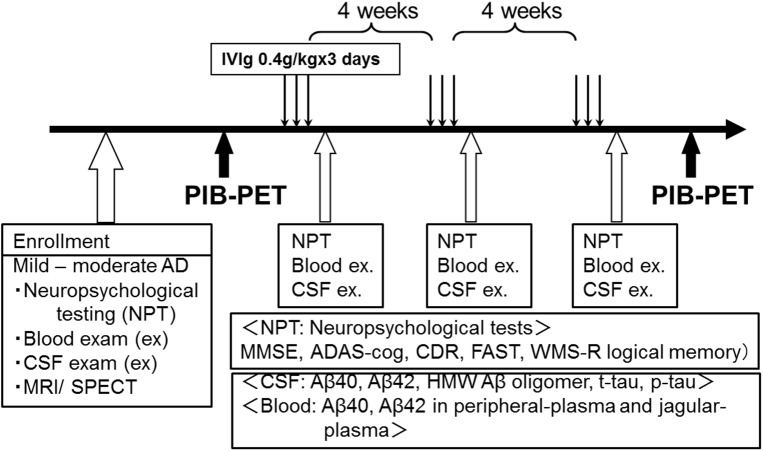
Our study was designed as an open label trial of IVIg in patients with mild and moderate AD that included 2 months of active treatment followed by at least two years of observation with neuropsychological tests. The study complied with the Declaration of Helsinki and was approved by the University Ethics Committee on 15th Aug in 2008 (Kyoto Prefectural University of Medicine, 602–8566, Kyoto, Japan). The trial was conducted in accordance with Good Clinical Research practice. This study has been retrospectively registered at UMIN (University hospital Medical Information Network) Clinical Trials Registry on 16th May 2016, because trial registration was not common in Japan when the trial was planned. The registration identification number is UMIN000022319 (http://www.umin.ac.jp/ctr/index-j.htm). Participants were recruited from Jul 2008 to Jan 2011. Written informed consent was obtained from the participants as well as caregivers for publication of their individual details and accompanying images in this manuscript. Five patients with AD were enrolled, based on the criteria described in the following subsection. All of the participants were given three cycles of IVIg in eight weeks (0.4 g/kg daily of Venoglobulin IH (Japan Blood Products Organization, Tokyo, Japan) for 3 days every four weeks). IVIg infusions were administrated through a 22 gauge intravenous catheter placed in a vein of the left arm. IVIg was mixed and prepared up to 2h prior to administration. Certified infusion pumps were employed to regulate rates of IVIg delivery. Total infusion times were 4–6 h per session depending on the patient’s body mass (Fig 1). Infusion process was performed under careful observation by certificated neurologists (TK, MK, RI, AT, or TT). Enrollment of patients, clinical assessment, treatment with IVIg, and specimen collection were performed at Kyoto Prefectural University of Medicine. PIB-PET was performed at Osaka City University graduate school of medicine. Participants were followed for at least 2 years (Fig 2). No financial reward was offered to the participants. (Research implementation plan and TREND statement check list of this study are in S1–S3 Files)
Fig 1. Trial schedule (A).
Fig 2. Flow diagram of the trial (B).
Clinical assessments
All subjects underwent neurological examinations including cognitive testing and examinations of blood (from peripheral and internal jugular veins) and CSF at baseline and at every IVIg treatment thereafter. Brain magnetic resonance imaging (MRI) (a 1.5 Tesla MRI unit, Philips Intera Achieva Pulser scanner) was carried out at baseline.
PIB-PET scans were performed at one month before the first treatment and within four weeks after the final treatment. Inclusion criteria included a diagnosis of probable AD by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS-ARDRA) criteria, Aβ deposition in PIB-PET, stable doses of approved AD treatment for at least 3 months, ability to comply with the study protocol, and availability of a suitable caregiver. Subjects were excluded if they had vascular dementia as detected by MRI, known allergy to IVIg therapy, IgA deficiency, renal failure, untreated vascular risk factors, unstable co-morbid medical conditions, or were receiving other investigational AD therapies or certain psychoactive medications. Subjects were also screened to exclude the presence of significant depression, psychosis and other neuropsychiatric disorders. Serum viscosity, IgA levels, renal function, thyroid function, complete blood count, metabolic profile, and hepatitis virus titers were checked at baseline. Subsets of these tests were repeated throughout the study for safety purposes. MMSE, Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog), Clinical Dementia Rating (CDR), Functional Assessment Staging (FAST) and Wechsler Memory Scale Revised (WMS-R) logical memory testing was performed by the same rater at baseline and just before each cycle of IVIg. Thereafter, MMSE was carried out every 3 or 6 months throughout the following observational period (at least two years). Adverse events in accordance with the definition of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline were recorded.
Sample collection
Sample collection was performed at a fixed time in the morning to avoid any effect from fluctuation of Aβ levels during the day [17]. Blood samples were drawn with 22 gauge needles simultaneously from the right internal jugular vein under ultrasound guidance and from the right median cubital vein through venous puncture, and then immediately transferred into the EDTA-containing tubes used to collect plasma samples. CSF was obtained through lumbar puncture at the L3/L4 or L4/L5 interspace, according to a standard protocol. Tubes were centrifuged at 1000 x g, plasma and CSF separated, aliquoted into 2 ml of polypropylene vials, and stored at– 80°C until time of analysis. Blood and CSF sampling were done at baseline, just before each cycle of IVIg, and at post IVIg (at least one month after the final cycle of treatment).
Biomarker analyses
Aβ42, t-tau and p-tau concentration in CSF were quantified by Luminex assays with INNO-BIA AlzBio3 (INNOGENETICS Inc, Ghent, Belgium), as described by the manufacturer. Levels of HWM Aβ oligomers in CSF were measured with the BAN50- Single Antibody Sandwich–ELISA (BAN50 SAS-ELISA) as described previously [16]. This ELISA system employs the same monoclonal antibody (BAN50) for both capture and detection of the oligomers and cannot detect Aß monomers because the capture antibody would then occupy the only antibody-binding site available. This system does, however, detect Aß oligomers because they have multiple binding sites. This ELISA is specific only for HMW Aß oligomers of 10-20mer in size. Neither low molecular weight Aß oligomers nor Aß monomer is not detected [16]. Levels of HMW Aβ oligomers are shown as arbitrary units based on the concentration of the synthetic ‘multiple-antigenic’ peptide used for calibration of this ELISA. The signal per one arbitrary unit of this standard has been estimated to correspond to 1.54–5.0 pM of HMW Aß42 oligomers [18]. Aβ40 and Aβ42 in plasma were measured with INNO-BIA plasma Aß kits (INNOGENETICS Inc, Ghent, Belgium).
PET scan
11C-PIB was synthesized using 2-(4’-aminophenyl)-6-hydroxybenzothiazole as the labeled precursor molecule. After an intravenous injection of 11C-PIB (150–300 MBq), a dynamic 60 min list-mode emission scan was acquired in the three-dimensional mode without arterial sampling using an Eminence-B PET scanner (Shimadzu Corp., Kyoto, Japan). According to the study performed by Lopresti et. al. [19], in which the frame summation of the dynamic images was recorded for 40–60 min., Logan graphical analysis was used for determining the regional counts (distribution volume ratio, DVR = binding potential + 1) using the cerebellum as the reference region. For this purpose, the cortical lesions occurring in the frontal, parietal, and lateral temporal lobes, and the precuneus regions were selected. The mean cortical DVR (MCDVR) was the mean of the DVR values of these lesions [20].
Statistics
Differences between biomarker levels at baseline and post treatment were tested by a Wilcoxon signed-rank test using SPSS software version 23. Statistical significance was defined as p < 0.05. Statistical power for the test (1-β) was calculated by postulating their effect sizes were medium (0.5) using G*Power 3 software.
Results
Demographics
Five patients (3 male, 2 female) with probable AD participated in this study. At baseline, their ages ranged from 54 to 67 years (mean 60.8 years). MMSE scores ranged from 8 to 25 (mean 18.6). ADAS-cog scale ranged from 3.6 to 33.3 (mean 15.7). All subjects were receiving stable doses of a cholinesterase inhibitor (donepezil) for at least 3 months prior to enrolling in the study (Table 1). The median length of follow-up was 34 months (30–50).
Table 1. Baseline characteristics of patients.
| Patient No. | Sex | Age | Duration of symptoms (years) | Use of choline esterase inhibitor | MMSE | ADAS-cog |
|---|---|---|---|---|---|---|
| 1 | M | 63 | 3 | Donepezil 5mg/day 24 months |
8 | 33.3 |
| 2 | F | 63 | 1 | Donepezil 3mg/day 18 months |
17 | 11.7 |
| 3 | F | 67 | 1 | Donepezil 5mg/day 3 months |
23 | 18.3 |
| 4 | M | 57 | 3 | Donepezil 5mg/day 11 months |
20 | 11.7 |
| 5 | M | 54 | 1 | Donepezil 5mg/day 12 months |
25 | 3.6 |
| Mean±SD | 60.8±5.2 | 18.6±6.7 | 15.7±11.1 |
SD: standard deviation
Adverse events
IVIg infusions were well tolerated in the majority of cases. However, case 5 was admitted with a first attack of epileptic seizure seven days after the first cycle of IVIg. Neurological examination revealed no focal signs. Routine blood and CSF examinations were all within normal ranges, and brain MRI did not show any signs of encephalitis or amyloid-related imaging abnormalities (ARIA). Electroencephalogram on the 3rd hospital day showed no epileptic discharges. He did not have any history of previous epilepsy. At that time, there was a possibility that his epileptic seizure might be related to the IVIg treatment, and further administration of IVIg was accordingly discontinued. Although we discontinued IVIg, he developed a second generalized tonic-clonic seizure 3 months later. We therefore consider that his seizure is not related to IVIg therapy, but is caused by AD itself [21]. Other minor events including headaches (in case 3) that were judged to be treatment-related were benign and resolved spontaneously.
MMSE
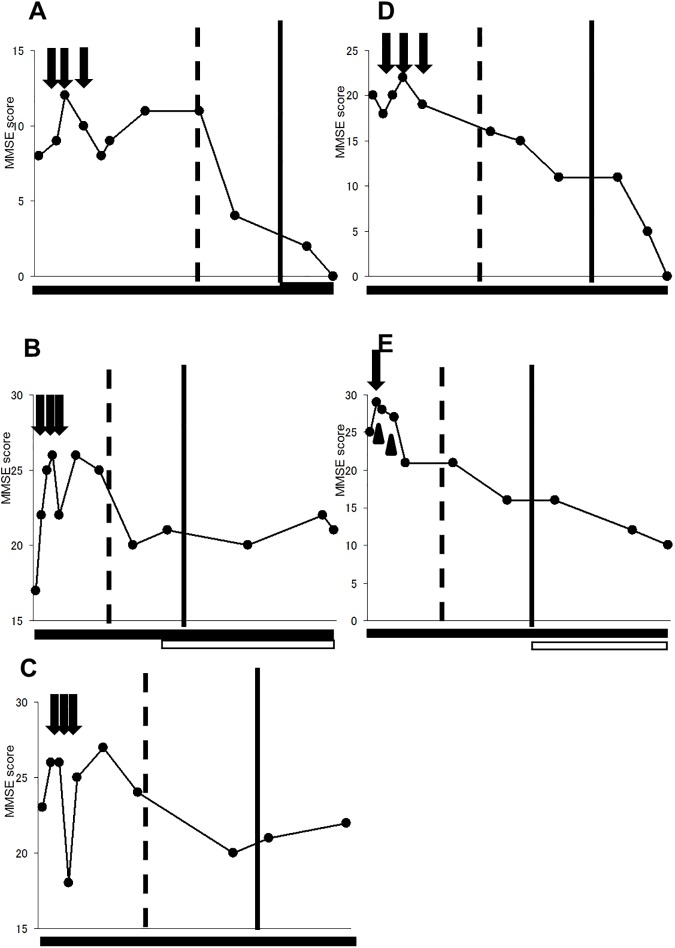
MMSE scores in patients during the period of treatment and the observational period are shown in the Fig 3. Changes of other cognitive scaling (ADAS-cog, CDR, FAST and WMS-R logical memory II) are summarized in Table A in S4 File. All patients including case 5 who had epilepsy and received incomplete treatment showed temporary improvement in MMSE scores during and/or after the three cycles of IVIg compared with their scores at the baseline. During one year after the treatment, during which the symptomatic drug treatments for AD in each patient were not changed, three (case 1. 2. 3) of four patients who received three cycles of IVIg therapy maintained improved MMSE scores compared with those at the baseline. These three patients are referred to as ‘good responders’ to IVIg in the following description. Case 4 who showed continuous deterioration of MMSE score after the treatment is referred as a ‘poor responder’ to IVIg. We could not judge the response to IVIg in case 5 due to the suspension of treatment, and in this case the MMSE score constantly deteriorated during the observational period.
Fig 3. The MMSE scores over the observational period.
A, B, C, D, and E show the MMSE changes in cases 1, 2, 3, 4, and 5, respectively. Arrows indicate IVIg treatments. Arrow heads indicate seizure attacks in case 5. Dashed lines and solid lines indicate 1 and 2 years after the first cycle of IVIg treatment in each patient. Bars below the graphs show the use of currently available symptomatic drugs for AD. Black bar: donepezil (thickness of bars indicates dosage of donepezil; thin bar = 3mg per day, medium bar = 5mg per day, bold bar = 8mg per day). White bar: memantine.
PIB-PET
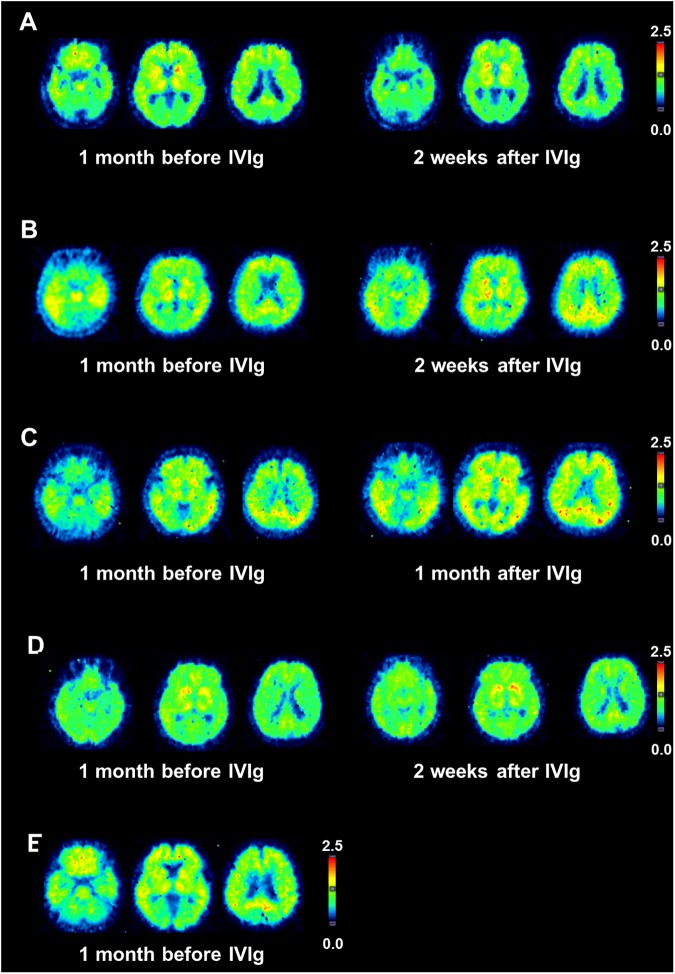
Aβ deposition measured by PIB-PET DVR map in each patient is shown in Fig 4. In all cases, PIB retention is observed in the frontal, posterior cingulate, striatum, parietal, and lateral temporal areas, as described previously [22]. There was no apparent change in distribution pattern before and after the treatment. The MCDVR was 1.38 (before) / 1.31 (after) in case 1, 1.53 (before) and 1.69 (after) in case 2, 1.79 (before) / 1.69 (after) in case 3, 1.36 (before) /1.39 (after) in case 4, and 1.55 (before) in case 5. Rate of changes for PIB retention in all participants after the treatment (calculated by the formula of MCDVR (after)–MCDVR (before)/ MCDVR (before)) were within the measurement error range in our institute (±10%). Therefore, we interpreted that no significant change in PIB retention was observed in any patient after the treatment compared with baseline.
Fig 4. Transaxial PIB-PET scans before and after the IVIg treatment.
DVR maps in each patient are shown. A, B, C, D, and E represent case 1, 2, 3, 4, and 5, respectively. Rate for PIB retention after the treatment was –6.7% in case 1,+10% in case 2, -5.6% in case 3 and +2.2% in case 4.
CSF and plasma biomarkers
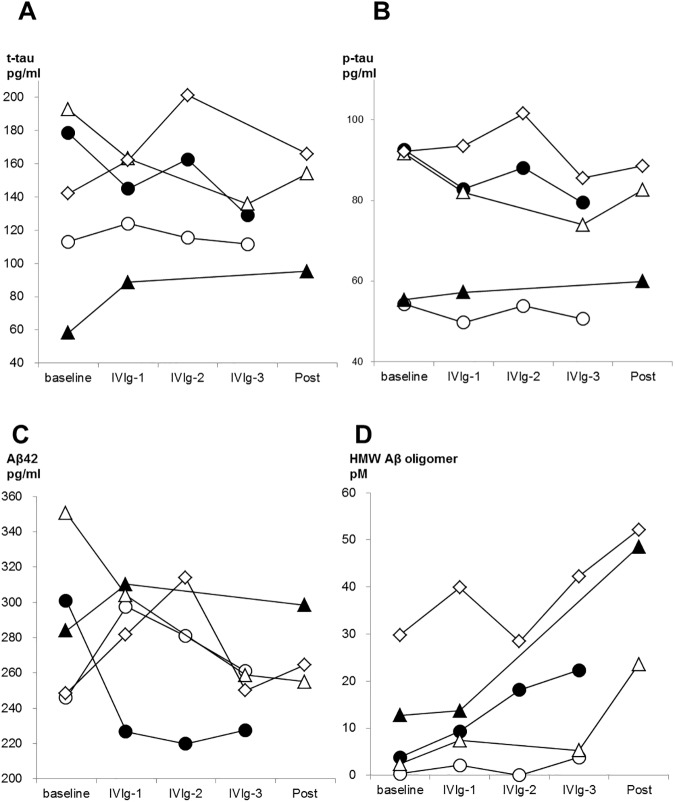
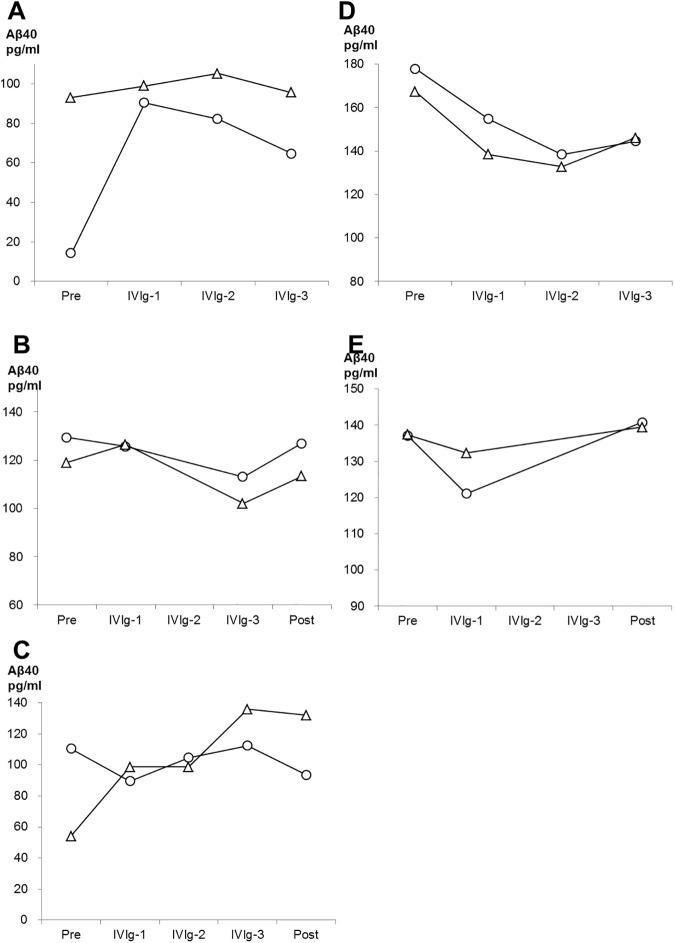
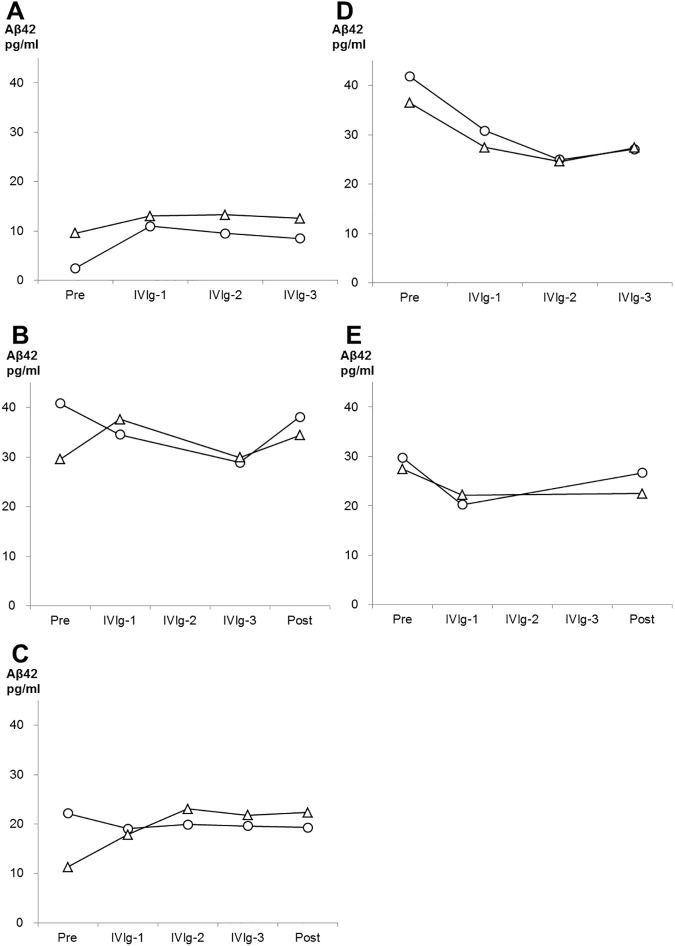
Levels of t-tau, p-tau, Aβ42, and HMW Aβ oligomers in CSF are shown in Fig 5. T-tau and Aβ42 did not show any significant difference compared to baseline, at every cycle of IVIg, and at post treatment. On p-tau, four of five patients showed lower p-tau levels after the treatment than baseline, although the difference did not reach statistical significance (P = 0.315). (It is possible that difference did not reach significance due to our small sample size, because statistical power in this analysis was insufficient (0.106).) The level of HMW Aβ oligomers was significantly increased after the treatment (P = 0.043). Note, in case 1 and 4 who refused CSF examination at post treatment, the values at the third cycle of treatment were used for analysis above described. Regarding peripheral and jugular-plasma, the IVIg treatment altered neither Aβ40 nor Aβ42 levels (S1 Fig). As shown in the Figs 6 and 7, Aβ40 or Aβ42 levels in jugular-plasma were not always higher than those in peripheral-plasma at baseline, although the jugular vein is a major drainage route from the brain. Interestingly, in three good responders, Aβ40 and Aβ42 levels were elevated in jugular-plasma after the treatment. Furthermore, these increased Aβ40 and Aβ42 levels in jugular-plasma were maintained during the whole treatment period including post IVIg in cases 1 and 3. Case 2 showed a transient increase of these markers in the first cycle of IVIg. This elevation was more evident in Aβ42 than that in Aβ40. On the other hand, the poor responder (case 4) or the participant with suspension of the treatment (case 5) did not show any increase of Aβ levels in jugular-plasma. In contrast, the levels of Aβ40 and Aβ42 in peripheral-plasma over the treatment did not change in relation to reactivity to IVIg. (The raw data of the biomarkers are summarized in Table B,C,D,E,F,and G in S4 File)
Fig 5. Changes of CSF biomarkers over the period of treatment.
Case 1, 2, 3 (good responders), 4 (a poor responder) and 5 (case with discontinued treatment) are represented by white circles, white triangles, white squares, black circles, and black triangles, respectively. A: t-tau B: p-tau C: Aβ42 D: HMW Aβ oligomers.
Fig 6. Changes of Aβ40 in peripheral-plasma and jugular-plasma over the period of treatment in the participants.
Levels of Aβ40 in peripheral-plasma and jugular-plasma in each participant are represented by white circles and white triangles, respectively. The data from cases 1, 2, 3 (good responders), 4 (a poor responder) and 5 (case with discontinued treatment) are shown in A, B, C, D, and E, respectively.
Fig 7. Changes of Aβ42 in peripheral-plasma and jugular-plasma over the period of treatment in the participants.
Levels of Aβ42 in peripheral-plasma and jugular-plasma in each participant are represented by white circles and white triangles, respectively. The data from cases 1, 2, 3 (good responders), 4 (a poor responder) and 5 (case with discontinued treatment) are shown in A, B, C, D, and E, respectively.
Discussion
Our first major finding is that the levels of HMW Aβ oligomers as measured by the BAN50 SAS-ELISA were significantly increased after treatment in all participants. Regarding monomeric Aβ species, we found that IVIg therapy altered the levels of neither Aβ40 nor Aβ42 in CSF, which is in accord with previous results [23]. Aβ oligomers in CSF have not been adopted as a biomarker in previous studies of IVIg or other immunotherapies for AD due to the poor reliability of measurement. The BAN50 SAS-ELISA that we have developed is one of the few systems available that can reliably and reproducibly detect HMW Aβ oligomers in CSF [24]. The result was reproduced using the same set of antibody in one following study [25]. Our data suggest that HMW Aβ oligomers could be a better CSF biomarker to reflect the anti-amyloid effects of IVIg in the brain than Aβ40 or Aβ42. The increase in HMW Aβ oligomers in CSF on IVIg treatment could be due to the disaggregation of Aβ fibrils by the anti-Aβ antibodies contained in IVIg [26]. Moreover, we should note the other possibility that an increased signal in the BAN50-SAS ELISA could also be derived not from genuine ‘pathological’ Aβ oligomers (consisting only of Aβ), but also from complexes composed of multiple copies of Aβ in association with other molecules which, in theory at least, would also be detected by this ELISA [16, 24]. IVIg treatment is reported to increase the levels of anti-Aβ antibodies in CSF [11]. Therefore, pseudo (or non-pathological) oligomer complexes consisting of multiple copies of Aβ cross-linked by such antibodies could possibly be detected in this study.
Three of the five patients had preserved cognitive function more than one year after the IVIg treatment and all three of them showed sustained or transiently increased Aβ40 and Aβ42 levels in jugular-plasma after the treatment. IVIg has been reported not to alter the levels of Aβ40 or Aβ42 in peripheral-plasma [23], which is in agreement with our result that Aβ species in peripheral-plasma did not change in response to treatment. These results suggest that the levels of Aβ in peripheral-plasma are not a good measure for estimation of the efflux of Aβ from the brain in immunotherapy of AD. The levels of Aβ species in jugular-plasma after passive immunization therapy for AD have not been measured previously. Our results imply that Aβ species in jugular-plasma will provide a more direct and precise biomarker for estimation of Aβ clearance from the brain in the study of IVIg or other passive immunotherapies than those in peripheral-plasma. Moreover, our data provide some supportive evidence that IVIg may be more beneficial in AD in cases with efficient clearance of Aβ from the brain to the jugular vein than in those with poor clearance of Aβ.
In this study, the levels of Aβ in jugular-plasma were not always higher than those in peripheral-plasma, even though the internal jugular veins drain most of the outflow from the brain and so would be expected to be a major source of brain-derived Aβ. We suppose that this unexpected result is probably explained by the following two factors. The first is that Aβ is also produced from peripheral sources outside of the brain. Plasma Aβ is derived from not only the central nervous system, but also from the liver or from arteries with atherosclerosis [27]. Such systemically derived plasma Aβ might play a confounding role in this study. The second factor is the possibility that drainage of Aβ from the brain could be mainly or partly via another route instead of the jugular vein. Recent reports show that there are drainage pathways of brain interstitial Aβ along the cerebral vessels into cervical lymph nodes, and finally superior vena cava via the thoracic duct [28, 29]. Such lymphatic drainage might explain the imbalance between Aβ levels in peripheral and jugular-plasma.
We also found that Aβ deposition as measured by PIB-PET was not altered by the IVIg treatment. It is still controversial whether passive immunization can improve PIB retention [8, 30]. Moreover, regarding IVIg therapy, Relkin et. al. reported no significant changes in PIB retention for 6 months in the brains of 12 patients with AD treated with IVIg [31]. Moreover, one report in a transgenic mouse model of AD showed that naturally occurring autoantibodies against Aβ preferentially bind to Aβ oligomers but fail to bind to monomers or fibrils and therefore, improved memory but did not readily clear senile plaques from the brains of transgenic mice [32]. The lack of reduction in PIB retention in our study is consistent with these previous results. These facts suggest that disaggregation of Aβ fibril by the IVIg therapy has a too small to reduce Aβ deposition in the brain in such a short period and, therefore, that PIB retention is not a good marker to reflect the anti-amyloid effects of IVIg.
Conclusions
The present study has found that treatment with IVIg resulted in an increase in the levels of HMW Aβ oligomers, as measured by the BAN50 SAS-ELISA, in all participants; Aβ40 and Aβ42 levels in jugular-plasma showed a transient or sustained increase after treatment in three of five patients, which correlated with preserved cognitive function, whereas Aβ levels in peripheral-plasma did not correlate with any treatment response; and PIB retention was not changed after IVIg treatment. These results suggest that the levels of HMW Aβ oligomers in CSF give a better indication of the anti-amyloid effects of IVIg than more conventional measures of CSF Aβ; Aβ in jugular-plasma possibly provides a more direct and precise measure of clearance of Aβ from the brain in immunotherapy trials than peripheral-plasma; and PIB-PET appears to be unsuitable for estimation of the anti-amyloid effects of immunotherapy. Although the small sample size in our study limits these conclusions, these findings do have positive implications for development of a surrogate biomarker to measure the efficacy of the immunotherapy, including IVIg therapy, for future clinical studies of AD.
Supporting information
Case 1, 2, 3 (good responders), 4 (a poor responder) and 5 (case with discontinued treatment) are represented by white circles, white triangles, white squares, black circles, and black triangles, respectively. n.s: not significant. A: Aβ40 in peripheral-plasma B: Aβ40 in jugular-plasma C: Aβ42 in peripheral-plasma D: Aβ42 in jugular-plasma.
(PPT)
(PDF)
(PDF)
(DOC)
Table A in S4 File. Summary of the clinical course after IVIg therapy in the 5 patients. Table B in S4 file. Changes for t-tau concentration in CSF (pg/ml). Table C in S4 File. Changes for p-tau concentration in CSF (pg/ml). Table D in S4 File. Changes for Aβ42 concentration in CSF (pg/ml). Table E in S4 File. Changes for HMW Aβ oligomers levels in CSF (pM). Table F in S4 File. (a). Changes for Aβ40 concentration in peripheral-plasma (pg/ml) (b). Changes for Aβ40 concentration in jugularl-plasma (pg/ml). Table G in S4 File. (a). Changes for Aβ42 concentration in peripheral-plasma (pg/ml) (b). Changes for Aβ42 concentration in jugularl-plasma (pg/ml).
(DOCX)
Acknowledgments
The authors wish to thank Takeda Pharmaceutical Company Limited for the generous gifts of the monoclonal antibody BAN50 and its SAS-ELISA kit.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant to MN from Mitsubishi Tanabe Pharma Corporation (exsiting Japan Blood Product Organization). (Grant number: 20-437). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–16. doi: 10.1016/S1474-4422(10)70119-8 [DOI] [PubMed] [Google Scholar]
- 2.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–5. doi: 10.1073/pnas.151261398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Disaggregation of Alzheimer beta-amyloid by site-directed mAb. Proc Natl Acad Sci U S A. 1997;94:4109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada K, Yabuki C, Seubert P, Schenk D, Hori Y, Ohtsuki S, et al. Abeta immunotherapy: intracerebral sequestration of Abeta by an anti-Abeta monoclonal antibody 266 with high affinity to soluble Abeta. J Neurosci. 2009;29:11393–8. doi: 10.1523/JNEUROSCI.2021-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levites Y, Das P, Price RW, Rochette MJ, Kostura LA, McGowan EM, et al. Anti-Abeta42- and anti-Abeta40-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model. J Clin Invest. 2006;116:193–201. doi: 10.1172/JCI25410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, et al. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation. 2004;1:24 doi: 10.1186/1742-2094-1-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–21. doi: 10.1056/NEJMoa1312889 [DOI] [PubMed] [Google Scholar]
- 8.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–33. doi: 10.1056/NEJMoa1304839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold R, Stangel M, Dalakas MC. Drug Insight: the use of intravenous immunoglobulin in neurology—therapeutic considerations and practical issues. Nat Clin Pract Neurol. 2007;3:36–44. doi: 10.1038/ncpneuro0376 [DOI] [PubMed] [Google Scholar]
- 10.Dodel RC, Du Y, Depboylu C, Hampel H, Frolich L, Haag A, et al. Intravenous immunoglobulins containing antibodies against beta-amyloid for the treatment of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75:1472–4. doi: 10.1136/jnnp.2003.033399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Relkin NR, Szabo P, Adamiak B, Burgut T, Monthe C, Lent RW, et al. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging. 2009;30:1728–36. doi: 10.1016/j.neurobiolaging.2007.12.021 [DOI] [PubMed] [Google Scholar]
- 12.Fillit H, Hess G, Hill J, Bonnet P, Toso C. IV immunoglobulin is associated with a reduced risk of Alzheimer disease and related disorders. Neurology. 2009;73:180–5. doi: 10.1212/WNL.0b013e3181ae7aaf [DOI] [PubMed] [Google Scholar]
- 13.Du Y, Dodel R, Hampel H, Buerger K, Lin S, Eastwood B, et al. Reduced levels of amyloid beta-peptide antibody in Alzheimer disease. Neurology. 2001;57:801–5. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–22. doi: 10.1016/j.cell.2012.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeffler DA. Intravenous immunoglobulin and Alzheimer's disease: what now? J Neuroinflammation. 2013;10:70 doi: 10.1186/1742-2094-10-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukumoto H, Tokuda T, Kasai T, Ishigami N, Hidaka H, Kondo M, et al. High-molecular-weight beta-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J. 2010;24:2716–26. doi: 10.1096/fj.09-150359 [DOI] [PubMed] [Google Scholar]
- 17.Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology. 2007;68:666–9. doi: 10.1212/01.wnl.0000256043.50901.e3 [DOI] [PubMed] [Google Scholar]
- 18.Kasai T, Tokuda T, Taylor M, Nakagawa M, Allsop D. Utilization of a multiple antigenic peptide as a calibration standard in the BAN50 single antibody sandwich ELISA for Abeta oligomers. Biochem Biophys Res Commun. 2012;422:375–80. doi: 10.1016/j.bbrc.2012.04.146 [DOI] [PubMed] [Google Scholar]
- 19.Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46:1959–72. [PubMed] [Google Scholar]
- 20.Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–47. doi: 10.1038/sj.jcbfm.9600146 [DOI] [PubMed] [Google Scholar]
- 21.Scarmeas N, Honig LS, Choi H, Cantero J, Brandt J, Blacker D, et al. Seizures in Alzheimer disease: who, when, and how common? Arch Neurol. 2009;66:992–7. doi: 10.1001/archneurol.2009.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009 [DOI] [PubMed] [Google Scholar]
- 23.Dodel R, Rominger A, Bartenstein P, Barkhof F, Blennow K, Forster S, et al. Intravenous immunoglobulin for treatment of mild-to-moderate Alzheimer's disease: a phase 2, randomised, double-blind, placebo-controlled, dose-finding trial. Lancet Neurol. 2013;12:233–43. doi: 10.1016/S1474-4422(13)70014-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasai T, Tokuda T, Taylor M, Kondo M, Mann DM, Foulds PG, et al. Correlation of Abeta oligomer levels in matched cerebrospinal fluid and serum samples. Neurosci Lett. 2013;551:17–22. doi: 10.1016/j.neulet.2013.06.029 [DOI] [PubMed] [Google Scholar]
- 25.Herskovits AZ, Locascio JJ, Peskind ER, Li G, Hyman BT. A Luminex assay detects amyloid beta oligomers in Alzheimer's disease cerebrospinal fluid. PLoS One. 2013;8(7):e67898 Epub 2013/07/12. PubMed Central PMCID: PMCPmc3699502. doi: 10.1371/journal.pone.0067898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frenkel D, Balass M, Katchalski Katzir E, Solomon B. High affinity binding of monoclonal antibodies to the sequential epitope EFRH of beta-amyloid peptide is essential for modulation of fibrillar aggregation. J Neuroimmunol. 1999;95:136–42. [DOI] [PubMed] [Google Scholar]
- 27.Roher AE, Esh CL, Kokjohn TA, Castano EM, Van Vickle GD, Kalback WM, et al. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers Dement. 2009;5:18–29. doi: 10.1016/j.jalz.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carare RO, Hawkes CA, Weller RO. Afferent and efferent immunological pathways of the brain. Anatomy, function and failure. Brain Behav Immun. 2014;36:9–14. Epub 2013/10/23. doi: 10.1016/j.bbi.2013.10.012 [DOI] [PubMed] [Google Scholar]
- 30.Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–72. doi: 10.1016/S1474-4422(10)70043-0 [DOI] [PubMed] [Google Scholar]
- 31.Relkin N, Vallabhajosula S, Mosconi L, Kothari P, de Leon M, Tsui W, et al. Use of PIB-PET in a double-blind, placebo-controlled Alzheimer's disease (AD) immunotherapy trial. J Nucl Med. 2008;49: Supplement1:36. [Google Scholar]
- 32.Dodel R, Balakrishnan K, Keyvani K, Deuster O, Neff F, Andrei-Selmer LC, et al. Naturally occurring autoantibodies against beta-amyloid: investigating their role in transgenic animal and in vitro models of Alzheimer's disease. J Neurosci. 2011;31:5847–54. doi: 10.1523/JNEUROSCI.4401-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Case 1, 2, 3 (good responders), 4 (a poor responder) and 5 (case with discontinued treatment) are represented by white circles, white triangles, white squares, black circles, and black triangles, respectively. n.s: not significant. A: Aβ40 in peripheral-plasma B: Aβ40 in jugular-plasma C: Aβ42 in peripheral-plasma D: Aβ42 in jugular-plasma.
(PPT)
(PDF)
(PDF)
(DOC)
Table A in S4 File. Summary of the clinical course after IVIg therapy in the 5 patients. Table B in S4 file. Changes for t-tau concentration in CSF (pg/ml). Table C in S4 File. Changes for p-tau concentration in CSF (pg/ml). Table D in S4 File. Changes for Aβ42 concentration in CSF (pg/ml). Table E in S4 File. Changes for HMW Aβ oligomers levels in CSF (pM). Table F in S4 File. (a). Changes for Aβ40 concentration in peripheral-plasma (pg/ml) (b). Changes for Aβ40 concentration in jugularl-plasma (pg/ml). Table G in S4 File. (a). Changes for Aβ42 concentration in peripheral-plasma (pg/ml) (b). Changes for Aβ42 concentration in jugularl-plasma (pg/ml).
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.