Abstract
We analyzed the utility of Revised International staging system (RISS) in an unselected cohort of newly diagnosed multiple myeloma (NDMM; cohort 1), and relapsed/refractory multiple myeloma (RRMM; cohort 2) patients. Cohort 1 included 1900 patients seen within 90 days of diagnosis, from 2005 to 2015, while cohort 2 had 887 patients enrolled in 23 clinical trials at Mayo Clinic. The overall survival (OS) and progression-free survival (PFS) was calculated from the time since diagnosis or trial registration. The median estimated follow up was 5 and 2.3 years for Cohorts 1 and 2, respectively. Among 1067 patients evaluable in Cohort 1, the median OS and PFS was 10 and 2.8 years for RISS stage I, 6 and 2.7 years for RISS stage II and 2.6 and 1.3 years for RISS stage III (P<0.0001). Among 456 patients evaluable in Cohort 2, the median OS and PFS was 4.3 and 1.1 years for RISS stage I, 2 and 0.5 years for RISS stage II and 0.8 and 0.2 years for RISS stage III (P<0.0001). In conclusions, RISS gives a better differentiation of NDMM as well as RRMM patients into three survival subgroups and should be used to stratify patients in future clinical trials.
Introduction
Multiple myeloma (MM) is a very heterogeneous disease with nearly a quarter of patients dying within 3 years of diagnosis and others maintaining durable disease control for more than 10 years.1, 2 For this reason, various prognostic factors and staging systems have been developed to predict the disease outcomes. The Durie/Salmon staging sytem used hemoglobin, calcium level, the type and level of monoclonal protein and number of bone lesions to predict myeloma cell tumor burden and long-term outcomes.3 Subsequently, the International Staging System (ISS) was developed, which identified three patient groups with different survival based on serum β2- microglobulin (β2m) and serum albumin. High serum β2m reflected high tumor burden and reduced renal function, while low serum albumin is thought to be mediated by the effects of inflammatory cytokines like interleukin-6 (IL-6).4
However, ISS did not incorporate one of the most important prognostic factors in MM, namely chromosomal abnormalities (CA). In patients with newly diagnosed MM (NDMM), high-risk disease is characterized by the presence of del (17p), t(4; 14) (p16;q32) or t(14;16)(q32;q23) detected by interphase fluorescent in situ hybridization.5, 6 High serum lactate dehydrogenase (LDH) has been linked to shorter OS in MM and likely reflects disease aggressiveness and drug resistance, and may also be an indicator of extramedullary disease.7, 8 The revised ISS stage (Revised International staging system (RISS)) was developed by pooling data from 4445 patients with NDMM enrolled on 11 international trials. It combines the ISS with high-risk CA [del (17p), t(4; 14) (p16; q32) or t(14; 16) (q32; q23)] and serum LDH to classify patients into three risk groups. The 5-year overall survival (OS) of patients with stage I, II and III RISS was 82, 62 and 40, while the 5-year progression-free survival (PFS) was 55%, 36% and 24%, respectively.9
Unlike ISS, RISS has been derived from data obtained only from patients enrolled in clinical trials. Clinical trials almost always exclude patients with serious comorbidities and poor performance making it less applicable to real-life scenario.10, 11 Finally, RISS development only used data from previously untreated symptomatic MM, and its utility in relapsed disease is less clear.4, 9 Hence, we examined the utility of RISS in an unselected cohort of patients with NDMM as well as in a selected group of patients with relapsed/refractory MM (RRMM).
Patients and methods
Patients
The purpose of our study was to analyze the clinical utility of the RISS in patients with NDMM in a non-clinical trial setting and in patients with RRMM. To address these issues, we studied two cohorts of patients. The first cohort (NDMM cohort; cohort 1) included 1900 consecutive patients with NDMM seen at Mayo Clinic within 90 days of diagnosis, between January 2005 and December 2015. Their clinical and laboratory data were collected by chart review and analyzed retrospectively. The second cohort (RRMM cohort; cohort 2) included 887 patients with RRMM enrolled in different clinical trials at Mayo Clinic.
ISS and RISS
ISS stage I is defined as serum β2m <3.5 mg/l and serum albumin level ⩾3.5 g/dl, stage III is defined as serum β2m>5.5 mg/l and stage II includes all remaining patients.4 The RISS stage I includes patients with ISS stage I with no high-risk CA [del(17p) and/or t(4;14) and/or t(14;16)], and normal LDH level (less than the upper limit of normal range), stage III includes patients with ISS stage III and either high-risk CA or high LDH level, and stage II includes all the other possible combinations.9 The value of all four variables (β2m, albumin, CA and LDH) was available for 1067 patients in the NDMM cohort, though RISS could be calculated in 1352 patients on the basis on the availability of either CA or LDH. RISS staging was analyzed in 456 assessable cases in the RRMM cohort with complete data.
Prognostic risk score
We also examined a simpler approach that utilized equal weight for each of the poor prognostic factors. The variables used to calculate the RISS stage were assigned a value of either 0, if favorable, or 1, if unfavorable as follows: β2m (<5.5 mg/l versus ⩾5.5 mg/l), albumin (⩾3.5 g/dl versus <3.5 g/dl), LDH (⩽upper limit of normal range versus >upper limit of normal range), high-risk translocation (absence versus presence) and deletion 17p (absence versus presence). The values of these four variables were then added together to arrive at a final score.
Statistical analysis
The primary end point was OS, defined as the time from diagnosis (NDMM cohort) or trial registration (RRMM cohort) until death from any cause or until the patient was last known to be alive. The secondary end point of PFS was defined as the time from diagnosis or trial registration until progression or death as a result of any cause or until the last date the patient was known to be progression free, whichever occurred first. The OS and PFS curves were estimated using the Kaplan–Meier method and their differences were analyzed using the two-sided log-rank test. All reported P-values were two sided at the conventional 5% significance level. Data were analyzed with SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics and treatments
In the NDMM cohort, the median age was 65.0 years (range, 22–95 years) and 59.9% were male. Among these, 1751 patients (92.2%) received novel drugs (immunomodulatory drugs (IMIDs) or proteasome inhibitors (PIs)) as induction or initial therapy with or without autologous stem cell transplant and maintenance treatment. Overall, RISS could be calculated in 71.2% (n=1352) of patients; among which 229 patients (17.0%) were RISS stage I, 938 patients (69.4%) were RISS stage II and 185 patients (13.7%) were RISS stage III.
In RRMM cohort, the median age was 65.0 years (range, 32–90 years) and 59.6% were males. Six-hundred fifty-four patients (73.7%) received novel drugs as part of trial therapy. Overall, RISS could be calculated in 51.4% (n=456) of patients; among which 104 patients (22.8%) were RISS stage I, 294 patients (64.5%) were RISS stage II and 58 patients (12.7%) were RISS stage III. The baseline characteristics of both the cohorts are listed in Table 1.
Table 1. Baseline characteristics for Cohorts 1 and 2.
| Characteristic |
Number of patients (%) |
|
|---|---|---|
| Cohort 1 (N=1900) | Cohort 2 (N=887) | |
| Age, years | ||
| ⩽65 | 1009 (53.1%) | 465 (52.4%) |
| >65 | 891 (46.9%) | 422 (47.6%) |
| Sex | ||
| Male | 1137 (59.9%) | 529 (59.6%) |
| Female | 763 (40.2%) | 358 (40.4%) |
| ISS stage | ||
| I | 481 (25.0%) | 235 (26.5%) |
| II | 653 (34.4%) | 377 (42.5%) |
| III | 501 (26.4%) | 178 (20.1%) |
| Missing | 265 (14.0%) | 97 (10.9%) |
| High-risk CA by iFISH | ||
| Absent | 1071 (56.4%) | 346 (39.0%) |
| Present | 297 (16.0%) | 126 (14.2%) |
| Missing | 532 (28.0%) | 415 (46.8%) |
| LDH level | ||
| Normal | 1258 (66.2%) | 673 (75.9%) |
| Elevated | 268 (14.1%) | 182 (20.5%) |
| Missing | 374 (19.7%) | 32 (3.6%) |
| Treatment | ||
| IMIDs | 1304 (68.6%) | 458 (51.6%) |
| PIs | 877 (46.2%) | 196 (22.1%) |
| ASCT | 247 (13%) | 0 (0%) |
| Other | 0 (0%) | 233 (26.3%) |
| Missing | 9 (0.47%) | 0 (0%) |
Abbreviations: ASCT, autologous stem cell transplant; CA, chromosomal abnormalities; iFISH, fluorescent in situ hybridization; IMID, immunomodulatory drugs; ISS, International Staging System; LDH, lactate dehydrogenase; PIs, proteasome inhibitors.
RISS staging and prognostication
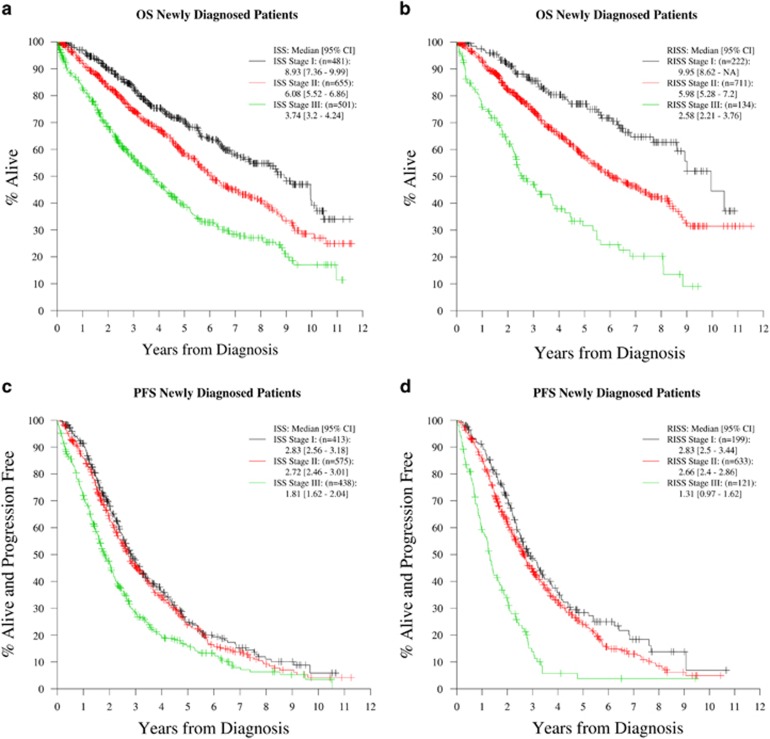
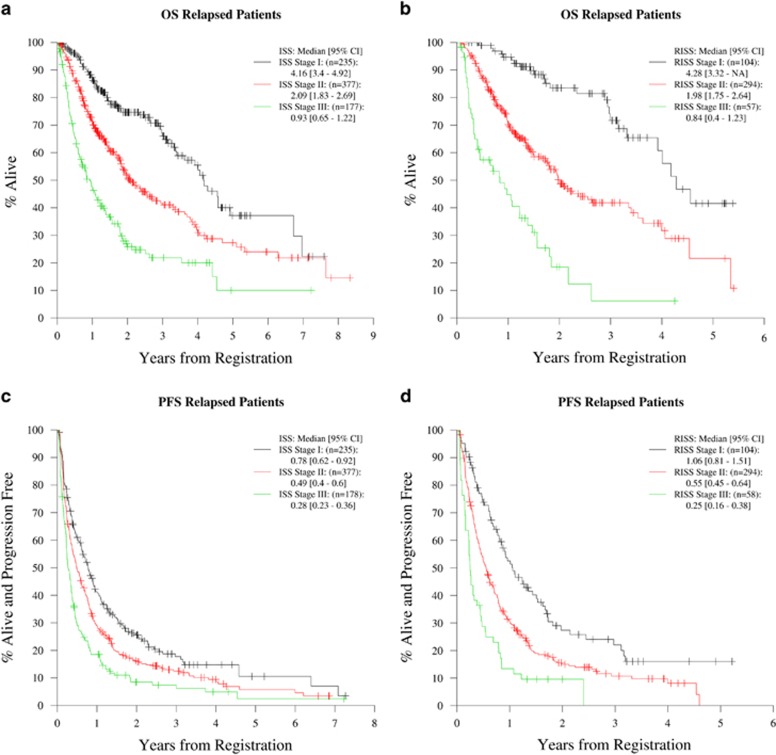
The median estimated follow up was 5 years (95% confidence interval (CI); 4.8, 5.5 years) for NDMM cohort and 2.3 years (interquartile range; 2.1, 2.6 years) for the RRMM cohort. The main analysis included 1067 assessable cases in the NDMM cohort and 456 assessable cases in the RRMM cohort with RISS calculated on the basis of complete data on all four variables. The median and 5-year estimates of OS and PFS for the NDMM cohort based on ISS (Figures 1a and c) and RISS (Figures 1b and d) stages are depicted in Table 2. The outcomes according to RISS staging done on a total of 1352 patients in whom RISS could be calculated on the basis of availability of either CA or LDH is depicted in the Supplementary Table 1. The median and 2-year estimates of OS and PFS for the RRMM cohort according to the ISS (Figures 2a and c) and RISS staging (Figures 2b and d) are listed in Table 3.
Figure 1.
OS and PFS curves for different ISS and RISS stages among patients with NDMM (Cohort 1). (a) OS curves for all ISS stages in patients with NDMM. (b) OS curves for all RISS stages in patients with NDMM. (c) PFS curves for all ISS stages in patients with NDMM. (d) PFS curves for all RISS stages in patients with NDMM.
Table 2. OS and PFS analysis across three different diagnostic scores in newly diagnosed patients (Cohort 1).
| Diagnostic test | Cohort | Median OS years (95% CI) | 5-Year OS % (95% CI) | Median PFS years (95% CI) | 5-Year PFS % (95% CI) |
|---|---|---|---|---|---|
| ISS | ISS-I | 8.9 (7.36–9.99) | 70.4 (65.2–75.1) | 2.83 (2.56–3.18) | 25.3 (20.2–30.7) |
| ISS-II | 6.08 (5.52–6.86) | 58.5 (53.8–62.9) | 2.72 (2.46–2.98) | 23.7 (19.6–27.9) | |
| ISS-III | 3.74 (3.20–4.24) | 38.5 (33.3–43.6) | 1.81 (1.62–2.04) | 16.0 (12.2–20.2) | |
| Total | 5.74 (5.48–6.36) | 55.7 (52.8–58.5%) | 2.46 (2.32–2.61) | 21.8 (19.2–24.4) | |
| RISS | RISS-I | 9.95 (8.62–NA) | 77 (69.4–82.9) | 2.83 (2.5–3.44) | 28.4 (21–36.2) |
| RISS-II | 5.98 (5.28–7.2) | 57.1 (52.5–61.5) | 2.66 (2.4–2.86) | 24.1 (20.1–28.3) | |
| RISS-III | 2.58 (2.21–3.76) | 31.6 (22.1–41.5) | 1.31 (0.97–1.62) | 3.8 (0.9–10.5) | |
| Total | 6.29 (5.60–7.20) | 58 (54.3–61.6) | 2.5 (2.32–2.67) | 22.5 (19.3–25.9) | |
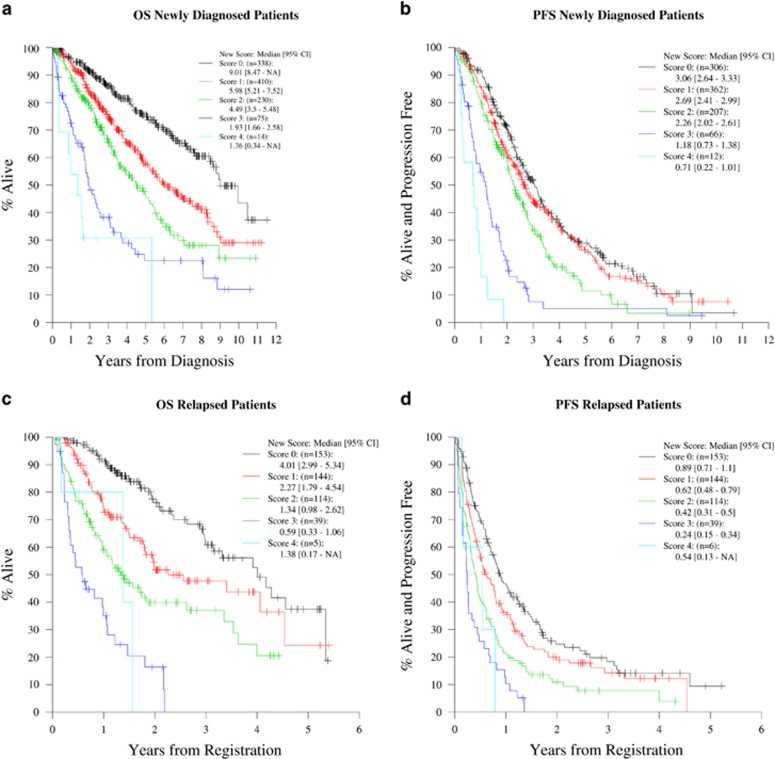
| New Score | 0 | 9.01 (8.47–NA) | 75.5 (69.4–80.6) | 3.06 (2.64–3.33) | 28.9 (23.0–35.1) |
| 1 | 5.98 (5.21–7.52) | 57.9 (51.7–63.6) | 2.69 (2.41–2.99) | 26.3 (20.8–32.0) | |
| 2 | 4.49 (3.50–5.48) | 45.4 (36.8–53.7) | 2.26 (2.02–2.61) | 11.5 (6.1–18.6) | |
| 3 | 1.93 (1.66–2.58) | 22.5 (12.7–34.1) | 1.18 (0.73–1.38) | 5.0 (1.0–14.3) | |
| 4 | 1.36 (0.34–NA) | 30.8 (9.5–55.4) | 0.79 (0.22–1.01) | 0 | |
| Total | 6.29 (5.60–7.20) | 58.0 (54.3–61.6) | 2.5 (2.32–2.67) | 22.5 (19.3–25.9) |
Abbreviations: CI, confidence interval; ISS, International Staging System; NA, not applicable; OS, overall survival; PFS, progression-free survival; RISS, Revised International Staging System.
Figure 2.
OS and PFS curves for different ISS and RISS stages among patients with RRMM (Cohort 2). (a) OS curves for all ISS stages in patients with RRMM. (b) OS curves for all RISS stages in patients with RRMM. (c) PFS curves for all ISS stages in patients with RRMM. (d) PFS curves for all RISS stages in patients with RRMM.
Table 3. OS and PFS analysis across three different diagnostic scores in relapsed/refractory patients (Cohort 2).
| Diagnostic Test | Cohort | Median OS years (95% CI) | 2-year OS % (95% CI) | Median PFS years (95% CI) | 2-year PFS % (95% CI) |
|---|---|---|---|---|---|
| ISS | ISS-I | 4.16 (3.40–4.92) | 74.6 (67.6–80.4) | 0.78 (0.62–0.92) | 25.6 (19.7–31.8) |
| ISS-II | 2.09 (1.83–2.69) | 50.8 (44.7–56.5) | 0.49 (0.40–0.60) | 15.9 (12.2–20.0) | |
| ISS-III | 0.93 (0.65–1.22) | 25.9 (18.7–33.6) | 0.28 (0.23–0.36) | 8.5 (4.7–13.7) | |
| Total | 2.35 (1.93–2.93) | 52.2 (48.1–56.2) | 0.47 (0.41–0.56) | 17.1 (14.3–20.0) | |
| RISS | RISS-I | 4.28 (3.32–NA) | 83.5 (72.9–90.2) | 1.06 (0.81–1.51) | 27.4 (18.1–37.5) |
| RISS-II | 1.98 (1.75–2.64) | 48.7 (41.4–55.5) | 0.55 (0.45–0.64) | 15.0 (10.8–19.9) | |
| RISS-III | 0.83 (0.40–1.23) | 18.5 (8.1–32.3) | 0.25 (0.16–0.38) | 9.6 (3.5–19.2) | |
| Total | 2.37 (1.96–3.15) | 52.2 (48.1–56.2) | 0.60 (0.49–0.70) | 17.0 (13.3–21.1) | |
| New Score | 0 | 4.01 (2.99–5.34) | 76.2 (66.5–83.4) | 0.89 (0.71–1.10) | 24.7 (17.3–32.8) |
| 1 | 2.27 (1.79–4.54) | 51.7 (41.3–61.2) | 0.62 (0.48–0.79) | 18.9 (12.4–26.5) |
Abbreviations: CI, confidence interval; ISS, International Staging System; OS, overall survival; PFS, progression-free survival; RISS, Revised International Staging System.
We also looked at the differences in outcomes in patients belonging to various RISS stages among patients diagnosed between 2005 and 2010 as compared with those diagnosed between 2011 and 2015. The median OS was 9.8 years (95% CI; 8.6 years, not reached) versus not reached for patients belonging to RISS stage I (P=0.45), 5.9 years (95% CI; 5.2, 6.7 years) versus 5.4 years (95% CI; 5.4 years, not reached) for patients belonging to RISS stage II (P=0.13), and 2.7 years (95% CI; 2.2, 3.6 years) versus 3.2 years (95% CI; 1.9 years, not reached) for patients belonging to RISS stage III (P=0.6). The median PFS among patients diagnosed from 2005 to 2010 versus those diagnosed from 2011 to 2015 was 2.8 years (95% CI; 2.5, 3.6 years) versus 3.2 years (95% CI; 2.3, 3.4 years) for patients belonging to RISS stage I (P=0.56), 2.3 years (95% CI; 2.1, 2.6 years) versus 2.7 years (95% CI; 2.5, 3 years) for patients belonging to RISS stage II (P=0.45), and 1.3 years (95% CI; 0.9, 1.6 years) versus 1.4 years (95% CI; 1.1, 2.1 years) for patients belonging to RISS stage III (P=0.55). Hence, although the median OS and PFS has improved in 2011–2015 as compared with 2005–2010 among all RISS stages; however, the difference is not statistically significant.
Prognostic risk score
The variables used to determine the RISS stage were used to calculate a prognostic risk score in both the cohorts as described in Methods. Among NDMM, there were 1067 assessable patients for whom the complete data for all the four variables were available. Among these patients, 31.7% (n=338), 38.4% (n=410), 21.6% (n=230), 7.0% (n=75) and 1.3% (n=14) had risk scores of 0, 1, 2, 3 and 4 respectively. The median and 5-year OS (Figure 3a) and PFS (Figure 3b) estimates for this cohort are shown in Table 2.
Figure 3.
OS and PFS curves for different prognostic risk scores among patients with NDMM (Cohort 1) and RRMM (Cohort2). (a) OS curves for all risk scores in patients with NDMM. (b) PFS curves for all risk scores in patients with NDMM. (c) OS curves for all risk scores in patients with RRMM. (d) PFS curves for all risk scores in patients with RRMM.
In the RRMM cohort, there were 456 assessable patients for whom the complete data for all the four variables were available. Among these patients, 33.6% (n=153) had a final risk score of 0, 31.6% (n=144) had a score of 1, 25.0% (n=114) had a score of 2, 8.6% (n=39) had a score of 3 and only 1.3% (n=6) had a final risk score of 4. The median and 2-year OS (Figure 3c) and PFS (Figure 3d) estimates for this cohort are listed in Table 3.
Discussion
In this study, we depicted that the RISS staging system is a simple and easily applicable prognostic model, which gives a better distinction of patients with NDMM as well as RRMM into three survival groups. Patients with NDMM (outside clinical trials) with RISS stage I, II and III had a 5-year OS rate of 76.3%, 55.7% and 29.5%, respectively, while patients with RRMM with RISS stage I, II and III had a 2-year OS rate of 83.5, 48.7 and 18.5. We also demonstrated that as compared with patients with NDMM diagnosed between 2005 and 2010, those diagnosed between 2011 and 2015, have better OS and PFS among all RISS groups, though the results are not statistically significant. In addition, we also showed that a simpler approach giving equal weight to each of the four prognostic variables can also be used to stratify patients with NDMM and RRMM.
Although ISS staging system is a powerful prognostic tool reflecting the patient's status and the tumor burden, it does not account for the biological factors which have a major role in disease evolution and resistance to treatment. The CA not only depict the prognosis of patients with myeloma, but also affect clinical presentation and management strategies.12 Also, high serum LDH has a major impact on the survival of myeloma patients even when they belong to a low or intermediate ISS subgroup.13 Hence, the incorporation of CA and LDH levels into ISS to formulate RISS staging system is highly relevant.
In addition, it should be noted that serum β2m levels may be elevated in several benign conditions such as chronic inflammation, liver disease, renal dysfunction and some acute viral infections, apart from being prognostic in lymphoproliferative malignancies, especially MM.14 Hence, although the criteria for ISS stage I is very specific, any patient with elevated serum β2m falls in ISS stage III. Based on the RISS staging system, 13.7% of NDMM patients belong to the poor prognostic RISS stage III. Interestingly, Shaughnessy et al. identified a 70-gene subset as a predictor of outcome after studying the gene expression profile of 532 patients with NDMM and found that the high-risk score was present in 13% of patients with shorter durations of complete remission, event-free survival (EFS), and OS.15
The International Myeloma Working Group (IMWG) report on RISS analyzed a large sample size of 4445 patients with NDMM enrolled onto 11 international clinical trials. The Surveillance, Epidemiology and End Results analysis from 2008 to 2012 states that MM is most frequently diagnosed among people aged 65–74 years with 61.8% of patients aged ⩾65 years.16 This elderly population is not well represented in the IMWG report with only 35% of patients were older than 65 years. In our study, 46.89% of patients with NDMM in Cohort 1 were aged more than 65 years. In addition, all patients analyzed in IMWG study received novel agents with or without autologous stem cell transplant as part of their upfront treatment. Our study, however, has included all patients seen at Mayo Clinic over a span of 10 years from 2005 onwards. Hence, 149 patients (7.84%) did not receive novel agents or autologous stem cell transplant for their initial treatment. As in the report by IMWG, the majority of patients (69.33% in our study versus 62% in IMWG study), belonged to intermediate risk category. This distribution aids in better survival differentiation among the 3 RISS stages.
Randomized clinical trials represent a final step in evaluating the efficacy of any new treatment. However, <3% of adult cancer patients participate in clinical trials in the United States.17 Trials may not be available for patients willing to participate, or when they are available, patients are not enrolled because they do not meet trial eligibility criteria.18, 19 Usually, patients are excluded on basis of criteria pertaining to age, comorbidities and performance status.20 Various studies have demonstrated positive evidence that participation in trials improves outcomes.20, 21, 22 Even after matching patients for age, stage, de novo presentation, and treatment, trial patients could still benefit from changes in behavior or outlook associated with being under observation (the ‘Hawthorne' effect) or from care that is administered according to strict protocol.23, 24, 25 Therefore, it was very important to assess the clinical utility of the RISS staging system in real-life patients outside clinical trials.
MM remains an incurable disease and nearly all patients with the disease relapse and eventually succumb to refractory disease. The extent of disease at relapse, the type and response to previous therapy as well as the time of relapse affect prognosis of such patients. Kumar et al. analyzed the outcomes of 286 patients with relapsed MM, who were refractory to bortezomib and were relapsed following, refractory to or ineligible to receive, an IMiD based on ISS stage at time of enrollment (T0) in the study. He showed that the ISS stage was prognostic for OS following T0 with median survivals of 12, 8 and 4 months for ISS stages 1, 2 and 3, respectively.26 However, the RISS staging system was yet to be validated in patients with RRMM.
In addition, we demonstrated that as compared with patients with NDMM diagnosed between 2005 and 2010, the median OS and PFS in those diagnosed between 2011 and 2015, have improved for RISS stage I, II as well as III, although the results are not statistically significant. Previously, a retrospective analysis of 1038 patients also showed that the median OS increased from 4.6 years to 6.1 years for 2001–2005 cohort versus 2006–2010 cohort (P=0.02). The improvement in outcomes was linked to use of one or more novel agents like bortezomib, lenalidomide and thalidomide.1 In the United States, four drugs (panobinostat, ixazomib, aratumumab and elotuzumab) were approved for the treatment of MM in 2015.27 Hence, we expect that the impact of incorporation of these agents will be seen over time.
The retrospective nature and single institutional experience of this study does limit the scope of its conclusions. However, after reviewing the literature, we concluded that this is one of the largest known series reported on these patient populations. Our study not only validated the RISS in patients with NDMM as well as RRMM but also explored a new prognostic risk score using all the four prognostic variables.
Conclusion
In conclusion, MM is a heterogeneous disease and requires a simple, reliable and easily applicable staging system that combines clinical and biological disease related prognostic factors and can be applied for universal patient classification. The RISS combines the prognostic power of high-risk CA and LDH with ISS score and gives a better differentiation of MM patients into three survival subgroups. In this study, we showed that the difference in survival outcomes retain statistical significance in an unselected cohort of patients with NDMM as well as in a group of patients with RRMM enrolled in various clinical trials. Hence, it should be used to stratify patients in future clinical trials to guide towards more effective personalized treatment.
We also found that though the median OS and PFS has improved in 2011–2015 as compared with 2005–2010 among all RISS stages; however, the difference is not statistically significant. In addition, we analyzed a simpler approach giving equal weight to each of the four prognostic variables, which can also be used to stratify patients with NDMM and RRMM. Gene expression profiling and assessment of minimal residual disease by multiparametric flow cytometry or molecular methods are currently being studied for detailed analysis and prognostication of MM and may be incorporated in future staging algorithms.
Acknowledgments
This publication (or project) was made possible by the CTSA Grant UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely our responsibility and do not necessarily represent the official views of the National Institutes of Health.
Author contributions
NT and SKK designed the study, collected the data, analyzed the data, wrote the first draft and approved the final version of the manuscript. SVR, MQL, AD, FKB, MAG, SRH, NL, RSG, DD, PK, YL, YLH, ALF, MAH, SRZ, JAL, WIG and SJR provided patient management, critically reviewed the first draft and approved the final version of the manuscript. BL and AP provided help with statistical analysis, critically reviewed the first draft and approved the final version of the manuscript.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
The authors declare no conflict of interest.
Supplementary Material
References
- Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 2014; 28: 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV. Myeloma today: disease definitions and treatment advances. Am J Hematol 2016; 91: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 1975; 36: 842–854. [DOI] [PubMed] [Google Scholar]
- Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23: 3412–3420. [DOI] [PubMed] [Google Scholar]
- Avet-Loiseau H, Li C, Magrangeas F, Gouraud W, Charbonnel C, Harousseau JL et al. Prognostic significance of copy-number alterations in multiple myeloma. J Clin Oncol 2009; 27: 4585–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia 2009; 23: 2210–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos MA, Barlogie B, Smith TL, Alexanian R. High serum lactate dehydrogenase level as a marker for drug resistance and short survival in multiple myeloma. Ann Intern Med 1991; 115: 931–935. [DOI] [PubMed] [Google Scholar]
- Moreau P, Cavo M, Sonneveld P, Rosinol L, Attal M, Pezzi A et al. Combination of international scoring system 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem-cell transplantation at high risk of early MM progression-related death. J Clin Oncol 2014; 32: 2173–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L et al. Revised International Staging System for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol 2015; 33: 2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastaniah W, Elimam N, Abdalla K, Felimban S, Abrar MB. Comparison of clinical trial versus non-clinical trial treatment outcomes of childhood acute lymphoblastic leukemia using comparable regimens. Hematology 2016; 21: 175–181. [DOI] [PubMed] [Google Scholar]
- Unger JM, Barlow WE, Martin DP, Ramsey SD, LeBlanc M, Etzioni R et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst 2014; 106: dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan AM, Rajkumar SV. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J 2015; 5: e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E, Katodritou E, Roussou M, Pouli A, Michalis E, Delimpasi S et al. High serum lactate dehydrogenase adds prognostic value to the international myeloma staging system even in the era of novel agents. Eur J Hematol 2010; 85: 114–119. [DOI] [PubMed] [Google Scholar]
- Bethea M, Forman DT. Beta 2-microglobulin: its significance and clinical usefulness. Ann Clin Lab Sci 1990; 20: 163–168. [PubMed] [Google Scholar]
- Shaughnessy Jr JD, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood 2007; 109: 2276–2284. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. SEER stat fact sheets: myeloma. Available at: http://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 15 March2016.
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004; 291: 2720–2726. [DOI] [PubMed] [Google Scholar]
- Javid SH, Unger JM, Gralow JR, Moinpour CM, Wozniak AJ, Goodwin JW et al. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316). Oncologist 2012; 17: 1180–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde CN, Springer BC, Butler B, White MS, Atkins J. Factors influencing enrollment in clinical trials for cancer treatment. South Med J 1999; 92: 1189–1193. [DOI] [PubMed] [Google Scholar]
- Elting LS, Cooksley C, Bekele BN, Frumovitz M, Avritscher EB, Sun C et al. Generalizability of cancer clinical trial results: prognostic differences between participants and nonparticipants. Cancer 2006; 106: 2452–2458. [DOI] [PubMed] [Google Scholar]
- Edwards SJ, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J. Ethical issues in the design and conduct of randomised controlled trials. Health Technol Assess 1998; 2: i-vi 1–132. [PubMed] [Google Scholar]
- Vist GE, Hagen KB, Devereaux PJ, Bryant D, Kristoffersen DT, Oxman AD. Systematic review to determine whether participation in a trial influences outcome. BMJ 2005; 330: 1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger JM, Barlow WE, Martin DP, Ramsey SD, Leblanc M, Etzioni R et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst 2014; 106: dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunholtz DA, Edwards SJL, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a ‘trial effect'. J Clin Epidemiol 2001; 54: 217–224. [DOI] [PubMed] [Google Scholar]
- Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet 1993; 342: 1317–1322. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia 2012; 26: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan AM, Kumar SK. New investigational drugs with single-agent activity in multiple myeloma. Blood Cancer J 2016; 6: e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.