Dear Sir,
We previously reported the therapeutic potential of pevonedistat (TAK-924/MLN4924), a novel inhibitor of protein neddylation, in patients with acute myeloid leukemia (AML).1 Pevonedistat is a small-molecule inhibitor of the neural cell developmentally downregulated 8 (NEDD8)-activating enzyme, which processes NEDD8 for binding to target substrates.2, 3, 4, 5 The most characterized NEDD8 target in cells are the Cullin-RING E3 ubiquitin ligases (CRLs), which direct the degradation of specific substrates through the proteasome (for example, p27, CDT1, Nrf-2).6 In the absence of NEDD8, CRL substrates accumulate causing antiproliferative effects in AML.1 In a phase I trial (C15003 study), patients with relapsed/refractory myelodysplastic syndrome or AML were treated with 1-h infusions of pevonedistat every 21 or 28 days across five schedules. On Schedule A, dose escalation commenced at 25 mg/m2 on Days 1, 3 and 5 in a standard ‘3+3' manner to determine the maimum tolerated dose (MTD). Following this, four additional schedules were evaluated with pevonedistat: Schedule B (Days 1, 4, 8 and 11 starting at 147 mg/m2); Schedule C (Days 1, 8 and 15 starting one dose level lower than the MTD determined for Schedule A (44 mg/m2)); Schedule D (Days 1, 3 and 5 stating at 45 mg/m2 to be combined with azacitidine 75 mg/m2 on Days 1–5 and 8–9); and Schedule E (Days 1, 3 and 5 at fixed dose of 50 mg/m2). Schedules A–C and E were administered every 21 days, whereas schedule D was administered every 28 days. Adverse events (AEs) on Schedules A and B were taken into account when designing Schedules C–E; based on Schedule A MTD, an expansion dose of 50 mg/m2 was selected for Schedule E. Results for Schedules A and B were published by Swords et al.,7 and the reported MTDs were 59 and 83 mg/m2, respectively. Hepatotoxicity was dose limiting for Schedule A and multiorgan failure (MOF) for Schedule B. The objective response rate in patients treated at or below the MTD was 17% for Schedule A and 10% for Schedule B, confirming single agent activity in refractory AML patients.8 Considering the promising safety and activity of pevonedistat reported thus far,8, 9, 10 we carried out a comprehensive safety analysis of all 72 patients treated across five schedules (A–E) in the C15003 study.
Seventy-two patients were evaluable for toxicity: Schedule A (n=27), Schedule B (n=26), Schedule C (n=2), Schedule D (n=1), and Schedule E (n=16). Most patients had AML (N=66, 92%), 5 had myelodysplastic syndrome (7%) and 1 patient had ALL (1%). Median age was 65.5 years (range: 19–84). The most frequently reported all-grade AEs were: pyrexia (49%), diarrhea (43%), decreased appetite (35%), peripheral edema (33%), dyspnea and fatigue (each 32%), febrile neutropenia (31%) and nausea (31%). These were generally mild and transient. The most common reported laboratory toxicities were elevations in aspartate aminotransferase (25%) and alanine transaminase (24%). Treatment-related myelosuppression was infrequent with thrombocytopenia reported in 18% of patients. Fifteen (21%) patients discontinued the study owing to AEs (Table 1). Overall, 56 (78%) patients experienced at least 1 serious adverse event (SAE): Schedule A=20 (74%), Schedule B=20 (77%), Schedule C=2 (100%), Schedule D=1 (100%), and Schedule E=13 (81%). The most common SAE overall was febrile neutropenia reported in 17 (24%) patients (A=8, B=6 and E=3). Other SAEs occurring in >2 patients were pneumonia (n=12, 17%), disease progression (n=9, 13%), fever (n=6, 8%), sepsis (n=6, 8%), intracranial hemorrhage (n=5, 7%), hypotension (n=4, 6%), gastrointestinal hemorrhage (n=3, 4%) and mental status changes (n=3, 4%). There were 28 (39%) on-study deaths and 4 of these deaths were due to sepsis (1 patient on Schedule A at 78 mg/m2), MOF (2 patients on Schedule B at 110 mg/m2 and 147 mg/m2) and cardiopulmonary failure related to leukastasis (1 patient on Schedule D at 45 mg/m2). Eighteen deaths, including three of the four deaths due to MOF/cardiopulmonary failure occurred during Cycle 1. The on-study deaths unrelated to pevonedistat (n=24) occurred owing to underlying disease (n=11), sepsis (n=3), intracranial hemorrhage (n=5), pneumonia (n=3), and 1 case each of pulmonary hemorrhage and pancytopenia.
Table 1. Treatment-emergent adverse events (AE).
| AE, n (%) | Schedule Aa (n=27) | Schedule B (n=26) | Schedule C (n=2) | Schedule D (n=1) | Schedule E (n=16) | Total (n=72) |
|---|---|---|---|---|---|---|
| Any AEb | 27 (100) | 26 (100) | 2 (100) | 1 (100) | 16 (100) | 72 (100) |
| Pyrexia | 12 (44) | 16 (62) | 0 | 0 | 7 (44) | 35 (49) |
| Diarrhea | 14 (52) | 9 (35) | 2 (100) | 0 | 6 (38) | 31 (43) |
| Anorexia | 11 (41) | 7 (27) | 1 (50) | 0 | 6 (38) | 25 (35) |
| Peripheral edema | 11 (41) | 6 (23) | 1 (50) | 0 | 6 (38) | 24 (33) |
| Fatigue | 9 (33) | 9 (35) | 0 | 0 | 5 (31) | 23 (32) |
| Dyspnea | 6 (22) | 10 (38) | 0 | 1 (100) | 6 (38) | 23 (32) |
| FN | 11 (41) | 8 (31) | 0 | 0 | 3 (19) | 22 (31) |
| Nausea | 10 (37) | 7 (27) | 0 | 0 | 5 (31) | 22 (31) |
| Chills | 8 (30) | 11 (42) | 0 | 0 | 1 (6) | 20 (28) |
| Dizziness | 11 (41) | 4 (15) | 1 (50) | 0 | 4 (25) | 20 (28) |
| Myalgia | 8 (30) | 7 (27) | 1 (50) | 0 | 4 (25) | 20 (28) |
| Increased AST | 9 (33) | 3 (12) | 1 (50) | 0 | 5 (31) | 18 (25) |
| Increased ALT | 8 (30) | 4 (15) | 1 (50) | 0 | 4 (25) | 17 (24) |
| Headache | 5 (19) | 7 (27) | 1 (50) | 0 | 4 (25) | 17 (24) |
| Epistaxis | 6 (22) | 6 (23) | 0 | 0 | 5 (31) | 17 (24) |
| Vomiting | 8 (30) | 5 (19) | 1 (50) | 0 | 2 (13) | 16 (22) |
| Cough | 7 (26) | 6 (23) | 0 | 0 | 2 (13) | 15 (21) |
| Pneumonia | 5 (19) | 4 (15) | 2 (100) | 0 | 4 (25) | 15 (21) |
| Any treatment-related grade ⩾3 AEc | 16 (59) | 20 (77) | 2 (100) | 1 (100) | 12 (75) | 51 (71) |
| Low platelets | 2 (7) | 2 (8) | 0 | 0 | 0 | 4 (6) |
| MI | 0 | 1 (4) | 0 | 1 (100) | 0 | 2 (3) |
| FNd | 0 | 2 (8) | 0 | 0 | 0 | 2 (3) |
| Increased AST | 2 (7) | 0 | 0 | 0 | 0 | 2 (3) |
| Increased ALT | 1 (4) | 0 | 0 | 0 | 1 (6) | 2 (3) |
| Increased bilirubin | 0 | 1 (4) | 0 | 0 | 1 (6) | 2 (3) |
| Hypoxia | 1 (4) | 1 (4) | 0 | 0 | 0 | 2 (3) |
| Hypotension | 1 (4) | 1 (4) | 0 | 0 | 0 | 2 (3) |
| MOF | 0 | 2 (8) | 0 | 0 | 0 | 2 (3) |
| Fatigue | 2 (7) | 0 | 0 | 0 | 0 | 2 (3) |
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; FN, febrile neutropenial; MI, myocardial infarction; MOF, multiorgan failure.
Schdeule A (dose escalation cohort, Days 1, 3, 5); Schedule B (dose escalation cohort, Days 1, 4, 8, 11); Schedule C (weekly dosing schedule); Schedule D (combination with azacitidine); Schedule E (dose expansion cohort, Days 1, 3, 5).
Any AE occurring in >20% of patients overall.
Any treatment-related grade ⩾3 AE occurring in >1 patient overall.
Bacteremia occurred in a total of 3 patients on study (1 on Schedule A and 2 on Schedule E) (4%) and septic shock in 1 patient on Schedule C (1%); these events were reported as not related to pevonedistat.
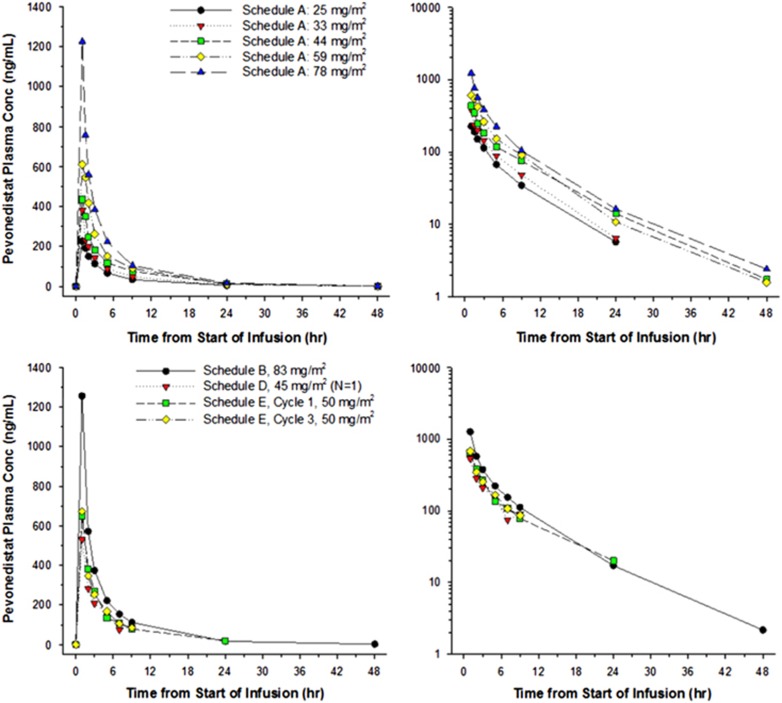
Overall, there were 10 dose-limiting toxicities (DLTs) in 6 patients: transaminase elevation (Schedule A, 78 mg/m2); orthostatic hypotension (Schedule B, 110 mg/m2); cardiac failure (Schedule B, 147 mg/m2); MOF (Schedule B, 147 mg/m2); rash (Schedule E, 50 mg/m2); and elevated alanine transaminase (Schedule E, 50 mg/m2). With the exception of 1 DLT, all events occurred within 2 days of first exposure to pevonedistat. There were no DLTs at doses <50 mg/m2, and all Grade 4 DLTs occurred at the highest dose of pevonedistat administered (147 mg/m2). Mean pevonedistat plasma concentration–time profiles obtained on Day 1 of Cycle 1 (and Cycle 3 on Schedule E) are shown in Figure 1. No obvious differences in exposures were observed between Cycles 1 and 3, suggesting that pevonedistat PK remains stationary over time. Of the 55 response evaluable patients, there were 2 complete remissions (4%) on Schedule A, and 5 partial remissions (9%) across Schedule A (n=2), Schedule B (n=2) and Schedule E (n=1). The overall complete remission/partial remission rate was 13%. Thirty-four patients (62%) had stable disease after treatment with ⩾4 courses of pevonedistat (Schedule A=14, Schedule B=13, Schedule E=7). All responses were in patients with AML.
Figure 1.
Mean pevonedistat plasma concentration–time profiles. Serial samples were obtained on Day 1 of Cycle 1 (and Day 1 of Cycle 3 on Schedule E) following a 1-h intravenous infusion and presented graphically (linear and semi-log plots, left and right panels, respectively).
The principal serious and potentially drug-related toxicities noted in this trial were hepatotoxicity and sepsis syndromes with MOF. The exact mechanisms accounting for the hepatotoxicity observed in some patients treated with pevonedistat is unclear. In vitro RNA interference to disrupt NEDD8 conjugation induces shape changes in HEK293 and HeLa cells (edema and swelling). This effect may relate to the accumulation of the cullin-dependent GTPase RhoA, which regulates cytoskeleton proteins.11 This effect is reproducible with pevonedistat treatment in vitro and is reversible by RhoA knockdown. It is interesting to speculate that transient leakage of transaminases from edematous hepatocytes in treated patients may have accounted for some of the laboratory toxicities observed in this study. A more compelling perspective on pevonedistat-induced liver toxicity was presented by Wolenski et al.,12 who demonstrated that treatment with pevonedistat lowered the activation threshold for tumor necrosis factor (TNF)-mediated cell death, by conferring cellular sensitivity to low concentrations of TNF-α. Patients with higher circulating levels of TNF-α (typically the case during sepsis) treated with pevonedistat in our study may have been more prone to the tissue-damaging effects of this cytokine. This mechanism may have contributed to the MOF events observed. It should be noted that MOF was rare in this trial and commonly occurred in heavily pretreated AML patients treated at higher doses of pevonedistat (other than the 1 patient on Schedule D (45 mg/m2) who died of MOF from leukostasis, there were no MOF events at doses of pevonedistat ⩽83 mg/m2). In order to study potential links with cytokine signaling and systemic inflammatory syndromes, we measured plasma cytokines (interleukin-1β and TNF-α) in treated patients. All the samples from patients treated on Schedule E were below the detectable limits of the assay. None of these patients had MOF events. Cytokine data were not available for patients who had developed MOF (Schedules A and B), which was a limitation of our study.
The most common AE reported was pyrexia (n=35, 49%) and pneumonia was the most common infection (n=15, 21%). Establishing the contribution of pevonedistat to these toxicities is challenging. However, there have been a number of reports describing the effects of impaired protein neddylation on innate immunity. For example, the perforin family of pore-forming proteins (the perforins), key bactericidal effector molecules of the innate immune system, require ubiquitin side chains for function. McCormack et al.13 demonstrated that direct small interfering RNA knockdown of CRLs reduced bactericidal activity of perforin proteins by blocking their ubiquitination. The same phenotype was achieved with pharmacological inhibition of CRLs using pevonedistat. Mice treated with pevonedistat and subsequently inoculated with salmonella died faster from sepsis and organ failure, compared with untreated controls. Despite these interesting observations, none of the patients with MOF on this study had documented bacterial infections. Others have shown that the antigen-presenting ability of dendritic cells and macrophages is markedly impaired by disrupting the neddylation of target substrates either genetically or chemically.14 This effect suggests a potential use for pevonedistat in the management of autoimmune disorders and graft versus host disease. Elucidation of the immunomodulatory effects of pevonedistat will help to further define both the promise and limitation of his new agent. In summary, the most serious toxicity was observed at higher doses of pevonedistat. For this reason, doses beyond 100 mg/m2 are not being considered for further investigation and no cases of drug-related MOF have been noted at doses currently in development. Pevonedistat combination studies for patients with myeloid malignancies are ongoing.9, 15
Acknowledgments
This research was supported by Takeda Pharmaceuticals, Inc. This study is registered with ClinicalTrials.gov, number NCT00677170. RTS received support from grant NIH 1KL2TR000461.
Author contributions
RTS, HPE, DJD, JA, MM, HF, BJD, FA, HS, FJG and BCM were involved in the conception and design of the study. RTS, JW, HPE, DJD, JA, MM, ZH, HS, HF, FS, BJD, FJG and BCM were responsible for the collection and assembly of data. RTS, HPE, DJD, JA, MM, ZH, FA, HS, HF, FS, BJD, FJG and BCM were involved in data analysis and interpretation. All authors were responsible for writing/reviewing the draft manuscript, and all authors approved the final draft manuscript.
Footnotes
ZH, HS, HF, FS and BJD are employees of Takeda. The other authors declare no conflict of interest.
References
- Swords RT, Kelly KR, Smith PG, Garnsey JJ, Mahalingam D, Medina E et al. Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood 2010; 115: 3796–3800. [DOI] [PubMed] [Google Scholar]
- Milhollen MA, Narayanan U, Soucy TA, Veiby PO, Smith PG, Amidon B. Inhibition of NEDD8-activating enzyme induces rereplication and apoptosis in human tumor cells consistent with deregulating CDT1 turnover. Cancer Res 2011; 71: 3042–3051. [DOI] [PubMed] [Google Scholar]
- Milhollen MA, Thomas MP, Narayanan U, Traore T, Riceberg J, Amidon BS et al. Treatment-emergent mutations in NAEbeta confer resistance to the NEDD8-activating enzyme inhibitor MLN4924. Cancer Cell 2012; 21: 388–401. [DOI] [PubMed] [Google Scholar]
- Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 2009; 458: 732–736. [DOI] [PubMed] [Google Scholar]
- Soucy TA, Smith PG, Rolfe M. Targeting NEDD8-activated cullin-RING ligases for the treatment of cancer. Clin Cancer Res 2009; 15: 3912–3916. [DOI] [PubMed] [Google Scholar]
- Chiba T, Tanaka K. Cullin-based ubiquitin ligase and its control by NEDD8-conjugating system. Curr Protein Pept Sci 2004; 5: 177–184. [DOI] [PubMed] [Google Scholar]
- Swords RT, Erba HP, DeAngelo DJ, Bixby DL, Altman JK, Maris M et al. Pevonedistat (MLN4924), a first-in-class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukaemia and myelodysplastic syndromes: a phase 1 study. Br J Haematol 2015; 169: 534–543. [DOI] [PubMed] [Google Scholar]
- Shah JJ, Jakubowiak AJ, O'Connor OA, Orlowski RZ, Harvey RD, Smith MR et al. Phase I study of the novel investigational NEDD8-activating enzyme inhibitor pevonedistat (MLN4924) in patients with relapsed/refractory multiple myeloma or lymphoma. Clin Cancer Res 2016; 22: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swords R, Savona M, Maris M, Erba H, Hua Z, Faessel H et al First-in-class NAE inhibitor pevonedistat (MLN4924), in combination with azacitadine for acute myeloid leukemia (AML) patients considered unfit for conventional chemotherapy: results from the C15009 trial. European Hematology Association, abstract 4024 2014.
- Sarantopoulos J, Shapiro G, Roger B, Cohen R, Clark J, Kauh J et al. Phase I study of the investigational NEDD8-activating enzyme inhibitor pevonedistat (TAK-924/MLN4924) in patients with advanced solid tumors. Clin Cancer Res 2016; 22: 847–857. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang Z, Meng M, Zhao Y, Dong N, Yan H et al. Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Mol Cell 2009; 35: 841–855. [DOI] [PubMed] [Google Scholar]
- Wolenski F, Fisher C, Sano T, Wyllie S, Cicia L, Gallacher M et al. The NAE inhibitor MLN4924 synergizes with TNF-a to induce death through apoptosis and necroptosis in a rat hepatoma cell line. Cell Death Discov 2015; 1: 15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack RM, Lyapichev K, Olsson ML, Podack ER, Munson GP. Entericpathogens deploy cell cycle inhibiting factors to block the bactericidal activity of Perforin-2. Elife 2015; 4: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson N, Toubai T, Kapeles S, Sun Y, Oravecz-Wilson K, Tamaki H et al. Neddylation plays an important role in the regulation of murine and human dendritic cell function. Blood 2013; 122: 2062–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chen S, Zhang Y, Kmieciak M, Leng Y, Li L et al. The NAE inhibitor pevonedistat interacts with the HDAC inhibitor belinostat to target AML cells by disrupting the DDR. Blood 2016; 127: 2219–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]