Abstract
Introduction
Maternal obesity creates an adverse intrauterine environment, negatively impacts placental respiration, is associated with a higher incidence of pregnancy complications and programs the offspring for disease in adult life in a sexually dimorphic manner. We defined the effect of maternal obesity and fetal sex on pro- and anti-oxidant status in placenta and placental mitochondria.
Methods
Placental villous tissue was collected at term via c-section prior to labor from four groups of patients based on fetal sex and prepregnancy/1st trimester body mass index: lean - BMI 22.1±0.3 (6 male, 6 female) and obese - BMI 36.3±0.4 (6 male, 6 female). Antioxidant enzyme activity, mitochondrial protein carbonyls, nitrotyrosine residues, total and nitrated superoxide dismutase (SOD) and nitric oxide synthesis were measured.
Results
Maternal obesity was associated with decreased SOD and catalase activity, and total antioxidant capacity (TAC), but increased oxidative (protein carbonyls) and nitrative (nitrotyrosine) stress in a sexually dimorphic manner. Placentas of lean women with a male fetus had higher SOD activity and TAC (p<0.05) than other groups whereas obese women with a male fetus had highest carbonyls and nitrotyrosine (p<0.05). Glutathione peroxidase and thioredoxin reductase activity increased with obesity, significantly with a male fetus, perhaps as a compensatory response.
Conclusion
Maternal obesity affects oxidative stress and antioxidant activity in the placenta in a sexually dimorphic manner. The male fetus of a lean women has the highest antioxidant activity, a protection which is lost with obesity perhaps contributing to the increased incidence of adverse outcomes with a male fetus.
Keywords: Oxidative stress, antioxidants, obesity, mitochondria, placenta
Introduction
The prevalence of maternal obesity (body mass index (BMI) > 30) has been steadily increasing both in the US [1] and worldwide [2]. Maternal obesity deleteriously affects maternal health and increases delivery complications, as well as negatively impacting short term and long term health outcomes in the offspring [3]. As BMI increases, pregnant women have an increased incidence of complications such as preeclampsia, gestational diabetes, cesarean sections, stillbirths, and preterm deliveries [4], as well as increased healthcare costs due to increased admission of newborns to the neonatal intensive care unit [5–7] and longer hospital stays [8]. Newborns born to obese mothers have an increased prevalence of cleft palates, spina bifida, and congenital heart defects [4, 9, 10]. The challenge of intrauterine stress associated with maternal obesity can disrupt normal placental function thus impacting fetal growth and development by mechanisms that remain largely unknown [3].
The physiologic balance between production of ROS and scavenging by intracellular antioxidants is critical to cell survival. In mammalian cells, including trophoblast, a variety of antioxidant mechanisms operate to control ROS production and action [11]. The primary antioxidant is superoxide dismutase (SOD), which dismutates superoxide to hydrogen peroxide, and is found in both cytosol (SOD1) and mitochondria (SOD2). Glutathione peroxidases (GPx’s) found in both the mitochondrial matrix and cytoplasm, and catalase (CAT) found in peroxisomes are part of a secondary defense mechanism catalyzing the conversion of hydrogen peroxide to water. These antioxidant enzymes operate in a coordinated manner to defend against ROS propagation and action. Pregnancy has been characterized as a state of oxidative stress, an imbalance of ROS molecules and antioxidant scavenging capabilities, due to increased placental mitochondrial activity and subsequent generation of reactive oxygen species (ROS) [12]. Oxidative stress was further increased in pregnancies complicated by preeclampsia and gestational diabetes, where obesity is a predisposing factor [12]. Placentas from preeclamptic women showed reduced enzymatic activity of SOD, GPx, and thioredoxin reductase (TrxR), as well as increased lipid peroxidation, an oxidative stress marker [13, 14]. However there were no differences in another marker of oxidative stress, protein carbonyls [14]. Whereas one study of placentas from pregnancies complicated by gestational diabetes showed increased SOD activity and protein carbonyls but no differences in GPx activity [15] another study of gestational diabetes reported no differences in placental SOD and GPx activities but decreased CAT and antioxidant potential together with increased lipid peroxidation and xanthine oxidase activity [16]. Since it has been well established that obesity alone increases oxidative stress markers in the non-pregnant state [17], the aim of the present study was to examine the effect of maternal obesity on oxidative stress in the placenta in uncomplicated pregnancies.
In our previous study of the effect of maternal obesity on placental miR-210 expression we observed evidence of sexual dimorphism as miR-210 expression was increased in placentas of obese women with female but not male fetuses compared with placentas from lean women with a female fetus [18]. Other studies have demonstrated that male animals have higher oxidant levels under hypertensive [19] and obese [20] conditions. Therefore in the present study we investigated the role of sex as a biological variable on oxidative stress and antioxidant status in placenta and predicted sex-dependent outcomes with maternal obesity.
We have previously observed that as maternal adiposity increases there was a parallel increase in placental ROS generation [21] and nitrotyrosine residues [22], thus indicating both oxidative and nitrative stress occur in the placenta in obese women. Despite mitochondria being a major source of ROS and shown to cause oxidative stress during normal [12] and complicated pregnancies [23], little is known about the effect of maternal obesity on mitochondria in the placenta. Based on previous evidence, we also hypothesized that with maternal obesity superoxide and nitric oxide would be increased in placental mitochondria resulting in peroxynitrite generation leading to nitration of mitochondrial proteins thus causing further decreased antioxidant defense mechanisms.
Methods
Ethical Approval and Study Participants
Placentas were collected from the Labor and Delivery Unit at University Hospital San Antonio under a protocol approved by the Institutional Review Board of the University of Texas Health Science Center San Antonio, with informed consent from the patients. Placentas were collected from patients with no history of smoking or drug use at c-section at term from uncomplicated pregnancies in the absence of labor. Prepregnancy/1st trimester body mass index (BMI) was used to determine adiposity, defined as lean (BMI 18.5–24.9) or obese (BMI 30–40). Following delivery, villous tissue was randomly sampled, flash frozen, and stored at −80°C.
Assays
Tissue was homogenized in isolation buffer (0.25Msucrose, 10mMTris-HCl, 1mMEDTA, pH7.4) with protease inhibitors (Roche, Indianapolis, IN). Placental homogenates were assayed according to the manufacturer’s recommendation for SOD, GPx, TrxR and CAT activity, total antioxidant capacity (TAC), and nitric oxide (NO) (Cayman Chemical, Ann Arbor, MI).
Mitochondrial preparation
Placental mitochondria were extracted using differential centrifugation. Homogenates were centrifuged at 1,500xg for 15min at 4°C, the supernatant was removed and transferred into a new tube then centrifuged at 16,000xg for 10min at 4°C to pellet the mitochondria. The pellet was resuspended in resolving buffer (0.25Msucrose, 1mMTris-HCl, 1mMEDTA, pH 7.8) and centrifuged at 12,000xg for 10min at 4°C. The pellet was washed with isolation buffer by centrifuging at 12,000xg for 10min at 4°C. The pellet was then suspended in 2% CHAPS in TBS and agitated for 2hr at 4°C to lyse mitochondria. Mitochondrial extracts were stored at −80°C.
Protein Carbonyl Assay
Centrifugation steps were the same as described above. However, mitochondria were extracted in the buffer recommended by the manufacturer for protein carbonyl (Cayman Chemical, Ann Arbor, MI). Resuspended mitochondria pellets were sonicated to lyse the mitochondria and stored at −80°C.
Protein Assays
Protein was quantified from placental and mitochondrial extracts using Bradford protein analysis (Bio-Rad, Hercules, CA).
Western Blotting
Placental mitochondria extracts (15μg) were loaded onto 4–20%Tris-HCl gel, electrophoresed, and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were blocked with 5%BSA in PBS for 1hr before being probed with nitrotyrosine (1:1000) or MnSOD (1:500) 1° antibody (Millipore, Temecula, CA), and normalized to voltage-dependent anion channel protein (VDAC, 1:1000) (Abcam, Cambridge, MA) overnight at 4°C. Membranes were washed with 10%Tris-buffered saline with Tween20 (TBST), incubated with appropriate horseradish peroxidase 2° antibody (Cell Signaling, Danvers, MA) in 1%milk+TBST for 2hr at RT, then visualized using enhanced chemiluminescence (Roche, Indianapolis, IN).
Immunoprecipitation of nitrated proteins
Placental mitochondria extracts (200μg) were incubated with anti-nitrotyrosine antibody(1:50) and EZview Red Protein A Affinity Gel (Sigma, St. Louis,MI). The immunoprecipitated nitrated proteins were then electrophoresed as previously described prior to western blotting with antibody to MnSOD and band density normalized to IgG used as the loading control..
Statistics
Results are expressed as mean ± SEM where n is the number of individual placental samples studied. Student’s t-test was used to compare differences between lean and obese groups. Comparisons between groups were made using a two-way ANOVA with maternal BMI and fetal sex as factors. If mean values were found to be significantly different (p<0.05), a Student-Newman-Keuls post hoc test was applied to analyze differences between treatments. p<0.05 indicates statistical significance between groups (n values = number of placentas). All statistical analysis was performed using Sigma Stat Version 2.03 (Systat Software, San Jose, CA).
Results
By design pre-pregnancy or first trimester BMI was significantly different between the groups. The subjects were predominantly Hispanic. There were no differences in gestational age or birth weight between groups (Table 1).
Table 1.
Clinical Characteristics of Subjects.
| LEAN | OBESE | |
|---|---|---|
| Pre-pregnancy/1st trimester BMI (kg/m2) | 22.1 ± 0.3 | 36.3 ± 0.4 * |
| Gestational Age at delivery (weeks) | 39.0 ± 0.2 | 40.0 ± 0.2 |
| Birthweight (gms) | 3451 ± 113 | 3731 ± 92 |
| Fetal Sex (F/M) | 6/6 | 6/6 |
Values are mean ± SEM. (* = p<0.05 vs lean)
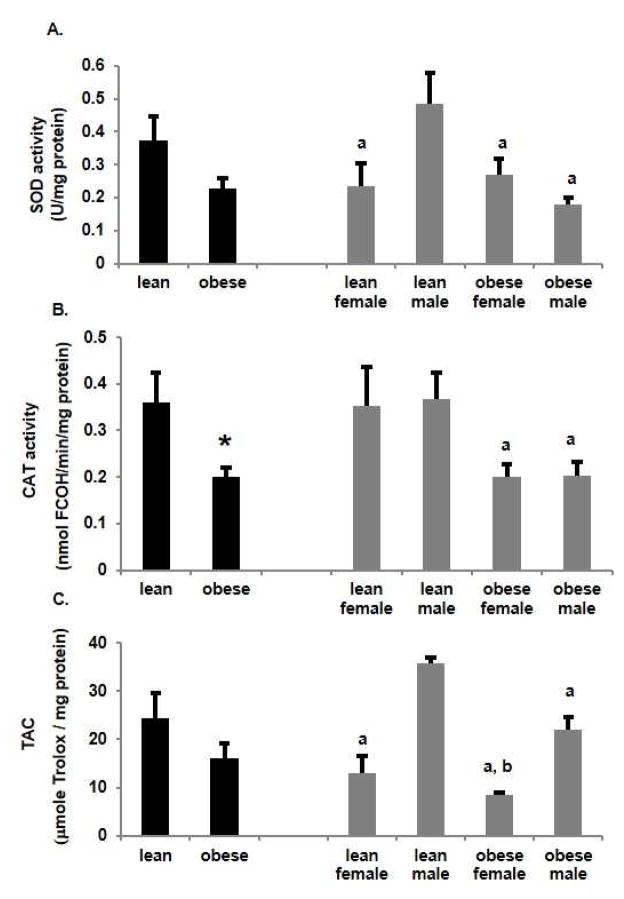
To assess the antioxidant status of placental homogenates between lean and obese groups, SOD and CAT activity and TAC were measured. Interestingly SOD, CAT (p<0.05) and TAC (Figure 1A, 1B, 1C respectively) were lower in placentas from obese women. Of note, placentas from lean women with a male fetus had significantly (p<0.05) higher SOD activity than placentas from lean women with a female fetus. Whereas SOD activity was significantly (p<0.05) reduced in placentas from obese women with a male fetus compared to lean women with a male fetus, there was no effect of obesity on placental SOD activity with a female fetus. CAT activity was not different between male and female placentas of lean women however it was significantly reduced with obesity in placentas of either male or female fetuses (Figure 1B). Differences in TAC between groups (Figure 1C) paralleled the observations seen with SOD activity. Thus, TAC was significantly (p<0.05) higher in placentas from lean women with a male fetus compared to a female fetus. In addition, TAC was significantly (p<0.05) decreased with obesity in women with a male fetus compared to lean women with a male fetus. There was no effect of obesity on placental TAC with female fetuses
Figure 1.
Effect of obesity on antioxidant activity in the placenta of lean and obese women. A. Superoxide dismutase (SOD) activity was expressed as SOD U/mg protein. Values are mean ± SEM. (a = p<0.05 vs lean male, n=5). B. Catalase (CAT) activity was measured as nmol formaldehyde (FCOH)/min/mg protein. Values are mean ± SEM. (* = p<0.05 vs lean, a = p<0.05 vs lean male, n=5). C. Total antioxidant capacity (TAC) was measured as μmole Trolox/mg protein in lean and obese women. Values are mean ± SEM. (a = p<0.05 vs lean male, b = p<0.05 vs obese male, n=5).
In addition to CAT, GPx and TrxR scavenge hydrogen peroxide [24]. In placental homogenates, both GPx and TrxR activity (Figure 2A, B) increased with obesity, although the effect was only significant for GPx. Surprisingly, the increase in GPx activity with obesity was totally accounted for by a significant (p<0.05) increase in placentas from a male fetus with no change from the lean state with a female fetus. TrxR activity also significantly (p<0.05) increased with obesity in placentas with a male fetus vs their lean counterparts and vs placentas from obese women with a female fetus. TrxR activity however was also significantly (p<0.05) greater in placentas from lean women with female fetus compared to lean women with a male and to obese women with a female fetus.
Figure 2.
Effect of obesity on selenoproteins in placenta of lean and obese women. A. Glutathione peroxidase (GPx) activity was measured as nmol NADP+/min/mg protein. Values are mean ± SEM. (a = p<0.05 vs obese male, n=6). B. Thioredoxin reductase (TrxR) activity was measured as nmol 2-nitro-5-thiobenzoate (NTB)/min/mg protein. Values are mean ± SEM. (a = p<0.05 vs lean male, b = p<0.05 vs obese female, n=5)
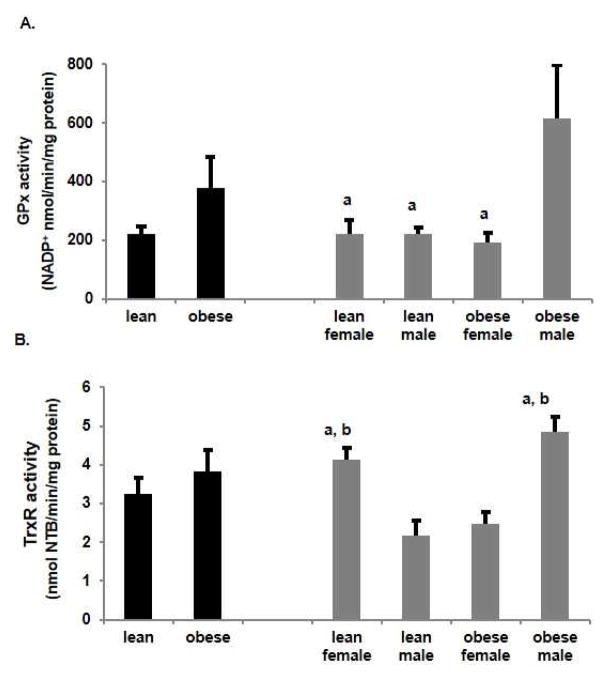
Since we previously showed increased ROS in the placenta with obesity [21], placental mitochondrial ROS were assessed. Protein carbonyls, which can be used as an indirect measure of oxidative stress were significantly (p<0.05) increased in placental mitochondria with obesity (Figure 3). Further stratification of data by fetal sex revealed that this increase with obesity could be accounted for by a significant increase in placentas of a male fetus, with no effect of obesity if the fetus was female. These data with oxidized protein are the reciprocal of the measurements of antioxidant enzyme activity, SOD, CAT and of TAC.
Figure 3.
Effect of obesity on oxidative stress in placental mitochondria. Protein carbonyls were measured as mmol/mg protein in lean and obese women. Values are mean ± SEM. (a = p<0.05 vs lean male, n=5)
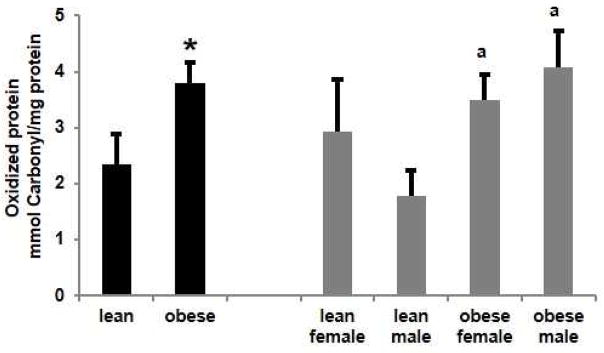
Increased synthesis of NO and superoxide can lead to the generation of peroxynitrite and so NO generation was measured in placenta homogenates. The effect of obesity and fetal sex on NO generation (Figure 4A) paralleled changes in protein carbonyls, being significantly (p<0.05) increased with obesity. Nitric oxide was significantly (p<0.05) higher in placentas from obese women with a male fetus than obese women with a female fetus and both lean groups.
Figure 4.
Effect of obesity on placental nitric oxide (NO) generation and mitochondrial nitrative stress. A. NO generation was measured as Total nitrate/nitrite levels (pmol/mg protein) in lean and obese women. Values are mean ± SEM. (* = p<0.05 vs lean, a = p<0.05 vs obese male, n=5). B. Western analysis of placental mitochondrial nitrotyrosine (3-NT) residues. Density for each band was normalized to its loading control (VDAC). Values are mean ± SEM. (* = p<0.05 vs lean, a = p<0.05 vs lean female, b = p<0.05 vs lean male).
Nitrotyrosine residues are an indirect measure of peroxynitrite generation and an indicator of nitrative stress. We have previously reported increased nitrotyrosine residues in the placenta with obesity [22]. Mitochondrial nitrotyrosine residues, assayed by western blot and normalized to voltage-dependent anion channel (VDAC) (Figure 4B), were significantly (p<0.05) increased with obesity in placentas with either a male or female fetus compared to their respective lean counterparts. The NO and nitrotyrosine data suggest that increased generation of NO with obesity leads to a parallel increase in peroxynitrite.
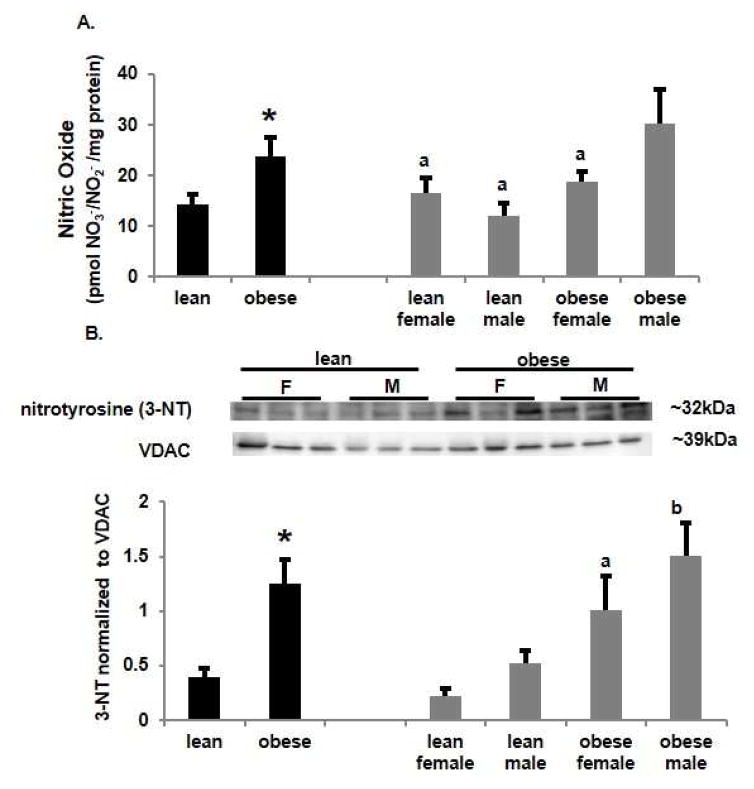
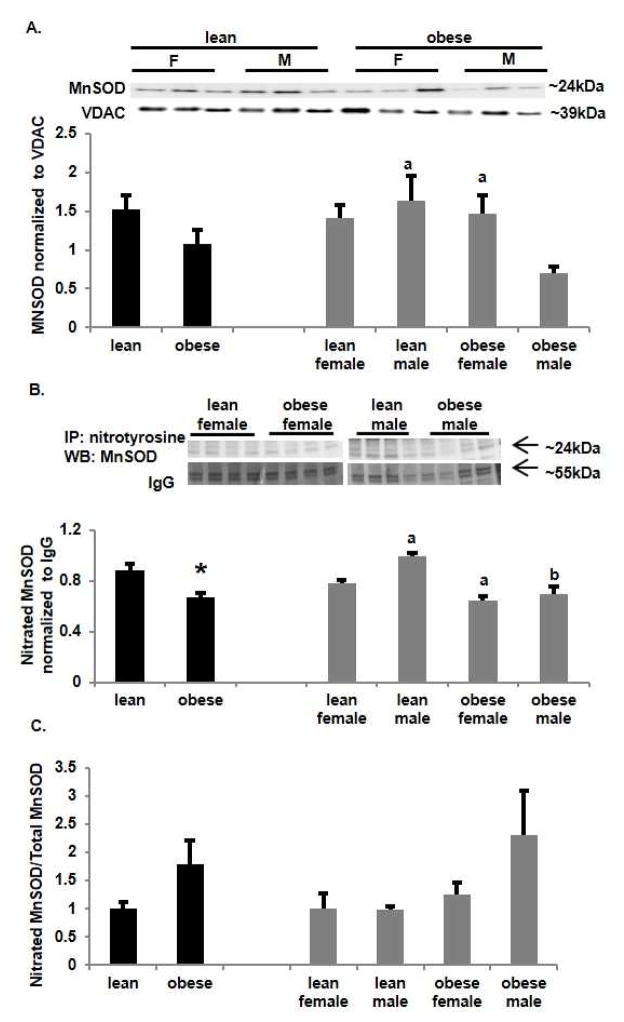
Manganese superoxide dismutase (MnSOD) is the mitochondrial isoform and is readily nitrated resulting in loss of enzymatic activity [25]. We found no overall significant differences in MnSOD protein expression in mitochondrial extracts between placentas of lean and obese women (Figure 5A). However again there was a sexually dimorphic effect with MnSOD protein being significantly decreased (p<0.05) in placentas from obese women with a male fetus vs a female fetus and compared to lean women with a male fetus. Nitrated mitochondrial proteins were immunoprecipitated and probed with an antibody for MnSOD by western blot (Figure 5B). Levels of nitrated MnSOD paralleled those of total MnSOD being significantly (p<0.05) decreased with obesity. Nitrated MnSOD was significantly (p<0.05) higher in placentas from lean women with a male fetus compared to female fetuses and placentas from obese women. The ratio of nitrated MnSOD to total MnSOD (Figure 5C) showed no significant difference across groups although there was a trend to increase in nitrated MnSOD with obesity with the effect predominantly being in obese women with a male fetus. As nitration of MnSOD decreases catalytic activity [25] the combination of decreased MnSOD protein and increased MnSOD nitration with maternal obesity may downregulate the ability of SOD to scavenge ROS.
Figure 5.
Effect of obesity induced nitrative stress on mitochondrial manganese superoxide dismutase (MnSOD). A. Western analysis of placental mitochondrial MnSOD. Density of each band was normalized to its loading control voltage-dependent anion channel (VDAC). Values are mean ± SEM. (a = p<0.05 vs obese male, n=6). B. Western analysis following immunoprecipitation of placental mitochondrial nitrated MnSOD. Density of each band was normalized to its loading control IgG from the immunoprecipitation. Values are mean ± SEM. (a = p<0.05 vs lean female, b = p<0.05 vs lean male, n=6). C. Ratio of nitrated MnSOD to Total MnSOD. Values are mean ± SEM. (n=6)
Discussion
Increased placental oxidative stress has been shown with preeclampsia and gestational diabetes [12–16] and with maternal obesity, which increases the risk of preeclampsia and gestational diabetes [21, 22, 26]. However the effect on antioxidants in these studies were variable being increased [26], decreased [27], or not different [22], perhaps due to different patient populations, different levels of adiposity, and inclusion of both male and female placentas. We have addressed this variability by studying obesity in the absence of other medical conditions, analyzing the data based on maternal BMI and fetal sex, as well as using mitochondrial extracts. Additional strengths of the study include defined conditions for collection of placental tissue at cesarean section in the absence of labor to avoid generation of oxidative stress, random sampling of tissue and matching for gestational age.
Stratification by fetal or placental sex reveals some interesting points. Firstly in the lean women the male placenta has increased antioxidant defenses as illustrated by greater SOD activity and TAC. However this advantage is lost with maternal obesity where SOD and CAT activity are both reduced and no different from that seen in the female placenta of an obese woman. TAC is also reduced compared to lean women but is still higher than the female placenta of an obese woman. Male fetuses are known to be at increased risk of adverse outcomes compared to the female fetus [28, 29] potentially due to decreased antioxidant defenses in situations such as obesity and the greater response, e.g. the change in antioxidant enzymes, to the adverse environment. Recently in a mouse study of sex-specific placental responses to chronic hypoxia [30], male placentas were less susceptible to oxidative stress than females and the offspring were less growth restricted. Our results reinforce the case that all future studies of the placenta in adverse pregnancy conditions such as preeclampsia, diabetes, and IUGR should consider the effect of placental sex.
Interestingly the activity of the antioxidant selenoprotein enzymes, glutathione peroxidase and thioredoxin reductase, show different patterns of change with obesity and with fetal sex compared to SOD, CAT, and TAC. GPx and TrRx activity were significantly increased in placentas from obese women with a male fetus. A possible explanation for this result is that since there are two pathways that scavenge hydrogen peroxide, the SOD-CAT pathway is prone to nitration and downregulated, an increase in GPx and TrRx could compensate for this effect. Interestingly, upregulation of GPx and TrRx expression was seen in several tissues of obese pigs fed a high fat diet [31] while reduced selenium levels are found in plasma of obese children [32] and morbidly obese women [33]. Regional differences in soil selenium levels have been related to the inverse association reported between selenium intake and the incidence of preeclampsia [34], a state of oxidative stress [12–14]. Our data do illustrate that not all antioxidant enzymes are regulated or respond to oxidative stress in the same way. Enzymatic generation of ROS occurs at specific sites within cells and their radius of action is governed by their short half-lives [12]. The expression of antioxidant enzymes is similarly compartmentalized within the cell and each may respond differently depending on the ROS that are generated locally and need to be inactivated.
The downregulation of specific antioxidant enzyme activity and total antioxidant capacity, could subsequently result in oxidative and nitrative stress as evidenced by increased protein carbonyls and nitrotyrosine residues respectively in placental mitochondria which we have previously reported in whole placental tissue with maternal obesity [21, 22]. Nitration of antioxidant enzymes such as MnSOD participates in a vicious feedback cycle resulting in a further buildup of reactive oxygen species thus consuming the antioxidant defenses of the placenta. This may be an important effect of maternal obesity that could result in nitration of mitochondrial proteins [35] and the decrease placental mitochondrial respiration we have reported with obesity [21].
We continue to demonstrate sexual dimorphism in the placenta as previously reported [18, 36]. Sexual dimorphism of antioxidants and markers of oxidative and nitrative stress has also been demonstrated in the human placenta in response to glucocorticoid exposure in preterm deliveries [37] where male placentas had a higher pro-oxidant state (increased protein carbonyls, lipid hydroperoxides, and nitrotyrosine), but lower glutathione peroxidase activity when compared to female placentas [37]. These studies indicate that antioxidant defense mechanisms are decreased in the male in the setting of an adverse event, preterm labor, and may be responsible for increased mortality/morbidity and poor fetal outcomes in males [28–30]. In addition, a fetal sheep study using male-female twins demonstrated organ specific sexual dimorphism of antioxidant expression [38] with increased SOD activity in male brains and female livers, and increased glutathione content (reduced and oxidized) in male liver and skeletal muscle. Sexually dimorphic expression of the selenoproteins including GPx has also been reported in murine tissues [39]. Several studies have shown sexual dimorphism of gene expression in the human placenta [40–42] and in response to adverse environments e.g. maternal distress [43]. In murine models obesity alters placental morphology, cell proliferation and inflammation [44] and maternal diet alters placental gene expression [45] and epigenetic systems [46] in a sexually dimorphic manner. Maternal dietary n-3 polyunsaturated fatty acid administration gave sexually dimorphic changes in gene expression in placentas in mice [47] and in humans [41]. The underlying basis for these sexually dimorphic differences in gene expression in placenta remains unknown. The sexually dimorphic genes appear to arise both from autosomes and sex chromosomes [40]. While there is evidence that sex steroids can affect antioxidant enzymes [39, 48] and oxidative stress [49], there is a paucity of data on differences in placental sex steroids in adverse environments. Overall there are a limited number of studies focusing on the sexual dimorphism of the pro- and anti-oxidant status of the fetus, hence more studies are needed to clarify the mechanisms involved.
Highlights.
In the lean woman the placenta of a male fetus has the highest antioxidant enzyme activity
Maternal obesity is associated with decreased placental antioxidant enzyme activity
In the obese woman the placenta of a male has the highest oxidative and nitrative stress
Selenoprotein antioxidant enzymes may increase as a compensatory response to oxidative stress.
Maternal obesity affects placental oxidative stress but in a sexually dimorphic manner which may contribute to increased adverse outcomes for the male fetus
Acknowledgments
Funding: This work was supported by the National Institutes of Health R01 HL075297 and T32 HL007446-32.
Abbreviations
- SOD
superoxide dismutase
- CAT
catalase
- GPx
glutathione peroxidase
- TrxR
thioredoxin reductase
- TAC
total antioxidant capacity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev Med. 2013;56(6):372–8. doi: 10.1016/j.ypmed.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989–2007. Int J Obes (Lond) 2010;34(3):420–8. doi: 10.1038/ijo.2009.250. [DOI] [PubMed] [Google Scholar]
- 3.Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1(4):170–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103(2):219–24. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 5.Perlow JH, Morgan MA, Montgomery D, Towers CV, Porto M. Perinatal outcome in pregnancy complicated by massive obesity. Am J Obstet Gynecol. 1992;167(4 Pt 1):958–62. doi: 10.1016/s0002-9378(12)80019-6. [DOI] [PubMed] [Google Scholar]
- 6.Galtier-Dereure F, Montpeyroux F, Boulot P, Bringer J, Jaffiol C. Weight excess before pregnancy: complications and cost. Int J Obes Relat Metab Disord. 1995;19(7):443–8. [PubMed] [Google Scholar]
- 7.Bianco AT, Smilen SW, Davis Y, Lopez S, Lapinski R, Lockwood CJ. Pregnancy outcome and weight gain recommendations for the morbidly obese woman. Obstet Gynecol. 1998;91(1):97–102. doi: 10.1016/s0029-7844(97)00578-4. [DOI] [PubMed] [Google Scholar]
- 8.Isaacs JD, Magann EF, Martin RW, Chauhan SP, Morrison JC. Obstetric challenges of massive obesity complicating pregnancy. J Perinatol. 1994;14(1):10–4. [PubMed] [Google Scholar]
- 9.Mills JL, Troendle J, Conley MR, Carter T, Druschel CM. Maternal obesity and congenital heart defects: a population-based study. Am J Clin Nutr. 2010;91(6):1543–9. doi: 10.3945/ajcn.2009.28865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madsen NL, Schwartz SM, Lewin MB, Mueller BA. Prepregnancy body mass index and congenital heart defects among offspring: a population-based study. Congenit Heart Dis. 2013;8(2):131–41. doi: 10.1111/j.1747-0803.2012.00714.x. [DOI] [PubMed] [Google Scholar]
- 11.Fridovich I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann N Y Acad Sci. 1999;893:13–8. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- 12.Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122(4):369–82. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 13.Madazli R, Benian A, Aydin S, Uzun H, Tolun N. The plasma and placental levels of malondialdehyde, glutathione and superoxide dismutase in pre-eclampsia. J Obstet Gynaecol. 2002;22(5):477–80. doi: 10.1080/0144361021000003573. [DOI] [PubMed] [Google Scholar]
- 14.Vanderlelie J, Venardos K, Clifton VL, Gude NM, Clarke FM, Perkins AV. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta. 2005;26(1):53–8. doi: 10.1016/j.placenta.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Coughlan MT, Vervaart PP, Permezel M, Georgiou HM, Rice GE. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta. 2004;25(1):78–84. doi: 10.1016/S0143-4004(03)00183-8. [DOI] [PubMed] [Google Scholar]
- 16.Biri A, Onan A, Devrim E, Babacan F, Kavutcu M, Durak I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta. 2006;27(2–3):327–32. doi: 10.1016/j.placenta.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30(3):400–18. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 18.Muralimanoharan S, Guo C, Myatt L, Maloyan A. Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity. Int J Obes (Lond) 2015;39(8):1274–81. doi: 10.1038/ijo.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan JC, Sasser JM, Pollock JS. Sexual dimorphism in oxidant status in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R764–8. doi: 10.1152/ajpregu.00322.2006. [DOI] [PubMed] [Google Scholar]
- 20.Nickelson KJ, Stromsdorfer KL, Pickering RT, Liu TW, Ortinau LC, Keating AF, Perfield JW., 2nd A comparison of inflammatory and oxidative stress markers in adipose tissue from weight-matched obese male and female mice. Exp Diabetes Res. 2012;2012:859395. doi: 10.1155/2012/859395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mele J, Muralimanoharan S, Maloyan A, Myatt L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am J Physiol Endocrinol Metab. 2014;307(5):E419–25. doi: 10.1152/ajpendo.00025.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts VH, Smith J, McLea SA, Heizer AB, Richardson JL, Myatt L. Effect of increasing maternal body mass index on oxidative and nitrative stress in the human placenta. Placenta. 2009;30(2):169–75. doi: 10.1016/j.placenta.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Walsh SW. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta. 1998;19(8):581–6. doi: 10.1016/s0143-4004(98)90018-2. [DOI] [PubMed] [Google Scholar]
- 24.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26(1):1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 25.MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA. 1996;93(21):11853–8. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malti N, Merzouk H, Merzouk SA, Loukidi B, Karaouzene N, Malti A, Narce M. Oxidative stress and maternal obesity: feto-placental unit interaction. Placenta. 2014;35(6):411–6. doi: 10.1016/j.placenta.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Saben J, Lindsey F, Zhong Y, Thakali K, Badger TM, Andres A, Gomez-Acevedo H, Shankar K. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35(3):171–7. doi: 10.1016/j.placenta.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res. 2012;71(3):305–10. doi: 10.1038/pr.2011.50. [DOI] [PubMed] [Google Scholar]
- 29.Binet ME, Bujold E, Lefebvre F, Tremblay Y, Piedboeuf B. Role of gender in morbidity and mortality of extremely premature neonates. Am J Perinatol. 2012;29(3):159–66. doi: 10.1055/s-0031-1284225. [DOI] [PubMed] [Google Scholar]
- 30.Matheson H, Veerbeek JH, Charnock-Jones DS, Burton GJ, Yung HW. Morphological and molecular changes in the murine placenta exposed to normobaric hypoxia throughout pregnancy. J Physiol. 2016;594(5):1371–88. doi: 10.1113/JP271073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao H, Li K, Tang JY, Zhou JC, Wang KN, Xia XJ, Lei XG. Expression of Selenoprotein Genes Is Affected by Obesity of Pigs Fed a High-Fat Diet. J Nutr. 2015;145(7):1394–401. doi: 10.3945/jn.115.211318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blazewicz A, Klatka M, Astel A, Korona-Glowniak I, Dolliver W, Szwerc W, Kocjan R. Serum and urinary selenium levels in obese children: a cross-sectional study. J Trace Elem Med Biol. 2015;29:116–22. doi: 10.1016/j.jtemb.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Alasfar F, Ben-Nakhi M, Khoursheed M, Kehinde EO, Alsaleh M. Selenium is significantly depleted among morbidly obese female patients seeking bariatric surgery. Obes Surg. 2011;21(11):1710–3. doi: 10.1007/s11695-011-0458-2. [DOI] [PubMed] [Google Scholar]
- 34.Vanderlelie J, Perkins AV. Selenium and preeclampsia: A global perspective. Pregnancy Hypertens. 2011;1(3–4):213–24. doi: 10.1016/j.preghy.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33(11):1451–64. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 36.Muralimanoharan S, Gao X, Weintraub S, Myatt L, Maloyan A. Sexual dimorphism in activation of placental autophagy in obese women with evidence for fetal programming from a placenta-specific mouse model. Autophagy. 2016;12(5):752–69. doi: 10.1080/15548627.2016.1156822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stark MJ, Hodyl NA, Wright IM, Clifton VL. Influence of sex and glucocorticoid exposure on preterm placental pro-oxidant-antioxidant balance. Placenta. 2011;32(11):865–70. doi: 10.1016/j.placenta.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Al-Gubory KH, Garrel C. Sex-specific divergence of antioxidant pathways in fetal brain, liver, and skeletal muscles. Free Radic Res. 2016;50(3):366–73. doi: 10.3109/10715762.2015.1130224. [DOI] [PubMed] [Google Scholar]
- 39.Riese C, Michaelis M, Mentrup B, Gotz F, Kohrle J, Schweizer U, Schomburg L. Selenium-dependent pre- and posttranscriptional mechanisms are responsible for sexual dimorphic expression of selenoproteins in murine tissues. Endocrinology. 2006;147(12):5883–92. doi: 10.1210/en.2006-0689. [DOI] [PubMed] [Google Scholar]
- 40.Buckberry S, Bianco-Miotto T, Bent SJ, Dekker GA, Roberts CT. Integrative transcriptome meta-analysis reveals widespread sex-biased gene expression at the human fetal-maternal interface. Mol Hum Reprod. 2014;20(8):810–9. doi: 10.1093/molehr/gau035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedlmeier EM, Brunner S, Much D, Pagel P, Ulbrich SE, Meyer HH, Amann-Gassner U, Hauner H, Bader BL. Human placental transcriptome shows sexually dimorphic gene expression and responsiveness to maternal dietary n-3 long-chain polyunsaturated fatty acid intervention during pregnancy. BMC Genomics. 2014;15:941. doi: 10.1186/1471-2164-15-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci USA. 2006;103(14):5478–83. doi: 10.1073/pnas.0508035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mina TH, Raikkonen K, Riley SC, Norman JE, Reynolds RM. Maternal distress associates with placental genes regulating fetal glucocorticoid exposure and IGF2: Role of obesity and sex. Psychoneuroendocrinology. 2015;59:112–22. doi: 10.1016/j.psyneuen.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Kim DW, Young SL, Grattan DR, Jasoni CL. Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol Reprod. 2014;90(6):130. doi: 10.1095/biolreprod.113.117259. [DOI] [PubMed] [Google Scholar]
- 45.Mao J, Zhang X, Sieli PT, Falduto MT, Torres KE, Rosenfeld CS. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci USA. 2010;107(12):5557–62. doi: 10.1073/pnas.1000440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabory A, Ferry L, Fajardy I, Jouneau L, Gothie JD, Vige A, Fleur C, Mayeur S, Gallou-Kabani C, Gross MS, Attig L, Vambergue A, Lesage J, Reusens B, Vieau D, Remacle C, Jais JP, Junien C. Maternal diets trigger sex-specific divergent trajectories of gene expression and epigenetic systems in mouse placenta. PLoS One. 2012;7(11):e47986. doi: 10.1371/journal.pone.0047986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones ML, Mark PJ, Waddell BJ. Maternal omega-3 fatty acid intake increases placental labyrinthine antioxidant capacity but does not protect against fetal growth restriction induced by placental ischaemia-reperfusion injury. Reproduction. 2013;146(6):539–47. doi: 10.1530/REP-13-0282. [DOI] [PubMed] [Google Scholar]
- 48.Pajovic SB, Saicic ZS. Modulation of antioxidant enzyme activities by sexual steroid hormones. Physiol Res. 2008;57(6):801–11. doi: 10.33549/physiolres.931377. [DOI] [PubMed] [Google Scholar]
- 49.Miller AA, De Silva TM, Jackman KA, Sobey CG. Effect of gender and sex hormones on vascular oxidative stress. Clin Exp Pharmacol Physiol. 2007;34(10):1037–43. doi: 10.1111/j.1440-1681.2007.04732.x. [DOI] [PubMed] [Google Scholar]