Abstract
Objective:
To examine longitudinal trajectories of white matter organization in pediatric moderate/severe traumatic brain injury (msTBI) over a 12-month period.
Methods:
We studied 21 children (16 M/5 F) with msTBI, assessed 2–5 months postinjury and again 13–19 months postinjury, as well as 20 well-matched healthy control children. We assessed corpus callosum function through interhemispheric transfer time (IHTT), measured using event-related potentials, and related this to diffusion-weighted MRI measures of white matter (WM) microstructure. At the first time point, half of the patients with TBI had significantly slower IHTT (TBI-slow-IHTT, n = 11) and half were in the normal range (TBI-normal-IHTT, n = 10).
Results:
The TBI-normal-IHTT group did not differ significantly from healthy controls, either in WM organization in the chronic phase or in the longitudinal trajectory of WM organization between the 2 evaluations. In contrast, the WM organization of the TBI-slow-IHTT group was significantly lower than in healthy controls across a large portion of the WM. Longitudinal analyses showed that the TBI-slow-IHTT group experienced a progressive decline between the 2 evaluations in WM organization throughout the brain.
Conclusions:
We present preliminary evidence suggesting a potential biomarker that identifies a subset of patients with impaired callosal organization in the first months postinjury who subsequently experience widespread continuing and progressive degeneration in the first year postinjury.
Traumatic brain injury (TBI) is associated with substantial mortality and morbidity in children. Demyelination of white matter (WM) that is commonly found post-TBI1,2 can have adverse cognitive repercussions.3,4 In children, this disruption to myelin is compounded, as the brain is still maturing, and myelination continues well beyond age 30.5,6 With diffusion-weighted MRI (DWI), we can identify WM disruptions postinjury. Prior work has shown lower WM organization in TBI,7,8 suggesting disrupted myelin.
Interhemispheric transfer time (IHTT) is the time required for signals to traverse the cerebral hemispheres through the corpus callosum (CC). It is an index of callosal functional organization; longer IHTT indicates slower information transfer. Both children and adults show slower IHTT following TBI.9,10 We measured IHTT using visual event-related potentials (ERPs), through EEG scalp recordings. We previously published a study integrating ERP and DWI data in TBI,11 finding a subgroup of patients in the first months postinjury who show disrupted functional and structural organization of the CC and impaired cognitive performance.9,11 Here we present longitudinal analyses using data collected when patients returned for follow-up approximately 12 months after the initial evaluation. We hypothesized that the subgroup of children with moderate/severe TBI (msTBI) showing disrupted WM organization at the first assessment would continue to show disruption, while those who did not would continue to track well with healthy controls.
METHODS
The methods are similar to those reported in our prior articles with this dataset.7,11
Participants.
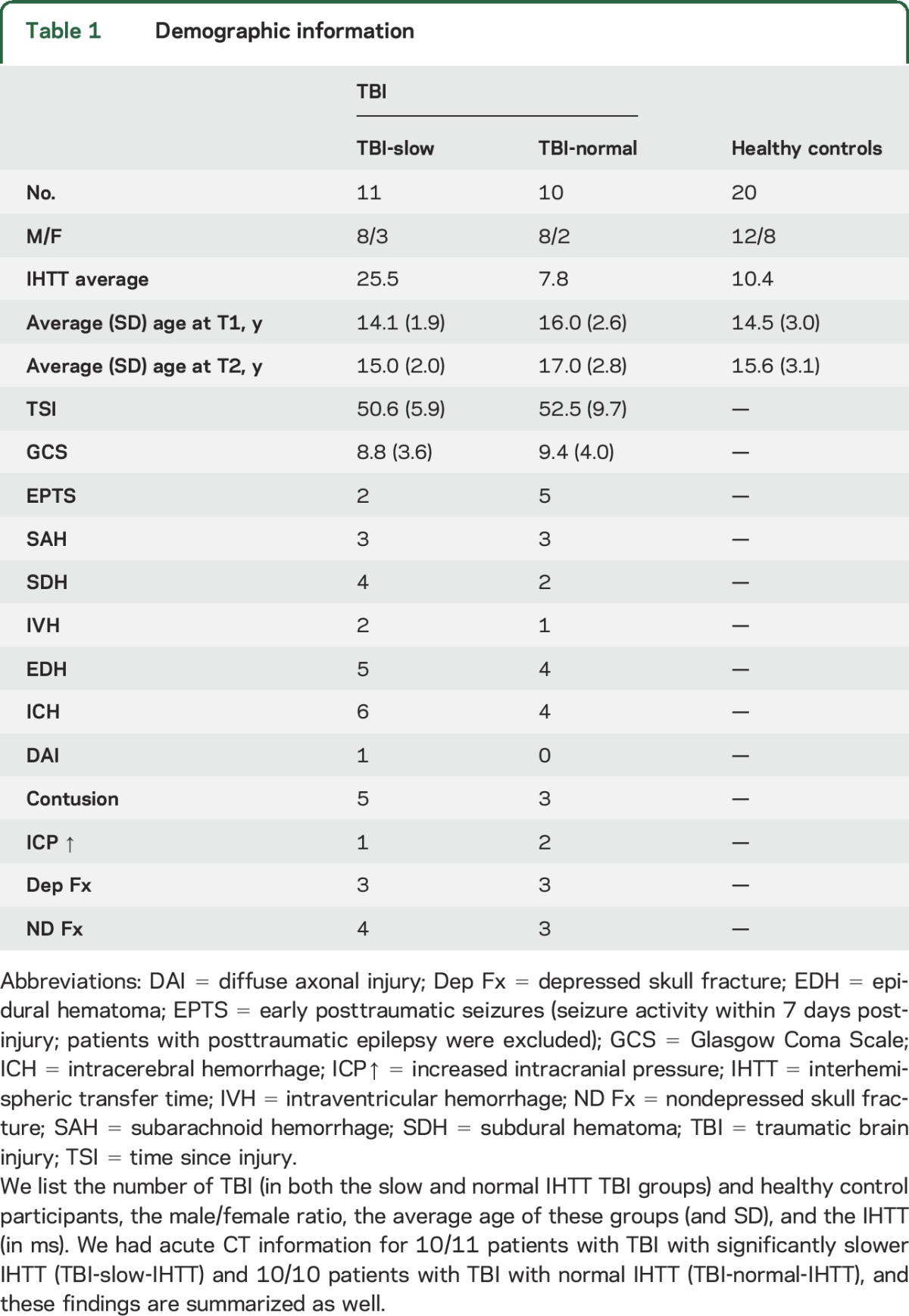
We recruited patients with TBI from 4 pediatric intensive care units (PICUs) from level 1 and level 2 trauma centers in Los Angeles County. Parents of patients were given an institutional review board (IRB)–approved pamphlet concerning the study and discussed it with a study representative. Those interested in participating gave permission to investigators to contact the family after discharge from the PICU. Of those parents who gave permission to be contacted, 35% ended up participating in the study. Healthy controls, matched for age, sex, and educational level, were recruited from the community through flyers, magazines, and school postings. We studied 21 children (16 M/5 F) with msTBI in the postacute phase (2–5 months postinjury) and again 12 months later, as well as 20 healthy control children (12 M/8 F) also assessed twice. Cross-sectional results from our initial analysis of the postacute phase were published previously.11 The current analyses focus on the follow-up in the chronic phase and the change from the postacute to chronic time points. Details on injury mechanisms and CT findings can be found in the supplemental data at Neurology.org. The demographics (table 1) of our sample are consistent with existing epidemiologic information on moderate/severe pediatric/adolescent TBI, both in the male to female ratio and in the mechanisms of injury.12 Inclusion and exclusion criteria are detailed in the supplemental data.
Table 1.
Demographic information
Standard protocol approvals, registrations, and patient consents.
All participants or their legal guardians signed informed consent for participation in the study. Recruitment, consent processes, and study activities were approved by the University of California, Los Angeles (UCLA) IRB.
Cognitive performance.
Our cognitive performance score is a summary measure from tests assessing multiple domains known to be affected in TBI.13 Further details are reported in the supplemental data.
Scan acquisition.
Participants were scanned on 3T Siemens (Munich, Germany) Trio MRI scanners with whole brain anatomical and 72-gradient DWI. DWI was acquired with the following acquisition parameters: GRAPPA mode, acceleration factor phase encoding 2, repetition time/echo time 9,500/87 ms, field of view 256 × 256 mm, isotropic voxel size 2 mm. Seventy-two images were collected per participant: 8 b0 and 64 diffusion-weighted (b = 1,000 s/mm2).
Scan comparison.
Halfway through the study, scanning moved from the UCLA Brain Mapping Center to the Staglin IMHRO Center for Cognitive Neuroscience (Staglin). Both scanners were 3T Siemens Trio scanners, and the protocol was maintained. Further details on how this was addressed can be found in the supplemental data.
Tractography and fiber clustering.
We used a tract-extraction method developed in our laboratory, automated multi-atlas tract extraction (autoMATE),14–16 which has been robustly tested in TBI.7,17,18 AutoMATE has been described fully in prior articles.14–16 Further preprocessing details are reported in the supplemental data.
As part of autoMATE, 5 WM tract atlases were constructed from healthy young adults' (20–30 years old) high angular resolution diffusion imaging data, as detailed previously.14–16 The atlas, based on the Eve brain atlas,19 includes 18 major WM tracts: the anterior thalamic radiation (left and right: atr_l and atr_r), corticospinal tract (left and right: cst_l and cst_r), cingulum (left and right: cgc_l and cgc_r), inferior fronto-occipital fasciculus (left and right: ifo_l and ifo_r), inferior longitudinal fasciculus (left and right: ilf_l and ilf_r), arcuate fasciculus (left only: arc_l, as the right arcuate is too asymmetric for population studies to be practical20), fornix, and corpus callosal tracts divided into 6 segments: frontal, precentral gyrus, postcentral gyrus, parietal, temporal, and occipital. Details about fiber clustering can be found in the supplemental data. Fractional anisotropy (FA) is the degree to which water is diffusing preferentially in one direction (along axons). Mean diffusivity (MD) (also called apparent diffusion coefficient) is a related measure of the average diffusivity across all 3 primary eigenvectors and typically increases when FA decreases. Radial diffusivity (RD) is the average of the eigenvalues corresponding to the 2 nonprimary eigenvectors, and axial diffusivity is the eigenvalue corresponding to the primary eigenvector. We extracted tract-based FA, MD, RD, and axial diffusivity at this point for group comparison.
ERP recording.
EEG was recorded during the postacute phase (2–5 months postinjury) while participants completed a computerized, pattern-matching task with bilateral field advantage.9 A BIOSEMI system was used to acquire ERPs. Visual ERPs were recorded, synchronized to the onset of the pattern presentation. Further details on how IHTT was computed from this information can be found in the supplemental data.
As detailed previously,9,11 there was a bimodal distribution in IHTT in the TBI group, with half of patients having significantly impaired IHTT, while the other patients with TBI had scores within the range of the healthy controls. The cutoff between the groups was determined as the point that optimized the balance between sensitivity and specificity: 18 ms. IHTT longer than this was outside the range of the healthy controls. In our prior cross-sectional studies, we found that these 2 TBI subgroups differed in cognitive function and WM structural organization9,11 at 2–5 months post-TBI. Importantly, the TBI-slow-IHTT and TBI-normal-IHTT groups do not differ in time since injury (p = 0.61), injury severity (measured by Glasgow Coma Scale score) (p = 0.73), or any injury variables available to us from CT. The TBI-normal-IHTT group had a higher incidence of early posttraumatic seizures, but this was not significant (p = 0.12). This can be seen in table 1. We additionally mapped the location and extent of the lesions in each group, shown in figure e-1. Within this subset with longitudinal data analyzed here, there was an age difference between patients with TBI with significantly slower IHTT (TBI-slow-IHTT) and patients with TBI with normal IHTT (TBI-normal-IHTT). Our prior study included a larger cohort, and there were no differences in age or sex distribution between the groups.11 Therefore, age is not a significant explanatory variable for these differences. In addition, the age difference has a minimal effect on the longitudinal analyses conducted in this article, as the interscan interval was consistent across participants and we covaried for age in all analyses.
Statistical analysis.
Details on the statistical models used can be found in the supplemental data.
RESULTS
Chronic differences.
We grouped patients with msTBI into 2 groups based on their postacute IHTT (2–5 months postinjury): TBI-slow-IHTT and TBI-normal-IHTT. We then compared TBI-slow-IHTT to healthy controls and TBI-normal-IHTT to healthy controls, running multiple linear regression to examine their element-wise WM organization in the chronic phase (13–19 months postinjury), to see if the WM differences found in the postacute phase normalized or persisted. We covaried for age, sex, scanner, and between-session intervals. These were corrected for multiple comparisons across all points (false discovery rate, q < 0.05). In the TBI-normal-IHTT vs healthy control comparison, we found no significant differences in FA, MD, or axial diffusivity, and minimal differences in RD (0.018% of points tested, so no figure included).
When we compared the TBI-slow-IHTT vs the healthy control group, we found large differences in FA, MD, and RD. We found lower FA and higher MD and RD in the TBI-slow-IHTT group compared to healthy controls. The significant clusters were extensive, especially for the MD and RD analyses. The spatial extent of these results is greater than our original results in the postacute phase.11 These can be seen in table e-1 and figure 1.
Figure 1. Group differences in along-tract white matter organization.
Along-tract differences in mean diffusivity between patients with traumatic brain injury with significantly slower interhemispheric transfer time (n = 11) and healthy controls (n = 20), run using linear regression (critical p = 0.019). The colors correspond to the –log10 p value of the regression, as shown in the legend. Results are shown from the anterior, posterior, superior, inferior, right, and left orientations.
Longitudinal changes.
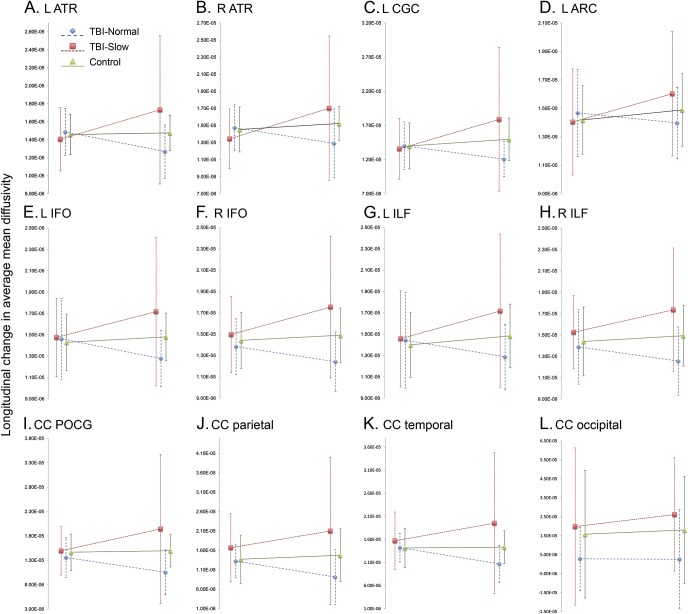
When comparing across groups, our aim was to localize portions of tracts with greatest group level diffusivity measurement differences and we therefore mapped out these differences on a point-by-point level. However, when evaluating longitudinal differences, we wanted to avoid biases and noise from potential imprecisions in intraparticipant registration and look more globally at whether the entire tract was affected, which could possibly lead to more clinically significant behavioral and cognitive differences. Therefore, our longitudinal analysis focused on tract-average differences. WM measures (FA, MD, axial diffusivity, and RD) were averaged along a tract at both time points, and the difference was calculated. Examining the change in tract average measures between TBI-slow-IHTT and healthy controls and between TBI-normal-IHTT and healthy controls, we again found different patterns in the 2 TBI groups. The TBI-slow-IHTT group had significantly altered longitudinal trajectories, with the TBI-slow-IHTT group showing increases in average MD, RD, and axial diffusivity in 13 of the 18 tracts over the 12 months, while the healthy controls showed decreases in these tracts over the same period of time. In contrast, we found no significant differences between the TBI-normal-IHTT and healthy controls. The longitudinal trajectory of the TBI-normal-IHTT subgroup did not deviate from the healthy controls. We chart the changes in average MD across tracts showing significant group differences in longitudinal changes in table e-2 and figure 2. These scatterplots show the group averaged MD values after the effects of age, sex, scanner, and interscan interval have been regressed out. Although not shown, we also examined a model including time since injury and found similar effects.
Figure 2. Longitudinal change in tract average mean diffusivity (MD) across patients with traumatic brain injury (TBI) with significantly slower interhemispheric transfer time (IHTT) (TBI-slow-IHTT), patients with TBI with normal IHTT (TBI-normal-IHTT), and healthy controls.
We chart the group average MD for the TBI-slow-IHTT (n = 11), TBI-normal-IHTT (n = 10), and healthy controls (n = 20). Age, sex, scanner, and interscan interval have been regressed out of the MD calculations, and averages have been shifted by the same variable to be in the positive range. Group averages are shown for the postacute and chronic time points, along with SDs, and linear trend lines. The error bars indicate SD. Only tracts showing significant group differences in the change in MD over time are shown. (A) L ATR, (B) R ATR, (C) L CGC, (D) L ARC, (E) L IFO, (F) R IFO, (G) L ILF, (H) R ILF, (I) CC POCG, (J) CC parietal, (K) CC temporal, (L) CC occipital. ATR = anterior thalamic radiation; CGC = cingulum; ARC = arcuate; CC = corpus callosum; IFO = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; POCG = corpus callosum-post-central gyrus segment.
We also ran IHTT as a continuous variable, but did not find any associations that survived correction for multiple comparisons.
We map the longitudinal changes in each group along tracts as well in figure 3. These are still images from movies: TBI-slow-IHTT (video 1), TBI-normal-IHTT (video 2) and healthy control (video 3).
Figure 3. Longitudinal changes in along-tract mean diffusivity (MD) in patients with traumatic brain injury (TBI) with significantly slower interhemispheric transfer time (IHTT) (TBI-slow-IHTT), patients with TBI with normal IHTT (TBI-normal-IHTT), and healthy controls.
The group-averaged maps are shown for both time points, across TBI-slow-IHTT (n = 11), TBI-normal-IHTT (n = 10), and healthy controls (n = 20). Approximately 12 months passed between the beginning and ending time point. As indicated in the legend, blue areas have the lowest MD and therefore the highest white matter (WM) organization, while red areas have the highest MD. The healthy controls show minimal decreases in MD. The TBI-slow-IHTT group shows widespread increases in MD. The TBI-normal-IHTT group shows a mixture.
Cognitive results.
We previously found9,11 that the groups differed significantly in cognitive performance postacutely. In the current study, the TBI-slow-IHTT group had significantly poorer cognitive performance than healthy controls (p = 0.026), and the TBI-normal-IHTT group was intermediate, and not significantly different from the other 2 groups postacutely. In the chronic evaluations, again the TBI-slow-IHTT group had significantly poorer cognitive performance than healthy controls (p = 0.0036), with the TBI-normal-IHTT intermediate and not significantly different from the other 2 groups. We did not find a significant longitudinal change in cognitive performance in any group, and there were no significant correlations between change in cognitive performance and change in WM organization. The cognitive performance score is composed of age-normalized scores, however, so this is not unexpected. In addition, examining groups as small as 10 participants, this was an underpowered analysis.
DISCUSSION
There is a wide range of outcomes following msTBI. Individual differences in acute injury severity account for a relatively modest amount of the variance in long-term cognitive outcomes.13,21 Our incomplete understanding of the predictors of postinjury outcome hampers clinicians' ability to identify those patients who would benefit most from intensive neurorehabilitation. Our prior work found that patients with TBI could be divided into 2 groups based on their IHTT (the time for information to traverse the hemispheres via the posterior CC) at 2–5 months postinjury9,11 and these groups differed in structural WM organization and cognitive function. Here we conducted longitudinal analyses showing that the group differences persist through the first 18 months postinjury, becoming more widespread, as the TBI-normal-IHTT group recovers while the TBI-slow-IHTT group experiences progressive decline. Our IHTT task potentially can identify after a few months postinjury patients at higher risk for a poor outcome neurologically and cognitively. This finding clearly needs to be replicated in a wider cohort, but it raises the possibility of a second window for intervention, to reduce the long-term functional morbidity of TBI in children.
Using the cutoff for the IHTT established in the postacute phase, we had 11 participants in the TBI-slow-IHTT group and 10 participants in the TBI-normal-IHTT group with longitudinal data. There were minimal differences between the TBI-normal-IHTT group and the 20 healthy controls in the chronic phase, and they did not differ in longitudinal changes in tract organization (figure 2). While we did not perform along-tract analyses of longitudinal changes, we did chart the group-averaged along-tract MD for the 3 groups at both time points (figure 3). In the TBI-normal-IHTT group, we see decreases in MD, potentially indicating recovering myelin. These along-tract averages can be skewed by outliers, so we have included images of the along-tract SD within groups (figure e-2). Some of the areas with the highest variance included the occipital projections of the splenium. The variance was higher in these regions across groups, which could indicate that diffusion is not accurately represented with a tensor in these areas. The variance in these areas needs to be kept in mind when interpreting results as possible indications of recovery.
Comparing the TBI-slow-IHTT group to healthy controls, we found widespread disruptions in WM organization in the chronic evaluation, pictured in figure 1, more extensive than differences in the postacute phase.11 We also found that this disruption was progressive, as seen in figure 2. Across 13 tracts, we found significant group differences in the change in average MD, as well as RD and axial diffusivity. Healthy controls showed minimal change in MD, while the TBI-slow-IHTT group had marked increases in MD across these 13 tracts. Higher MD indicates poor WM organization, and increasing MD over time reflects a progressive loss of WM organization. This could be interpreted as progressive demyelination, but a neuroinflammatory response could also increase MD22,23 and is a problematic secondary injury in TBI.24 Excess tissue water due to inflammation can also register as an increase in MD.25 Inflammation is a necessary and healthy response to injury, but if excessive or prolonged, it can become pathologic and cause further damage.24 Prior studies in adults have found that the inflammatory response can continue for decades postinjury, which is associated with poorer outcome.26 The time course of inflammation in children is not well described, but it may affect the postinjury trajectory of WM in some patients.27,28 Future studies will investigate whether a differential inflammatory response explains the group differences we have found.
Longitudinal studies of pediatric TBI are few, but critical for understanding the postinjury trajectory of brain structure and function. Maturation is a nonlinear process with several critical windows for neural and cognitive development.29 Adolescence is a significant period for cognitive development, which is supported by WM maturation.30 The 2 prior longitudinal pediatric TBI studies using different approaches found evidence to support both recovery and continuing degenerative processes.31,32 We examined longitudinal changes in WM organization following msTBI using tractography. Tractography allows us to localize results more accurately, and make more meaningful connections with cognitive effects, as we know the affected tracts, and what regions they connect. These prior studies found evidence for both recovery and progressing disruption in their dataset. We similarly found this mixture, but with the added dimension of subgroups within the TBI patient group.
One limitation of this study is the small sample size, decreasing our power to examine trends within the TBI patient group. Attrition is an issue in longitudinal studies, but there were no significant differences between patients who continued and those who did not. These results clearly need to be replicated in larger samples. We plan to recruit large participant groups to replicate these results and hope other research groups will consider adding an IHTT measure to their study to test for replication. Another limitation is with DWI. The DWI signal detects the diffusion of water in the brain, and mathematically models WM tracts and WM organization, but it cannot identify the neuropathology causing disruptions in WM organization. Decreases in FA are often interpreted as demyelination, but inflammation can also affect this signal. These processes likely co-occur in the brain postinjury. With multimodal data, such as including magnetic resonance spectroscopy, which yields neural metabolites that give additional information about inflammation, we may be able to better resolve this question.
We discovered a potential biomarker that at 2–5 months postinjury predicted the trajectories of changes in WM organization over the next 9–16 months. The subgroup of patients with impaired WM organization in the first months postinjury showed progressive decline throughout the WM during this period, while other patients showed signs of recovery. This is a preliminary result from a small sample, and needs to be replicated by additional datasets, but the consistency of the differences between the slow and normal IHTT subgroup across modalities, including cognitive,9 structural MRI (Dennis et al., unpublished, 2017), diffusion MRI, and our preliminary analyses of functional MRI (task and resting-state), and magnetic resonance spectroscopy give us confidence that it is not an imaging artifact. We have previously hypothesized that, for some patients, local disruption dominates initially but gradually spreads and becomes a global phenomenon. This process has been termed connectomal diaschisis—changes in the connectome involving areas distant from the lesion.33 It is noteworthy that a functional measure of WM organization (IHTT) predicted the course of structural changes in WM organization. Brain structural and functional organization are an outcome, not a cause, and are not interventional targets, but may identify patients who may benefit from more aggressive treatment.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Alma Martinez and Alma Ramirez for assisting with participant recruitment and study coordination and the participants and their families for contributing their time to this study.
GLOSSARY
- AutoMATE
automated multi-atlas tract extraction
- CC
corpus callosum
- DWI
diffusion-weighted MRI
- ERP
event-related potential
- FA
fractional anisotropy
- IHTT
interhemispheric transfer time
- IRB
institutional review board
- MD
mean diffusivity
- msTBI
moderate/severe traumatic brain injury
- PICU
pediatric intensive care unit
- RD
radial diffusivity
- TBI
traumatic brain injury
- UCLA
University of California, Los Angeles
- WM
white matter
Footnotes
Supplemental data at Neurology.org
Editorial, page 1386
AUTHOR CONTRIBUTIONS
E.L.D. wrote the initial manuscript and all authors revised the manuscript. M.U.E., T.B., A.O., R.M., C.B., J.J., C.C.G., and R.F.A. designed the study, recruited participants, and collected the imaging and cognitive data. E.L.D., F.R., J.V.R., Y.J., R.F.A., and P.M.T. analyzed the data. E.L.D.: data analysis, data interpretation, initial manuscript drafting. F.R.: data analysis, manuscript revision. M.E.: data acquisition, data interpretation, manuscript revision. T.B.: study concept and design, data interpretation, manuscript revision. R.V.: data analysis, manuscript revision. J.E.V.-R.: data analysis, data interpretation, manuscript revision. Y.J.: tool development, data interpretation, manuscript revision. A.O.: data acquisition, data interpretation, manuscript revision. R.M.: study concept and design, data acquisition, manuscript revision. C.B.: study concept and design, data acquisition, manuscript revision. J.J.: study concept and design, data acquisition, manuscript revision. C.G.: study concept and design, data interpretation, manuscript revision. P.T.: study concept and design, data interpretation, manuscript revision. R.A.: study concept and design, study supervision, data interpretation, manuscript revision.
STUDY FUNDING
This study was supported by the NICHDS (R01 HD061504). E.L.D. is supported by a grant from the NINDS (K99 NS096116). E.L.D., F.R., Y.J., J.V., and P.T. are also supported by NIH grants to P.T.: U54 EB020403, R01 EB008432, R01 AG040060, and R01 NS080655. C.C.G. is supported by the UCLA BIRC, NS027544, NS05489, UCLA Steve Tisch BrainSPORT Program, and Easton Foundation. Scanning was supported by the Staglin IMHRO Center for Cognitive Neuroscience. The Recovery After Pediatric Brain Injury Study (RAPBI) was supported by NIH (R01 HD061504, K99 NS096116, U54 EB020403, R01 EB008432, R01 AG040060, R01 NS080655, NS027544, NS05489), the UCLA BIRC, the UCLA Steve Tisch BrainSPORT Program, the Easton Foundation, and the Staglin IMHRO Center for Cognitive Neuroscience.
DISCLOSURE
E. Dennis, F. Rashid, M. Ellis, T. Babikian, R. Vlasova, J. Villalon-Reina, Y. Jin, A. Olsen, R. Mink, C. Babbitt, and J. Johnson report no disclosures relevant to the manuscript. C. Giza reports consultant fees from NFL-Neurological Care Program, NHLPA, Pearson PLC, Alcobra, and Neural Analytics; serves on the advisory panel for the Major League Soccer, NCAA, US Soccer Federation, and California State Athletic Commission; and has received speaker fees from Medical Education Speakers Network. P. Thompson and R. Asarnow report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Flygt J, Djupsjo A, Lenne F, Marklund N. Myelin loss and oligodendrocyte pathology in white matter tracts following traumatic brain injury in the rat. Eur J Neurosci 2013;38:2153–2165. [DOI] [PubMed] [Google Scholar]
- 2.Ng HK, Mahaliyana RD, Poon WS. The pathological spectrum of diffuse axonal injury in blunt head trauma: assessment with axon and myelin strains. Clin Neurol Neurosurg 1994;96:24–31. [DOI] [PubMed] [Google Scholar]
- 3.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 2007;130:2508–2519. [DOI] [PubMed] [Google Scholar]
- 4.Ewing-Cobbs L, Prasad MR, Swank P, et al. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage 2008;42:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartzokis G, Lu PH, Tingus K, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging 2010;31:1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochunov P, Williamson DE, Lancaster J, et al. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging 2012;33:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis EL, Jin Y, Villalon-Reina J, et al. White matter disruption in moderate/severe pediatric traumatic brain injury: advanced tract-based analyses. Neuroimage Clin 2015;7:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol 2013;34:2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis MU, Marion SD, McArthur DL, et al. The UCLA study of children with moderate-to-severe traumatic brain injury: event-related potential measure of interhemispheric transfer time. J Neurotrauma 2016;33:990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathias JL, Bigler ED, Jones NR, et al. Neuropsychological and information processing performance and its relationship to white matter changes following moderate and severe traumatic brain injury: a preliminary study. Appl Neuropsychol 2004;11:134–152. [DOI] [PubMed] [Google Scholar]
- 11.Dennis EL, Ellis MU, Marion SD, et al. Callosal function in pediatric traumatic brain injury linked to disrupted white matter integrity. J Neurosci 2015;35:10202–10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keenan HT, Bratton SL. Epidemiology and outcomes of pediatric traumatic brain injury. Dev Neurosci 2006;28:256–263. [DOI] [PubMed] [Google Scholar]
- 13.Babikian T, Asarnow R. Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology 2009;23:283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Y, Shi Y, Zhan L, et al. Automatic clustering of white matter fibers in brain diffusion MRI with an application to genetics. Neuroimage 2014;100:75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin Y, Shi Y, Zhan L, et al. Labeling white matter tracts in HARDI by fusing multiple tract atlases with applications to genetics. Proceeding 10th IEEE ISBI. San Francisco, 2013;512–515. [DOI] [PMC free article] [PubMed]
- 16.Jin Y, Shi Y, Zhan L, et al. Automatic population HARDI white matter tract clustering by label fusion of multiple tract atlases. Multimodal Brain Image Anal 2012;7508:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dennis EL, Jin Y, Kernan C, et al. White matter integrity in traumatic brain injury: effects of permissible fiber turning angle. Proceeding 12th IEEE ISBI. New York, 2015;930–933. [DOI] [PMC free article] [PubMed]
- 18.Dennis EL, Jin Y, Kernan CL, et al. White matter integrity in traumatic brain injury: effects of permissible fiber turning angle. Proceeding 12th IEEE International Symposium Biomedical Imaging. Brooklyn, 2015. [DOI] [PMC free article] [PubMed]
- 19.Zhang Y, Zhang J, Oishi K, et al. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage 2010;52:1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catani M, Allin MP, Husain M, et al. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA 2007;104:17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran LM, Babikian T, Del Piero L, et al. The UCLA study of predictors of cognitive functioning following moderate/severe pediatric traumatic brain injury. JINS 2016;22:512–519. [DOI] [PubMed] [Google Scholar]
- 22.Pasternak O, Westin CF, Bouix S, et al. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci 2012;32:17365–17372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daianu M, Mendez MF, Baboyan VG, et al. An advanced white matter tract analysis in frontotemporal dementia and early-onset Alzheimer's disease. Brain Imaging Behav 2016;10:1038–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol 2013;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics 2007;4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol 2011;70:374–383. [DOI] [PubMed] [Google Scholar]
- 27.Dennis EL, Hua X, Villalon-Reina J, et al. Tensor-based morphometry reveals volumetric deficits in moderate/severe pediatric traumatic brain injury. J Neurotrauma 2016;33:840–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dennis EL, Prasad G, Babikian T, et al. Adaptive algorithms to map how brain trauma affects anatomical connectivity in children. 11th International Symposium on Medical Information Processing and Analysis (SIPAIM 2015). Cuenca, Ecuador: International Society for Optics and Photonics; 2015.
- 29.Babikian T, Merkley T, Savage RC, Giza CC, Levin H. Chronic aspects of pediatric traumatic brain injury: review of the literature. J Neurotrauma 2015;32:1849–1860. [DOI] [PubMed] [Google Scholar]
- 30.Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci 2004;16:1227–1233. [DOI] [PubMed] [Google Scholar]
- 31.Wu TC, Wilde EA, Bigler ED, et al. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev Neurosci 2010;32:361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilde EA, Ayoub KW, Bigler ED, et al. Diffusion tensor imaging in moderate-to-severe pediatric traumatic brain injury: changes within an 18 month post-injury interval. Brain Imaging Behav 2012;6:404–416. [DOI] [PubMed] [Google Scholar]
- 33.Carrera E, Tononi G. Diaschisis: past, present, future. Brain 2014;137:2408–2422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.