Abstract
Objective:
To assess the prevalence and the specificity of leptomeningeal enhancement (LME) on postcontrast T2–fluid-attenuated inversion recovery (FLAIR) MRI in multiple sclerosis (MS) compared to a variety of inflammatory and noninflammatory neurologic conditions assessed in 2 academic research hospitals.
Methods:
On 3T postcontrast T2-FLAIR images, the presence of focal gadolinium enhancement was evaluated in the leptomeningeal compartment in 254 people with non-MS neurologic conditions or neurotropic viral infections. Based on their clinical diagnosis, patients were grouped as follows: (1) other-than-MS inflammatory neurologic diseases; (2) noninflammatory neurologic diseases; (3) human T-lymphotropic virus (HTLV)–infected; (4) HIV-infected; (5) healthy volunteers.
Results:
LME was detected in 56/254 non-MS cases (22%) vs 74/299 (25%) of MS cases. LME was nearly 4-fold more frequent in non-MS inflammatory neurologic conditions (18/51 cases, 35%) than in noninflammatory neurologic conditions (3/38, 8%) and healthy volunteers (5/66, 8%). The highest prevalence of LME was detected in HTLV infection (17/38 cases, 45%), particularly in the setting of HTLV-associated myelopathy (14/25 cases, 56%). LME also frequently occurred in HIV infection (13/61 cases, 21%). Unlike in MS, LME is not associated with lower brain and cortical volumes in non-MS inflammatory neurologic conditions, including HTLV and HIV infection.
Conclusions:
Despite its relevance to MS pathogenesis and cortical pathology, LME is not specific to MS, occurring frequently in non-MS inflammatory neurologic conditions and especially in those patients with HTLV-associated myelopathy. Overall, this strengthens the notion that LME localizes inflammation-related focal disruption of the blood–meninges barrier and associated scarring.
The breakdown of the blood–leptomeningeal barrier in vessels traversing the subarachnoid space is associated with meningeal inflammation in a variety of neurologic diseases, especially those with an inflammatory or infectious etiology. In a fashion complementary to the more invasive analysis of CSF, postcontrast T2-weighted fluid-attenuated inversion recovery (T2-FLAIR) MRI has recently shown potential in uncovering this specific feature of meningeal inflammation, with greater sensitivity than conventional T1-weighted MRI.1–6
Recently, we reported high prevalence of perivascular leptomeningeal enhancement (LME) on 3T 3D postcontrast T2-FLAIR in a cohort of 299 people with multiple sclerosis (MS): ∼25% had at least one CSF-restricted area of LME (nearly 40% of those with a primary progressive disease course).4 Histopathologic assessment in 2 autopsy cases disclosed clusters of perivascular lymphocytes and macrophages spatially associated with subpial cortical demyelination, and a correlation with cortical atrophy lent support to the hypothesis that focal LME is related to meningeal inflammation and cortical damage in MS.4
In this cross-sectional study of participants in clinical research protocols in 2 academic research hospitals, we assessed the prevalence and specificity of LME on 3T postcontrast T2-FLAIR in a non-MS cohort (n = 254), comparing the results to our previously described MS cohort (n = 299).4 Our goal was to explore the often-overlooked disruption of the blood–leptomeningeal barrier in other-than-MS neurologic diseases (both inflammatory and noninflammatory) and in infection with neurotropic viruses, in particular HTLV and HIV.
METHODS
From late 2010 to early 2016, imaging, laboratory, and clinical data were prospectively collected under institutional review board–approved protocols in 254 adults from 2 academic research hospitals: the NIH Clinical Center (Bethesda, MD) and the San Raffaele Hospital (Milan, Italy). For the purpose of LME evaluation on postcontrast T2-FLAIR images, participants were grouped according to clinical diagnosis, as follows:
Fifty-one individuals with inflammatory and immune-mediated neurologic diseases other than MS spectrum, including neuromyelitis optica spectrum disorder, immune-mediated encephalitis, immune-mediated cerebellar ataxia, systemic inflammatory diseases with white matter (WM) MRI abnormalities not suggestive for MS, and Susac syndrome
Thirty-eight individuals with noninflammatory neurologic diseases, including small vessel disease, migraine, neurodegenerative diseases, and compressive myelopathy
Thirty-eight individuals infected with HTLV, including 25 with a diagnosis of HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP) according to the published WHO diagnostic criteria and 13 with asymptomatic HTLV infection; 18 people with HAM/TSP were untreated, 4 were enrolled in clinical trials of experimental agents at the NIH, 1 was treated with methotrexate, 1 was treated with interferon-β-1a, and 1 was treated with oral prednisone; no asymptomatic HTLV carrier was treated
Sixty-one individuals with HIV infection on antiretroviral therapy and with plasma viral load <200 copies/mL
Sixty-six healthy volunteers (enrolled in NIH-approved studies allowing gadolinium injection); only 2 healthy volunteers overlapped with the cohort in our previous study4
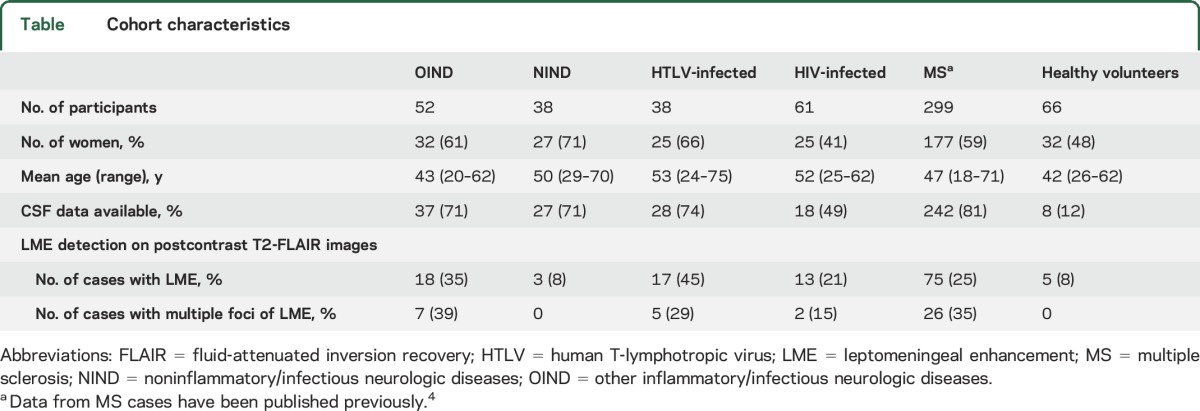
Demographic data are described in the table.
Table.
Cohort characteristics
MRI studies were performed on 4 3T MRI scanners: 2 Philips Intera scanners (Philips Medical Systems, the Netherlands), a Siemens Skyra scanner (Siemens AG, Germany), and a General Electric Signa HDx scanner (GE Healthcare, USA). In all scans, 3D T2-FLAIR images were acquired after postcontrast T1-weighted images. Only scans with 3D T2-FLAIR images obtained at least 10 minutes after IV injection of a single dose (0.1 mmol/kg) of gadolinium-based contrast material were included, as previously described.4
LME was identified on postcontrast T2-FLAIR by an investigator (M.A.) who had participated in our prior study and who used the same criteria for evaluation4: (1) LME was defined as signal intensity within the subarachnoid space substantially greater than that of brain parenchyma and brighter on postcontrast than on precontrast T2-FLAIR images (available here in 81% of cases); and (2) high-signal regions adjacent to dural venous sinuses, basal meninges, and large subarachnoid veins were excluded a priori. When present, LME was classified according to number of foci and associated enhancement on postcontrast T1-weighted images. The presence or absence of contrast-enhancing WM lesions was also assessed on postcontrast T1-weighted images.
Volumes of the brain and cerebral cortex, normalized to the intracranial volume, were obtained using Lesion-TOADS and SPECTRE software on precontrast T1-weighted images, as previously described.7
When available, CSF results (leukocyte count, total protein, and the presence of CSF-restricted oligoclonal immunoglobulin G bands) were recorded. The time between lumbar puncture (LP) and MRI was highly variable, and only 3 scans occurred within a week after the LP. In no case did we observe signs of pachymeningeal diffuse and contiguous enhancement that are commonly associated with LP.
Statistics.
A general linear model on squared age values, adjusted for unequal variances, assessed differences in age among different disease groups as well as a potential effect of participant age on LME. Statistical comparison of LME occurrence across groups was based on a logistic regression model corrected for age. The association between LME and normalized brain as well as cortical volumes was evaluated in disease groups with high LME occurrence (inflammatory and immune-mediated neurologic diseases, HTLV, and HIV infections) using 2 linear heteroscedastic models, including age as covariate. For CSF results, we verified the hypothesis of homogeneous odds ratios (ORs) across disease groups and used a Cochran-Mantel-Haenszel χ2 test with continuity correction to compute the common OR.
Standard protocol approvals, registrations, and patient consents.
The study received approval from an ethical standards committee on human experimentation in each of the 2 academic research hospitals, and written informed consent was obtained from all participants.
RESULTS
Focal LME was detected in 56/254 non-MS cases (22%) vs 74/299 (25%) of previously published MS cases.4 Irrespective of the clinical diagnosis, the main morphologic features of the detected non-MS LME resembled those previously described in MS4: (1) location in the leptomeninges in proximity to one or more vessels; (2) prevalently nodular or linear in shape; and (3) more frequently supratentorial than infratentorial. In the non-MS cohort, LME was found as a single focus in 42/56 cases (75%) and multiple foci in 14/56 (25%). In 73 of 99 identified foci of LME (74%), minimal hyperintense signal, generally in proximity to a meningeal vessel, was also seen on postcontrast T1-weighted images. There was no association between the presence of LME and brain parenchymal enhancing lesions (only 9 of 254 individuals had evidence of contrast enhancement within the brain parenchyma). People with enhancing foci in the leptomeninges were slightly older than those without (mean age 51.2 and 46.1, respectively; p = 0.004); this was consistent across all groups.
Overall, LME was ∼4-fold more frequent in inflammatory and immune-mediated neurologic conditions (18/51 cases, 35%, figure 1) than in non-inflammatory neurologic conditions (3/38 cases, 8%) and healthy volunteers (5/66 cases, 8%, figure e-1 at Neurology.org). The highest prevalence of LME was detected in HTLV infection (17/38 cases, 45%), especially in HAM/TSP (14/25 cases, 56%, figure 2) vs asymptomatic HTLV carriers (3/13 cases, 23%). LME was also frequently found in HIV infection (13/61 cases, 21%). Among patients with LME, multiple foci were more frequently seen in inflammatory and immune-mediated neurologic conditions as well as in HTLV infection. See the table for further details.
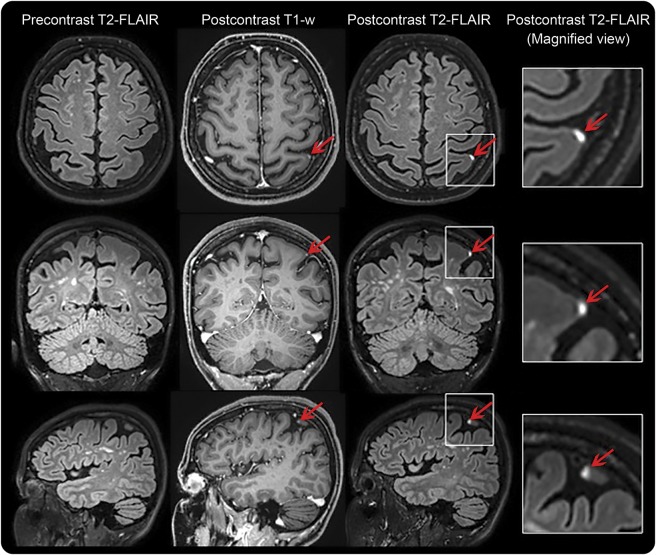
Figure 1. Leptomeningeal contrast enhancement in representative cases.
Leptomeningeal enhancement in a 42-year-old woman with Behçet disease, celiac disease, and multiple white matter lesions. Leptomeningeal enhancement (arrows) corresponds to foci of high signal intensity within the subarachnoid space on postcontrast 3D T2–fluid-attenuated inversion recovery (FLAIR) images at 3T MRI, but not on the corresponding precontrast T2-FLAIR. Postcontrast T1-weighted images show subtle abnormal signal that would not routinely be classified as enhancement. Leptomeningeal enhancement shares common morphologic features among different neurologic and neuroinfectious conditions.
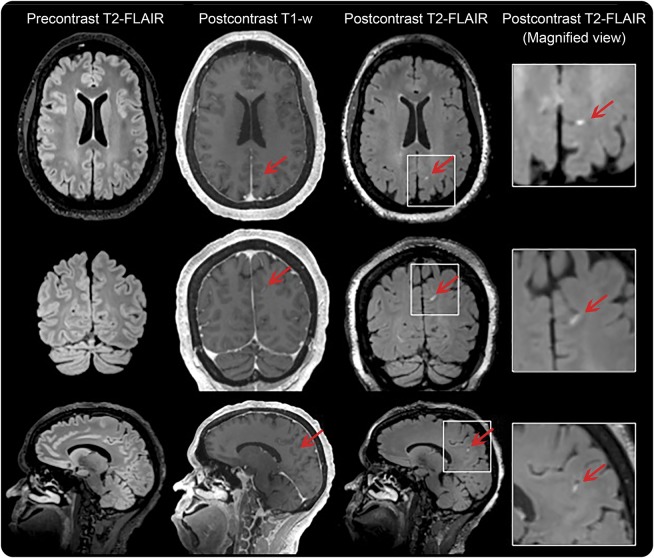
Figure 2. Leptomeningeal enhancement in a 47-year-old woman with human T-lymphotropic virus–1–associated myelopathy/tropical spastic paraparesis, disease duration 20 years, who required 2 walking aids to walk ∼20 m without resting.
FLAIR = fluid-attenuated inversion recovery.
The logistic regression model showed overall significance (likelihood ratio test = 51.3, degrees of freedom = 6, p < 0.0001), with no evidence of model lack of fit (deviance statistics = 551.5, degrees of freedom = 546, p = 0.43). The OR of LME for each disease group (compared to healthy volunteers) is shown in figure 3. Among groups, inflammatory/immune-mediated neurologic conditions, HTLV, and MS showed a significant increase in odds of LME occurrence (p = 0.0007, p = 0.001, and p = 0.02, respectively).
Figure 3. Odds ratios of leptomeningeal enhancement in disease groups compared to healthy controls.
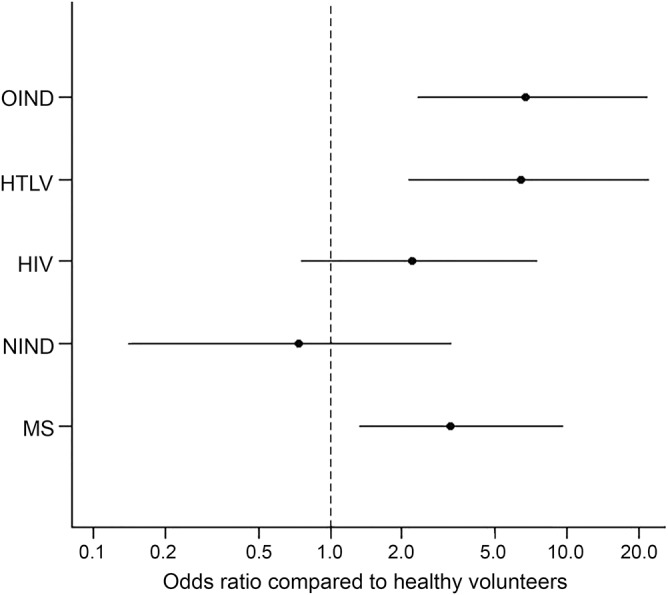
HTLV = human T-lymphotropic virus; MS = multiple sclerosis; NIND = noninflammatory/noninfectious neurologic diseases; OIND = other inflammatory/infectious neurologic diseases.
In those disease groups showing high occurrence of LME, age-corrected normalized brain and cortical volumes were not significantly different in individuals with and without LME (inflammatory and immune-mediated neurologic conditions, p = 0.14, p = 0.09, respectively; HTLV, p = 0.54, p = 0.07, respectively; and HIV, p = 0.56, p = 0.34, respectively).
In 118 of 254 cases (46%), results of CSF examination were available for comparison. Considering the clinical diagnosis in the statistical model, CSF protein (common OR 1.28, 95% confidence interval [CI] 0.44–3.75) and leukocyte count (common OR 1.66, 95% CI 0.46–6.00) were not different in individuals with and without LME. There was no association with CSF-restricted oligoclonal immunoglobulin bands (common OR 0.78, 95% CI 0.20–3.03), which were detected in only 42/87 available cases (48%).
DISCUSSION
In this cross-sectional study, we explored the prevalence and specificity of LME on 3T postcontrast T2-FLAIR images, screening an academic clinical research-based sample of individuals with a variety of neurologic conditions as well as controls. We found that subtle and focal abnormalities of the blood–meninges barrier were frequently observed in inflammatory and immune-mediated neurologic conditions as well as in individuals infected with neurotropic viruses (HTLV and HIV). Furthermore, the low frequency of LME in controls and in individuals without underlying inflammatory neurologic disease provides additional support to the recent notion that CSF-restricted enhancement on postcontrast T2-FLAIR images, when present, is an expression of breakdown of the blood–meningeal barrier, related directly to ongoing inflammation or post-inflammatory scarring, as might occur in traumatic brain injury.8
This interpretation is in line with the role of the leptomeninges as a relay and modulatory gate for peripheral immune cells in health and in a variety of immunopathologic processes that lead to focal blood–meningeal barrier impairment. In viral meningoencephalitis, including that related to HIV, involvement of the leptomeninges is a key pathologic feature. In MS, in which immunologic tolerance toward CNS myelin antigens fails, LME is associated with pathologically detected subpial cortical demyelination, cortical atrophy, and clinical disease progression.4
Among all pathologic conditions explored here, foci of LME were most frequently detected in HTLV (45% of cases) and, remarkably, in the majority (56%) of HAM/TSP cases. In the few reported autopsy cases of HAM/TSP, despite the fact that the most striking pathologic changes were found in the spinal cord, perivascular lymphocyte and monocyte infiltration in thickened leptomeninges was observed in the forebrain.5,9–13 However, this finding was considered to be unrelated to parenchymal tissue damage and the overall clinical picture. In this context, future assessment of the predictive value of LME for the development of HAM/TSP in HTLV-infected individuals would be of great research interest and, perhaps, diagnostic relevance.
Unlike in MS,4,14 LME was not significantly associated with lower brain and cortical volume in this heterogeneous cohort of inflammatory and immune-mediated neurologic conditions or individuals with neurotropic viruses (HTLV and HIV). This is not surprising, as these diseases are characterized by distinct immunologic events occurring within the leptomeninges and disease-specific CSF cytokine/chemokine profiles that might not necessarily trigger the cortical damage and thinning described in MS.4,14
In line with our previous study,4 we did not detect any significant association between LME and routine CSF analysis (total protein, leukocyte count, and CSF-restricted oligoclonal bands), suggesting that LME can identify subtle and focal abnormalities of the blood–meningeal barrier. In future studies, it would be important to assess whether specific cytokine/chemokine profiles in the CSF might correlate with LME in MS vs other neurologic conditions as well as to standardize the timing occurring between MRI acquisition and CSF collection.
Leptomeningeal compartment contrast enhancement is not specific to MS but occurs frequently in non-MS inflammatory and immune-mediated neurologic conditions and, remarkably, in patients with HTLV-associated myelopathy. Despite this, the link between LME and cortical thinning may prove a distinct feature of MS that requires future in-depth correlation analysis with CSF immunologic profiles. The association between LME and spinal cord atrophy, particularly in HAM/TSP, would be useful to ascertain. Overall, this cross-sectional study strengthens the potential role of postcontrast T2-FLAIR MRI for assessing inflammation within the leptomeningeal compartment.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the study participants; the NINDS Neuroimmunology and Neuro-HIV Clinics for recruiting and evaluating the patients and for coordinating the scans; the NIH Functional Magnetic Resonance Imaging Facility; Sally Steinbach and Sonya Steele for coordinating patients with HIV and healthy volunteers, respectively; and Antonella Iadanza for assistance with 3T MRI data acquisition.
GLOSSARY
- CI
confidence interval
- FLAIR
fluid-attenuated inversion recovery
- HAM/TSP
human T-lymphotropic virus–associated myelopathy/tropical spastic paraparesis
- HTLV
human T-lymphotropic virus
- LME
leptomeningeal enhancement
- LP
lumbar puncture
- MS
multiple sclerosis
- OR
odds ratio
- WM
white matter
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Drs. Absinta and Reich: study concept and design. Drs. Absinta and Vuolo, J. Ohayon, and Drs. Nair, Martinelli, Scotti, Falini, Smith, and Cortese: acquisition of data. Drs. Absinta and Vuolo, M.P. de Alwis, A. Meani, and Dr. Reich: analysis and interpretation. Drs. Absinta, Reich, Jacobson, Nath, Cortese, and Filippi: critical revision of the manuscript for important intellectual content. Drs. Absinta and Reich: study supervision.
STUDY FUNDING
The Intramural Research Program of NINDS supported this study.
DISCLOSURE
M. Absinta was partially supported by a National Multiple Sclerosis Society (NMSS) postdoctoral fellowship, award FG 2093-A-1. I. Cortese, L. Vuolo, G. Bhagavtheeshwaran, P. Manori de Alwis, J. Ohayon, and A. Meani report no disclosures relevant to the manuscript. V. Martinelli received honoraria for lectures/consulting services or travel grants from Genzyme, Biogen, Merck Serono, Novartis, Bayer, and Teva. R. Scotti, A. Faliini, B. Smith, A. Nath, and S. Jacobson report no disclosures relevant to the manuscript. M. Filippi received personal compensation for activities with Merck-Serono, Genmab, Biogen-Dompé, Bayer-Schering, and Teva Neuroscience as a consultant, speaker, and advisory board member. He received also research support from Merck-Serono, Biogen-Dompé, Bayer-Schering, Teva Neuroscience, and Fondazione Italiana Sclerosi Multipla (FISM). D. Reich received research support from collaborations with the Myelin Repair Foundation and Vertex Pharmaceuticals. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Mamourian AC, Hoopes PJ, Lewis LD. Visualization of intravenously administered contrast material in the CSF on fluid-attenuated inversion-recovery MR images: an in vitro and animal-model investigation. AJNR Am J Neuroradiol 2000;21:105–111. [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths PD, Coley SC, Romanowski CA, Hodgson T, Wilkinson ID. Contrast-enhanced fluid-attenuated inversion recovery imaging for leptomeningeal disease in children. AJNR Am J Neuroradiol 2003;24:719–723. [PMC free article] [PubMed] [Google Scholar]
- 3.Parmar N, Albisetti M, Berry LR, Chan AK. The fibrinolytic system in newborns and children. Clin Lab 2006;52:115–124. [PubMed] [Google Scholar]
- 4.Absinta M, Vuolo L, Rao A, et al. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology 2015;85:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso A, Eisele P, Ebert AD, et al. Leptomeningeal contrast enhancement and blood-CSF barrier dysfunction in aseptic meningitis. Neurol Neuroimmunol Neuroinflamm 2015;2:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zurawski J, Lassmann H, Bakshi R. Use of magnetic resonance imaging to visualize leptomeningeal inflammation in patients with multiple sclerosis: a review. JAMA Neurol 2017;74:100–109. [DOI] [PubMed] [Google Scholar]
- 7.Shiee N, Bazin PL, Ozturk A, Reich DS, Calabresi PA, Pham DL. A topology-preserving approach to the segmentation of brain images with multiple sclerosis lesions. Neuroimage 2010;49:1524–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiara Ricciardi M, Bokkers RP, Butman JA, et al. Trauma-specific brain abnormalities in suspected mild traumatic brain injury patients identified in the first 48 hours after injury: a blinded magnetic resonance imaging comparative study including suspected acute minor stroke patients. J Neurotrauma 2017;34:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokota T, Yamada M, Furukawa T, Tsukagoshi H. HTLV-I-associated meningitis. J Neurol 1988;235:129–130. [DOI] [PubMed] [Google Scholar]
- 10.Araga S, Takahashi K, Ooi S. Subacute meningoencephalitis associated with human T-lymphotrophic virus type I (HTLV-I): report of a case. Acta Neurol Scand 1989;79:361–365. [DOI] [PubMed] [Google Scholar]
- 11.Kawano Y, Kira J. Chronic hypertrophic cranial pachymeningitis associated with HTLV-I infection. J Neurol Neurosurg Psychiatry 1995;59:435–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartier LM, Cea JG, Vergara C, Araya F, Born P. Clinical and neuropathological study of six patients with spastic paraparesis associated with HTLV-I: an axomyelinic degeneration of the central nervous system. J Neuropathol Exp Neurol 1997;56:403–413. [DOI] [PubMed] [Google Scholar]
- 13.Umehara F, Okada Y, Fujimoto N, Abe M, Izumo S, Osame M. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in HTLV-I-associated myelopathy. J Neuropathol Exp Neurol 1998;57:839–849. [DOI] [PubMed] [Google Scholar]
- 14.Zivadinov R, Ramasamy DP, Vaneckova M, et al. Leptomeningeal contrast enhancement is associated with progression of cortical atrophy in MS: a retrospective, pilot, observational longitudinal study. Mult Scler Epub 2016 Nov 4. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.