Abstract
Objective:
To characterize patients with myositis with HIV infection.
Methods:
All HIV-positive patients with myositis seen at the Johns Hopkins Myositis Center from 2003 to 2013 were included in this case series. Muscle biopsy features, weakness pattern, serum creatine kinase (CK) level, and anti–nucleotidase 1A (NT5C1A) status of HIV-positive patients with myositis were assessed.
Results:
Eleven of 1,562 (0.7%) patients with myositis were HIV-positive. Myositis was the presenting feature of HIV infection in 3 patients. Eight of 11 patients had weakness onset at age 45 years or less. The mean time from the onset of weakness to the diagnosis of myositis was 3.6 years (SD 3.2 years). The mean of the highest measured CK levels was 2,796 IU/L (SD 1,592 IU/L). On muscle biopsy, 9 of 10 (90%) had endomysial inflammation, 7 of 10 (70%) had rimmed vacuoles, and none had perifascicular atrophy. Seven of 11 (64%) patients were anti-NT5C1A-positive. Upon presentation, all had proximal and distal weakness. Five of 6 (83%) patients followed 1 year or longer on immunosuppressive therapy had improved proximal muscle strength. However, each eventually developed weakness primarily affecting wrist flexors, finger flexors, knee extensors, or ankle dorsiflexors.
Conclusions:
HIV-positive patients with myositis may present with some characteristic polymyositis features including young age at onset, very high CK levels, or proximal weakness that improves with treatment. However, all HIV-positive patients with myositis eventually develop features most consistent with inclusion body myositis, including finger and wrist flexor weakness, rimmed vacuoles on biopsy, or anti-NT5C1A autoantibodies.
Polymyositis (PM) and dermatomyositis are autoimmune muscle diseases characterized by the subacute development of symmetric proximal muscle weakness, elevated creatine kinase (CK) levels, myositis autoantibodies, and, in the case of dermatomyositis, characteristic rashes.1,2 PM muscle biopsies frequently reveal lymphocytes surrounding and invading non-necrotic muscle fibers (i.e., primary inflammation) whereas perifascicular atrophy is the hallmark histologic feature of dermatomyositis. Patients with inclusion body myositis (IBM) typically present over the age of 50 years with slowly progressive asymmetric weakness preferentially affecting the quadriceps, wrist flexors, and finger flexor muscles, though proximal muscle weakness may also be observed.3 IBM muscle biopsies usually have evidence of primary inflammation, but can be differentiated from PM muscle biopsies by the presence of rimmed vacuoles in ∼85% of cases.4 IBM muscle biopsies frequently show amyloid with Congo red stain or inclusions with electron microscopy or immunostaining for ubiquitin or aggregated proteins (e.g., TDP-43, or p62).5–8 Furthermore, a majority of patients with IBM have antibodies recognizing cytosolic 5′ nucleotidase 1A (NT5C1A); these are not found in the vast majority of patients with PM.9 Distinguishing PM from IBM is of critical importance because the former disease is responsive to immunosuppression whereas the latter disease is largely refractory to treatment.10
Prior reports have suggested that patients with HIV infection may develop various forms of myositis (HIV-myositis), including PM,11–14 dermatomyositis,15 or IBM.14,16–18 However, the combination of detailed strength testing, comprehensive muscle biopsy analysis, and anti-NT5C1A testing has not been reported in the existing case series of patients with HIV-myositis. In this case series, we provide detailed descriptions of all 11 patients with HIV-myositis referred to the Johns Hopkins Myositis Center. These patients did not clearly fit well-established definitions of PM or IBM. Instead, most patients had overlapping features of both diseases at initial presentation, but over time, progressed into a pattern characteristic of IBM.
METHODS
Design.
All HIV-positive patients enrolled in the Johns Hopkins Myositis Center Longitudinal Cohort from 2003 through 2013 were included in this retrospective case series.
Patients with myositis.
All of the patients were evaluated as part of routine clinical care at the outpatient neurology clinic at the Johns Hopkins University Hospital or Johns Hopkins Bayview Medical Center in Baltimore, Maryland.
Control HIV-positive patients.
Sera from 21 HIV-positive patients without muscle disease were obtained from Dr. Ned Sacktor.
Assessment of neuromuscular disease.
Strength assessment was completed by 1 of 3 physicians (A.L.M., T.E.L., or L.C-S.) through manual muscle testing and graded by a locally modified and systematically used Medical Research Council (MRC) scale. For each muscle group tested, strength results were recorded in the following format: right/left. Distal finger flexor (i.e., flexor digitorum profundus) strength was tested by having patients flex the distal phalanx of each finger against resistance with the corresponding middle phalanx fixed. All patients had a myopathy workup, including EMG and clinical laboratory studies (CK); all but one patient had a muscle biopsy. An EMG was defined as consistent with myositis if there was both a myopathic recruitment pattern (i.e., early recruitment of polyphasic, short-duration, or low-amplitude motor unit action potentials) and spontaneous activity (i.e., fibrillation potentials or positive sharp waves).
Screening for anti-NT5C1A autoantibodies was performed by immunoblotting as previously described.19
Statistics.
All statistical analysis was performed using STATA 11 (StataCorp, College Station, TX). We examined pairwise correlations between dichotomous disease features including NT5C1A antibody status, immunotherapy at blood draw status, sex, race (black or white), presence of rimmed vacuoles, presence of primary inflammation, history of an AIDS-defining illness, and antiretroviral history at the time of diagnosis. Pairwise comparisons for dichotomous and continuous variables among groups were made using Fisher exact test and Wilcoxon rank-sum test, respectively: age at diagnosis of HIV and diagnosis of myositis, latency between HIV and weakness, mean of the highest measured CK levels, and length of follow-up. For this analysis, MRC strength scores were converted to a 10-point scale adapted from the manual muscle strength testing scale used by the International Myositis Assessment and Clinical Studies group20: 10 = MRC 5, 9 = MRC 4+, 8 = MRC 4, 7 = MRC 4−, 5 = MRC 3, 1.5 = MRC 2, and 0 = MRC 1 or 0. Because of the hypothesis-generating nature of this case series, we considered p < 0.05 to be significant.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Johns Hopkins Institutional Review Board and written informed consent was obtained from each patient.
RESULTS
Illustrative cases.
Case 1.
At age 57 years, a woman began to have trouble getting out of chairs and raising her arms above her head. She then developed difficulty swallowing and started to have falls. She was referred to a neuromuscular specialist, who noted proximal muscle weakness. Her CK was elevated at 4,476 IU/L and EMG was consistent with myositis. Muscle biopsy showed non-necrotic muscle fibers surrounded and invaded by lymphocytes but no rimmed vacuoles. After prebiopsy laboratory testing revealed that she was HIV-positive, she was started on treatment with abacavir, lamivudine, and zidovudine. On examination at our clinic, she was noted to have weakness of neck flexors (5−), arm abductors (4+/4+), wrist extensors (4+/4+), wrist flexors (4−/4−), distal finger flexors (4/4−), hip flexors (4−/4−), ankle dorsiflexors (4+/4+), and plantarflexors (4+/4+). She was started on prednisone and mycophenolate mofetil with a reduction of her CK level to 169 IU/L, normalization of her muscle strength, and resolution of her dysphagia. However, after discontinuation of immunosuppressive therapy for several months, she developed increasing CK levels to 702 IU/L and re-emergence of weakness restricted to wrist flexors (4+/4+) and distal finger flexors (4+/4+). She had positive testing for anti-NT5C1A autoantibodies.
Case 2.
A man was diagnosed with HIV infection at age 37 years and was a nonprogressor without antiretroviral treatment. Ten years later, while running 12 miles a day to train for a marathon, he developed muscle weakness; within months, he became unable to run 2 blocks. On initial evaluation at Johns Hopkins Neurology, he had weak deltoids (4+/4+), hip flexors (4/4), and ankle dorsiflexors (4−/4−). Laboratory results included a CD4 count of 1,188 cells/mm3, viral load of 26,864 copies/mL, and CK of 4,855 IU/L. EMG showed findings consistent with myositis and a quadriceps muscle biopsy revealed primary inflammation without rimmed vacuoles. After a few months of treatment with prednisone (60 mg/d) and azathioprine (100 mg/d), he regained 5/5 power in all muscles and his CK dropped to 350 IU/L. However, as the prednisone was tapered, his weakness returned and CK levels increased. After adjusting the azathioprine dose up to 200 mg/d, his CD4 counts declined to 105 cells/mm3; azathioprine was discontinued, and he was started on tenofovir-emtricitabine, atazanavir, ritonavir, and abacavir. He subsequently developed progressive weakness with elevated CK levels that did not improve with steroids, methotrexate, and IV immunoglobulin (IVIg). His examination showed full power in the deltoids and biceps; however, triceps, wrist flexors, and finger flexors were weak (∼4/4) with asymmetry. He had weakness of hip flexors (4/4), knee flexors (4/4), knee extensors (2/2), and ankle dorsiflexors (4/4). The serum CK was 752 IU/L. A repeat biceps muscle biopsy showed primary inflammation with rimmed vacuoles. Testing of a serum sample obtained near the time of his second biopsy revealed that he was anti-NT5C1A-positive.
Demographics and presentation of HIV-myositis.
Eleven out of 1,562 (0.7%) patients in the Johns Hopkins Myositis Center cohort were identified as HIV-positive; no HIV-positive patients with myositis had weakness that resolved following discontinuation of potentially toxic medications. The patients with HIV-myositis were 82% male, 60% black, and diagnosed with HIV at a mean age of 32 years (SD 12 years) (table 1). The mean age at weakness onset was 41 years (SD 9 years), with an average of 9 years (SD 10 years) from the time of HIV diagnosis until noticing weakness. Eight of 11 (73%) patients were age 45 or younger at the onset of weakness, and the mean time from the onset of weakness to the diagnosis of myositis was 3.6 years (SD 3.2 years). Interestingly, there was a bimodal distribution in the gap between HIV diagnosis and onset of muscle symptoms. Five of 11 patients experienced weakness within 3 years of HIV diagnosis, including 3 (cases 1, 8, and 9) in whom the weakness preceded and led to the HIV diagnosis; the rest did not notice weakness until 10 or more years later. Only one patient (case 9) had an AIDS-defining illness before, during, or after developing HIV-myositis. Among the 11 patients with HIV-myositis, 2 (18%) were coinfected with hepatitis C and 1 (9%) had evidence of active hepatitis B infection. In 4 patients (1, 2, 8, and 9), weakness developed before exposure to antiretroviral agents. We were unable to determine with certainty whether antiretroviral therapy preceded muscle weakness in 4 of the patients. Since many of the patients in this series were diagnosed with HIV and received their HIV care outside of the Hopkins system, we were unable to obtain complete documentation of antiretroviral use, which, in some cases, had been initiated decades earlier (available data can be found in table e-1 at Neurology.org). Thus, we were unable to make definite conclusions about the possible relationship of specific antiretroviral drugs to the development of myositis symptoms.
Table 1.
Demographics and clinical features of patients with HIV-myositis

The mean of the highest measured CK levels of patients with HIV-myositis was 2,796 IU/L (SD: 1,591 IU/L), and EMG revealed findings consistent with myositis in 10 of 11 (91%) patients (table 1). All patients presented with both symmetric proximal muscle weakness and distal weakness (including the finger flexors, wrist flexors, or ankle dorsiflexors). Eight patients had neck flexion weakness and 7 developed dysphagia.
Biopsy and autoantibody testing in HIV-myositis.
Muscle biopsy analysis revealed that 9 of 10 (88.9%) patients had endomysial inflammation; 3 out of 5 (60%) biopsies available for review at the Johns Hopkins Neuromuscular Pathology Laboratory had clear invasion of normal-appearing muscle fibers by inflammatory cells. Muscle biopsies from 6 of 10 (60%) had rimmed vacuoles characteristic of IBM (table 1). Of 6 patients with HIV-myositis, 3 (50%) had innumerable COX-negative fibers, a common finding seen in patients with IBM. TDP-43 immunostaining was performed on 1 patient (case 8) and p62 immunostaining on 3 patients; characteristic cytoplasmic aggregates were seen in all cases. No patient had perifascicular atrophy as seen in dermatomyositis. Seven of 11 (64%) patients had sera positive for the IBM-associated autoantibody recognizing NT5C1A, including all 3 patients who did not have rimmed vacuoles on biopsy. By comparison, only 2 out of 21 (9.5%) HIV-positive patients without muscle disease were anti-NT5C1A-positive (p = 0.001).
Comparing those with and without anti-NT5C1A autoantibodies, there were no differences in sex, race, muscle biopsy features (i.e., rimmed vacuoles and primary inflammation), mean of the highest measured CK levels, age at HIV diagnosis, age at weakness onset, immunosuppressive therapy, or latency between HIV diagnosis and onset of weakness.
Comparison of HIV-myositis patients with PM and IBM at initial presentation.
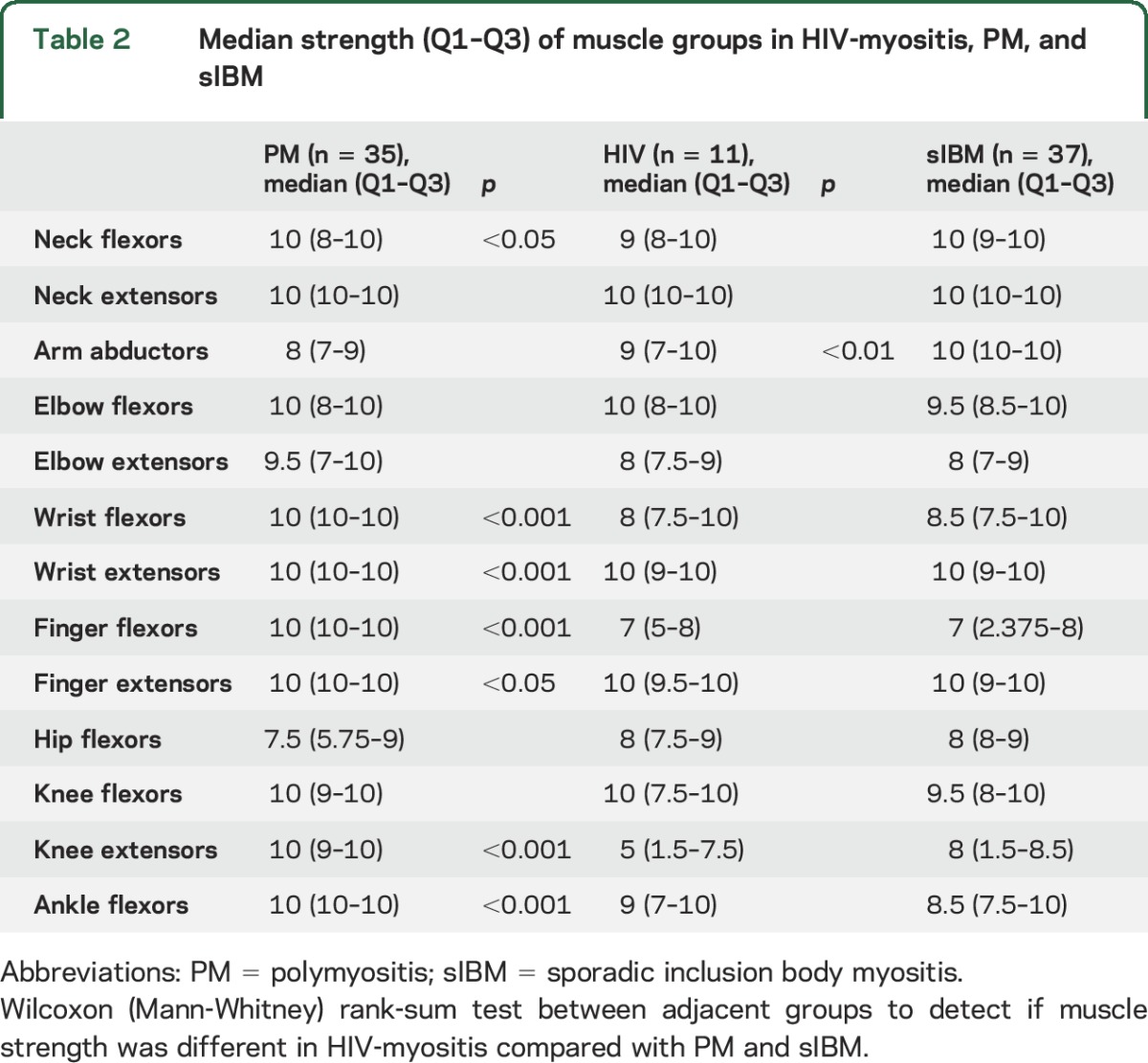
Although HIV-myositis patients have many clinical features of sporadic IBM, none of the 11 patients met ENMC 2011 criteria for probable or clinically defined IBM.21 Seven of 11 patients failed to meet diagnostic criteria for IBM due to age at onset <45, and 4 of 10 failed due to serum CK >12× the upper limit of normal. We compared the pattern of weakness in HIV-myositis at initial presentation with 37 patients with sporadic IBM and 35 patients with PM. As shown in table 2, the pattern of weakness for HIV-myositis is similar to that of sporadic IBM, with the exception of significantly more deltoid weakness in HIV-myositis.
Table 2.
Median strength (Q1–Q3) of muscle groups in HIV-myositis, PM, and sIBM
Longitudinal course of HIV-myositis.
Five patients with HIV-myositis were followed for more than 5 years, and each of these was treated with immunosuppressive therapy. In all 5 patients, there was an improvement in proximal arm strength, but finger flexor and quadriceps weakness characteristic of IBM remained or progressed (figure). Three patients (cases 5, 6, and 7) presented to the Myositis Center having tried immunosuppressive therapy with no reported improvement, 1 patient (case 8) tried IVIg for more than a year without improvement, and 2 patients (cases 10 and 11) have never been exposed to immunosuppressive therapy. To date, we have not followed untreated patients with HIV-myositis for long enough to determine whether proximal muscle weakness might improve spontaneously.
Figure. Evolution of muscle strength in patients with HIV-myositis followed longitudinally using locally weighted regression.
DISCUSSION
In this case series, we comprehensively describe the detailed clinical presentations, muscle biopsy findings, and anti-NT5C1A testing in patients with HIV-myositis. By the time of their most recent visit, none of the 11 patients described here fulfilled Bohan and Peter criteria for definite PM22,23 given the presence of distal weakness that was often asymmetric. Similarly, no patient fulfilled European Neuromuscular Centre 2011 criteria for definite or probable IBM for a variety of reasons, including lack of intracellular amyloid deposition, age at onset <45, or CK levels greater than 12 times the upper limit of normal. Indeed, the young age at onset (8 of 11 experienced weakness at age 45 or younger) especially distinguishes these patients with HIV-myositis from patients with typical IBM. Features most consistent with IBM included the long interval between the onset of muscle symptoms and the diagnosis of myositis, the eventual emergence of distal weakness in every patient, and either rimmed vacuoles on biopsy, positive anti-NT5C1A autoantibodies, or both. Conversely, in addition to young age, features more typical for PM in our series included markedly elevated CK levels and, in those patients followed longitudinally, improved proximal arm abduction strength following immunosuppression. No patient with HIV-myositis presented to our clinic with a skin rash characteristic of dermatomyositis. Also, no patient with HIV-myositis developed interstitial lung disease, Raynaud phenomenon, arthritis, or any systemic autoimmune diseases as are frequently found in association with PM. Overall, many patients initially presented with what could be characterized as a PM/IBM overlap that eventually evolved into an IBM phenotype.
Interestingly, a recent study from Japan revealed that hepatitis C infection may predispose patients to develop IBM.24 Although HIV-infected patients are at increased risk of hepatitis C coinfection, only 2 in our cohort of 11 patients with HIV-myositis were also infected with hepatitis C. Thus, hepatitis C and HIV are likely to be independent risk factors for developing myositis. Furthermore, the clinical features of hepatitis C-infected patients with IBM from Japan, including an age at onset around 65 years, were indistinguishable from those without infection. In contrast, the clinical features of patients with HIV-myositis in our cohort were not identical to our IBM control group. This suggests the possibility that hepatitis C and HIV infection may predispose patients to different forms of myositis.
Our finding that most patients with HIV-myositis have prominent features of IBM may be surprising given that the largest similar case series reported that all patients with HIV-myositis had PM. In that study, 13 HIV-positive patients were diagnosed with PM based on the presence of biopsy-proven PM in each one and proximal muscle weakness in the majority.13 As in our study, proximal muscle weakness improved during the course of longitudinal follow-up: 8 patients improved with immunosuppressive treatment and 4 had spontaneous remission of their muscle disease. While these patients were reported as having HIV-PM, the authors did not indicate whether rimmed vacuoles were observed. Furthermore, the presence or absence of distal weakness on initial presentation and at follow-up was not reported in that study. Along with the unavailability of anti-NT5C1A testing at the time of the prior study, the absence of information regarding rimmed vacuoles and distal weakness makes it difficult to determine whether some of those patients could have had features of IBM.
While we are aware of 7 other HIV-IBM cases reported in detail in the literature,16–18 none was described as presenting with proximal muscle weakness that improved during follow-up as we observed in several of the patients reported here. Of note, a recent study included detailed histologic analyses of 19 biopsies from patients with HIV-myositis14; 5 cases were pathologically classified as PM and 14 cases as IBM. However, since only minimal clinical information accompanied each biopsy specimen, these investigators could not comment on whether those patients who had biopsies consistent with PM had anti-NT5C1A autoantibodies or a pattern of weakness more consistent with IBM.
Although the numbers of patients studied was small, we did not find clinical differences between patients with HIV-myositis with and without anti-NT5C1A autoantibodies. Importantly, myositis can be the presenting feature of HIV infection, which occurred in 3 patients in this case series. One of these patients developed pneumocystis pneumonia after initiating immunosuppressive therapy for myositis prior to the diagnosis of HIV infection. Given this experience, we propose that HIV testing be performed in at-risk patients with an initial diagnosis of myositis, particularly those with clinical features of PM/IBM overlap; prophylactic antibiotics should be strongly considered in those who test positive and will be treated with immunosuppressive medications.
In this study, we found that all 11 HIV-positive patients in our cohort developed 2 or more of the following typical IBM features: weakness of wrist and finger flexors, rimmed vacuoles on muscle biopsy, and positive anti-NT5C1A antibodies. However, proximal muscle weakness was common and arm abduction strength improved following immunosuppressive therapy in some of our patients with HIV-myositis. Although we are unable to conclude whether improvement resulted from treatment or reflects the natural history of the disease, it may be reasonable to offer a trial of immunosuppressive therapy to patients with HIV-myositis who have significant proximal muscle weakness. Given the increased risk of using immunosuppressive medications in this population, we recommend that a trial of such medications only be attempted under the co-supervision of an infectious disease expert.
Supplementary Material
GLOSSARY
- CK
creatine kinase
- IBM
inclusion body myositis
- IVIg
IV immunoglobulin
- MRC
Medical Research Council
- NT5C1A
nucleotidase 1A
- PM
polymyositis
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Thomas E. Lloyd: drafting/revising the manuscript for content, study concept and design, analysis and interpretation of data, acquisition of data, statistical analysis, study supervision and coordination. E. Harlan Michele: drafting/revising the manuscript for content, analysis and interpretation of data, acquisition of data. Iago Pinal-Fernandez: drafting/revising the manuscript for content, analysis and interpretation of data, acquisition of data, statistical analysis. Lisa Christopher-Stine: drafting/revising the manuscript for content, acquisition of data. Katherine Pak: drafting/revising the manuscript for content, acquisition of data. Ned Sacktor: drafting/revising the manuscript for content, acquisition of data. Andrew L. Mammen: drafting/revising the manuscript for content, study concept and design, analysis and interpretation of data, acquisition of data, study supervision and coordination.
STUDY FUNDING
This research was supported in part by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH. T.E.L. is supported by R01 NS082563 and NS094239. L.C.-S. and the Myositis Research Database are supported by the Huayi and Siuling Zhang Discovery Fund.
DISCLOSURE
T. Lloyd, I. Pinal-Fernandez, and H. Michelle report no disclosures relevant to the manuscript. L. Christopher-Stine has received personal fees from Walgreens, Novartis, Idera Pharmaceuticals, Ono Pharma UK, Marathon Pharmaceuticals, Medimmune/AstraZeneca, and Mallinckrodt; is a consultant for Medimmune, Optioncare, and Mallinckrodt; is on the advisory boards of Mallinckrodt and Novartis; and has intellectual property interest in the HMGCR assay licensed to Inova Diagnostics. K. Pak and N. Sacktor report no disclosures relevant to the manuscript. A. Mammen holds a patent on an anti-HMGCR autoantibody test licensed to Inova Diagnostics but receives no royalties from this. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Rider LG, Miller FW. Deciphering the clinical presentations, pathogenesis, and treatment of the idiopathic inflammatory myopathies. JAMA 2011;305:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalakas MC. Inflammatory muscle diseases. N Engl J Med 2015;373:393–394. [DOI] [PubMed] [Google Scholar]
- 3.Griggs RC, Askanas V, DiMauro S, et al. Inclusion body myositis and myopathies. Ann Neurol 1995;38:705–713. [DOI] [PubMed] [Google Scholar]
- 4.Chahin N, Engel AG. Correlation of muscle biopsy, clinical course, and outcome in PM and sporadic IBM. Neurology 2008;70:418–424. [DOI] [PubMed] [Google Scholar]
- 5.Hiniker A, Daniels BH, Lee HS, Margeta M. Comparative utility of LC3, p62 and TDP-43 immunohistochemistry in differentiation of inclusion body myositis from polymyositis and related inflammatory myopathies. Acta Neuropathol Commun 2013;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubourg O, Wanschitz J, Maisonobe T, et al. Diagnostic value of markers of muscle degeneration in sporadic inclusion body myositis. Acta Myol 2011;30:103–108. [PMC free article] [PubMed] [Google Scholar]
- 7.Salajegheh M, Pinkus JL, Taylor JP, et al. Sarcoplasmic redistribution of nuclear TDP-43 in inclusion body myositis. Muscle Nerve 2009;40:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nogalska A, Terracciano C, D'Agostino C, King Engel W, Askanas V. p62/SQSTM1 is overexpressed and prominently accumulated in inclusions of sporadic inclusion-body myositis muscle fibers, and can help differentiating it from polymyositis and dermatomyositis. Acta Neuropathol 2009;118:407–413. [DOI] [PubMed] [Google Scholar]
- 9.Larman HB, Salajegheh M, Nazareno R, et al. Cytosolic 5'-nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann Neurol 2013;73:408–418. [DOI] [PubMed] [Google Scholar]
- 10.Benveniste O, Guiguet M, Freebody J, et al. Long-term observational study of sporadic inclusion body myositis. Brain 2011;134:3176–3184. [DOI] [PubMed] [Google Scholar]
- 11.Simpson DM, Bender AN. Human immunodeficiency virus-associated myopathy: analysis of 11 patients. Ann Neurol 1988;24:79–84. [DOI] [PubMed] [Google Scholar]
- 12.Illa I, Nath A, Dalakas M. Immunocytochemical and virological characteristics of HIV-associated inflammatory myopathies: similarities with seronegative polymyositis. Ann Neurol 1991;29:474–481. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RW, Williams FM, Kazi S, Dimachkie MM, Reveille JD. Human immunodeficiency virus-associated polymyositis: a longitudinal study of outcome. Arthritis Rheum 2003;49:172–178. [DOI] [PubMed] [Google Scholar]
- 14.Hiniker A, Daniels BH, Margeta M. T-cell-mediated inflammatory myopathies in HIV-positive individuals: a histologic study of 19 cases. J Neuropathol Exp Neurol 2016;75:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll MB, Holmes R. Dermatomyositis and HIV infection: case report and review of the literature. Rheumatol Int 2011;31:673–679. [DOI] [PubMed] [Google Scholar]
- 16.Cupler EJ, Leon-Monzon M, Miller J, Semino-Mora C, Anderson TL, Dalakas MC. Inclusion body myositis in HIV-1 and HTLV-1 infected patients. Brain 1996;119:1887–1893. [DOI] [PubMed] [Google Scholar]
- 17.Dalakas MC, Rakocevic G, Shatunov A, Goldfarb L, Raju R, Salajegheh M. Inclusion body myositis with human immunodeficiency virus infection: four cases with clonal expansion of viral-specific T cells. Ann Neurol 2007;61:466–475. [DOI] [PubMed] [Google Scholar]
- 18.Loutfy MR, Sheehan NL, Goodhew JE, Walmsley SL. Inclusion body myositis: another possible manifestation of antiretroviral-associated mitochondrial toxicity. AIDS 2003;17:1266–1267. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd TE, Christopher-Stine L, Pinal-Fernandez I, et al. Cytosolic 5'-nucleotidase 1A as a target of circulating autoantibodies in autoimmune diseases. Arthritis Care Res (Hoboken) 2016;68:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rider LG, Koziol D, Giannini EH, et al. Validation of manual muscle testing and a subset of eight muscles for adult and juvenile idiopathic inflammatory myopathies. Arthritis Care Res (Hoboken) 2010;62:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose MR; Group EIW. 188th ENMC international workshop: inclusion body myositis, 2–4 December 2011, Naarden, The Netherlands. Neuromuscul Disord 2013;23:1044–1055. [DOI] [PubMed] [Google Scholar]
- 22.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–347. [DOI] [PubMed] [Google Scholar]
- 23.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403–407. [DOI] [PubMed] [Google Scholar]
- 24.Uruha A, Noguchi S, Hayashi YK, et al. Hepatitis C virus infection in inclusion body myositis: a case-control study. Neurology 2016;86:211–217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



