Abstract
Objective:
With the emergency department (ED) being a high-risk site for diagnostic errors, we sought to estimate ED diagnostic accuracy for identifying acute cerebrovascular events.
Methods:
MEDLINE and Embase were searched for studies (1995–2016) reporting ED diagnostic accuracy for ischemic stroke, TIA, or subarachnoid hemorrhage (SAH). Two independent reviewers determined inclusion. We identified 1,693 unique citations, examined 214 full articles, and analyzed 23 studies. Studies were rated on risk of bias (QUADAS-2). Diagnostic data were extracted. We prospectively defined clinical presentation subgroups to compare odds of misdiagnosis.
Results:
Included studies reported on 15,721 patients. Studies were at low risk of bias. Overall sensitivity (91.3% [95% confidence interval (CI) 90.7–92.0]) and specificity (92.7% [91.7–93.7]) for a cerebrovascular etiology was high, but there was significant variation based on clinical presentation. Misdiagnosis was more frequent among subgroups with milder (SAH with normal vs abnormal mental state; false-negative rate 23.8% vs 4.2%, odds ratio [OR] 7.03 [4.80–10.31]), nonspecific (dizziness vs motor findings; false-negative rate 39.4% vs 4.4%, OR 14.22 [9.76–20.74]), or transient (TIA vs ischemic stroke; false discovery rate 59.7% vs 11.7%, OR 11.21 [6.66–18.89]) symptoms.
Conclusions:
Roughly 9% of cerebrovascular events are missed at initial ED presentation. Risk of misdiagnosis is much greater when presenting neurologic complaints are mild, nonspecific, or transient (range 24%–60%). This difference suggests that many misdiagnoses relate to symptom-specific factors. Future research should emphasize studying causes and designing error-reduction strategies in symptom-specific subgroups at greatest risk of misdiagnosis.
A recent US National Academy of Medicine report highlights that medical misdiagnosis is a major public health problem likely to affect almost everyone at least once in his or her lifetime, sometimes with devastating consequences.1 Estimates suggest that more than 12 million Americans are misdiagnosed each year, up to half of whom may have serious preventable harms as a result.2 Even conservative estimates that consider only hospital deaths suggest that misdiagnosis accounts for at least 40,000–80,000 deaths annually in the United States.3 Less is known about the aggregate burden of misdiagnosis-related morbidity, although it is estimated that, among hospital adverse events, 47% of diagnostic errors result in serious disability.4 A national analysis of closed malpractice claims suggests that serious disability from diagnostic error is probably at least as common as death.5 The worldwide burden is likely even higher.
The emergency department (ED) is a high-risk site for preventable errors.6 Among adverse events in the ED deemed negligent, most are diagnostic failures, and more than half of closed malpractice claims in emergency medicine are diagnosis-related.7 The majority of these relate to inappropriate discharge, with half of the patients released from the ED having, in retrospect, met criteria for admission.6 The spectrum of ED diagnostic errors seen in closed-claims malpractice cases is broad, but some studies suggest that ED misdiagnoses may be unevenly distributed and disproportionate for neurologic conditions. A recent study of 242 closed ED claims found that 3 of the top 5 diagnoses were neurologic (stroke, meningitis, spinal epidural abscess), together accounting for 20% of diagnostic claims.7 In this study, acute stroke claims were more than twice as common as those for acute myocardial infarction (13.4% vs 5.4%).7 Such results accord with hospital records analyses indicating that deaths due to cerebrovascular events result from diagnostic error far more frequently than those due to myocardial infarction (45% vs 1%, p < 0.001).8
Stroke is a leading cause of major long-term disability in the United States and an enormous source of global disease burden.9 Among major diagnostic errors reported by physicians, stroke is the fourth most common.10 Closed-claims analyses focused on neurologic conditions find failure to diagnose accounts for the majority of errors, stroke is the most common misdiagnosis, and more than 20% occur in the ED.11 A recent cross-sectional analysis using linked inpatient and ED visit records across 9 US states suggested the frequency of an initial misdiagnosis in the preceding 30 days was between 1.2% and 12.7% of all hospital stroke admissions.12 Disproportionally higher odds were found for patients presenting with headache or dizziness, suggesting that presenting symptoms may be an important predictor of misdiagnosis risk.12
Nevertheless, controversy exists over the true rate of stroke misdiagnosis in the ED.13,14 Reported misdiagnosis rates with acute cerebrovascular disease range from as low as 4%15 to as high as 64%,16 depending on the clinical population under study. While it is clear that these rates compare unfavorably to myocardial infarction (ED misdiagnosis rate ∼2%),17 more precise estimates would help clarify the burden of harms from misdiagnosis and could help identify subgroups for which misdiagnosis-reduction interventions should be sought.
In an effort to rigorously address this knowledge gap, we conducted a systematic review of observational studies of diagnostic accuracy for cerebrovascular disorders in the ED. We hypothesized that errors would be more frequent in patients with milder, nonspecific, or transient cerebrovascular manifestations.
METHODS
Data sources and searches.
We searched MEDLINE (via PubMed) and Embase for articles using text words and controlled-vocabulary terms related to misdiagnosis or diagnosis-focused research studies (appendix e-1 at Neurology.org). We limited our search to articles published since 1995 and reporting on patients from 1995 or later, since advances in neuroimaging and stroke therapy in the early 1990s substantially altered expectations for diagnosis of patients with possible cerebrovascular disease. Our search was updated through February 26, 2016.
Study selection.
Articles were selected by 2 independent raters using predetermined inclusion criteria and a structured process (appendix e-1). Our focus was studies examining the accuracy of cerebrovascular diagnosis in the ED by ED physicians. We sought to eliminate studies in which patients were diagnosed largely by prehospital providers, advanced practice providers, or consultant neurologists, although full details of the specific providers involved in ED diagnosis were not available for some studies (appendix e-2). We calculated interrater agreement on full-text inclusion using Cohen kappa.
Data extraction and quality assessment.
Quality of evidence was assessed with respect to our primary outcome measures for diagnostic accuracy (appendix e-3). We excluded studies with a low diagnostic reference standard and further assessed the risk of bias or applicability concerns for each included study using QUADAS-2 criteria (appendix e-3). Information abstracted from each eligible article included the type of study conducted, the number of research participants, and the number of true-positive, false-positive, true-negative, and false-negative diagnoses, if available. When possible, we extracted data on the nature of false-positive and false-negative ED diagnoses. For 10 studies, we attempted to contact the first or corresponding author for additional study information. Seven authors responded and 5 provided additional information.
Data synthesis and analysis.
We report the accuracy of ED diagnosis overall and by cerebrovascular condition. While several studies reported on patients with intracerebral hemorrhage (ICH) as well, we did not attempt to analyze those patients separately since no numbers on false negatives and false positives could be retrieved. We calculated sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) for ED diagnoses for any cerebrovascular condition. Although properly considered proportions rather than rates, we use here the more common terminology, defining the false-negative rate as 1 − sensitivity, false-positive rate as 1 − specificity, false discovery rate as 1 − NPV, and false omission rate as 1 − PPV (appendix e-1).
When only partial results from a particular study were reported (e.g., no data on patients without stroke or false positives), we excluded that study only from the relevant calculations (e.g., specificity, PPV), but not the remaining calculations (e.g., sensitivity, NPV) (appendix e-4). Where studies included data both from an ED population and other populations (e.g., primary care or prehospital), we included only results from the ED population. We present proportions and, where appropriate, 95% confidence intervals (CIs). Tests of heterogeneity were conducted based on the Cochran Q test. We assessed for possible trends in diagnostic accuracy parameters over time using weighted linear regression with weights equal to 1 over the estimated standard error of the accuracy parameters (equivalent to the χ2 test for trend).
Three prospectively defined subgroups were analyzed separately. Those with milder (subarachnoid hemorrhage [SAH] without vs with altered mental status), nonspecific (dizziness vs motor manifestations), or transient (TIA vs ischemic stroke) symptoms were compared using odds ratios (OR).
The Cohen kappa, CI, heterogeneity statistics, trend analyses, and OR were calculated using R v3.2.4 (Foundation for Statistical Computing, Vienna, Austria) by a PhD biostatistician. This study is reported in accordance with PRISMA guidelines.
RESULTS
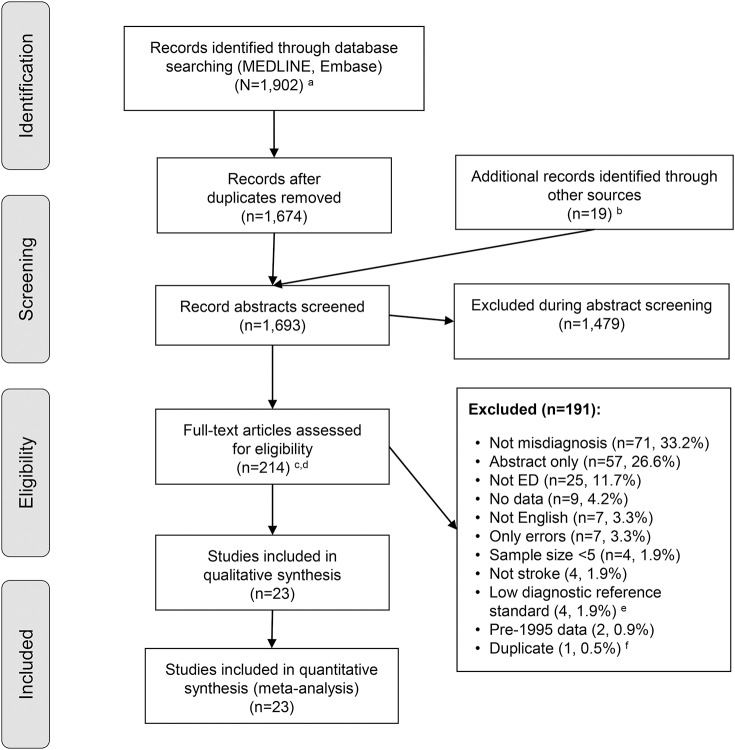
Our search identified 1,693 unique citations, of which 1,479 (87.4%) were excluded at the abstract level. Our independent raters had good to excellent initial agreement on inclusion of full-text manuscripts (kappa values 0.7–0.8, appendix e-1). After resolving initial disagreements, 23/214 (11%) studies were considered eligible (figure 1), representing 1.4% of the total. Four studies with low diagnostic reference standard were among those excluded (appendix e-1). Among the 23 studies included in the final meta-analysis, the quality of the diagnostic reference standard with respect to the primary outcome measures of ED diagnostic accuracy was judged high in 4 and moderate in 19 studies (appendix e-3). The risk of bias and applicability concerns using the QUADAS-2 rating system was judged high or unclear in 0 (n = 8), 1 (n = 14), 2 (n = 1), or more than 2 (n = 0) of the 7 QUADAS-2 bias/applicability categories (appendix e-3).
Figure 1. Citation search and selection flow diagram.
aMEDLINE was accessed via PubMed; Embase was accessed via embase.com. bHand search of citation lists from selected studies and investigator files identified 19 additional manuscripts for review. cAbstracts coded as yes or maybe by at least one reviewer were included in full-text review. dAfter full-text evaluation by 2 reviewers, any differences were resolved by discussion and adjudication by a third, independent reviewer. eDiagnostic reference standard was low in 4 studies (see appendix e-1). fOne study was removed because of duplicate data (see appendix e-1). ED = emergency department.
Included studies (n = 23) reported on 15,721 unique patients (8,975 stroke/TIA, 1,941 SAH, 697 ICH, 212 unspecified hemorrhage, 30 undetermined cerebrovascular events, 3,866 cerebrovascular mimics). In 2 studies, the breakdown between stroke and TIA (n = 851) was not provided.18,19 By contacting authors, we were able to remove duplicate, partially overlapping results from 2 related reports.15,20
Aggregate sensitivity was 91.3% (95% CI 90.7%–92.0%) (false-negative rate 8.7%) and specificity was 92.7% (91.7%–93.7%) (false-positive rate 7.3%) (table 1). For sensitivity (n = 13 studies), the I2 statistic for heterogeneity was 98.6%, indicating high variation across studies due to heterogeneity (p < 0.001, Cochran Q test). For specificity (n = 3 studies), the I2 statistic for heterogeneity was 99.8%, indicating high variation across studies due to heterogeneity (p < 0.001). Forest plots are provided in figure 2 for sensitivity, specificity, NPV, and PPV. Analysis for time effects identified no significant trend in diagnostic accuracy over the defined study period. Heterogeneity across studies appeared to correlate with differences in included stroke subpopulations. Table 2 shows subgroup analyses for patients with ischemic stroke, TIA, and SAH. Diagnostic accuracy parameters for individual studies are shown in appendix e-4.
Table 1.
Overall emergency department (ED) diagnostic accuracy for acute cerebrovascular events
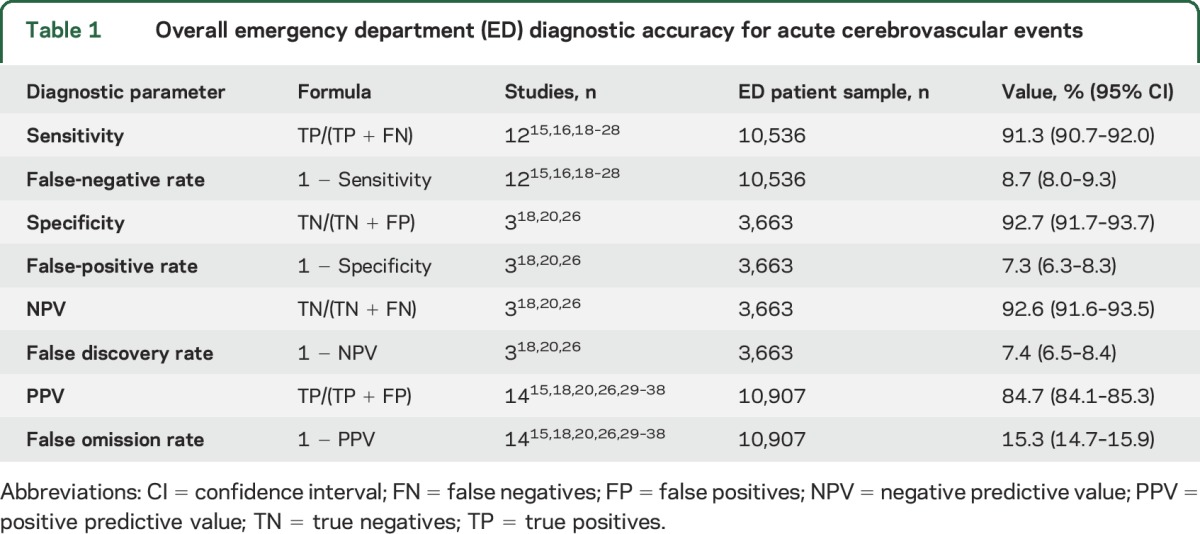
Figure 2. Emergency department (ED) diagnostic accuracy for acute cerebrovascular events by study: Sensitivity, specificity, positive predictive values (PPV), and negative predictive values (NPV).
Forest plots show sensitivity, specificity, PPV, and NPV (mean [95% confidence interval]) in ED diagnosis of cerebrovascular events. Results are shown by study and pooled. Note significant heterogeneity across studies, discussed in the text.
Table 2.
Emergency department (ED) diagnostic accuracy by type of cerebrovascular event

Selected studies permitted comparison of diagnostic accuracy for those with mild, nonspecific, and transient symptoms (table 3). These prospectively defined subgroups were at significantly higher risk of a missed diagnosis (range 24%–60%) than their counterparts with more severe, specific, or persistent deficits. The aggregate false-negative rate was 29.4% (24.8%–33.9%) for subtle vs 4.3% (3.8%–4.9%) for obvious presentations, OR 9.16 (7.09–11.84). The aggregate false discovery rate was 58.9% (52.5%–65.2%) for subtle vs 26.1% (22.3%–29.9%) for obvious presentations, OR 4.06 (2.92–5.63). We could not calculate a false-positive rate because these studies did not report true negatives.
Table 3.
Variation in likelihood of misdiagnosis based on subtle vs obvious clinical phenotype
Initial ED diagnoses in missed cases (false negatives) were available for 352 of 647 patients. Migraine and nonmigrainous headaches (26.1%) and vertigo/dizziness (14.5%) were the most frequent initial diagnostic categories (appendix e-5). Among these, 81.5% (n = 75/92) of cases initially misdiagnosed as headache received a final diagnosis of SAH, while 100% (n = 51/51) of cases initially diagnosed as vertigo/dizziness turned out to be ischemic strokes or TIAs. Correct final diagnoses in stroke mimics initially misdiagnosed as stroke (false positives) were available for 966 of 1,208 patients. Seizures (16.7%), vertigo/dizziness (9.4%), and migraine (8.1%) were the most frequent final diagnoses (appendix e-5).
Misdiagnoses were also noted to vary in nonprospectively defined subgroups. For ischemic stroke, increased risk of misdiagnosis was associated with demographic factors (younger age,25,38 lack of history of vascular risk factors33), provider cognitive factors (imprecise history taking),33 and systems factors (ED assessment in nonteaching [community] hospitals38 or in hospitals without a neurology residency24). Four studies stratified misdiagnoses in ischemic stroke by age, demonstrating higher rates of missed strokes in patients younger than 35 years (relative to those aged 35–49 years)25 and younger age among those found to have stroke/TIA mimics (mean/median age 47–65 years vs 65–73 years27,29,38). Two studies found that posterior circulation strokes were missed more often than anterior circulation strokes (37% vs 16%, p < 0.00128; OR 3.78 [1.87–7.63]27). Two other studies identified a tendency for lower NIH Stroke Scale (NIHSS) scores in missed strokes (mean 2.8 vs 6.4, p = 0.07628; median 3 vs 6, p = 0.2127). For SAH, increased risk of misdiagnosis was associated with demographic factors (<12 years of education),22 disease factors (small volume of SAH),22 and systems factors (ED assessment in nonteaching [community] hospitals increased risk 2.1-fold [1.02–4.44]).21 The greater risk of missed SAH in nonteaching hospitals could not be explained by decreased availability of CT imaging.21
DISCUSSION
Our study suggests that ED diagnosis of cerebrovascular events is fairly accurate but misdiagnosis still occurs at a nontrivial rate (false-negative rate = 8.7%; false-positive rate = 7.3%), even in the era of modern neuroimaging. Given that there are roughly 1.2 million cerebrovascular events each year (800,000 strokes and 200,000–500,000 TIAs),39 the estimated false-negative rate likely corresponds to more than 100,000 missed events each year in the United States alone. Given the greater risks of harm associated with false-negative diagnoses,40 ED thresholds for considering a stroke diagnosis may be too high. Importantly, our prospectively defined subgroup analyses found that patients with less obvious manifestations of cerebrovascular disease were far more likely to be missed (29.4% vs 4.3%, false-negative rate) and also overcalled (58.9% vs 26.1%, false discovery rate), indicating that these subgroups should be the principal targets for interventions seeking to reduce cerebrovascular disease misdiagnosis.
Our results point to isolated dizziness and headache symptoms as the most common clinical contexts for missed cerebrovascular disease. For dizziness, where the primary misdiagnosis in stroke patients is vestibular neuritis or other peripheral-vestibular disorder,41 misdiagnosis is understandably more frequent without limb weakness.42 For headache, where the primary misdiagnosis in patients with SAH is migraine/other benign headache,21 misdiagnosis is understandably more frequent without altered mental state.23 These findings reinforce large-scale studies using administrative data that show strong temporal associations between ED treat-and-release visits for benign (presumably isolated) dizziness or headaches and subsequent inpatient hospital stroke admissions.12,43 Two related associations are the increased risk of missed posterior circulation stroke (typically presenting with dizziness, headache, and other nonclassical stroke symptoms44) and stroke with low NIHSS (known to be associated with posterior circulation strokes45). When atypical symptoms are also transient, diagnostic accuracy may decline precipitously. Posterior circulation TIAs most commonly present with transient, isolated vertigo44; in such cases, the missed-stroke rate at first medical contact may rise as high as 90%.44
There are probably multiple reasons for high cerebrovascular disease misdiagnosis rates among those with milder, nonspecific, or transient clinical manifestations. The simplest is the low signal-to-noise ratio, since dangerous causes are far outnumbered by benign ones in patients presenting common symptoms (e.g., headaches or dizziness) absent obvious red flags for cerebrovascular disease. Another is that those with transient neurologic symptoms usually appear well at the time of assessment without residual neurologic symptoms or signs, rendering bedside examination and confirmatory tests such as neuroimaging far less effective in diagnosis. There is also evidence that cognitive errors (including knowledge gaps, misconceptions, flawed mental models, and bias among providers) play an important role.42 For example, the absence of cognitive, speech, or motor findings appears to place stroke patients at considerably higher risk of being missed,19 presumably because such clinical findings are mistakenly considered sine qua non accompaniments for stroke.42 Frequent use of an outdated paradigm for diagnosing dizziness is another contributor.42,46 False reassurance by negative CT brain imaging is also a factor.42
Demographic risk factors for cerebrovascular misdiagnosis include having less than 12 years of education,22 being a woman, being a minority, and, in particular, being younger (age <45 years).12 Sex and race increase stroke misdiagnosis risk by 20%–30%12; proposed explanations for these disparities in care quality include more atypical clinical manifestations among women and minorities as well as implicit bias in providers. Age is particularly noteworthy as youth increases stroke misdiagnosis risk nearly 7-fold (patients 18–45 years vs those >75 years).12 This is not surprising since, among younger patients, the prevalence of strokes is low and the prevalence of stroke mimics high, again reducing the signal-to-noise ratio. Although fewer in total number, stroke misdiagnoses in younger patients can be devastating40 and likely have profound societal consequences given the high economic costs of death or disability, including long-term care.47
Systems risk factors for cerebrovascular disease misdiagnosis are less well-studied, but include evaluation at nonacademic, community hospital EDs or those without a neurology residency. Variability across hospitals was shown to be substantial in the 2 studies that assessed it, with hospital-specific rates of misdiagnosis ranging widely from 0% to 100% for SAH21 and 0.8% to 6.4% for stroke/TIA.38 Our systematic review deliberately focused on the accuracy of initial ED diagnoses rendered by ED physicians. Some studies suggest patient evaluation by board-certified neurologists or even neurology residents may be linked to a lower risk of stroke misdiagnosis.24,38 However, neurologists generally assess patients after evaluation is already in progress and usually have access to additional data. Furthermore, misdiagnoses still occur even when specialists perform the initial ED assessment. This is particularly true for patients with nonclassical symptoms such as dizziness, with neurologic accuracy reported as low as 74.2% (sensitivity 86.5%; specificity 65.3%).48 It is also possible that specialists may trade greater sensitivity for lower specificity, given that patients who receive IV thrombolysis (who typically have obvious, classical stroke manifestations), generally under the direction of a specialist, are found to have stroke mimics at rates of 2.8%–14.0%.49–51
The real-world effects of these errors on patient outcomes is incompletely understood, since most studies of diagnostic accuracy use cross-sectional designs without longitudinal follow-up. Nevertheless, available studies suggest the effects of misdiagnosis could be profound. One reviewed study found that a third of missed strokes would have been eligible for recombinant tissue plasminogen activator treatment28 and another found 4-fold higher mortality (OR 4.4 [1.8–10.5]) after missed stroke.27 It has been estimated that, of the roughly 45,000–75,000 missed strokes in patients presenting with dizziness to US EDs annually, perhaps 15,000–25,000 have preventable major stroke after missed opportunities to promptly treat minor stroke/TIA.43 Although crude SAH mortality in a population-based sample was lower among those misdiagnosed (presumably because of a milder illness spectrum),21 when adjusted for initial severity, the odds of death or severe disability at 1 year in patients with mild SAH who were initially misdiagnosed vs correctly diagnosed at first contact was 3-fold higher (OR 3.1 [1.2–7.7]).22 This indicates that cerebrovascular disease misdiagnosis, even in cases with milder initial presentations, is probably associated with nontrivial, preventable harms to patients.
Whether these cerebrovascular disease error rates are acceptable or unacceptable is a matter for public debate, although the absolute misdiagnosis rate is probably high enough to envision systems-oriented solutions that could produce a measurable reduction in misdiagnosis or misdiagnosis-related harms at reasonable cost. Routine access to ED neurologists, suggested by some,13 is probably impractical, although telemedicine could facilitate greater access.52 Indiscriminate use of expensive neuroimaging (CT/MRI) is neither accurate enough53 nor likely to be cost-effective.54,55 For common neurologic symptoms such as dizziness or headaches, cannot-miss-diagnosis reminder checklists and symptom-oriented diagnostic decision support have been proposed as possible solutions3 and deserve further exploration. Although not all patients can be definitively diagnosed by bedside assessment alone, risk stratification on the basis of particular symptom details (e.g., abruptness of headache onset, onset during exertion)56,57 or subtle signs (e.g., eye movement findings in those with acute dizziness)58 into high- or low-risk groups is often possible, even when less obvious neurologic manifestations are lacking.41 Such approaches offer the potential to provide cost-effective reductions in misdiagnosis-related harms,54 potentially facilitated by the use of novel technologies. It is likely, however, that improved diagnosis will also require some degree of culture change,59 with enhanced communication and teamwork among ED physicians, neurologists, and other providers.59,60
Our study has limitations. It is possible that we missed evidence related to cerebrovascular misdiagnoses. We reviewed only the English language literature and ICH was not an explicit part of our search. Publication bias could have favored reports indicating a higher (or lower) misdiagnosis rate. Some studies were of modest quality (definitions, standards, reporting), and diagnostic uncertainty expressed by ED physicians (e.g., “suspected TIA” or “stroke vs seizure”) was inconsistently handled across studies. In some studies, neurology residents contributed to the initial ED diagnosis along with emergency physicians,29,34–36,38 or neurologists were consulted by telephone.28,38 Because most studies did not report on how quickly diagnostic errors were recognized or the exact timing of these errors relative to imaging decisions, it is difficult to know if some errors simply reflected an initial, rough diagnostic assessment or a reasoned decision in an uncertain case to consult neurology, rather than directly ordering an MRI. These methodologic issues could have led to either overestimation or underestimation of misdiagnosis rates. We had large aggregate sample sizes, but a relatively small number of studies for some of our measures; the narrowness of our CIs may therefore overstate the precision of our results. Source studies did not systematically explore risk factors and causes for misdiagnosis (including variation at the individual provider level), limiting inferences about ideal solutions.
Our systematic review indicates that cerebrovascular disorders are misdiagnosed in the ED at nontrivial rates. There may be more than 100,000 missed cerebrovascular events each year in the United States alone. These misdiagnoses likely confer important risks of harm to patients from missed treatment opportunities and represent yet another unmeasured aspect of health disparities for women and minorities. There appears to be substantial variation in misdiagnosis rates based on clinical presentation, with milder, nonspecific, and transient symptoms carrying the greatest risk of misdiagnosis. Patients are most likely to receive an accurate cerebrovascular diagnosis in the presence of persistent cognitive or motor deficits and least likely to be accurately diagnosed when presenting with transient symptoms or isolated dizziness, vertigo, or headaches, especially if they are young. The most frequently identified stroke mimics mistakenly called strokes (7.3% false positives) were seizures, migraines, and inner ear disorders, which should be considered in the differential diagnosis. Future research to reduce cerebrovascular disease misdiagnosis or mitigate harms from diagnostic error should begin by focusing on these high-risk for misdiagnosis subgroups. These studies should define the effects of misdiagnosis on clinical outcomes and identify practical systems-oriented interventions to reduce them.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Morgenstern for his assistance in clarifying data on behalf of the BASIC stroke project and Dr. Nakajima, Dr. Schrock, and Dr. Uchino for providing additional datasets for this meta-analysis.
GLOSSARY
- CI
confidence interval
- ED
emergency department
- ICH
intracerebral hemorrhage
- NIHSS
NIH Stroke Scale
- NPV
negative predictive value
- OR
odds ratio
- PPV
positive predictive value
- SAH
subarachnoid hemorrhage
Footnotes
Supplemental data at Neurology.org
Editorial, page 1390
AUTHOR CONTRIBUTIONS
Alexander A. Tarnutzer: coded abstract and full-text studies, led analysis and interpretation of data, critically reviewed and edited the manuscript, and saw and approved the final version. Seung-Han Lee: coded abstract and full-text studies, critically reviewed and edited the manuscript, and saw and approved the final version. Karen Robinson: helped conceptualize the study, designed and conducted the literature search strategy, oversaw drafting of the Methods section, critically reviewed and edited the manuscript, and saw and approved the final version. Zheyu Wang: conducted all analysis and oversaw the interpretation, critically reviewed and edited the manuscript, and saw and approved the final version. Jonathan Edlow: helped develop study protocols, coded abstract and full-text studies, helped abstract data, analyzed results, critically reviewed and edited manuscript, and saw and approved the final version. Dr. Edlow reviews medical malpractice cases involving patients with neurologic emergencies for both defense and plaintiff firms. David Newman-Toker: performed or directly oversaw all aspects of study from conception through completion (principal investigator), led analysis and interpretation of data, authored primary manuscript draft (except Methods) and all major revisions, and saw and approved the final version. Dr. Newman-Toker had full access to all the data in the study and had final responsibility for the decision to submit for publication; he reviews medical malpractice cases involving patients with neurologic emergencies for both defense and plaintiff firms, conducts funded research related to stroke misdiagnosis, and has been loaned research equipment by 2 commercial companies (GN Otometrics and Interacoustics).
STUDY FUNDING
The preparation of this manuscript was supported by grants from the NIH (NCRR K23 RR17324, NIDCD U01 DC013778) and the Agency for Healthcare Research and Quality (AHRQ HS017755). Neither funding agency was involved in design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript.
DISCLOSURE
A. Tarnutzer, S. Lee, K. Robinson, and Z. Wang report no disclosures relevant to the manuscript. J. Edlow conducts medicolegal case reviews. D. Newman-Toker conducts medicolegal case reviews and funded research related to stroke misdiagnosis and has been loaned research equipment by 2 commercial companies (GN Otometrics and Interacoustics). Go to Neurology.org for full disclosures.
REFERENCES
- 1.National Academies of Sciences Engineering, and Medicine. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 2015. [Google Scholar]
- 2.Singh H, Giardina TD, Meyer AN, Forjuoh SN, Reis MD, Thomas EJ. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med 2013;173:418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman-Toker DE, Pronovost PJ. Diagnostic errors: the next frontier for patient safety. JAMA 2009;301:1060–1062. [DOI] [PubMed] [Google Scholar]
- 4.Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients: results of the Harvard Medical Practice Study II. N Engl J Med 1991;324:377–384. [DOI] [PubMed] [Google Scholar]
- 5.Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf 2013;22:672–680. [DOI] [PubMed] [Google Scholar]
- 6.Vinen J. Incident monitoring in emergency departments: an Australian model. Acad Emerg Med 2000;7:1290–1297. [DOI] [PubMed] [Google Scholar]
- 7.Troxel, the Doctor's company [online]. Available at: thedoctors.com/KnowledgeCenter/Publications/TheDoctorsAdvocate/Diagnostic-Error-in-Medical-Practice-by-Specialty. Accessed November 14, 2016.
- 8.Dubois RW, Brook RH. Preventable deaths: who, how often, and why? Ann Intern Med 1988;109:582–589. [DOI] [PubMed] [Google Scholar]
- 9.Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol 2009;8:345–354. [DOI] [PubMed] [Google Scholar]
- 10.Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med 2009;169:1881–1887. [DOI] [PubMed] [Google Scholar]
- 11.Glick TH, Cranberg LD, Hanscom RB, Sato L. Neurologic patient safety: an in-depth study of malpractice claims. Neurology 2005;65:1284–1286. [DOI] [PubMed] [Google Scholar]
- 12.Newman-Toker DE, Moy E, Valente E, Coffey R, Hines AL. Missed diagnosis of stroke in the emergency department: a cross-sectional analysis of a large population-based sample. Diagnosis 2014;1:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caplan LR. Dizziness: how do patients describe dizziness and how do emergency physicians use these descriptions for diagnosis? Mayo Clin Proc 2007;82:1313–1315. [DOI] [PubMed] [Google Scholar]
- 14.Edlow JA, Rothman RE, Barsan WG. What do we really know about neurological misdiagnosis in the emergency department? Mayo Clin Proc 2008;83:253–254. [DOI] [PubMed] [Google Scholar]
- 15.Morgenstern LB, Lisabeth LD, Mecozzi AC, et al. A population-based study of acute stroke and TIA diagnosis. Neurology 2004;62:895–900. [DOI] [PubMed] [Google Scholar]
- 16.Lever NM, Nystrom KV, Schindler JL, Halliday J, Wira C III, Funk M. Missed opportunities for recognition of ischemic stroke in the emergency department. J Emerg Nurs 2013;39:434–439. [DOI] [PubMed] [Google Scholar]
- 17.Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000;342:1163–1170. [DOI] [PubMed] [Google Scholar]
- 18.Moulin T, Sablot D, Vidry E, et al. Impact of emergency room neurologists on patient management and outcome. Eur Neurol 2003;50:207–214. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima M, Hirano T, Uchino M. Patients with acute stroke admitted on the second visit. J Stroke Cerebrovasc Dis 2008;17:382–387. [DOI] [PubMed] [Google Scholar]
- 20.Kerber KA, Brown DL, Lisabeth LD, Smith MA, Morgenstern LB. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department: a population-based study. Stroke 2006;37:2484–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermeulen MJ, Schull MJ. Missed diagnosis of subarachnoid hemorrhage in the emergency department. Stroke 2007;38:1216–1221. [DOI] [PubMed] [Google Scholar]
- 22.Kowalski RG, Claassen J, Kreiter KT, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA 2004;291:866–869. [DOI] [PubMed] [Google Scholar]
- 23.Mayer PL, Awad IA, Todor R, et al. Misdiagnosis of symptomatic cerebral aneurysm. Prevalence and correlation with outcome at four institutions. Stroke 1996;27:1558–1563. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed W, Bhattacharya P, Chaturvedi S. Early access to a neurologist reduces the rate of missed diagnosis in young strokes. J Stroke Cerebrovasc Dis 2013;22:e332–e337. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharya P, Nagaraja N, Rajamani K, Madhavan R, Santhakumar S, Chaturvedi S. Early use of MRI improves diagnostic accuracy in young adults with stroke. J Neurol Sci 2013;324:62–64. [DOI] [PubMed] [Google Scholar]
- 26.Whiteley WN, Wardlaw JM, Dennis MS, Sandercock PA. Clinical scores for the identification of stroke and transient ischaemic attack in the emergency department: a cross-sectional study. J Neurol Neurosurg Psychiatry 2011;82:1006–1010. [DOI] [PubMed] [Google Scholar]
- 27.Richoz B, Hugli O, Dami F, Carron PN, Faouzi M, Michel P. Acute stroke chameleons in a university hospital: risk factors, circumstances, and outcomes. Neurology 2015;85:505–511. [DOI] [PubMed] [Google Scholar]
- 28.Arch AE, Weisman DC, Coca S, Nystrom KV, Wira CR III, Schindler JL. Missed ischemic stroke diagnosis in the emergency department by emergency medicine and neurology services. Stroke 2016;47:668–673. [DOI] [PubMed] [Google Scholar]
- 29.Scott PA, Silbergleit R. Misdiagnosis of stroke in tissue plasminogen activator-treated patients: characteristics and outcomes. Ann Emerg Med 2003;42:611–618. [DOI] [PubMed] [Google Scholar]
- 30.Broadley SA, Thompson PD. Time to hospital admission for acute stroke: an observational study. Med J Aust 2003;178:329–331. [DOI] [PubMed] [Google Scholar]
- 31.Harbison J, Hossain O, Jenkinson D, Davis J, Louw SJ, Ford GA. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke 2003;34:71–76. [DOI] [PubMed] [Google Scholar]
- 32.Ferro JM, Falcao I, Rodrigues G, et al. Diagnosis of transient ischemic attack by the nonneurologist: a validation study. Stroke 1996;27:2225–2229. [DOI] [PubMed] [Google Scholar]
- 33.Ferro JM, Pinto AN, Falcao I, et al. Diagnosis of stroke by the nonneurologist: a validation study. Stroke 1998;29:1106–1109. [DOI] [PubMed] [Google Scholar]
- 34.Jeng J-S, Huang Z-S, Chang Y-C, et al. Misdiagnosis of acute cerebrovascular disease: experience from a hospital-based stroke registry in Taiwan (SCAN-V). Acta Neurol Taiwan 1998;7:185–192. [Google Scholar]
- 35.Leys D, Lucas C, Devos D, et al. Misdiagnoses in 1250 consecutive patients admitted to an acute stroke unit. Cerebrovasc Dis 1997;7:284–288. [Google Scholar]
- 36.Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis 2008;26:630–635. [DOI] [PubMed] [Google Scholar]
- 37.Schrock JW, Glasenapp M, Victor A, Losey T, Cydulka RK. Variables associated with discordance between emergency physician and neurologist diagnoses of transient ischemic attacks in the emergency department. Ann Emerg Med 2012;59:19–26. [DOI] [PubMed] [Google Scholar]
- 38.Uchino K, Massaro L, Hammer MD. Transient ischemic attack after tissue plasminogen activator: aborted stroke or unnecessary stroke therapy? Cerebrovasc Dis 2010;29:57–61. [DOI] [PubMed] [Google Scholar]
- 39.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics: 2012 update: a report from the American Heart Association. Circulation 2012;125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savitz SI, Caplan LR, Edlow JA. Pitfalls in the diagnosis of cerebellar infarction. Acad Emerg Med 2007;14:63–68. [DOI] [PubMed] [Google Scholar]
- 41.Tarnutzer AA, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ 2011;183:E571–E592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerber KA, Newman-Toker DE. Misdiagnosing dizzy patients: common pitfalls in clinical practice. Neurol Clin 2015;33:565–575, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newman-Toker DE. Missed stroke in acute vertigo and dizziness: it is time for action, not debate. Ann Neurol 2016;79:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paul NL, Simoni M, Rothwell PM, Oxford Vascular S. Transient isolated brainstem symptoms preceding posterior circulation stroke: a population-based study. Lancet Neurol 2013;12:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin-Schild S, Albright KC, Tanksley J, et al. Zero on the NIHSS does not equal the absence of stroke. Ann Emerg Med 2011;57:42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanton VA, Hsieh YH, Camargo CA Jr, et al. Overreliance on symptom quality in diagnosing dizziness: results of a multicenter survey of emergency physicians. Mayo Clin Proc 2007;82:1319–1328. [DOI] [PubMed] [Google Scholar]
- 47.Synhaeve NE, Arntz RM, Maaijwee NA, et al. Poor long-term functional outcome after stroke among adults aged 18 to 50 years: follow-up of transient ischemic attack and stroke patients and unelucidated risk factor evaluation (FUTURE) study. Stroke 2014;45:1157–1160. [DOI] [PubMed] [Google Scholar]
- 48.Royl G, Ploner CJ, Leithner C. Dizziness in the emergency room: diagnoses and misdiagnoses. Eur Neurol 2011;66:256–263. [DOI] [PubMed] [Google Scholar]
- 49.Winkler DT, Fluri F, Fuhr P, et al. Thrombolysis in stroke mimics: frequency, clinical characteristics, and outcome. Stroke 2009;40:1522–1525. [DOI] [PubMed] [Google Scholar]
- 50.Chernyshev OY, Martin-Schild S, Albright KC, et al. Safety of tPA in stroke mimics and neuroimaging-negative cerebral ischemia. Neurology 2010;74:1340–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vroomen PC, Buddingh MK, Luijckx GJ, De Keyser J. The incidence of stroke mimics among stroke department admissions in relation to age group. J Stroke Cerebrovasc Dis 2008;17:418–422. [DOI] [PubMed] [Google Scholar]
- 52.Bladin CF, Cadilhac DA. Effect of telestroke on emergent stroke care and stroke outcomes. Stroke 2014;45:1876–1880. [DOI] [PubMed] [Google Scholar]
- 53.Saber Tehrani AS, Kattah JC, Mantokoudis G, et al. Small strokes causing severe vertigo: frequency of false-negative MRIs and nonlacunar mechanisms. Neurology 2014;83:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newman-Toker DE, McDonald KM, Meltzer DO. How much diagnostic safety can we afford, and how should we decide? A health economics perspective. BMJ Qual Saf 2013;22(suppl 2):ii11–ii20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saber Tehrani AS, Coughlan D, Hsieh YH, et al. Rising annual costs of dizziness presentations to U.S. emergency departments. Acad Emerg Med 2013;20:689–696. [DOI] [PubMed] [Google Scholar]
- 56.Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med 2000;342:29–36. [DOI] [PubMed] [Google Scholar]
- 57.Perry JJ, Stiell IG, Sivilotti ML, et al. Clinical decision rules to rule out subarachnoid hemorrhage for acute headache. JAMA 2013;310:1248–1255. [DOI] [PubMed] [Google Scholar]
- 58.Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke 2009;40:3504–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas DB, Newman-Toker DE. Diagnosis is a team sport: partnering with allied health professionals to reduce diagnostic errors: a case study on the role of a vestibular therapist in diagnosing dizziness. Diagnosis 2016;3:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newman-Toker DE, Perry JJ. Acute diagnostic neurology: challenges and opportunities. Acad Emerg Med 2015;22:357–361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.