Abstract
Patient: Male, 61
Final Diagnosis: Strongyloides stercolaris-associated diarrhea
Symptoms: Diarrhea • epigastric pain • nausea • weight loss
Medication: Ivermectin
Clinical Procedure: Colonic biopsies
Specialty: Infectious Diseases
Objective:
Rare disease
Background:
Strongyloides stercoralis infection is endemic in subtropical and tropical regions but is reported rather sporadically in temperate countries. In the USA, the highest rates of infection are from the southeastern states, predominantly among immigrants. There is paucity of case reports on S. stercoralis infection among HIV-infected patients who were born and raised in the USA.
Case Report:
A 61-year-old male with known HIV infection (CD4 count: 235 cells/uL, undetectable HIV RNA, on antiretroviral therapy) presented with a 3-month history of diarrhea. He was initially diagnosed to have diarrhea secondary to norovirus and later with Escherichia coli. He was treated with levofloxacin but the diarrhea persisted. Stool PCR, Clostridium difficile enzyme-linked immunoassay, cryptosporidium and giardia antigen, cyclospora and isospora smear, and fecal microscopy were all negative. Peripheral blood eosinophil count was 1,000 eosinophils/mcL. Colonic biopsies revealed fragments of S. stercoralis larvae within the crypts. The patient was treated with ivermectin with improvement of symptoms. Social history revealed that he was born and raised in the northeastern USA. He was a daily methamphetamine user and engaged in anal sex with men. He denied travel to endemic areas, except for a visit to Japan more than 30 years ago.
Conclusions:
Our case highlights that S. stercoralis may be an underdiagnosed/under-reported cause of chronic diarrhea among HIV-infected patients. What makes this case peculiar is that the patient was born and raised in the continental USA, absence of recent travel to endemic areas, and relatively high CD4 counts. Parasitic infections, such as S. stercoralis, should be considered among HIV-infected patients with persistent diarrhea and eosinophilia regardless of ethnicity or recent travel history.
MeSH Keywords: HIV Infections, Strongyloides stercoralis, United States
Background
Strongyloides stercoralis is a parasitic infection that is endemic in subtropical and tropical regions but is reported rather sporadically in temperate countries [1]. In the USA, the highest rates of infection are from the southeastern states, predominantly among immigrants. Interestingly, there is a paucity of case reports on S. stercoralis infection among HIV-infected patients who were born and raised in the USA.
We present a rare case S. stercoralis associated diarrhea in a patient with HIV who was on antiretroviral therapy and who had relatively high CD4 counts. The patient had a negative fecal examination and was diagnosed through a colonoscopy biopsy. We searched the literature and cite cases of S. stercoralis infection among HIV-infected patients residing in the USA and discuss latest treatments and diagnostic modalities.
Case Report
A 61-year-old male with HIV infection (CD4 count of 235 cells/µL and undetectable HIV viral load) on antiretroviral therapy with emtricitabine-tenofovir, atazanavir, raltegravir, and ritonavir; presented to the emergency department (ED) of our hospital with a three-month history of diarrhea.
The patient had been told that he had a norovirus infection three months before presentation to our hospital, during which he developed diarrhea with mucous consistency and trace blood. The diarrhea improved but did not completely abate. When its severity increased, the patient directed himself to a different institution, where he was admitted for five days with the diagnosis of Escherichia coli gastroenteritis. He was discharged on a twelve-day course of oral levofloxacin. After six days on outpatient antibiotic therapy, the patient presented to our ED due to persistence of diarrhea. He complained of numerous non-bloody liquid bowel movements accompanied by nausea and epigastric pain. He had lost five pounds of weight over the last three months. He denied fever, vomiting, respiratory distress, wheezing, night sweats, or diaphoresis.
Further history revealed that he was born and raised in Connecticut. He moved to Philadelphia to complete his university degree and eventually established himself in New York where he used to work for the fashion industry. He was diagnosed with HIV in 1985, which he attributed to unprotected sex with men. He denied history of AIDS-defining illnesses but reported recent treatment for gonorrhea and chlamydia urethritis. The patient also disclosed that he had engaged in unprotected anal sex with multiple partners from different ethnicities, mainly Hispanic. He had no recent travel history, but had been to Japan 30 years ago. He had also traveled previously to France, Italy, and Turkey. His last trip out of the country was to Greece three years prior to his current presentation. He had a remote history of intravenous drug use and admitted to daily use of crystal methamphetamine. His past medical history was also significant for borderline personality disorder.
On arrival to the hospital, the patient was afebrile and had a blood pressure of 96/62 mm Hg with a heart rate of 87 beats per minute, and normal respiratory rate and oxygen saturation. He appeared cachectic, pale, and fatigued. He was alert and oriented with no nuchal rigidity and no focal neurologic deficits. No oropharyngeal rash was seen. Respiratory and cardiovascular examinations were unremarkable. His abdomen was soft but with diffuse tenderness on deep palpation. There was no abdominal distension or ascites.
Initial laboratory work disclosed mild hypokalemia (3.3 mmol/L), hypomagnesemia (1.4 mg/dL, hypocalcemia (6.2 mg/dL), hypoalbuminemia (2.6 g/dL), and anemia and eosinophilia (1,000 eosinophils/mcL). He had otherwise normal creatinine, aspartate aminotransferase, alanine aminotransferase, total bilirubin, lipase, and white blood cell count. The only imaging test performed was a computed tomography (CT) of the abdomen with intravenous contrast, which showed circumferential wall thickening of the rectosigmoid area with mild stranding in the adjacent fat and multiple small perirectal lymph nodes, suggestive of mild proctocolitis of the rectosigmoid.
Intravenous (IV) fluids were administered and electrolytes were repleted. He was started on ciprofloxacin 800 mg IV twice daily and metronidazole 500 mg IV three times daily. Diagnostic workup for infectious disease and inflammatory bowel disease were sent: sedimentation rate, stool pathogens by PCR, Clostridium difficile EIA, cryptosporidium, giardia antigen, and cyclospora and isospora. His sedimentation was 12 mm/hour and only the fecal leukocyte count test was positive.
Because the patient’s symptoms were only minimally improving with current management, a colonoscopy was performed. During the colonoscopy, the rectal examination revealed normal sphincter tone without palpable rectal or anal abnormalities. Diffuse moderate inflammation characterized by edema, erythema, and confluent ulcerations was found in the rectum and in the recto-sigmoid colon. Biopsies were taken with cold forceps for histology. Bowel preparation was inadequate, with copious quantities of semi-solid stool all along the sigmoid colon to the cecum. Stool samples were collected for ova and parasite examination but were found to be negative.
Colonic biopsies revealed fragments of larvae/worm within the crypts compatible with S. stercoralis along with increased lymphocytes and eosinophils in the background (Figures 1–3). Rectal biopsy showed colonic mucosa, a polypoid fragment with chronic inflammation, ulceration and possible pseudo-membranes with acute inflammation.
Figure 1.
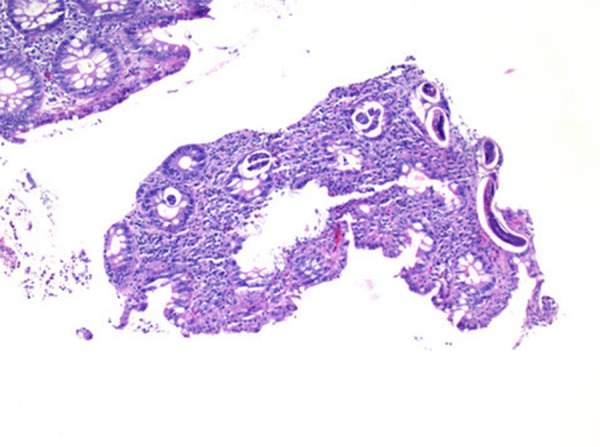
Sigmoid colon with larvae in crypts (H&E 100×).
Figure 2.

Sigmoid colon biopsy larvae (H&E 400×).
Figure 3.

Sigmoid colon larvae; background plasma cells, lymphocytes, and eosinophils (H&E 200×).
The patient’s symptomatology improved after therapy was switched to a 10-day regimen of ivermectin 12,000 mcg by mouth daily.
Discussion
Clinical presentation of Strongyloides infection
Strongyloidiasis is a parasitic disease that affects tens of millions of people worldwide. S. stercoralis is the pathogen responsible for the most common form of the disease, whereas S. fuelleborni is responsible for the so-called “swollen belly syndrome” in infants from Papua New Guinea and sub-Saharan Africa [1]. We present the case of an HIV-infected individual, born and raised in the northeastern USA, who was admitted for S. stercoralis related diarrhea and for whom the main potential risk factors were anal intercourse and a distant history of travel to Japan.
Most S. stercoralis infections occur secondary to skin contact with contaminated soils. However, transmission of S. stercoralis through solid organ transplantation, nosocomial spread, oral-anal sex, and rectal intercourse have also been reported [1]. Risk factors associated with S. stercoralis infection are HIV infection, low socioeconomic status, alcoholism, white race, and male gender [2,3].
In the classic infective cycle, soils become contaminated by free-living filariform larvae originating from human feces. They can infect their host through contact with exposed skin and subsequently reach the pulmonary alveolar spaces via afferent circulation. Once in the lungs, the larvae can ascend towards the pharynx to be swallowed into the small intestine, where they develop into adult worms [1]. What makes S. stercoralis a unique helminth is that, while embedded in the mucosa, the adult female worm reproduces through parthenogenesis [4]. Twenty-five to 30 days after infection, embryonated eggs reach the lumen and rapidly hatch to release non-infective rhabditiform larvae that are excreted with the feces often resulting in contamination of the soil. An alternative cycle happens when rhabditiform larvae turn into pathogenic filariform larvae while they are still inside the host intestinal tract. In that stage, they can penetrate the colonic mucosa or perianal skin, perpetuating the parasitism for decades through a mechanism known as autoinfection [1,5].
In acute strongyloidiasis infection, dermatologic irritation at the site of entrance followed by respiratory symptoms and gastrointestinal discomfort can be observed. Once in the chronic stage of the disease, most patients are either asymptomatic or suffer from non-specific gastrointestinal symptoms such as intermittent diarrhea, constipation, vomiting, or borborygmus. Other symptoms that may be associated with chronic infection are respiratory distress mimicking asthma, pruritus ani, and unexplained rash (larva currens rash and urticarial rash) [2]. In a study performed by Nabha et. al, gastrointestinal symptoms did not differ between S. stercoralis seropositive and seronegative AIDS patients, with eosinophilia the only test found to have some utility in the diagnosis of patients not taking steroids (p=0.02) [6] On the other hand, a study performed by Hochberg et al. found a significant association between weight loss and strongyloidiasis in HIV infected patients (OR 3.1, 95% 1.4–7.2) [7].
An acceleration of the autoinfective cycle can lead to hyperinfection syndrome, where affected individuals present signs and symptoms attributable to a severe increase in the body-larval burden. This may be associated with disseminated disease, which implies the direct involvement of any organ aside from the pulmonary autoinfective cycle. Gram-negative bacteremia represents a common complication secondary to gastrointestinal translocation [2,8].
In our patient, weight loss, diarrhea, and abdominal pain were the predominant features. There were no signs of disseminated disease and larval burden did not appear to be elevated. We believe that the improvement of our patient’s symptoms with ivermectin treatment points towards symptomatic infection rather than an incidental finding. This could be a case of either subacute or chronic infection. As it has been described in other case reports, clinical disease can appear as late as 60 years after the initial infection [6].
Several studies have found an association between HIV and increased risk for S. stercoralis infection [3,9]. Data coming from clinical and autopsy series in Africa, South America, and the USA showed very rare cases of S. stercoralis dissemination and hyperinfection syndrome despite vast numbers of people with co-infections. This led to the dismantling of the old belief that S. stercoralis dissemination and hyperinfection syndrome was an AIDS-defining illness [2,3,9,10]. The two main risk factors for developing life-threatening hyperinfection syndrome are the use of corticosteroids and co-infection by human T-lymphotropic virus type 1 (HLTV-1) [9]. Interestingly, among HIV-infected individuals, those who lack eosinophilia during hyperinfection seem to have worse prognosis [2,11].
Prevalence and distribution of strongyloidiasis in the USA
S. stercoralis can be found in any continent except Antarctica [5]. Tropical and subtropical regions are the most affected, with a prevalence of 85% in lower socioeconomic population [4,5]. In the USA, rural Appalachia and some areas of the southeast are endemic regions. However, most reported infections have involved travelers and veterans returning from endemic areas outside of the USA as well as immigrant populations living in northern urban centers [7].
A study on global distribution and risk factors of S. stercoralis published in 2013 by Schär et al. reported the following data on prevalence rates of strongyloidiasis in the USA: 40% (95% CI, 37.8%–43%) among immigrants, 49.2% (95% CI 0.1–99.9%) in hospitalized individuals and 2.7% (95% CI, 2.4–3%) in the community [3]. Twenty-two surveys were included in the analysis and test sensitivity was taken into account. High hospital rates seem to be related to low power studies but also to higher prevalence of at-risk individuals and use of high-sensitivity diagnostic methods when compared to community-based studies [3].
While there is a lack of national data regarding S. stercoralis prevalence in HIV-infected individuals living in the USA, it is presumed that most of this population acquired the parasitic infection either from their country of origin or through person to person contact in USA territory. An article from 1987 reported a relatively high prevalence of strongyloidiasis (3.9%) among sexually-active homosexual men in the USA [6]. More recently, in the study performed by Hochberg et al. in 2011, 26% of the 128 foreign-born HIV-infected individuals in the USA were found to have antibodies against S. stercoralis [7]. A different study conducted by Nabha et al. in 2012 in a non-endemic urban USA AIDS cohort found that 25% of the study participants were strongyloides seropositive by CrAg-ELISA testing. Interestingly, only 68% of the participants were foreign-born [6]. This data suggests that person-to-person sexual contact may be a significant route of transmission of S. stercoralis parasitism in non-endemic areas of the USA, and we believe that this may be a plausible source of infection in our patient’s case.
Including our case, we were able to identify only 12 cases of HIV-infected patients diagnosed with strongyloidiasis in the continental USA [4,5,8,10–15]. Cases where the exact location of diagnosis was not specified but the authors were from a hospital in the continental USA were also included (Table 1) [12,13]. In our review of the literature, nine patients suffered from hyperinfection syndrome [4,5,8,10–12,14], two from disseminated disease [13,15] and our case was the only published case report of possible subacute/chronic infection. Among the cases cited, clinical presentation was diverse. Respiratory and gastrointestinal symptoms were the most common presenting complaints. All the patients (except our patient), had AIDS. Only one of four patients with eosinophilia and hyperinfection syndrome survived. Exposure to steroids was reported in two cases. One patient had received steroid therapy in the setting of Pneumocystis pneumonia treatment [12]. The other patient developed hyperinfection syndrome and survived after treatment. One patient was on chemotherapy for the treatment of Burkitt lymphoma and died from disseminated disease [15]. Seven patients developed concomitant bacteremia or meningitis; all were fatal cases [8,10,13–15]. Diagnosis was achieved through different techniques. Stool analysis was positive in only four patients. Overall, a 75% death rate was observed.
Table 1.
Reported cases of HIV-seropositive patients with Strongyloides stercoralis infection in United States.
| R. | Age (yr), Country of origin, sexual orientation | Time since arrival to US (yr) | Signs and symptoms | Clinical Sd [2] | CD4 count (T cell/µL)/VL (copies/mL) | ART therapy | Eosinophilia (>400 cells/µL) | Steroids | Coinfections/complications | SS Isolation | Initial therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [4] | 49, Liberia, Unknown | 4 yr | Abdominal pain, pedal edema | HI | 59/Unknown | No | No | No | None | Ascitic fluid, Stool | TBZ (2 w) | Alive |
| [8] | 40, Columbia, Unknown | Unknown | Dyspnea, cough, weight loss | HI | Unknown | No | No | Unknown | E. coli meningitis, Klebsiella pneumoniae sepsis | Sputum | TBZ (10 d) | Death |
| [5] | 41, Hispanic, Unknown | Unknown | Fever, dyspnea, abdominal pain, vomiting, diarrhea | HI | 31/123,000 | Yes | Yes | No | None | Duodenal biopsy | IVM (14 d) | Alive |
| [11] | 60, North America, Homosexual | N/A | Epigastric pain, diarrhea | HI | 194/Unknown | Yes | No | No | None | Duodenal biopsy/BAL | TBZ (3 d) | Death |
| [10] | 34, Puerto Rico, Unknown | 15 yr | Pleuritic chest pain, cough, abdominal pain, diarrhea | HI | Unknown | No | Unknown | No | Strep. pneumoniae and Proteus mirabilis sepsis, Proteus mirabilis meningitis | Postmortem | None | Death |
| [10] | 40, Puerto Rico, Homosexual | 10 yr | Cough, fever, diarrhea | HI | 34/Unknown | Unknown | Yes | No | Klebsiella pneumoniae sepsis, failure of initial TBZ course | Sputum, Stool | TBZ (≥35 d) | Death |
| [10] | 49, Puerto Rico, Unknown | 30 yr | Pleuritic chest pain, cough, fever, weight loss | HI | 36/Unknown | No | Yes | No | Candidemia, pulmonary tuberculosis, ARDS | Sputum, Stool | TBZ (59 d) | Death |
| [12] | 28, Unknown, Unknown | Unknown | Dyspnea, Vomiting-abdominal pain, anorexia | HI | Unknown | Unknown | Unknown | Yes | None | Sputum, stool | TBZ (2 w) | Alive |
| [13] | 41, Unknown, Unknown | Unknown | Vomiting, anorexia, fever, headache | DS | 50/Unknown | No | Unknown | No | E. coli and Pseudomonas aeruginosa meningitis | Skin biopsy, sputum, nasogastric fluid | TBZ (28 d) IVM (6d) | Death |
| [14] | 30, Haiti, Unknown | Unknown | Fever/AMS | HI | 72/Unknown | Unknown | No | No | Streptococcus faecalis meningitis | BAL | TBZ (2 d) | Death |
| [15] | 45, Puerto Rico, Unknown | 22 yr | 3rd nerve palsy, urinary retention, Babinski | DS | Unknown | No | Yes | Yes | E. coli bacteremia | CSF | TBZ | Death |
| CR | 61, North America, Homosexual | N/A | Abdominal pain, diarrhea. | CS | 235/undetectable | Yes | No | No | No | Colonic biopsy | IVM (10 d) | Alive |
R. – references; yr – year; SS – S. stercoralis; HI – hyperinfection; TBZ – thiobendazole; CR – case report; CS – chronic Strongyloidiasis; IVM – ivermectine; DS – disseminated Strongyloidiasis; BAL – Bronchoalveolar lavage; CSF – Cerebrospinal fluis, AMS – altered mental status; E. coli – Escherichia coli.
Patient received chemotherapy.
Updates on the diagnosis of Strongyloides infection
Our review of the literature revealed the challenge of diagnosing S. stercoralis using stool microscopy. Early diagnosis of S. stercoralis is important because most helminth-related deaths in the USA are due to this parasite [5]. Detection of Strongyloides larvae is the gold standard for diagnosis; however, it lacks sensitivity due to low and intermittent shedding in stools. A single stool examination only detects about 30% of cases and therefore repeated stool examinations are recommended with an increase in sensitivity to 50% once three stool samples have been examined, and further increased to almost 100% sensitivity when seven stool samples are examined [16].
To further increase the sensitivity of stool testing, a new fecal antigen test was developed by Goncalves et al. utilizing ELISA to detect S. venezuelensis coproantigens in fecal samples of rats. This possibly represents the initial steps in the development of rapid coproantigen detection kits to diagnose strongyloides infection in humans [17].
Various serologic techniques have been developed during the last few decades to improve diagnostic sensitivity, including indirect immunofluorescence microscopy, gelatin particle agglutination, immunoblot, and enzyme-linked immunoassays (ELISA). ELISA has been used most extensively. Despite excellent sensitivity, results are often difficult to interpret due to an inability to distinguish between current, resolved, and past infections [18]. Serology can be falsely negative in cases of immunosuppression and in the presence of low titers [19], and falsely positive due to cross-reactivity with other helminths [18].
In the last few years, newer diagnostic tools and molecular methods have been developed to more efficiently and accurately diagnose Strongyloides infection. Newer techniques such as the luciferase immunoprecipitation system assay (LIPS) based on a 31-kDa recombinant antigen (NIE) from S. stercoralis and/or the recombinant S. stercoralis immunoreactive antigen (SsIR), the NIE-ELISA and CrudeAg-ELISA were all compared with comprehensive stool evaluation by Kwolewiecki et al. and have shown promise. NIE-LIPS showed the highest sensitivity and specificity of the serologic assays and demonstrated no cross-reactivity with other soil-transmitted helminths [20].
Real-time PCR directed to target the small subunit of the rRNA gene can now be applied to stool samples [21]. When compared to traditional parasitological and microscopic methods, quantitative PCR has an up to eight times increase in diagnostic yield [22–24]. However, high cost and its unavailability outside the laboratory setting are some factors limiting its utility.
In 2016, Lodh et al. showed for the first time that parasite DNA could be detected in filtered urine via PCR. They compared PCR on urine residue with current standard stool examination techniques. This method detected a significantly higher number of cases of S. stercoralis compared with standard stool examination techniques. However, in 6.4% of cases, larvae was seen in the stool, but no DNA was amplified from urine. This method is promising but needs further validation [25].
Updates on the treatment of Strongyloides infection
The drug of choice for treatment of strongyloidiasis is ivermectin, with albendazole as an alternative medication. Back in the 1960s, thiabendazole was commonly used for the treatment of S. stercoralis. However, thiabendazole is now rarely used due to the availability of more efficacious and better tolerated medications [26,27].
In the immunocompromised patient with S. stercoralis hyperinfection or disseminated disease, a crucial part of the treatment resides on reversal of immunosuppression. This means that in some cases, the dose of immunosuppressant therapy is decreased or the use of steroids is tapered off or discontinued.
When patients with hyperinfection or disseminated disease are not able to take oral medications due to altered mental status or ileus, several non-FDA approved forms of administrations had been tried, most of them coming from experiences in veterinary medicine. The possible regimens include subcutaneous [28,29], rectal [30], or parenteral ivermectin formulation.
Some experts recommend that the optimal length of treatment is between five to seven days for disseminated disease using either ivermectin monotherapy or ivermectin with albendazole. The alternative to a fixed-length therapy is to adjust the duration until the clinical symptoms completely resolve and daily stool examinations have been negative for at least two weeks (the duration of one autoinfection cycle). In some immunocompromised patients, a prolonged course of ivermectin therapy may be warranted [31].
In cases of refractory strongyloidiasis, combined longer-term ivermectin and albendazole has been effective [32]. Monthly doses of ivermectin could be given for at least six months in patients with ongoing immunosuppression that had hyperinfection syndrome. It is recommended that blood cultures be obtained in the setting of hyperinfection syndrome, and empiric broad spectrum antibiotics to cover enteric gram-negative bacteria should be considered.
Cyclosporine is an immunosuppressant medication given to patients mainly to prevent organ rejection after transplantation. Interestingly, it was discovered that it had anti-Strongyloides effect when it was used to induce immunosuppression in dogs infected with S. stercoralis. Subsequent studies showed that cyclosporine eradicated S. ratti from rats [33]. A later study reported that a single dose given to rats reduced the number of larvae in the stools by approximately 50% [34]. Although it has been demonstrated to have anti-helminthic effects, the use of cyclosporine for treatment of S. stercoralis infection in humans is currently not recommended primarily because of its immunosuppressive effects.
Other promising agents include amino-acetonitrile derivatives (AADs). AADs are a class of low molecular mass compounds bearing different aryloxy and aroyl moieties on an amino-acetonitrile core. They offer a class of novel synthetic compounds with high activity against gastrointestinal nematodes, including resistant isolates [35]. Its use has been limited to livestock/veterinary medicine.
Conclusions
We present a rare case of S. stercoralis infection in an HIV-infected patient. What makes our case peculiar is that most case reports of S. stercoralis infection are from patients with low CD4 count and from immigrants from endemic countries. In contrast, our patient had a relatively high CD4 count, undetectable viral load, and was born and raised in the northeastern USA. Carriers of S. stercoralis may remain asymptomatic for many years. Although it is possible that our patient may have been infected with S. stercoralis from his previous travels, anal sex with other men may also be a plausible route of transmission.
The diagnosis of S. stercoralis infection remains a challenge. Our patient’s stool ova and parasite examination was unrevealing and the diagnosis of S. stercoralis infection was found only through colonic biopsy. Clinicians must consider parasitic diseases such as S. stercoralis among HIV-infected patients with persistent diarrhea and eosinophilia, especially among men having sex with men, regardless of ethnicity or recent travel history.
Footnotes
Sources of support: Financial support for publication is subsidized by the Office of Graduate Medical Education of Yale New Haven Health-Bridgeport Hospital
References:
- 1.Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases Updated Edition. 7th ed. Philadelphia: Churchill Livingstone/Elsevier; 2015. [Google Scholar]
- 2.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17(1):208–17. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: Global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong IS, Zaidi SY, McEvoy P, Neafie RC. Diagnosis of Strongyloides stercoralis in a peritoneal effusion from an HIV-seropositive man. A case report. Acta Cytol. 2004;48(2):211–14. doi: 10.1159/000326318. [DOI] [PubMed] [Google Scholar]
- 5.Kakati B, Dang S, Heif M, et al. Strongyloides duodenitis: Case report and review of literature. J Natl Med Assoc. 2011;103(1):60–63. doi: 10.1016/s0027-9684(15)30246-7. [DOI] [PubMed] [Google Scholar]
- 6.Nabha L, Krishnan S, Ramanathan R, et al. Prevalence of Strongyloides stercoralis in an urban US AIDS cohort. Pathog Glob Health. 2012;106(4):238–44. doi: 10.1179/2047773212Y.0000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hochberg NS, Moro RN, Sheth AN, et al. High prevalence of persistent parasitic infections in foreign-born, HIV-infected persons in the United States. PLoS Negl Trop Dis. 2011;5(4):e1034. doi: 10.1371/journal.pntd.0001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maayan S, Wormser GP, Widerhorn J, et al. Strongyloides stercoralis hyperinfection in a patient with the acquired immune deficiency syndrome. Am J Med. 1987;83(5):945–48. doi: 10.1016/0002-9343(87)90656-5. [DOI] [PubMed] [Google Scholar]
- 9.Siegel MO, Simon GL. Is human immunodeficiency virus infection a risk factor for Strongyloides stercoralis hyperinfection and dissemination. PLoS Negl Trop Dis. 2012;6(7):e1581. doi: 10.1371/journal.pntd.0001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celedon JC, Mathur-Wagh U, Fox J, et al. Systemic strongyloidiasis in patients infected with the human immunodeficiency virus. A report of 3 cases and review of the literature. Medicine (Baltimore) 1994;73(5):256–63. doi: 10.1097/00005792-199409000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Lessnau KD, Can S, Talavera W. Disseminated Strongyloides stercoralis in human immunodeficiency virus-infected patients. Treatment failure and a review of the literature. Chest. 1993;104(1):119–22. doi: 10.1378/chest.104.1.119. [DOI] [PubMed] [Google Scholar]
- 12.Makris AN, Sher S, Bertoli C, Latour MG. Pulmonary Strongyloidiasis: An unusual opportunistic pneumonia in a patient with AIDS. Am J Roentgenol. 1993;161(3):545–47. doi: 10.2214/ajr.161.3.8352101. [DOI] [PubMed] [Google Scholar]
- 13.Martin SJ, Cohen PR, MacFarlane DF, Grossman ME. Cutaneous manifestations of Strongyloides stercoralis hyperinfection in an HIV-seropositive patient. Skinmed. 2011;9(3):199–202. [PubMed] [Google Scholar]
- 14.Kramer MR, Gregg PA, Goldstein M, et al. Disseminated Strongyloidiasis in AIDS and non-AIDS immunocompromised hosts: Diagnosis by sputum and bronchoalveolar lavage. South Med J. 1990;83(10):1226–29. doi: 10.1097/00007611-199010000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Dutcher JP, Marcus SL, Tanowitz HB, et al. Disseminated strongyloidiasis with central nervous system involvement diagnosed antemortem in a patient with acquired immunodeficiency syndrome and Burkitts lymphoma. Cancer. 1990;66(11):2417–20. doi: 10.1002/1097-0142(19901201)66:11<2417::aid-cncr2820661129>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen PB, Mojon M. Improved diagnosis of Strongyloides stercoralis by seven consecutive stool specimens. Zentralbl Bakteriol Mikrobiol Hyg A. 1987;263(4):616–18. doi: 10.1016/s0176-6724(87)80207-9. [DOI] [PubMed] [Google Scholar]
- 17.Gonçalves AL, Silva CV, Ueta MT, Costa-Cruz JM. A new faecal antigen detection system for Strongyloides venezuelensis diagnosis in immunosuppressed rats. Exp Parasitol. 2010;125(4):338–41. doi: 10.1016/j.exppara.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: A systematic review. PLoS Negl Trop Dis. 2013;7(1):e2002. doi: 10.1371/journal.pntd.0002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchi Blatt J, Cantos GA. Evaluation of techniques for the diagnosis of Strongyloides stercoralis in human immunodeficiency virus (HIV) positive and HIV negative individuals in the city of Itajai, Brazil. Braz J Infect Dis. 2003;7(6):402–8. doi: 10.1590/s1413-86702003000600008. [DOI] [PubMed] [Google Scholar]
- 20.Krolewiecki AJ, Ramanathan R, Fink V, et al. Improved diagnosis of Strongyloides stercoralis using recombinant antigen-based serologies in a community-wide study in northern Argentina. Clin Vaccine Immunol. 2010;17(10):1624–30. doi: 10.1128/CVI.00259-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verweij JJ, Canales M, Polman K, et al. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg. 2009;103(4):342–46. doi: 10.1016/j.trstmh.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 22.ten Hove RJ, van Esbroeck M, Vervoort T, et al. Molecular diagnostics of intestinal parasites in returning travellers. Eur J Clin Microbiol Infect Dis. 2009;28(9):1045–53. doi: 10.1007/s10096-009-0745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mejia R, Vicuña Y, Broncano N, et al. A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations. Am J Trop Med Hyg. 2013;88(6):1041–47. doi: 10.4269/ajtmh.12-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basuni M, Muhi J, Othman N, et al. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am J Trop Med Hyg. 2011;84(2):338–43. doi: 10.4269/ajtmh.2011.10-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lodh N, Caro R, Sofer S, et al. Diagnosis of Strongyloides stercoralis: Detection of parasite-derived DNA in urine. Acta Trop. 2016;163:9–13. doi: 10.1016/j.actatropica.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, et al. Efficacy and safety of ivermectin and thiabendazole in the treatment of Strongyloidiasis. Expert Opin Pharmacother. 2004;5(12):2615–19. doi: 10.1517/14656566.5.12.2615. [DOI] [PubMed] [Google Scholar]
- 27.Henriquez-Camacho C, Gotuzzo E, Echevarria J, et al. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. 2016;(1):CD007745. doi: 10.1002/14651858.CD007745.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacanowski J, Santos MD, Roux A, et al. Subcutaneous ivermectin as a safe salvage therapy in Strongyloides stercoralis hyperinfection syndrome: A case report. Am J Trop Med Hyg. 2005;73(1):122–24. [PubMed] [Google Scholar]
- 29.Turner SA, Maclean JD, Fleckenstein L, Greenaway C. Parenteral administration of ivermectin in a patient with disseminated strongyloidiasis. Am J Trop Med Hyg. 2005;73(5):911–14. [PubMed] [Google Scholar]
- 30.Tarr PE, Miele PS, Peregoy KS, et al. Case report: Rectal adminstration of ivermectin to a patient with Strongyloides hyperinfection syndrome. Am J Trop Med Hyg. 2003;68(4):453–55. [PubMed] [Google Scholar]
- 31.Segarra-Newnham M. Manifestations, diagnosis, and treatment of Strongyloides stercoralis infection. Ann Pharmacother. 2007;41(12):1992–2001. doi: 10.1345/aph.1K302. [DOI] [PubMed] [Google Scholar]
- 32.Pornsuriyasak P, Niticharoenpong K, Sakapibunnan A. Disseminated Strongyloidiasis successfully treated with extended duration ivermectin combined with albendazole: A case report of intractable strongyloidiasis. Southeast Asian J Trop Med Public Health. 2004;35(3):531–34. [PubMed] [Google Scholar]
- 33.Schad GA. Cyclosporine may eliminate the threat of overwhelming strongyloidiasis in immunosuppressed patients. J Infect Dis. 1986;153(1):178. doi: 10.1093/infdis/153.1.178. [DOI] [PubMed] [Google Scholar]
- 34.Armson A, Cunningham GA, Grubb WB, Mendis AH. Murine strongyloidiasis: The effects of cyclosporin A and thiabendazole administered singly and in combination. Int J Parasitol. 1995;25(4):533–35. doi: 10.1016/0020-7519(94)00115-5. [DOI] [PubMed] [Google Scholar]
- 35.Kaminsky R, Ducray P, Jung M, et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452(7184):176–80. doi: 10.1038/nature06722. [DOI] [PubMed] [Google Scholar]


