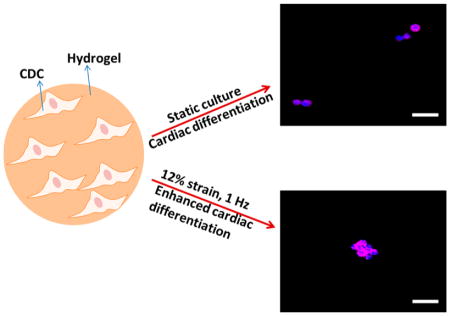
Abstract
Cardiac stem cell therapy has been considered as a promising strategy for heart tissue regeneration. Yet achieving cardiac differentiation after stem cell transplantation remains challenging. This compromises the efficacy of current stem cell therapy. Delivery of cells using matrices that stimulate the cardiac differentiation may improve the degree of cardiac differentiation in the heart tissue. In this report, we investigated whether elastic modulus of highly flexible poly(N-isopropylamide) (PNIPAAm)-based hydrogels can be modulated to stimulate the encapsulated cardiosphere derived cells (CDCs) to differentiate into cardiac lineage under static condition and dynamic stretching that mimics the heart beating condition. We have developed hydrogels whose moduli do not change under both dynamic stretching and static conditions for 14 days. The hydrogels had the same chemical structure but different elastic moduli (11, 21, and 40 kPa). CDCs were encapsulated into these hydrogels and cultured under either native heart-mimicking dynamic stretching environment (12% strain and 1 Hz frequency) or static culture condition. CDCs were able to grow in all three hydrogels. The greatest growth was found in the hydrogel with elastic modulus of 40 kPa. The dynamic stretching condition stimulated CDC growth. The CDCs demonstrated elastic modulus-dependent cardiac differentiation under both static and dynamic stretching conditions as evidenced by gene and protein expressions of cardiac markers such as MYH6, CACNA1c, cTnI, and Connexin 43. The highest differentiation was found in the 40 kPa hydrogel. These results suggest that delivery of CDCs with the 40 kPa hydrogel may enhance cardiac differentiation in the infarct hearts.
Keywords: myocardial infarction, poly(N-isopropylamide) hydrogel, dynamic stretching, cardiosphere derived cells, cardiac differentiation
Graphical Abstract
1. INTRODUCTION
Myocardial infarction (MI) is one of the major cardiovascular diseases that affect more than 8 million people in the United States alone.1 Following MI, the extensive cell death, cardiac extracellular matrix degradation, wall thinning, and cardiac fibrosis lead to the decrease of cardiac function. As the MI progresses from early to late stages, cardiac function further decreases causing heart failure. Unlike many other tissues, the damaged cardiac tissue cannot effectively regenerate by itself because cardiac stem cells in the heart spontaneously generate only a limited number of cardiomyocytes, and adult cardiomyocytes cannot proliferate.2 Stem/progenitor cell therapy has been considered as a promising strategy to regenerate the lost cardiac tissue to restore cardiac function.
Various cell types have been explored in clinical and preclinical models for cardiac therapy. Some stem cell types do not differentiate into cardiomyocytes to directly regenerate cardiac tissue, but can indirectly promote the regeneration. These cell types typically provide paracrine effects to augment the survival of resident cardiac cells, recruit endogenous stem cells, and vascularize the damaged heart tissue.3–5 Examples of these stem cells include bone marrow-derived stem cells6,7 and adipose-derived stem cells.8–11 Some stem cell types are capable of differentiating into cardiomyocytes to regenerate the cardiac tissue, leading to the restoration of heart function. These cells include cardiac stem/progenitor cells,12–16 pluripotent stem cell [embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs)]-derived cardiovascular progenitor cells,17–20 and cardiosphere-derived cells (CDCs).21–24 CDCs are harvested from the atria or ventricles of the heart and have fast ex vivo proliferation rate. They are able to differentiate into cardiomyocytes, smooth muscle cells and endothelial cells in vitro and in vivo.24 Compared to ethical issue associated with ESCs and possible teratoma formation risk of ESCs and iPSCs, CDCs stand out in stem/progenitor cell based therapy.24
To deliver CDCs into infarcted heart tissue, a common approach is to inject cell suspension directly to the damaged area.21–24 Yet the cell engraftment and cardiac differentiation have been found to be low, limiting the efficacy of the therapy. To increase cell engraftment and enhance cardiac differentiation, an appropriate cell carrier may be used. Injectable hydrogels are excellent candidates. These hydrogels can be readily injected into the tissue with minimal damage to the tissue. The hydrogels may be modified to improve cell adhesion, survival and cardiac differentiation. Different hydrogels have been injected into infarcted hearts, including fibrin,25 alginate,26 collagen,27 Matrigel,28 hyaluronic acid,29 chitosan,30 decellularized extracellular matrix,31 degradable poly(N-isopropylacrylamide) (PNIPAAm)-based hydrogels,32 poly-(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO),33 poly(D-lysine) (PDL),34 and MPEG-PCL-MPEG.35 Among these hydrogels, PNIPAAm-based hydrogels have the advantage of fast gelation, which can increase cell retention in the heart tissue;36–42 and convenient loading of cell/drugs below their sol–gel transition temperature (<32 °C). We have shown that these hydrogels support the survival and growth of bone marrow derived cells and CDCs.36–42
In this report, we developed a family of highly flexible poly(N-isopropylamide) (PNIPAAm)-based hydrogels, and investigated whether elastic modulus of can be modulated to stimulate the encapsulated CDCs to differentiate into cardiac lineage. Matrix modulus-driven cardiac differentiation is attractive as it is more convenient than commonly used coculture (with cardiomyocytes) approach21 and growth factor induction approach.43 It is expected that injection of CDCs with a hydrogel capable of differentiating them into cardiac lineage in vivo will enhance cardiac differentiation thus promoting cardiac regeneration. When delivered to the heart tissue, CDCs will experience not only matrix modulus but also cyclic stretching during heart beating. The dynamic mechanical signal has been found to affect stem cell differentiation into bone,44 cartilage,45 tendon,46 skeleton muscle47 and cardiac48 cells. It is possible that the cyclic stretching affects matrix modulus-driven CDC differentiation. Understanding this relation will allow us to determine whether the in vitro optimized elastic modulus for CDC cardiac differentiation will likely effectively differentiate into cardiac lineage after transplantation into infarcted hearts. In this work, we developed hydrogels whose moduli do not change under both dynamic mechanical training and static conditions for 14 days. CDCs were encapsulated into these hydrogels and cultured under either native heart-mimicking dynamic stretching environment or static condition. The effect of dynamic mechanical training on cardiac differentiation was investigated.
2. MATERIALS AND METHODS
2.1. Materials
All chemicals were purchased from Sigma-Aldrich unless otherwise stated. 2-hydroxyethyl methacrylate (HEMA) and acrylic acid (AAc) were passed through a column packed with inhibitor remover before use. N-isopropylacrylamide (NIPAAm) was purified by recrystallization in hexane for 3 times. β-Butyrolactone (Alfa Aesar) was used as received. BioFlex 6-well plates were purchased from Flexcell International Corporation. Iscove modified Dubecco’s media (IMDM) was purchased from Invitrogen. Fetal bovine serum (FBS) was obtained from Atalanta Biologicals.
2.2. Synthesis of Hydrogel Polymers
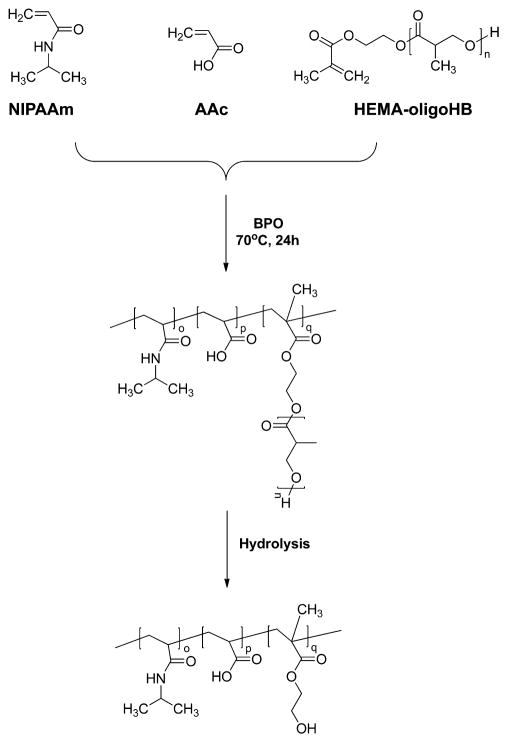
The hydrogel polymers were synthesized by copolymerization of NIPAAm, AAc and macromer HEMA-oligoHB based on HEMA and oligohydroxybutyrate (Scheme 1). The macromer was synthesized by HEMA-initiated ring-opening polymerization of β-butyrolactone using stannous (II) trifluoromethanesulfonate [Sn(OTf)2] as a catalyst.38–40,42 In brief, a 100 mL flask was charged with β-butyrolactone and HEMA. The flask was then immersed in a 110 °C oil bath to melt β-butyrolactone under the protection of nitrogen. The reaction was started following the addition of Sn(OTf)2 in THF/toluene (ratio 1/4). After 1 h, the reactant was precipitated in ice water. The precipitant was collected and redissolved in ethyl ester. The solution was dried with magnesium sulfate. After evaporation of ethyl ester under reduced pressure, the product was lyophilized. Structure of the macromer was confirmed by 1H NMR. When the feed ratio of HEMA and β-butyrolactone was controlled at 1:2, 1:4, and 1:6, the resulting macromers had HEMA to 2-hydroxybutyrate ratios of 1:2.1, 1:4.9, and 1:5.4, respectively.
Scheme 1.
Synthesis Route and Degradation Product of Hydrogel PNAH
The hydrogel polymers were synthesized by free radical polymerization. Benzoyl peroxide (BPO) was used as an initiator (Scheme 1).32,36–42 In brief, NIPAAm, AAc, macromer, and BPO were dissolved in dioxane and charged into a 250 mL 3-necked flask. The polymerization was conducted at 60 °C under the protection of nitrogen. After 16 h of reaction, the solution was precipitated in hexane. The polymer was first purified twice by dissolving in THF and precipitating in ethyl ether, and then vacuum-dried overnight. The polymers are abbreviated as PNAH(n), where n represents number of repeating units based on feed ratio.
2.3. Characterization of Hydrogel Polymers
Chemical composition of the synthesized polymers was determined by 1H NMR. Hydrogel solutions were obtained by dissolving the hydrogel polymers in Dulbecco’s modified phosphate buffer saline (DPBS) in an ice bath. The final concentration was 20 wt %. Lower critical solution temperatures (LCSTs) of the solutions were determined by DSC over a temperature range of 0–60 °C with a 10 °C/min heating rate. The solid hydrogels were formed after incubating the solutions in a 37 °C water bath.
Mechanical properties of the hydrogels were determined by a uniaxial mechanical test using an Instron tensile tester equipped with a 37 °C water bath.38–42 A 10 lb load cell and a displacement rate of 50 mm/min were used. Before testing, the hydrogels were incubated in 37 °C PBS for 1 day in order to reach an equilibrium state. To monitor hydrogel mechanical properties after degradation, we tested the hydrogels incubated in 37 °C PBS for 14 days. The elastic moduli of the hydrogels were calculated from the elastic deformation region of the stress–strain curves.
2.4. CDC Culture
Murine CDCs were isolated from atrial biopsy following the established protocol.49 Cells were cultured in a 175 cm2 tissue culture flask using IMDM supplemented with 10% FBS, 2% L-glutamine and 1% antibiotics. Cells were passaged when reaching 90% confluence. CDCs between passages 11 and 13 were used to encapsulate in the hydrogels.
2.5. CDC Encapsulation into Hydrogels and Dynamic Mechanical Training
To encapsulate CDCs into hydrogels, cells were typsinized from the culture flask and resuspended in PBS at a density of 40 million/mL. 0.2 mL of cell suspension was then mixed with 1 mL of hydrogel solution. The mixture was added into each well of the 6-well BioFlex culture plates. The plates were placed in a 37 °C incubator for gelation. After 45 min, the supernatant was replaced by culture medium (DPBS supplemented with 10% FBS, 2% L-glutamine and 1% antibiotics). The plates were then mounted to a Flexcell FX-5000 Bioreactor with a “heart” mimicking wave function (“Heart-P” wave, Figure 1). The strain and frequency were 12% and 1 Hz, respectively. Meanwhile, the cell/hydrogel constructs placed in the BioFlex culture plates without mechanical training will be the controls.
Figure 1.
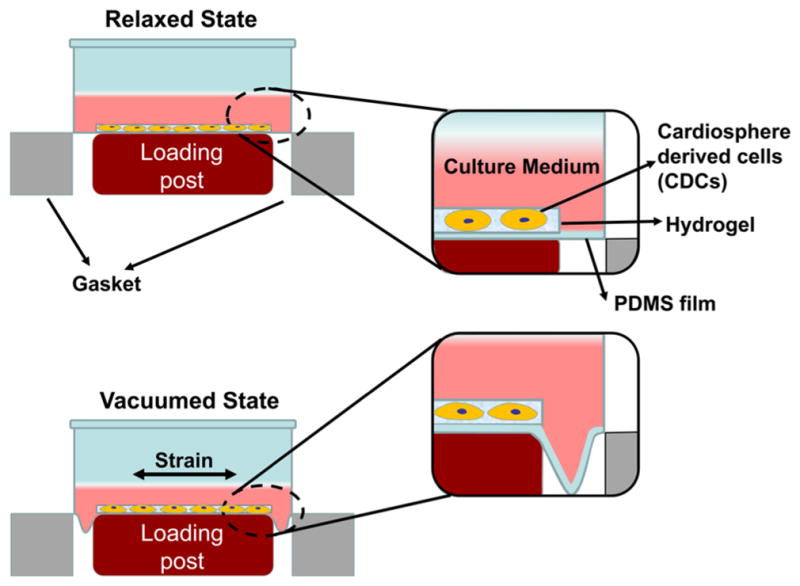
Scheme of dynamic training in Flexcell FX-5000 Bioreactor.
2.6. CDC proliferation in hydrogels
After 1, 7, and 14 days of culture, the CDC/hydrogel constructs were taken out from the plates and digested in papain solution at 60 °C for 24 h to release the double stranded DNA (dsDNA, for live cells). PicoGreen dsDNA kit (Invitrogen) was used to measure dsDNA content. For each group, the dsDNA content at each time point was normalized with that of day 1.
2.7. Differentiation of Encapsulated CDCs in Hydrogels under Static and Dynamic Mechanical Training Culture Conditions
CDC cardiac differentiation in the hydrogels under static and dynamic mechanical training conditions was characterized at the gene level by real time RT-PCR and at the protein level by immunohistochemistry. To characterize gene expression, we homogenized the CDC/hydrogel constructs in TRIzol (Sigma) following the manufacture’s protocol to collect RNA. The quality of total RNA was assessed by Nanodrop (Fisher). Then, 1 μg of RNA was used to reverse transcript into cDNA by a high capacity reverse transcription kit (ABI). Primers of forward and reverse pairs of myosin heavy chain alpha (MYH6), calcium channel, voltage-dependent, L type, alpha 1c (CACNA1c), and β-actin are listed in Table 1. Real-time RT-PCR was performed with Maxima SYBR Green/fluorescein master mix on an Applied Biosystem 7900 system. Fold differences were calculated using standard ΔΔCt method.38–40,42 Gene expression of CDCs cultured on 2D tissue culture flasks was used for normalization during the calculation.
Table 1.
Sequences and Tm Values of Primers Used for Real Time RT-PCR
| transcription | prime sequences | Tm (°C)a |
|---|---|---|
| MYH6 | forward GAGGAGATGCGAGATGAGAG | 61.6 |
| reverse CGGTTTGATCTTGAAGTAGAGC | 61.3 | |
| CACNA1c | forward CAGAAACTACAGGAGAAGAGG | 59.5 |
| reverse AAGAAGAGGATCAGGTTGGT | 60.5 | |
| β-Actin | forward AAGATCAAGATCATTGCTCCTC | 61.2 |
| reverse GGACTVATCGTACTCCTG | 59.5 |
Tm values were calculated by NIH PerlPrimer.
To characterize protein expression, we collected cell/hydrogel constructs after 14 days of culture and fixed in 4% paraformaldehyde for 1 h. The constructs were then washed by PBS for 3 times, embedded in optimal cutting temperature (OCT) solution, and sectioned with 10 μm thickness. The sections were blocked by 10% goat serum and permeablized by 0.3% Triton X-100 for 1 h. Primary antibody mouse antimouse cardiac troponin I (cTnI, Abcam), and rabbit antimouse connexin 43 (Cell Signaling) were used to incubate sections at 37 °C overnight, followed by incubating with secondary antibodies (Jackson ImmunoResearch). Finally, Hoechst 33342 was used to counter-stain cell nucleus. Sections without primary antibody incubation were used as negative controls. All images were taken by Olympus FV1000 confocal microscope.
2.8. Statistical Analysis
Data were reported as mean ± standard deviation. One way ANOVA with posthoc Tukey-Kramer test was used for data analysis. A statistical significance was considered when p < 0.05.
3. RESULTS
3.1. Hydrogel Polymer Composition and Thermosen-sitivity
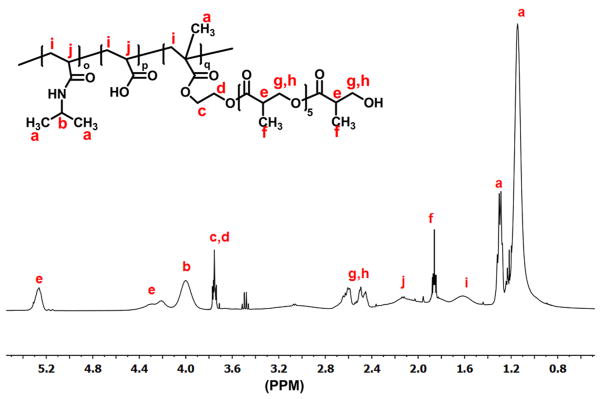
Hydrogel polymers were synthesized by free radical polymerization of NIPAAm, AAc, and macromer HEMA-oligoHB. Structure of the polymers was confirmed by 1H NMR. A typical 1H NMR spectrum exhibited characteristic peaks of NIPAAm, AAc and macromer units (Figure 2). The composition of the polymers was consistent with monomer feed ratio (Table 2). The hydrogels were thermosensitive. The three hydrogels PANH(2), PANH(4), and PANH(6) had LCSTs of 21.1, 18.9, and 16.1 °C, respectively (Figure 3). An increase in oligomer length from 2 HB units to 6 HB units decreased the LCST. The hydrogels remained flowable at temperatures below LCSTs, for example 4 °C (Figure 4a), whereas became solid gel at 37 °C (Figure 4b).
Figure 2.
1H NMR spectrum of synthesized hydrogel PANH(6).
Table 2.
Monomer Feed Ratio and Hydrogel Composition
| hydrogel | feed ratio | composition |
|---|---|---|
| PANH(2) | 86.0/4.0/10.0 | 86.0/4.0/10.0 |
| PANH(4) | 86.0/4.0/10.0 | 86.0/6.4/7.6 |
| PANH(6) | 86.0/4.0/10.0 | 86.0/6.9/7.1 |
Figure 3.
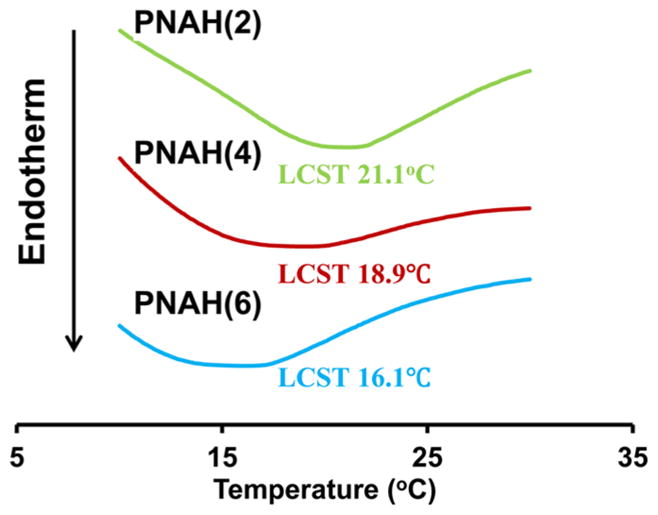
Lower critical solution temperature (LCST) of the hydrogel solutions based on three hydrogels. LCSTs were obtained from DSC curves.
Figure 4.
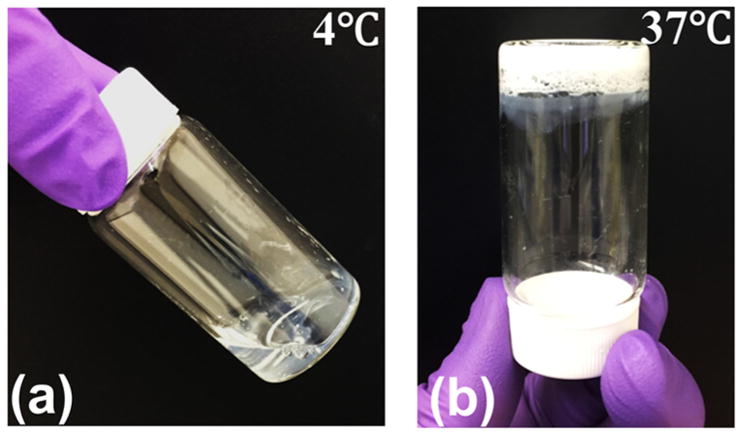
Macroscopic images of hydrogel PANH(6) before and after gelation: (a) flowable at 4 °C, (b) gelation at 37 °C.
3.2. Hydrogel Mechanical Properties with or without Dynamic Training
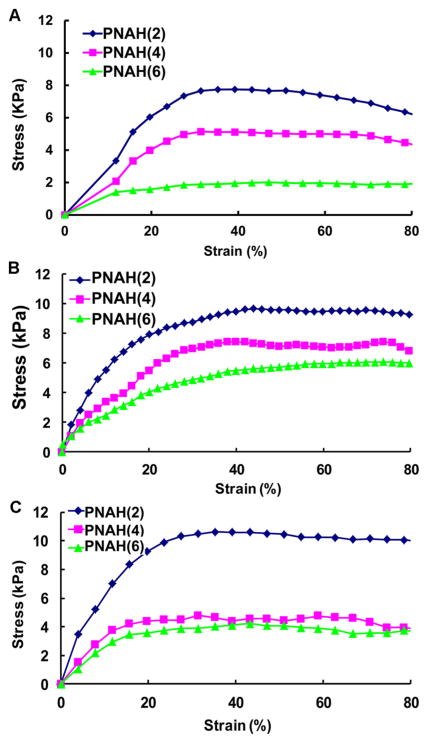
The hydrogels were stretchable at 37 °C (Figure 5a, b). Tensile testing results demonstrated that the breaking strain was greater than 300%, exceeding the strain limit of the testing instrument. The three hydrogels showed distinctively different tensile behaviors especially in the elastic deformation region (Figure 6A). The elastic modulus was dependent on the oligomer length (Table 3). An increase in number of HB units led to the decrease in elastic modulus.
Figure 5.
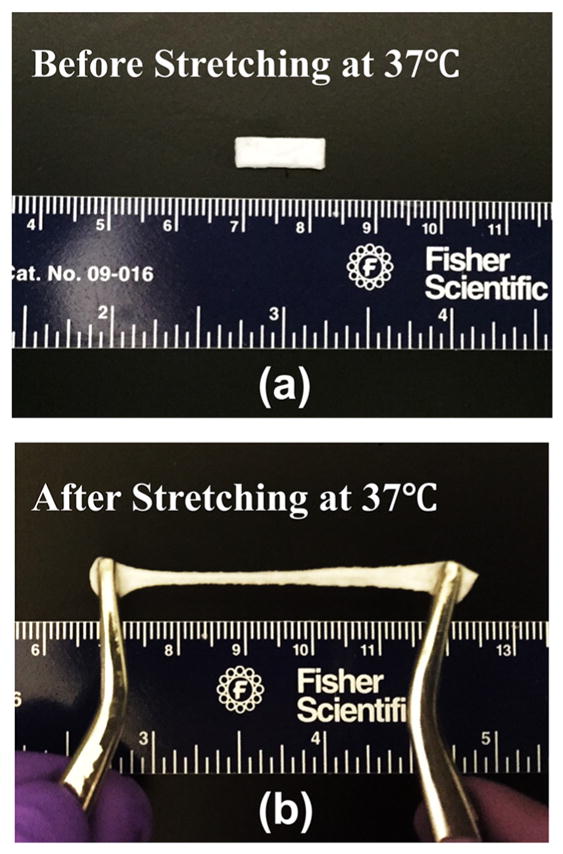
Macroscopic images of hydrogel PANH(6) (a) before and (b) after stretching at 37 °C.
Figure 6.
Stress–strain curves of hydrogels: (a) 1 day after gelation; (b) 14 days incubation in PBS under static condition; and (c) 14 days incubation in PBS under dynamic mechanical training. Only 80% of strain is shown in order to clearly demonstrate the difference in the elastic deformation region between three hydrogels.
Table 3.
Elastic Modulus of Hydrogels at Days 1 and 14 When Incubated in PBS at 37 °C with or without Dynamic Mechanical Training
| hydrogel | day 1 | day 14 (static) | day 14 (dynamic) |
|---|---|---|---|
| PANH(2) | 40.3 ± 9.8 | 43.8 ± 8.7 | 42.8 ± 2.2 |
| PANH(4) | 21.2 ± 1.2 | 24.4 ± 1.9 | 23.5 ± 3.9 |
| PANH(6) | 11.3 ± 1.0 | 16.6 ± 2.6 | 12.2 ± 2.2 |
To investigate whether hydrogel degradation and dynamic mechanical training have effects on elastic modulus, we performed tensile tests on hydrogels incubated in PBS under static and dynamic mechanical training conditions for 14 days, respectively. Under static condition, each of the three hydrogels exhibited the similar elastic modulus at day 14 and day 1 (p > 0.05. Figure 6B and Table 3). For the hydrogels that had mechanical training for 14 days, the elastic modulus of each hydrogel at day 14 was also similar to that at day 1 (p > 0.05). In addition, no significant difference was found for hydrogels with and without mechanical training (p > 0.05). These results demonstrate that hydrogel degradation and dynamic mechanical training during the 14-day period did not have impact on tensile properties.
3.3. CDC Proliferation in Hydrogels under Static and Dynamic Mechanical Training Conditions
CDCs were encapsulated in the hydrogels and cultured under either static condition or dynamic mechanical training condition to investigate the effect of mechanical training on cell proliferation. Cell number was quantified by dsDNA content (for live cells). Figure 7 demonstrates that cell dsDNA content increased during the 14-day culture period under both culture conditions (p < 0.01 for day 14 vs day 7 for all hydrogels). When comparing the dsDNA contents of the same hydrogels cultured under the static and dynamic mechanical training conditions, CDCs exhibited higher degree of proliferation under the dynamic mechanical training condition.
Figure 7.
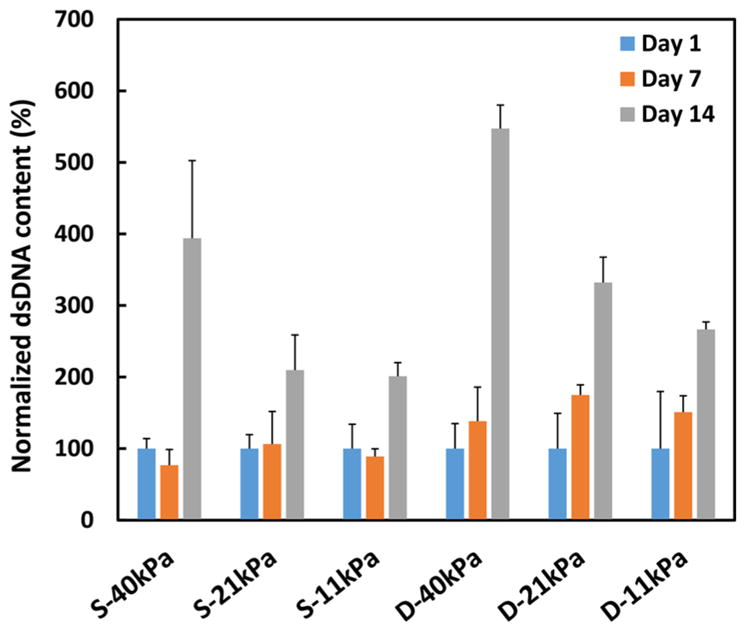
dsDNA content of CDCs in different hydrogels cultured under static (S) and dynamic mechanical training (D) conditions.
Besides culture conditions, hydrogel elastic modulus showed impact on CDC proliferation. CDCs demonstrated different proliferation pattern in the hydrogels with different elastic modulus. dsDNA contents in 40 kPa hydrogel were significantly higher at day 14 compared to those in 21 and 11 kPa hydrogels under both static (p < 0.01) and dynamic mechanical training (p < 0.05) conditions. These results indicate that 40 kPa hydrogel is optimal for CDC proliferation.
3.4. Cardiac Differentiation of Encapsulated CDCs under Static and Dynamic Mechanical Training Conditions
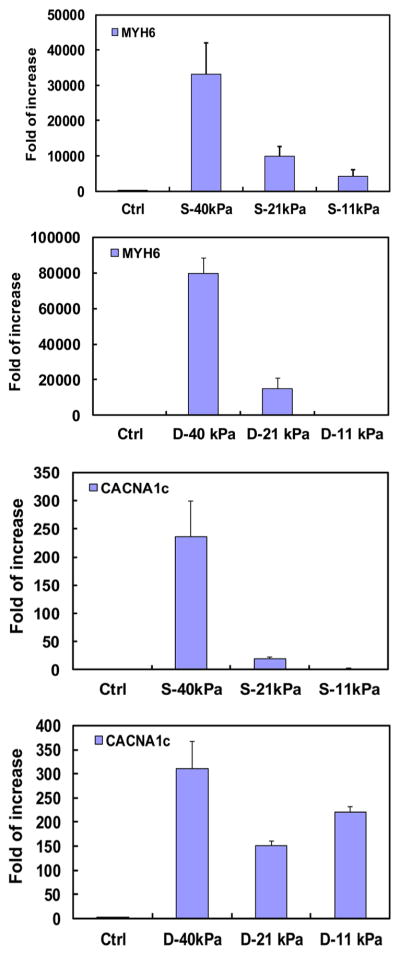
The cardiac differentiation of encapsulated CDCs was assessed at the gene level by real time RT-PCR and protein level by immunohistochemistry. Cardiac markers MYH6 and CACNA1c were used to characterize differentiation at the mRNA level. The two markers were significantly up-regulated in hydrogels with 21 and 40 kPa elastic modulus after 14 days of culture. The markers also demonstrated elastic modulus-dependent expression under static culture condition (Figure 8). The highest expressions were observed in the hydrogel with 40 kPa hydrogel (p < 0.05 for 40 kPa vs 21 and 11 kPa).
Figure 8.
Cardiac gene (MYH6 and CACNA1c) expressions of CDCs in different hydrogels cultured under static (S) and dynamic mechanical training (D) conditions.
Dynamic mechanical training condition showed impact on hydrogel elastic modulus-dependent CDC differentiation. For MYH6 marker, the dynamic mechanical training significantly upregulated the expression in 21 and 40 kPa hydrogels (p < 0.05, Figure 8), but not in 11 kPa hydrogel. For CACNA1c marker, the expression was substantially increased for 40 kPa hydrogel (p > 0.05), but significantly increased in 21 and 11 kPa hydrogels (p < 0.01). In addition, the expression in D-11 kPa hydrogel was greater than that in D-21 kPa. Overall, these results demonstrate that the highest cardiac differentiation was achieved in 40 kPa hydrogel and the dynamic mechanical training stimulated the cardiac differentiation.
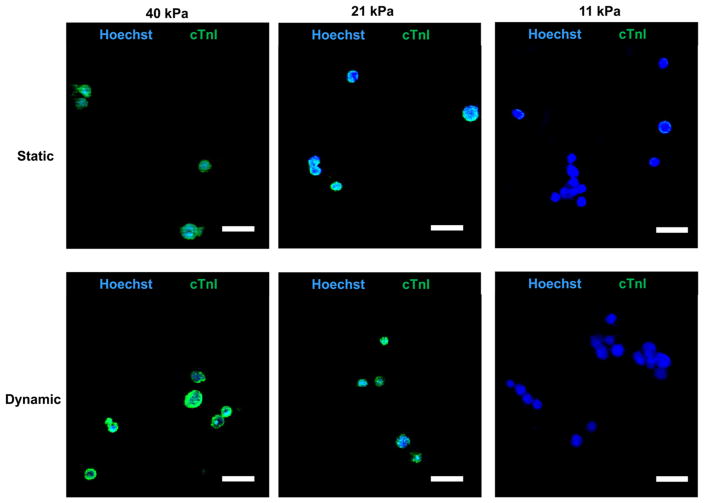
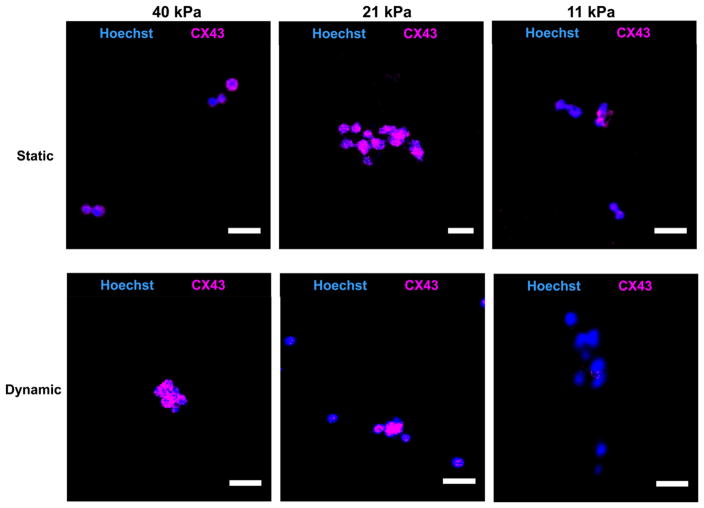
CDC cardiac differentiation was further characterized at the protein level by immunohistochemistry (Figures 9 and 10). Cells in the hydrogels were stained with cardiac markers cTnI and CX43. Under static culture condition, every cell in 21 and 40 kPa hydrogels expressed cTnI and CX43 markers, whereas only a few cells in the 11 kPa hydrogel expressed these markers. Dynamic mechanical training did not affect cTnI and CX43 expressions in 21 and 40 kPa hydrogels as every cell expressed both markers compared to that in static culture condition (Figures 9 and 10).
Figure 9.
cTnI immunostaining of CDCs in hydrogels cultured under static and dynamic mechanical training conditions. Scale bar 25 μm.
Figure 10.
Connexin 43 immunostaining of CDCs in hydrogels cultured under static and dynamic mechanical training conditions. Scale bar 25 μm.
4. DISCUSSIONS
Matrix modulus has been shown to stimulate stem cells to differentiate into cardiac lineages. For example, hydrogel modulus promoted cardiac differentiation of mesenchymal stem cells (MSCs),1 and embryonic stem cells50 in vitro. In these reports, cells were cultured under static condition, which is different from the dynamic environment in the native heart. It is unclear whether the dynamic environment affects matrix modulus-driven cardiac differentiation. In this report, we determined whether hydrogel elastic modulus-driven CDC cardiac differentiation is likely to effectively occur under cyclic stretching conditions mimicking that of the beating heart.
4.1. Hydrogel Mechanical Properties Change during Mechanical Training
Dynamic mechanical training is widely used in tissue engineering field to provide the similar mechanical cue as that in the real tissues to the cultured cells. To investigate the effect of dynamic mechanical training on matrix elastic modulus-driven stem cell differentiation, it is essential to ensure that the elastic modulus does not change during the mechanical training period compared to the static condition. We assessed the mechanical properties of different hydrogels with or without dynamic mechanical training after 14 days. In this report, 12% strain was used for mechanical training. This strain is in the elastic deformation range of all hydrogels (<20% strain) and therefore they can recover when unstretched. This strain is also similar to that of the heart tissue experiences during a beating cycle.51 Figure 6 and Table 2 demonstrate that the dynamic mechanical training did not significantly change elastic modulus of all three hydrogels. These hydrogels thus provided an ideal platform to investigate the effect of dynamic mechanical training on matrix elastic modulus-driven stem cell differentiation.
For biodegradable hydrogels, modulus may decrease during the degradation.52 For example, PEG hydrogels with fibrinogen as a cross-linker showed a dramatic decrease in compressive modulus after incubating in PBS and culture medium for 7 days. This was a result of fast hydrolytic degradation of fibrinogen cross-linker.53 In this work, the hydrogels with or without dynamic mechanical training showed the same elastic modulus at days 1 and 14, suggesting that hydrogel degradation did not alter elastic modulus (Figure 6 and Table 2). This is because the hydrogels based on NIPAAm, AAc, and macromer HEMA-oligoHB had slow degradation rate.42 It is also possible that the surface degradation mechanism is dominated during the hydrogel degradation. Hydrogel elastic modulus is likely to decrease when the molecular weight reduces during degradation. The surface degradation will decrease molecular weight of polymer chains on hydrogel surface without substantially changing molecular weight of polymer chains in the bulk. Therefore, the surface degradation mechanism allows the polymers in the bulk to maintain structural integrity. In the developed hydrogels, degradation is occurred in the side chain oligoHB. It may undergo surface degradation same as its high molecular weight polymer.54 The surface degradation mechanism is also indirectly evidenced by unchanged water content during the degradation [44.0 ± 5.1% (day 1) vs 44.1 ± 8.4% (day 14) for PANH(2); 38.6 ± 6.0% (day 1) vs 33.6 ± 5.2% (day 14) for PANH(4); 39.3 ± 1.2% (day 1) vs 33.3 ± 6.8% (day 14) for PANH(6)].
4.2. Proliferation of CDCs in Gels with Different Modulus under Static and Dynamic Cultures
When CDCs were encapsulated in the hydrogels, they were able to proliferate during a 14-day culture period. The greatest cell proliferation was observed for CDCs encapsulated in the 40 kPa hydrogel under both static and dynamic mechanical training conditions (Figure 7). This is consistent with our previous report where MSCs showed the greatest proliferation in hydrogels with similar modulus.40 It also indicates that the dynamic mechanical training did not affect the relationship between hydrogel modulus and cell proliferation. The dynamic mechanical training even stimulated CDC proliferation. The dsDNA contents in all three hydrogels under dynamic mechanical training condition were substantially higher than those in the static condition.
Dynamic stretching has been shown to affect cell proliferation. The mechanisms include DNA stimulation,55 enhanced focal adhesion56 and inhibition of apoptosis pathway under mechanical stimulation.57 Besides CDCs, many other cell types demonstrated enhanced proliferation under dynamic mechanical training condition. For example, human osteoblasts cultured under dynamic stretching condition (1000 μm strain at 1 Hz for 30 min per day) significantly increased cell number after 2 days.58 MSCs showed significantly higher cell density under 1% strain and 1 Hz condition than those cultured under static condition.59 Vascular smooth muscle cells proliferated significantly under constant mechanical stimulation compared to the static culture condition.60 The effects of mechanical stimulation to cell proliferation are also dependent on the frequency and strain applied. It was reported that osteoblasts showed a decrease in cell proliferation when frequency was higher than 1 Hz.61
4.3. Cardiac Differentiation of Encapsulated CDCs in Hydrogels under Static and Dynamic Culture
CDCs demonstrated hydrogel elastic modulus-dependent differentiation as confirmed at the mRNA and protein levels. Under the static culture condition, the expressions of MYH6 and CACNA1c at the mRNA level were increased with the increase of hydrogel elastic modulus from 11, 21 to 40 kPa (Figure 8). At the protein level, all of the cells in the 40 kPa hydrogel expressed cTnI and CX43 (Figures 9 and 10). These results are consistent with our previous report where CDCs in 40 kPa PNIPAAm-based hydrogel differentiated into cardiac lineage.38 The matrix modulus-driven CDC cardiac differentiation occurs not only in compact hydrogel but also in microporous scaffold. We have demonstrated that CDCs differentiated into cardiomyocytes in fibrous scaffold based on poly(ester urethane)urea and PNIPAAm hydrogel, and the most significant differentiation was in scaffold with elastic modulus of ~50–60 kPa.62 Besides CDCs, other cell types also showed matrix modulus-driven cardiac differentiation. MSCs had the highest cardiac differentiation in 65 kPa hydrogel based on NIPAAm, N-acryloxysuccinimide, AAc, and poly(trimethylene carbonate)-hydroxyethyl methacrylate.40 Embryonic stem cells demonstrated the greatest degree of cardiac differentiation in PEG hydrogel with compressive modulus of 322 Pa.50
CDCs cultured under dynamic mechanical training condition also showed elastic modulus-dependent cardiac differentiation (Figures 8–10). Similar to the static culture condition, the greatest differentiation under dynamic mechanical training condition was also in 40 kPa hydrogel. Comparing gene expressions of MYH6 and CACNA1c under static and dynamic mechanical training conditions, it was found that the dynamic mechanical training stimulated the cardiac differentiation in 40 kPa hydrogel (Figure 8). For hydrogels with elastic moduli of 11 and 21 kPa, the MYH6 expression under dynamic condition was higher in 21 kPa hydrogel than in 11 kPa, while the CACNA1c expression exhibited the opposite trend. The exact mechanism needs further exploration. However, the overall gene expression results demonstrate that 40 kPa hydrogel is preferred for CDC cardiac differentiation and the dynamic mechanical training stimulated the cardiac differentiation. At the protein level, every cell in 40 kPa hydrogel expressed cTnI and CX43 markers under the dynamic mechanical training condition (Figures 9 and 10).
Biomechanical cues have been shown to regulate cell proliferation and differentiation. For example, MSCs encapsulated in PEG hydrogels showed a significant upregulation of chondrogenic markers under dynamic compression condition.63 Addition of TGFβ1 in the culture medium further enhanced the expression. In contrast, Jacot et al. encapsulated MSCs in alginate hydrogels and cultured the constructs under dynamic compression. The results demonstrated the chrondrogenesis was significantly decreased.64 The discrepancy in these studies may result from different strain amplitude and frequency. In addition, mechanical cue type, such as uniaxial, biaxial, and equiaxial, has effect on cell proliferation and differentiation.65
Strain amplitude affects stem cell differentiation under dynamic stretching. MSCs showed a significant up-regulation of osteogenic markers when the stretching strain was less than 5%. However, increasing the strain to 10% or higher decreased the expressions.66 Huang et al. found that the optimal strain for MSC cardiac differentiation was between 10 and 15%.67 When the strain was greater than 20%, the cardiac gene expressions were down-regulated. In this work, 12% strain was used. It is similar to the strain a normal heart experiences during a beating cycle (10–15%).51 Our results demonstrated this strain stimulated CDC cardiac differentiation in 21 and 40 kPa hydrogels (Figures 8–10).
Strain frequency also plays an important role in cell differentiation. Shimko et al. reported that murine embryonic stem cells had a significant increase in cardiac marker expression under 3 Hz dynamic training than 1 Hz.68 This may be attributed to 3 Hz being more close to intrinsic heat beating rate than 1 Hz. The beating rate of a typical mouse heart is ~600 beat/min, which is equivalent to 10 Hz. In this work, we found that 1 Hz frequency stimulated murine CDCs to differentiate into cardiac lineage as the expressions of cardiac markers MYH6 and CACNA1c were significantly increased (Figures 8–10).
5. CONCLUSIONS
A family of thermosensitive hydrogels with tunable elastic modulus was developed as CDC carriers for cardiac cell therapy. The effect of hydrogel elastic modulus on CDC proliferation and cardiac differentiation was investigated under static and dynamic mechanical training conditions. CDCs showed the highest proliferation and differentiation in the 40 kPa hydrogel under both conditions. These results suggest that transplantation of CDCs using 40 kPa hydrogel may lead to optimal cardiac differentiation and regeneration.
Acknowledgments
This work was supported by National Science Foundation (1006734 and 1160122), National Institutes for Health (R01EB022018, R01HL124122, and R21EB021896), American Heart Association (15GRNT25830058 and 13GRNT17150041), National Science Foundation of China (81471788), and an Institute for Materials Research seed grant at The Ohio State University.
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics-2016 Update: A Report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Etzion S, Kedes LH, Kloner RA, Leor J. Myocardial Regeneration: Present and Future Trends. Am J Cardiovasc Drugs. 2001;1:233–244. doi: 10.2165/00129784-200101040-00002. [DOI] [PubMed] [Google Scholar]
- 3.Forrester JS, Makkar RR, Marban E. Long-Term Outcome of Stem Cell Therapy for Acute Myocardial Infarction: Right Results, Wrong Reasons. J Am Coll Cardiol. 2009;53:2270–2272. doi: 10.1016/j.jacc.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Garbern JC, Lee RT. Cardiac Stem Cell Therapy and the Promise of Heart Regeneration. Cell Stem Cell. 2013;12:689–698. doi: 10.1016/j.stem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen MR, Myerburg RJ, Francis DP, Cole GD, Marban E. Translating Stem Cell Research to Cardiac Disease Therapies: Pitfalls and Prospects for Improvement. J Am Coll Cardiol. 2014;64:922–937. doi: 10.1016/j.jacc.2014.06.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duran JM, Makarewich CA, Sharp TE, Starosta T, Zhu F, Hoffman NE, Chiba Y, Madesh M, Berretta RM, Kubo H, Houser SR. Bone-Derived Stem Cells Repair the Heart after Myocardial Infarction through Transdifferentiation and Paracrine Signaling Mechanisms. Circ Res. 2013;113:539–552. doi: 10.1161/CIRCRESAHA.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez EC, Kofidis T. Adult Stem Cells for Cardiac Tissue Engineering. J Mol Cell Cardiol. 2011;50:312–319. doi: 10.1016/j.yjmcc.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Mazo M, Planat-Benard V, Abizanda G, Pelacho B, Leobon B, Gavira JJ, Penuelas I, Cemborain A, Penicaud L, Laharrague P, Joffre C, Boisson M, Ecay M, Collantes M, Barba J, Casteilla L, Prosper F. Transplantation of Adipose Derived Stromal Cells is Associated with Functional Improvement in a Rat Model of Chronic Myocardial Infarction. Eur J Heart Failure. 2008;10:454–462. doi: 10.1016/j.ejheart.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Mazo M, Hernandez S, Gavira JJ, Abizanda G, Arana M, Lopez-Martinez T, Moreno C, Merino J, Martino-Rodriguez A, Uixeira A, Garcia de Jalon JA, Pastrana J, Martinez-Caro D, Prosper F. Treatment of Reperfused Ischemia with Adipose-Derived Stem Cells in a Preclinical Swine Model of Myocardial Infarction. Cell Transplant. 2012;21:2723–2733. doi: 10.3727/096368912X638847. [DOI] [PubMed] [Google Scholar]
- 10.Shevchenko EK, Makarevich PI, Tsokolaeva ZI, Boldyreva MA, Sysoeva VY, Tkachuk VA, Parfyonova YV. Transplantation of Modified Human Adipose Derived Stromal Cells Expressing VEGF165 Results in More Efficient Angiogenic Response in Ischemic Skeletal Muscle. J Transl Med. 2013;11:138. doi: 10.1186/1479-5876-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rigol M, Solanes N, Roura S, Roque M, Novensa L, Dantas AP, Martorell J, Sitges M, Ramirez J, Bayes-Genis A, Heras M. Allogeneic Adipose Stem Cell Therapy in Acute Myocardial Infarction. Eur J Clin Invest. 2014;44:83–92. doi: 10.1111/eci.12195. [DOI] [PubMed] [Google Scholar]
- 12.Hosoda T, Zheng H, Cabral-da-Silva M, Sanada F, Ide-Iwata N, Ogorek B, Ferreira-Martins J, Arranto C, D’Amario D, del Monte F, Urbanek K, D’Alessandro DA, Michler RE, Anversa P, Rota M, Kajstura J, Leri A. Human Cardiac Stem Cell Differentiation is Regulated by a Mircrine Mechanism. Circulation. 2011;123:1287–1296. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of Cardiac Stem Cells in Patients with Ischemic Cardiomyopathy: the SCIPIO Trial: Surgical Aspects and Interim Analysis of Myocardial Function and Viability by Magnetic Resonance. Circulation. 2012;126:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J. Intracoronary Delivery of Autologous Cardiac Stem Cells Improves Cardiac Function in a Porcine Model of Chronic Ischemic Cardiomyopathy. Circulation. 2013;128:122–131. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latham N, Ye B, Jackson R, Lam BK, Kuraitis D, Ruel M, Suuronen EJ, Stewart DJ, Davis DR. Human Blood and Cardiac Stem Cells Synergize to Enhance Cardiac Repair When Cotransplanted into Ischemic Myocardium. Circulation. 2013;128:S105–S112. doi: 10.1161/CIRCULATIONAHA.112.000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced Effect of Combining Human Cardiac Stem Cells and Bone Marrow Mesenchymal Stem Cells to Reduce Infarct Size and to Restore Cardiac Function after Myocardial Infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spater D, Abramczuk MK, Buac K, Zangi L, Stachel MW, Clarke J, Sahara M, Ludwig A, Chien KR. A HCN4+ Cardiomyogenic Progenitor Derived from the First Heart Field and Human Pluripotent Stem Cells. Nat Cell Biol. 2013;15:1098–1106. doi: 10.1038/ncb2824. [DOI] [PubMed] [Google Scholar]
- 18.Nsair A, Schenke-Layland K, Van Handel B, Evseenko D, Kahn M, Zhao P, Mendelis J, Heydarkhan S, Awaji O, Vottler M, Geist S, Chyu J, Gago-Lopez N, Crooks GM, Plath K, Goldhaber J, Mikkola HK, MacLellan WR. Characterization and Therapeutic Potential of Induced Pluripotent Stem Cell-Derived Cardiovascular Progenitor Cells. PLoS One. 2012;7:e45603. doi: 10.1371/journal.pone.0045603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudson J, Titmarsh D, Hidalgo A, Wolvetang E, Cooper-White J. Primitive Cardiac Cells from Human Embryonic Stem Cells. Stem Cells Dev. 2012;21:1513–1523. doi: 10.1089/scd.2011.0254. [DOI] [PubMed] [Google Scholar]
- 20.Foldes G, Mioulane M, Chahine MN. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Serve as in Vitro Model of Cardiac Hypertrophy. Heart. 2011;97:e7. doi: 10.1136/heartjnl-2011-300920b.29ret. [DOI] [PubMed] [Google Scholar]
- 21.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative Potential of Cardiosphere-Derived Cells Expanded from Percutaneous Endomyocardial Biopsy Specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 22.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marban L, Marban E. Direct Comparison of Different Stem Cell Types and Subpopulations Reveals Superior Paracrine Potency and Myocardial Repair Efficacy with Cardiosphere-Derived Cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maxeiner H, Mufti S, Krehbiehl N, Dulfer F, Helmig S, Schneider J, Boning A, Matejec R, Weigand MA, Schluter KD, Wenzel S. Interleukin-6 Contributes to the Paracrine Effects of Cardiospheres Cultured from Human, Murine and Rat Hearts. J Cell Physiol. 2014;229:1681–1689. doi: 10.1002/jcp.24613. [DOI] [PubMed] [Google Scholar]
- 24.Davis DR, Zhang YQ, Smith RR, Cheng K, Terrovitis J, Malliaras K, Li TS, White A, Makkar R, Marban E. Validation of the Cardiosphere Method to Culture Cardiac Progenitor Cells from Myocardial Tissue. PLoS One. 2009;4(9):e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christman KL, Lee RJ. Biomaterials for the Treatment of Myocardial Infarction. J Am Coll Cardiol. 2006;48:907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Mauricio RG, Acarregui A, Sanchez-Margallo FM, Crisostomo V, Gallo I, Hernandez RM, Pedraz JL, Orive G, Martin-Cancho MF. A Preliminary Approach to the Repair of Myocardial Infarction Using Adipose Tissue-Derived Stem Cells Encapsulated in Magnetic Resonance-Labelled Alginate Microspheres in a Porcine Model. Eur J Pharm Biopharm. 2013;84:29–39. doi: 10.1016/j.ejpb.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Suuronen EJ, Veinot JP, Wong S, Kapila V, Price J, Griffith M, Mesana TG, Ruel M. Tissue-Engineered Injectable Collagen-Based Matrices for Improved Cell Delivery and Vascularization of Ischemic Tissue Using CD133+ Progenitors Expanded from the Peripheral Blood. Circulation. 2006;114:I138–I144. doi: 10.1161/CIRCULATIONAHA.105.001081. [DOI] [PubMed] [Google Scholar]
- 28.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-Free Growth of Undifferentiated Human Embryonic Stem Cells. Nat Biotechnol. 2001;19(10):971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 29.Chang CY, Chan AT, Armstrong PA, Luo HC, Higuchi T, Strehin IA, Vakrou S, Lin X, Brown SN, O’Rourke B, Abraham TP, Wahl RL, Steenbergen CJ, Elisseeff JH, Abraham MR. Hyaluronic Acid-Human Blood Hydrogels for Stem Cell Transplantation. Biomaterials. 2012;33:8026–8033. doi: 10.1016/j.biomaterials.2012.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishihara M, Nakanishi K, Ono K, Sato M, Kikuchi M, Saito Y, Yura H, Matsui T, Hattori H, Uenoyama M, Kurita A. Photocrosslinkable Chitosan as a Dressing for Wound Occlusion and Accelerator in Healing Process. Biomaterials. 2002;23:833–840. doi: 10.1016/s0142-9612(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 31.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach JA, Preul M, Kinsey AM, DeMaria AN, Dib N, Christman KL. Safety and Efficacy of an Injectable Extracellular Matrix Hydrogel for Treating Myocardial Infarction. Sci Transl Med. 2013;5:173ra25. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujimoto KL, Ma Z, Nelson DM, Hashizume R, Guan J, Tobita K, Wagner WR. Synthesis, Characterization and Therapeutic Efficacy of a Biodegradable, Thermoresponsive Hydrogel Designed for Application in Chronic Infarcted Myocardium. Biomaterials. 2009;30:4357–4368. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bawa P, Pillay V, Choonara YE, du Toit LC. Stimuli-Responsive Polymers and Their Applications in Drug Delivery. Biomed Mater. 2009;4:022001. doi: 10.1088/1748-6041/4/2/022001. [DOI] [PubMed] [Google Scholar]
- 34.Crompton KE, Goud JD, Bellamkonda RV, Gengenbach TR, Finkelstein DI, Horne MK, Forsythe JS. Polylysine-Functionalised Thermoresponsive Chitosan Hydrogel for Neural Tissue Engineering. Biomaterials. 2007;28:441–449. doi: 10.1016/j.biomaterials.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 35.Jiang XJ, Wang T, Li XY, Wu DQ, Zheng ZB, Zhang JF, Chen JL, Peng B, Jiang H, Huang C, Zhang XZ. Injection of a Novel Synthetic Hydrogel Preserves Left Ventricle Function after Myocardial Infarction. J Biomed Mater Res, Part A. 2009;90:472–477. doi: 10.1002/jbm.a.32118. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Wang F, Roy S, Sen CK, Guan J. Injectable, Highly Flexible, and Thermosensitive Hydrogels Capable of Delivering Superoxide Dismutase. Biomacromolecules. 2009;10:3306–3316. doi: 10.1021/bm900900e. [DOI] [PubMed] [Google Scholar]
- 37.Guan J, Hong Y, Ma Z, Wagner WR. Protein-Reactive, Thermoresponsive Copolymers with High Flexibility and Biodegradability. Biomacromolecules. 2008;9:1283–1292. doi: 10.1021/bm701265j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z, Guo X, Guan J. An Oxygen Release System to Augment Cardiac Progenitor Cell Survival and Differentiation under Hypoxic Condition. Biomaterials. 2012;33:5914–5923. doi: 10.1016/j.biomaterials.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Guo X, Guan J. A Thermosensitive Hydrogel Capable of Releasing bFGF for Enhanced Differentiation of Mesenchymal Stem Cell into Cardiomyocyte-Like Cells under Ischemic Conditions. Biomacromolecules. 2012;13:1956–1964. doi: 10.1021/bm300574j. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Guo X, Palmer AF, Das H, Guan J. High-Efficiency Matrix Modulus-Induced Cardiac Differentiation of Human Mesenchymal Stem Cells inside a Thermosensitive Hydrogel. Acta Biomater. 2012;8:3586–3595. doi: 10.1016/j.actbio.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 41.Wang F, Li Z, Khan M, Tamama K, Kuppusamy P, Wagner WR, Sen CK, Guan J. Injectable, Rapid Gelling and Highly Flexible Hydrogel Composites as Growth Factor and Cell Carriers. Acta Biomater. 2010;6:1978–1991. doi: 10.1016/j.actbio.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Li Z, Li X, Fan Z, Liu Z, Xie X, Guan J. Regulating Myogenic Differentiation of Mesenchymal Stem Cells Using Thermosensitive Hydrogels. Acta Biomater. 2015;26:23–33. doi: 10.1016/j.actbio.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Ekhteraei-Tousi S, Mohammad-Soltani B, Sadeghizadeh M, Mowla SJ, Parsi S, Soleimani M. Inhibitory Effect of hsa-mir-590–5p on Cardiosphere-Derived Stem Cells Differentiation through Downregulation of TGFB Signaling. J Cell Biochem. 2015;116:179–191. doi: 10.1002/jcb.24957. [DOI] [PubMed] [Google Scholar]
- 44.Altman GH, Horan RL, Martin I, Farhadi J, Stark PR, Volloch V, Richmond JC, Vunjak-Novakovic G, Kaplan DL. Cell Differentiation by Mechanical Stress. FASEB J. 2002;16:270–272. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 45.Tagil M, Aspenberg P. Cartilage Induction by Controlled Mechanical Stimulation in Vivo. J Orthop Res. 1999;17:200–204. doi: 10.1002/jor.1100170208. [DOI] [PubMed] [Google Scholar]
- 46.Butler DL, Juncosa-Melvin N, Boivin GP, Galloway MT, Shearn JT, Gooch C, Awad H. Functional Tissue Engineering for Tendon Repair: a Multidisciplinary Strategy Using Mesenchymal Stem Cells, Bioscaffolds, and Mechanical Stimulation. J Orthop Res. 2008;26:1–9. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- 47.Tidball JG. Mechanical Signal Transduction in Skeletal Muscle Growth and Adaptation. J Appl Physiol. 2005;98:1900–1908. doi: 10.1152/japplphysiol.01178.2004. [DOI] [PubMed] [Google Scholar]
- 48.Heng BC, Haider H, Sim EK, Cao T, Ng SC. Strategies for Directing the Differentiation of Stem Cells into the Cardiomyogenic Lineage in Vitro. Cardiovasc Res. 2004;62:34–42. doi: 10.1016/j.cardiores.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 49.Li Z, Guo X, Matsushita S, Guan J. Differentiation of Cardiosphere-Derived Cells into a Mature Cardiac Lineage Using Biodegradable Poly(N-Isopropylacrylamide) Hydrogels. Biomaterials. 2011;32:3220–3232. doi: 10.1016/j.biomaterials.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 50.Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, Hubbell JA. Three-Dimensional Extracellular Matrix-Directed Cardioprogenitor Differentiation: Systematic Modulation of a Synthetic Cell-Responsive PEG-Hydrogel. Biomaterials. 2008;29:2757–2766. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Braunwald E, Sonnenblick EH, Ross J, Jr, Gault JH. Insights in Cardiovascular Physiology Derived from Muscle Mechanics. Am J Cardiol. 1967;20:705–711. [PubMed] [Google Scholar]
- 52.Anseth KS, Bowman CN, BrannonPeppas L. Mechanical Properties of Hydrogels and Their Experimental Determination. Biomaterials. 1996;17:1647–1657. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- 53.Kim PD, Peyton SR, VanStrien AJ, Putnam AJ. The Influence of Ascorbic Acid, TGF-Beta 1, and Cell-Mediated Remodeling on the Bulk Mechanical Properties of 3-D PEG-Fibrinogen Constructs. Biomaterials. 2009;30:3854–3864. doi: 10.1016/j.biomaterials.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 54.Angelini S, Cerruti P, Immirzi B, Santagata G, Scarinzi G, Malinconico M. From Biowaste to Bioresource: Effect of a Lignocellulosic Filler on the Properties of Poly(3-Hydroxybutyrate) Int J Biol Macromol. 2014;71:163–173. doi: 10.1016/j.ijbiomac.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 55.Sandy JR. DNA Changes in Mechanically Deformed Osteoblasts: a New Hypothesis. Br J Orthod. 1993;20:1–11. doi: 10.1179/bjo.20.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Grossi A, Yadav K, Lawson MA. Mechanical Stimulation Increases Proliferation, Differentiation and Protein Expression in Culture: Stimulation Effects are Substrate Dependent. J Biomech. 2007;40:3354–3362. doi: 10.1016/j.jbiomech.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Dimmeler S, Hermann C, Galle J, Zeiher AM. Upregulation of Superoxide Dismutase and Nitric Oxide Synthase Mediates the Apoptosis-Suppressive Effects of Shear Stress on Endothelial Cells. Arterioscler, Thromb, Vasc Biol. 1999;19:656–664. doi: 10.1161/01.atv.19.3.656. [DOI] [PubMed] [Google Scholar]
- 58.Kaspar D, Seidl W, Neidlinger-Wilke C, Ignatius A, Claes L. Dynamic Cell Stretching Increases Human Osteoblast Proliferation and CICP Synthesis but Decreases Osteocalcin Synthesis and Alkaline Phosphatase Activity. J Biomech. 2000;33:45–51. doi: 10.1016/s0021-9290(99)00171-2. [DOI] [PubMed] [Google Scholar]
- 59.Maul TM, Chew DW, Nieponice A, Vorp DA. Mechanical Stimuli Differentially Control Stem Cell Behavior: Morphology, Proliferation, and Differentiation. Biomech Model Mechanobiol. 2011;10:939–953. doi: 10.1007/s10237-010-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Birukov KG, Shirinsky VP, Stepanova OV, Tkachuk VA, Hahn AW, Resink TJ, Smirnov VN. Stretch Affects Phenotype and Proliferation of Vascular Smooth Muscle Cells. Mol Cell Biochem. 1995;144:131–139. doi: 10.1007/BF00944392. [DOI] [PubMed] [Google Scholar]
- 61.Kaspar D, Seidl W, Neidlinger-Wilke C, Beck A, Claes L, Ignatius A. Proliferation of Human-Derived Osteoblast-Like Cells Depends on the Cycle Number and Frequency of Uniaxial Strain. J Biomech. 2002;35:873–880. doi: 10.1016/s0021-9290(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 62.Xu Y, Patnaik S, Guo X, Li Z, Lo W, Butler R, Claude A, Liu Z, Zhang G, Liao J, Anderson PM, Guan J. Cardiac Differentiation of Cardiosphere-Derived Cells in Scaffolds Mimicking Morphology of the Cardiac Extracellular Matrix. Acta Biomater. 2014;10:3449–3462. doi: 10.1016/j.actbio.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, Seliktar D, Elisseeff J. Differential Response of Adult and Embryonic Mesenchymal Progenitor Cells to Mechanical Compression in Hydrogels. Stem Cells. 2007;25:2730–2738. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- 64.Thorpe SD, Buckley CT, Vinardell T, O’Brien FJ, Campbell VA, Kelly DJ. Dynamic Compression Can Inhibit Chondrogenesis of Mesenchymal Stem Cells. Biochem Biophys Res Commun. 2008;377:458–462. doi: 10.1016/j.bbrc.2008.09.154. [DOI] [PubMed] [Google Scholar]
- 65.Riehl BD, Park JH, Kwon IK, Lim JY. Mechanical Stretching for Tissue Engineering: Two-Dimensional and Three-Dimensional Constructs. Tissue Eng, Part B. 2012;18:288–300. doi: 10.1089/ten.teb.2011.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koike M, Shimokawa H, Kanno Z, Ohya K, Soma K. Effects of Mechanical Strain on Proliferation and Differentiation of Bone Marrow Stromal Cell Line ST2. J Bone Miner Metab. 2005;23:219–225. doi: 10.1007/s00774-004-0587-y. [DOI] [PubMed] [Google Scholar]
- 67.Huang Y, Zheng LS, Gong XH, Jia XL, Song W, Liu ML, Fan YB. Effect of Cyclic Strain on Cardiomyogenic Differentiation of Rat Bone Marrow Derived Mesenchymal Stem Cells. PLoS One. 2012;7:e34960. doi: 10.1371/journal.pone.0034960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitchell GF, Jeron A, Koren G. Measurement of Heart Rate and Q-T Interval in the Conscious Mouse. Am J Physiol. 1998;274:H747–H751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]