Abstract
Background
The long-term survival benefit of concurrent neoadjuvant chemoradiotherapy in patients with resectable esophageal cancer remains controversial. In the present study, we conducted a meta-analysis to assess these effectiveness.
Methods
We searched for most relevant studies published up to the end of August 2016, using Pubmed and web of knowledge. And additional articles were identified from previous meta-analysis. The hazard ratio (HR, for overall survival and progression free survival) or risk ratio (RR, for R0 resection) with its corresponding 95 % confidence interval (CI) were used to assess the pooled effect.
Results
Twelve articles including 1756 patients were included in the meta-analysis. Concurrent neoadjuvant chemoradiotherapy followed by surgery was associated with significantly improved overall survival (HR=0.76 , 95% CI= 0.68-0.86), progression survival (HR =0.69, 95% CI= 0.59-0.81), and R0 resection rate(RR =1.17, 95% CI= 1.03-1.33). Subgroup analysis suggested that concurrent neoadjuvant chemoradiotherapy could improve overall survival outcome for squamous cell carcinoma (HR=0.73, 95%CI=0.61-0.88) but not those for adenocarcinoma (HR=0.72, 95%CI=0.48-1.04).
Conclusion
Our findings suggested that concurrent neoadjuvant chemoradiotherapy was associated with a significant survival benefit in patients with esophageal cancer.
Keywords: neoadjuvant concurrent chemoradiotherapy, esophageal cancer, overall survival, R0 resection rate, progression-free survival
INTRODUCTION
With more than 456,000 newly diagnosed cases and 400,000 related deaths annually, esophageal cancer is the tenth most common cancer and the eighth leading cause of cancer-related deaths worldwide. [1, 2] Since most esophageal cancer patients are diagnosed at the advanced stages, the 5-year survival rate is less than 20%. [3] Despite surgical care and improvements in preoperative staging, surgery alone leads to relatively few long-term survivors. [4] A great number of patients who underwent esophagectomy continue to die as a result of tumor recurrence. [5]
Adjuvant therapies, with either radiotherapy or chemotherapy, have not shown survival any benefits. [5] This, along with the evident difficulties of administering radiotherapy and chemotherapy after resection for esophageal cancer, makes recent trials focus on the role of neoadjuvant treatment, especially the concurrent neoadjuvant chemoradiotherapy (NCRT). A recent meta-analysis showed that concurrent NCRT was associated with improved 1-, 3- and 5-year survival rate. [6] However, the outcomes of their study are pooled risk ratio (RR), which did not consider the survival time, Moreover, analysis for R0 resection and progression-free survival were not conducted.
Several Randomized clinical trials (RCTs) have investigated the effect of concurrent NCRT on operable esophageal cancer. However, these results are controversial. A meta-analysis pooling current literatures might be helpful for confirming such effect. Therefore, the aim of the current study was to determine whether concurrent NCRT plus surgery is superior to surgery alone for operable esophageal cancer.
RESULTS
Literature search and study characteristics
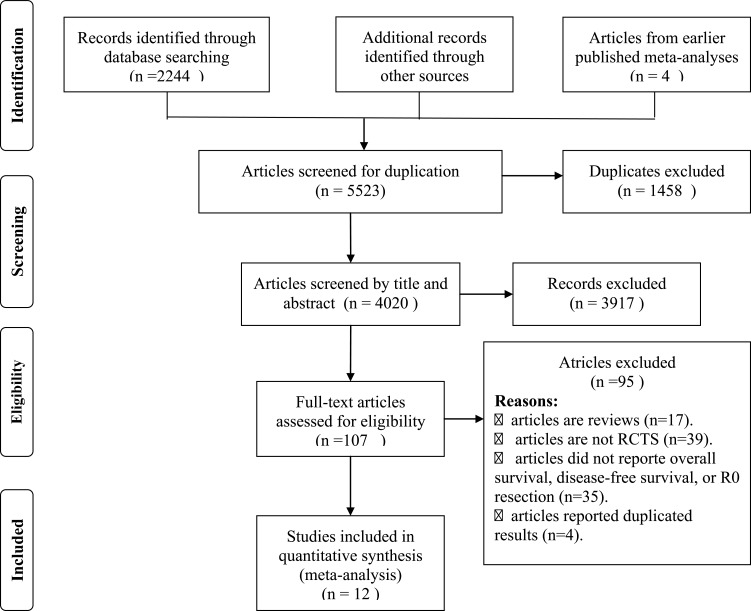
In total, 12 RCTs including 1756 patients (877patients were treated with concurrent NCRT plus surgery and 879 patients were treated with surgery alone) were included in the meta analysis. [7–18] The detailed processes of our literature search are displayed in Figure 1. Of these 12 studies, twelve studies reported the outcome of overall survival, six studies reported the R0 resection rate, and four studies reported the progression-free survival. The main characters of these studies are presented in Table 1.
Figure 1. The flow diagram of screened, excluded, and analyzed publications.
Table 1. Chemoradiotherapy regimens in randomized trials included in the meta-analysis.
| First author | Year | Sample size | Pathology | Neoadjuvant treatment schedule | ||
|---|---|---|---|---|---|---|
| NCRTS | SA | total | ||||
| Wlsh | 1996 | 58 | 55 | 113 | AC | Cis 75mg/m2 on days 7 and 42; FU 15 mg/kg on days 1-5 and 36-40 40 Gy in 15 fractions over 3weeks |
| Shapiro | 2015 | 178 | 188 | 366 | AC and SCC | Carboplatin (AUC 2 mg/mL per min) and paclitaxel (50 mg/m2 of body-surface area) were administered intravenously for five cycles, starting on days 1, 8, 15, 22, and 29. A total concurrent radiation dose of 41·4 Gy was given in 23 fractions of 1·8 Gy, on 5 days per week (excluding weekends), starting on the fi rst day of the first chemotherapy cycle. |
| Mariette | 2014 | 81 | 89 | 170 | AC and SCC | A total dose of 45 Gy was delivered in 25 fractions (five fractions per week) over 5 weeks. Chemotherapy was delivered concomitantly and composed of two cycles of fluorouracil (FU) and cisplatin. FU 800 mg/m2 per 24 hours was administered as a continuous infusion from days 1 to 4 and 29 to 32. Cisplatin 75mg/m2 was delivered by infusiononday 1 or 2 and againonday 29 or 30. |
| Lv | 2010 | 80 | 80 | 160 | SCC | Radiation was delivered in a total dose of 40 Gy (20 fractions at 2 Gy per fraction). For chemotherapy, 2 cycles were administered on days 1-3 and days 22-24 of radiotherapy. A paclitaxel (PTX)+cisplatin (DDP) regimen was used, including PTX (135 mg/m2 per day) administered as a short-term infusion on day 1 of each cycle, while DDP (20 mg/m2 per day) was delivered as a continuous infusion over 24 h on days 1-3 of each cycle. |
| Burmeister | 2005 | 128 | 128 | 256 | AC and SCC | Cis 80mg/m2 on day 1; FU 800 mg/m2 per day on days 1-4, 35 Gy in 15 fractions over 3 weeks |
| Cao | 2009 | 118 | 118 | 236 | SCC | Cis 20 mg/m2 per day on days 1–5; FU 500 mg/m2 per day on days 1–5; mitomycin 10mg/m2 per day on day 1 40 Gy, 2 Gy per fraction over 4weeks |
| Lee | 2004 | 51 | 50 | 101 | SCC | Cis 60 mg/m2 on days 1 and 22; FU 1000 mg/m2 per day on days 2–5. 45·6Gy, 1·2Gy per fraction over 28 days |
| Tepper | 2008 | 30 | 26 | 56 | AC and SCC | Cis 60mg/m2 on days 1 and 29; FU 1000 mg/m2 per day on days 1–4 and 29–32 50·4Gy, 1·8Gy per fraction over 5·6weeks |
| Natsugoe | 2006 | 22 | 23 | 45 | SCC | A total radiation dose of 40 Gy was applied, in 2-Gy fractions delivered 5 days/week for 4 weeks to the media stinum and neck. In the same period, intravenous chemotherapy was performed using cisplatin (7 mg over 2 h) and 5-fluorouracil (5-FU; 350 mg over 24 h). |
| Urba | 2007 | 50 | 50 | 100 | AC and SCC | Cis 20mg/m2 on days 1–5 and 17–21; FU 300 mg/m2 on days 1–21; vinblastine 1 mg/m2 on days 1–4 and 17–20 45 Gy, 1·5Gy per fraction over 3weeks |
| Hsu | 2013 | 46 | 38 | 84 | SCC | The chemotherapy regimen included 80 mg/m2 of cisplatin intravenously on day 1 followed by 600 mg/m2/day of 5-fluorouracil and 90 mg/m2/day of leucovorin given by continuous intravenous infusion on days 1–4, concurrent with 45.0–50.4 Gy of externalbeam radiation at 1.8–2.0 Gy per fraction. |
| Apinop | 1994 | 35 | 34 | 69 | SCC | Cis 100 mg/m2 on days 1 and 29; FU 1000 mg/m2 per day on days 1–4 and 29–32 40 Gy, 2 Gy per fraction over 4weeks |
NCRTS, neoadjuvant chemoradiotherapy followed by surgery; SA , surgery alone; SCC, squamous cell carcinoma; AC, adenocarcinoma; Cis, cisplatin; FU, fluorouracil
Primary outcome
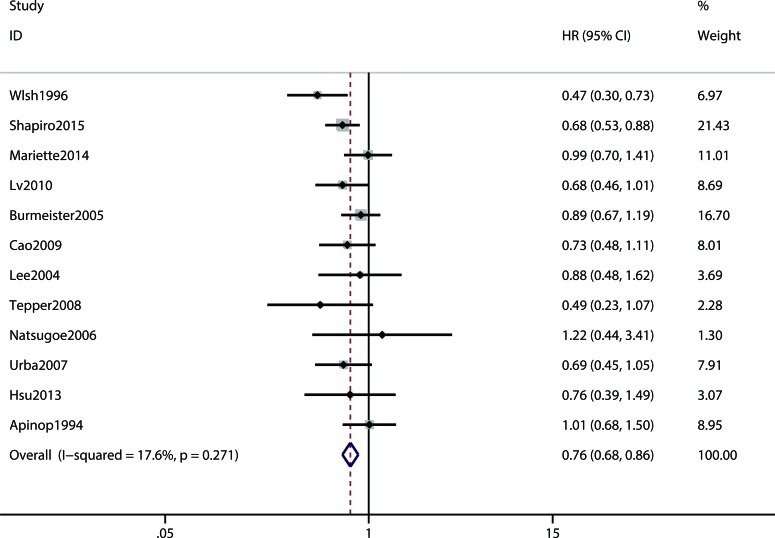
The primary outcome overall survival was reported in twelve RCTs. Compared with the surgery alone group, the pooled hazard ratio (HR) for the concurrent NCRT plus surgery group was 0.76 (95% CI 0.68-0.86) (Figure 2). As presented in Table 2, when we carried out the stratified analysis by geographical location, significant results were observed both in the west and east (HR = 0.75, 95%CI = 0.64-0.85 and HR = 0.82, 95%CI = 0.67-0.88, respectively). Furthermore, the subgroup analysis by histological type showed that concurrent NCRT plus surgery can improve squamous cell carcinoma (SCC) patients (HR = 0.73, 95%CI = 0.61-0.88), but not adenocarcinoma (AC) patients (HR = 0.72, 95%CI = 0.48-1.04) or AC+SCC patients (HR = 0.80, 95%CI = 0.62-1.04).
Figure 2. Meta analysis comparing the overall survival between neoadjuvant chemoradiotherapy plus surgery and surgery alone. CI, confidence interval; HR, hazard ratio.
Table 2. Subgroup analysis for overall survival of concurrent NCRT plus surgery vs. surgery alon.
| Subgroups | Included studies | Sample size | HR (95 % CI) | P value for heterogeneity | |
|---|---|---|---|---|---|
| NCRTS | SA | ||||
| Geographical location | |||||
| West | 6 | 525 | 536 | 0.74(0.64-0.85) | 0.081 |
| East | 6 | 352 | 343 | 0.82(0.67-1.00) | 0.714 |
| Histology | |||||
| SCC | 8 | 438 | 436 | 0.76(0.63-0.90) | 0.508 |
| AC | 3 | 272 | 274 | 0.72(0.48-0.1.08) | 0.021 |
| AC+SCC | 3 | 161 | 165 | 0.80(0.62-1.04) | 0.182 |
NCRTS, neoadjuvant chemoradiotherapy followed by surgery; SA , surgery alone; SCC, squamous cell carcinoma; AC, adenocarcinoma; HR, hazard ratio; CI: confidence interval.
Secondary outcomes
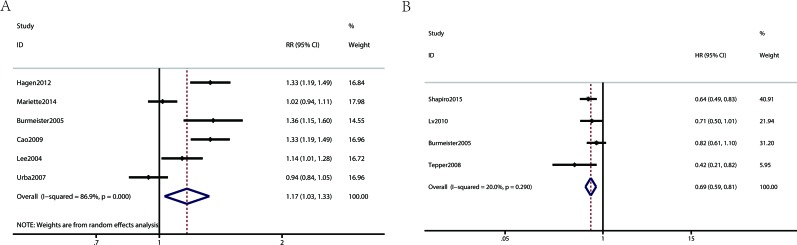
Figure 3 show Forest plots for the secondary outcomes, including the R0 resection rate and progression-free survival. Four RCTs reported the progression-free survival, indicating a statistically significant difference (HR 0.69, 95% CI 0.59-0.81, Figure 3B) for the concurrent NCRT plus surgery group compared to the surgery alone group. Six RCTs reported the R0 resection rate, indicating a statistically significant difference (RR 1.17, 95% CI 1.03-1.33, Figure 3A) for the concurrent NCRT plus surgery group compared to the surgery alone group.
Figure 3.
Meta analysis comparing the secondary outcomes of patients receiving neoadjuvant chemoradiotherapy plus surgery and surgery alone (A: R0 resection; B: progression-free survival).
Publication bias
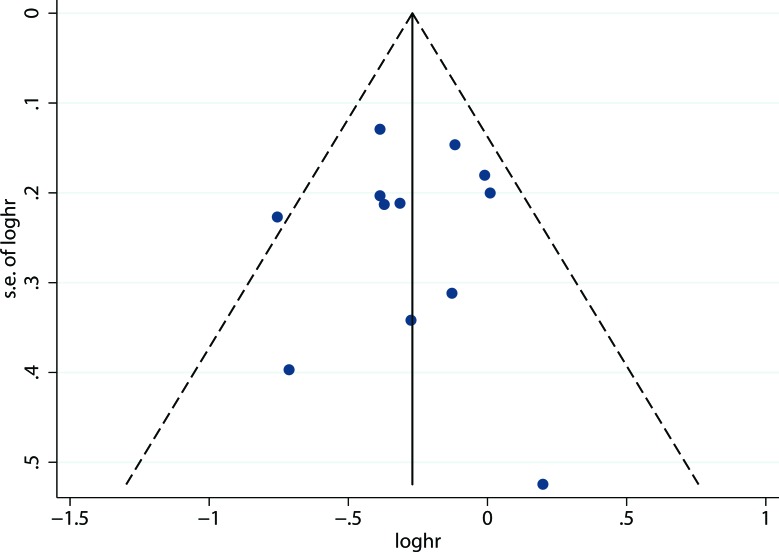
A funnel plot of the effect size for overall survival was found to be symmetrical (Figure. 4), indicating little publication bias. The P value based on Egger's test for overall survival was 0.990 (t = -0.01), which also showed no publication bias existed.
Figure 4. Funnel plot for publication bias of overall survival between neoadjuvant chemoradiotherapy plus surgery and surgery alone.
DISCUSSION
The results of present meta-analysis showed that concurrent NCRT was associated with improved overall survival, progression-free survival, and R0 resection rate in patients with esophageal cancer. And this is the largest meta-analysis to explore the survival benefit with concurrent NCRT plus surgery versus surgery alone.
Several potential explanations exist for the improved effects of concurrent NCRT. First, chemotherapy has a radio sensitizing effect, which can enhance the local effects of concurrent radiotherapy, reducing the possibility of tumor spreading from the primary tumor site prior to surgery. Second, concurrent chemoradiotherapy may have the ability to inhibit the proliferation of tumor cells in the primary lesion site, decreasing the length of preoperative treatment required.
Our subgroup analysis also demonstrated that concurrent NCRT plus surgery significantly improved overall survival outcome for patients in the west. Whereas in the east, concurrent NCRT plus surgery was only associated with improved overall outcome with a borderline significant. It might be ascribed the different genetic backgrounds and/or ethnicities of the participants, which should be further investigated with well-designed multicenter RCTs from different ethnics. Moreover, in order to verify the effect of concurrent NCRT, subgroup analysis based on histology was also introduced. Through this subgroup analysis, we found that concurrent NCRT plus surgery improved the overall survival outcome for SCC patients, but not for AC patients or AC+SCC patients. Several explanations may partly explain the reasons. Firstly, participants with SCC may gain more benefits from concurrent NCRT plus surgery than those with AC. Secondly, SCC patients have a greater potential to involve the superior mediastinum and cervical region than AC patients, which is more difficult for aggressive intrathoracic and cervical nodal dissection to be carried out. Besides, SCC is more sensitive to radiotherapy and chemotherapy than AC. [6] Thus, the addition of chemoradiotherapy to the treatment regime might have improved therapeutic outcome.
In order to determine the reasons that patients with resectable esophageal cancer could receive a survival advantage from concurrent NCRT, we chose the R0 resection rate as a secondary outcome. The final results revealed that the RR of R0 resection rate favored the group of concurrent NCRT, which approximated the results of previous study. [19] However, they included not only concurrent NCRT but also sequential NCRT, suggesting that the combination of concurrent and sequential NCRT rather than concurrent NCRT was the likely cause of improved R0 resection. In addition to R0 resection rate, our meta-analysis also investigated progression-free survival which is rarely published in previous meta-analysis. Compared with surgery alone, concurrent NCRT plus surgery significantly improved the progression-free survival. This finding also demonstrated that concurrent NCRT plus surgery could improve therapeutic outcomes.
Although subgroup analysis based on postoperative complications failed to be conducted, the included studies showed that treatment-related complications in the NCRT group had no significant difference from that of the surgery alone group [13, 17]. In addition, loss to follow-up was low (ranging from 0.4% to 4.2%), thus the result from our study is robust.
Several potential limitations of our meta-analysis should also be acknowledged. At first, since most studies included patients without clear identification of tumor-node-metastasis (TNM) classification, the subgroup based on TNM classification could not be achieved. In addition, as the primary endpoint was overall survival, the difference of complications between NRCT plus surgery group and surgery alone group was not clear.
In conclusion, concurrent NCRT improves overall survival, progression-free survival and R0 resection rate in patients with esophageal cancer. This improvement is statistically significant and clinically relevant for SCC but not for AC subtypes. Concurrent NCRT followed by surgery should be viewed as a standard care for patients with resectable esophageal squamous cell carcinoma. Further well-designed multicenter RCTs are warranted to verify the beneficial effect.
MATERIALS AND METHODS
Search strategy
A comprehensive literature search of Pubmed, and Web of Knowledge was conducted up to the end of August 2016. The following search formula were used: (([esophageal ] OR [oesophageal] OR [esophagus] OR [oesophagus]) AND ([neoplasms] OR [cancer] OR [carcinoma]) AND ([chemotherapy] OR [radiotherapy] OR [chemoradiotherapy] OR [combined modality therapy] OR [adjuvant]) AND [neoadjuvant]). The searches were limited to articles describing RCTS and published in English. In order to identify further additional studies, manual searching of reference lists was performed.
Study selection
Articles included should fit all the following criteria: (1) Studies were designed as RCTs; (2) compared concurrent NCRT with surgery alone were considered; (3) the outcome of interest was defined as overall survival, progression-free survival, or R0 resection rate; and (4) the sample size, hazard ratio (HR) and their 95 % confidence interval (CI), or data that would allow those findings to be inferred, was presented. If several publications reporting on the same population data met our criteria, the one with the longest follow-up period was selected.
Data extraction and outcome measures
The primary outcome was overall survival. The secondary outcomes were progression-free survival and R0 resection rate, which was defined by a tumor-free resection margin. Two authors (Baoxing Liu and Yacong Bo) independently extracted the following data from each eligible study, and discrepancies were resolved by a third investigator: first author, year of publication, number of patients randomized, and those who received chemoradiotherapy or surgery, HR for overall survival and progression-free survival, the total number of participants for each study, and the number of patients for R0 resection.
Statistical analysis
The meta-analysis was performed using STATA software (version 12.0; StatCorp, College Station, TX, USA) and P < 0.05 was considered as statistically significant. Overall survival and progression-free survival were measured with a hazard ratio (HR), while the R0 resection rate was measured using risk ratios (RR). If permitted, HR and the 95% confidence interval (CI) were obtained directly from the article; otherwise, they were calculated using the methods of Parmar, [20] Tierney, [21] and Williamson, [22] which use number of events and Kaplan-Meier survival curves to estimate the HR and 95% CI. The chi-square test and I2 test were used to assess heterogeneity, with P < 0.05 and/or I2 > 50% representing significant heterogeneity, and the random-effect model was selected. Otherwise, a fixed-effect model was applied [23]. Subgroup analyses were applied to evaluate potential effect modification of variables including geographic locations, and histological type (SCC and AC). Begger's Funnel plots and Egger's tests were performed to assess the publication bias [24, 25].
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Loomis D, Huang W, Chen G. The International Agency for Research on Cancer (IARC) evaluation of the carcinogenicity of outdoor air pollution: focus on China. Chinese Journal of Cancer. 2014;33:189–196. doi: 10.5732/cjc.014.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palladino-Davis AG, Mendez BM, Fisichella PM, Davis CS. Dietary habits and esophageal cancer. Diseases of the esophagus. 2015;28:59–67. doi: 10.1111/dote.12097. [DOI] [PubMed] [Google Scholar]
- 4.Kumagai K, Rouvelas I, Tsai JA, Mariosa D, Klevebro F, Lindblad M, Ye W, Lundell L, Nilsson M. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. The British journal of surgery. 2014;101:321–338. doi: 10.1002/bjs.9418. [DOI] [PubMed] [Google Scholar]
- 5.Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. The Lancet Oncology. 2007;8:545–553. doi: 10.1016/S1470-2045(07)70172-9. [DOI] [PubMed] [Google Scholar]
- 6.Wang DB, Zhang X, Han HL, Xu YJ, Sun DQ, Shi ZL. Neoadjuvant chemoradiotherapy could improve survival outcomes for esophageal carcinoma: a meta-analysis. Digestive diseases and sciences. 2012;57:3226–3233. doi: 10.1007/s10620-012-2263-8. [DOI] [PubMed] [Google Scholar]
- 7.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. The New England journal of medicine. 1996;335:462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro J, van Lanschot JJ, Hulshof MC, van Hagen P, van Berge Henegouwen MI, Wijnhoven BP, van Laarhoven HW, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. The Lancet Oncology. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 9.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. The New England journal of medicine. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 10.Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, Boige V, Pezet D, Robb WB, Le Brun-Ly V, Bosset JF, Mabrut JY, Triboulet JP, Bedenne L, Seitz JF. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. Journal of clinical oncology. 2014;32:2416–2422. doi: 10.1200/JCO.2013.53.6532. [DOI] [PubMed] [Google Scholar]
- 11.Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World journal of gastroenterology. 2010;16:1649–1654. doi: 10.3748/wjg.v16.i13.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, Ackland S, Gotley DC, Joseph D, Millar J, North J, Walpole ET, Denham JW. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. The Lancet Oncology. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 13.Cao XF, He XT, Ji L, Xiao J, Lv J. Effects of neoadjuvant radiochemotherapy on pathological staging and prognosis for locally advanced esophageal squamous cell carcinoma. Diseases of the esophagus. 2009;22:477–481. doi: 10.1111/j.1442-2050.2008.00910.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee JL, Park SI, Kim SB, Jung HY, Lee GH, Kim JH, Song HY, Cho KJ, Kim WK, Lee JS, Kim SH, Min YI. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Annals of oncology. 2004;15:947–954. doi: 10.1093/annonc/mdh219. [DOI] [PubMed] [Google Scholar]
- 15.Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D, Mayer R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. Journal of clinical oncology. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natsugoe S, Okumura H, Matsumoto M, Uchikado Y, Setoyama T, Yokomakura N, Ishigami S, Owaki T, Aikou T. Randomized controlled study on preoperative chemoradiotherapy followed by surgery versus surgery alone for esophageal squamous cell cancer in a single institution. Diseases of the esophagus. 2006;19:468–472. doi: 10.1111/j.1442-2050.2006.00615.x. [DOI] [PubMed] [Google Scholar]
- 17.Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. Journal of clinical oncology. 2001;19:305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 18.Hsu PK, Chien LI, Huang CS, Hsieh CC, Wu YC, Hsu WH, Chou TY. Comparison of survival among neoadjuvant chemoradiation responders, non-responders and patients receiving primary resection for locally advanced oesophageal squamous cell carcinoma: does neoadjuvant chemoradiation benefit all? Interactive cardiovascular and thoracic surgery. 2013;17:460–466. doi: 10.1093/icvts/ivt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kranzfelder M, Schuster T, Geinitz H, Friess H, Buchler P. Meta-analysis of neoadjuvant treatment modalities and definitive non-surgical therapy for oesophageal squamous cell cancer. The British journal of surgery. 2011;98:768–783. doi: 10.1002/bjs.7455. [DOI] [PubMed] [Google Scholar]
- 20.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. 2002;21:3337–3351. doi: 10.1002/sim.1303. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]